Abstract
Neutrophils possess multiple antimicrobial mechanisms that are critical for protection of the host against infection with extracellular microbes, such as the bacterial pathogen Staphylococcus aureus. Recruitment and activation of neutrophils at sites of infection is driven by cytokine and chemokine signals that directly target neutrophils via specific cell surface receptors. The IL-20 subfamily of cytokines has been reported to act at epithelial sites and contribute to psoriasis, wound healing, and anti-inflammatory effects during S. aureus infection. However, the ability of these cytokines to directly affect neutrophil function remains incompletely understood. Here, we show that human neutrophils altered their expression of IL-20 receptor chains upon migration and activation in vivo and in vitro. Such activation of neutrophils under conditions mimicking infection with S. aureus conferred responsiveness to IL-20 that manifested as modification of actin polymerization and inhibition of a broad range of actin-dependent functions, including phagocytosis, granule exocytosis, and migration. Consistent with the previously described homeostatic and anti-inflammatory properties of IL-20 on epithelial cells, the current studies provide evidence that IL-20 directly targets and inhibits key inflammatory functions of neutrophils during infection with S. aureus.
Introduction
Neutrophils are the most abundant leukocyte in humans and exhibit multiple antimicrobial mechanisms that are critical for protection of the host against infection with extracellular bacterial and fungal pathogens (1–3). For example, their central role in controlling and resolving infections with the bacterial pathogen Staphylococcus aureus is illustrated by the increased severity and frequency of these infections in patients with disorders of neutrophil number or function (4). S. aureus is a leading cause of infectious morbidity and mortality even in immunocompetent hosts. It most frequently displays its propensity to transition from a colonizer to a pathogen at epithelial barrier sites, such as the skin and lung, but it can also disseminate from these sites and cause severe invasive disease (5, 6).
Recruitment of neutrophils to epithelial sites of infection is driven by cytokine and chemokine signals that directly target neutrophils via specific cell surface receptors. These signals modulate a variety of neutrophil functions required for bacterial killing and clearance (2, 7–9). Among the pro-inflammatory factors produced by infected epithelium in response to S. aureus, TNFα and IL-8 are notably potent activators of neutrophils. TNFα enhances ROS production and degranulation, and IL-8 is a chemotactic factor that induces directional migration of neutrophils. These and other cytokines effect dynamic changes in the actin cytoskeleton of neutrophils to induce chemotaxis, arrest migration at the site of infection, enhance phagocytosis, and promote release of granules that contain pre-formed proteins that contribute to bacterial killing (9–15).
IL-19, IL-20, and IL-24, members of the IL-20 subfamily of cytokines, have been recognized to be active at epithelial sites in processes such as psoriasis and wound healing (16–18). We recently identified these cytokines as part of the epithelial response to S. aureus skin infection, contributing to the pathogenesis of infection by inhibiting the production of pro-inflammatory factors, such as interleukin (IL)-1β and IL-17, and thereby reducing neutrophil recruitment (19). In those studies, a species-specific pattern of induction for these cytokines was noted, with human cells producing IL-20 at high levels whereas IL-19 and IL-24 activity was more apparent in mice (19). The IL-20 subfamily cytokines signal through two distinct receptor complexes that consist of the common IL-20RB chain dimerized with either IL-20RA (Type I) or IL-22RA (Type II), and all three receptor chains have been identified in skin and lung tissues of humans (20–22). Expression of IL-20RB has not been reported in neutrophils, but expression of IL-20RA and IL-22RA has been described in synovial fluid cells, which consist mostly of neutrophils that have migrated to an inflammatory joint (23, 24). However, the functional consequences of expression of these receptors in neutrophils have not been well characterized.
Given the importance of neutrophils in the innate immune response to S. aureus infection in the skin and lung, and the documented production of IL-20 in response to such infections in the skin, we sought to better characterize the determinants and functional consequences of IL-20 receptor expression directly on neutrophils. Here, we report that human neutrophils alter their IL-20 receptor chain expression upon exposure to conditions that mimic those encountered during infection. We further identify that under these conditions human neutrophils respond to IL-20 by modifying actin polymerization and inhibiting a broad range of actin-dependent functions that include phagocytosis, granule exocytosis, and migration.
Materials and Methods
Ethics statement
All human sample collection and processing were performed with approval of the National Institute of Allergy and Infectious Disease IRB, which approved the associated clinical trial (NCT02262819). All subjects gave full consent to sample collection, and all participants provided their written consent to the research protocol.
Cell isolation and culture
Neutrophils were isolated from fresh heparinized venous blood obtained from healthy volunteers. All steps were performed at room temperature. Dextran was added to blood at a final concentration of 3% and erythrocytes were allowed to settle for 30 minutes. The leukocyte-rich supernatant was overlaid on Ficoll PLUS and spun at 400xg for 40 minutes. Remaining erythrocytes were removed with hypotonic lysis, and neutrophils were counted using a hemacytometer and suspended in neutrophil media (RPMI+1% human serum albumin and 20 mM HEPES) at a final concentration of 1×107 cells/mL. Purity of the final cell suspension was assessed by flow cytometry and ≥97% were CD66b+ CD45+ neutrophils and contained ≤0.1% CD14+ cells (monocytes). Cells were >99% viable by Trypan blue exclusion and were used immediately following isolation. For relevant experiments, neutrophils were treated with vehicle (DMSO diluted 1:2000) or inhibitors: SB 202190 (10 μM) or PD 98059 (30 μM) at 37°C for 30 minutes.
Human bronchoepithelial cell line BEAS2b was obtained from the American Type Culture Collection (ATCC CRL-9609). BEAS2b cells were maintained at no greater than 75% confluence in collagen coated flasks with complete bronchial epithelial growth medium (BEGM, Lonza) at 37°C and 5% CO2. For in vitro infection procedures, cells were seeded at low density in collagen coated 24 well plates and allowed to grow to 75% confluence before use.
Neutrophils that extravasated from circulation were obtained from healthy volunteers after induction of suction blisters as previously described (25). Briefly, blisters were induced on the forearm by suction and the epidermal roofs of the resulting blisters were removed. Chambers were placed around the dermal blister bases and filled with saline alone, or saline containing γ-irradiated strains of S. aureus from a healthy volunteer (SA1) or a patient with atopic dermatitis (SA2). Fluid from the chamber was removed after 24 hours, and cells were pelleted and resuspended in HBSS.
Flow cytometry for IL-20 receptor expression
All steps were performed with cold reagents and cells were kept on ice at all times. Cells were suspended in FACS buffer (Cell Staining Buffer, BioLegend) blocked with Human Fc block for 10 minutes prior to addition of antibodies for IL-20RA, IL-22RA (Abcam), IL-20RB (eBioscience), or species-appropriate isotype control antibodies at 10 μg/mL. Secondary and conjugate antibodies were added at concentrations according to manufacturer instructions: Brilliant Violent 421-anti-Rabbit, PE-anti-Rat, Alexa Fluor 647-anti-CD66b, FITC-anti-CD45 (BioLegend). Cell viability was determined by Zombie NIR Fixable Viability Kit (BioLegend) according to manufacturer instructions. Labeled cells were kept on ice and acquired on the flow cytometer (LSRFortessa, BD Biosciences) immediately. Analysis was performed with FlowJo software, cells were gated on singlets, and dead cells were excluded before gating on target populations.
Transwell migration assays
For assays where neutrophils migrated to BEAS2b cells or supernatants, the epithelial cells were grown in 24 well plates as described above and BEGM was removed the night before the experiment and replaced with minimal media (BEBM, Lonza). The following day, BEBM was replaced with fresh BEBM alone or BEBM with S. aureus USA300 (MOI 2). Infected BEAS2b cells were incubated for 2 hours before addition of transwell inserts (3.0 μm pore, Corning) in each well, to which 1.5×106 neutrophils were added. Neutrophils were allowed to migrate for three hours, at which point transwell inserts were removed and neutrophils were recovered from the bottom chamber by gentle pipetting. These cells were stained for IL-20 receptor and neutrophil markers as described above.
Alternatively, BEAS2b cells were incubated with S. aureus as described for four hours and supernatants were removed, sterile filtered (0.2 μm filter) and stored at −20°C until use. To remove IL-20 from supernatant, Protein G DynaBeads (ThermoFisher) were used according to manufacturer instructions with anti-IL-20 antibody (R & D Systems). Removal of IL-20 was confirmed by BioPlex assay (Bio-Rad).
To quantify neutrophil migration in response to BEAS2b cell supernatants or recombinant human (rh) IL-8 and/or rhIL-20 (R & D Systems), 24 well plates were coated with Coating Buffer: 20% autologous donor serum and 12 ng/mL ICAM-1 (R & D Systems) in HBSS with divalent cations (Cellgro) at 37°C for one hour and washed two times with HBSS. BEAS2b cell supernatant or neutrophil media containing 100 ng/mL rhIL-8 and/or 10 ng/mL rhIL-20 was added to the wells, with supernatant from uninfected cells or neutrophil media alone, respectively, used as negative controls. Neutrophils were allowed to migrate to BEAS2b cell supernatant for 3 hours or neutrophil media with recombinant human proteins for 1 hour. Neutrophils were then recovered from the lower chamber by gentle pipetting and counted manually using a hemacytometer. Each condition was performed in duplicate.
Infection of human neutrophils with S. aureus
The S. aureus USA300 LAC strain was kindly provided by M. Otto (NIAID, Bethesda, MD). S. aureus was grown to mid-exponential phase in BHI (BD Biosciences) from a refreshed overnight culture and washed with HBSS. Neutrophils were added to 48 well plates that were coated with Coating Buffer, at 1×106 cells per well in media. Neutrophils were either left unprimed or primed with TNFα at 10 ng/mL. For relevant experiments, cytochalasin D (10 μg/mL) was added. Neutrophils were then incubated for 30 minutes at 37°C. S. aureus was then added at MOI 1, diluted in either media alone or media containing 50 ng/mL rhIL-20, and plates were centrifuged at 1200 rpm for 8 minutes to synchronize phagocytosis.
Samples were taken in quadruplicate at multiple time points between 0.5–4 hours for CFU enumeration. Neutrophils were lysed by adding pH 11 dH2O to ensure lysis of phagosomes. Lysate was serially diluted in sterile PBS and plated on BHI agar plates that were then incubated at 37°C, and CFUs were enumerated the following day. To enumerate intracellular staphylococci, 100 μg gentamicin was added at indicated time points and plates were incubated on ice for 20 minutes. Cells were washed two times with ice cold PBS to remove excess antibiotic, and then lysed, serially diluted and CFU were enumerated as described. Samples were taken in duplicate.
Measurement of ROS production
Neutrophils were added to 96 well plates (5×105 cells/well) and primed and infected as described above. Each condition was set up in triplicate. Luminol (1 mM) was added to wells immediately following addition of S. aureus. The plate was incubated in a luminometer (Beckman Coulter DTX 880 Multimode Detector) set to 37°C and measurements were taken every four minutes for four hours.
Measurement of granule exocytosis
Supernatants were taken from neutrophils infected with S. aureus as described above and spun down at 8000 rpm for 5 minutes to ensure removal of cells. Primary (azurophilic) granule exocytosis was measured by neutrophil elastase activity assay. Supernatants were incubated at 37°C overnight with equal volume of the colorimetric substrate N-methoxysuccinyl-ala-ala-pro-val p-nitroanilide (1 mM), and the reaction was read at A405. Concentration of neutrophil elastase in samples was calculated based on a standard curve generated from recombinant neutrophil elastase.
Sandwich ELISAs for lactoferrin and MMP9 were used to measure secondary (specific) and tertiary (gelatinase) granule release, respectively. Antibody pairs for lactoferrin (capture: 265-1K1 and detection: HRP conjugate) and MMP9 (capture: IA5 and detection: biotinylated IIA5) were obtained from Thermo Fisher, and corresponding recombinant proteins were used as standards.
Zymograms were performed according to manufacturer (Invitrogen) instructions by separating samples on Novex 10% Zymogram Gelatin Protein gels, using renaturing buffer to renature proteins in the gel and developing buffer allowing digestion of gelatin to proceed overnight at 37°C. Areas of digested gelatin were visualized by Coomassie staining.
Western blotting
Neutrophil lysates were obtained from neutrophils infected with S. aureus as described above, incubated for 10 minutes at 37°C. Supernatant was aspirated from wells and lysis buffer (50 μL/well) was added to wells while the plate was on ice. Samples were stored at −80°C until use.
For SDS-PAGE, lysates were diluted in 6X sample buffer (0.5 M Tris, 10% SDS, 30% glycerol, 0.6M DTT, 0.012% bromophenol blue) and boiled for 10 minutes. After electrophoresis, proteins were transferred onto nitrocellulose membranes and blocked for one hour (Odyssey blocking buffer, Licor). Membranes were probed with antibodies for phospho-ERK1/2 (9101), total ERK1/2 (9102), phospho-p38 MAPK (9211) or phospho-ATF-2 (9221) from Cell Signaling Technology or Actin (ab8227) from Abcam. Corresponding secondary antibodies (anti-rabbit 680LT or anti-mouse 800CW; Licor) were added. Membranes were stripped and reprobed as needed to assess multiple targets. Immunoblots were imaged using a Licor scanner.
Immunofluorescence and confocal microscopy
For immunofluorescence by confocal microscopy, neutrophils were seeded on acid washed coverslips that were treated with Coating Buffer and placed in 24 well plates as described (26). Neutrophils were infected with S. aureus as described above and incubated at 37°C for 1 minute, supernatant was removed and cells were fixed with 4% paraformaldehyde at room temperature for 10 minutes. Cells were permeabilized with 0.1% Triton-X 100 for 10 minutes at room temperature and cells were stained with Alexa Fluor 488-phalloidin (Invitrogen) according to manufacturer instructions. After counterstaining with DAPI, coverslips were mounted with Prolong Gold (Invitrogen).
Confocal images were collected using a Leica DMI8000 confocal microscope (Leica Microsystems) enabled with 63× oil immersion objective NA 1.4. Images were acquired using constant laser intensity for Argon Laser and 488 nm wavelength for Alexa fluor 488. Photons were collected using constant photomultiplier electronic gain between the samples to quantify the differences in absolute fluorescence intensity levels from different conditions. Twenty-five fields of view from each condition were collected in an unbiased manner using an automated tiling method. Acquired images were further analyzed using Imaris image processing software (Bitplane USA) to quantitate the absolute total fluorescence intensity per cell. Cumulative intensity calculated from 25 fields of view were plotted as average mean intensity normalized to total number of cells.
Live imaging
For live imaging of neutrophils, Nunc Lab-Teck II Chambered Coverglass (8 well, ThermoFisher) was coated with Coating Buffer as described previously, and neutrophils were seeded at 5×105 cells/well. The slide was placed inside a heated chamber (37°C) on a Leica AF 6000 LX microscope, stimulations were added (GFP-expressing S. aureus with 100 ng/mL IL-8 +/− 10 ng/mL IL-20). Images were taken in three locations in each chamber every minute for at least 30 minutes.
Data Analysis
All data are shown as means ± SEM and are representative or compiled from at least three separate experiments using blood from different donors. Statistical differences were analyzed by t-test or Two-way ANOVA using GraphPad Prism 7 software.
Results
Human neutrophils express IL-20RB when activated
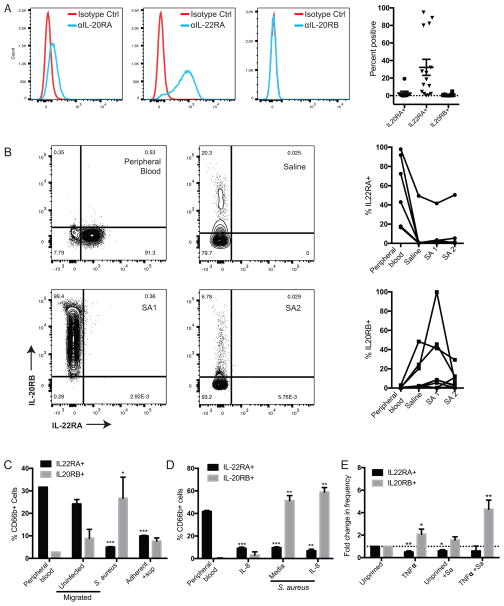
To assess IL-20 receptor expression on human neutrophils, we first examined neutrophils isolated from peripheral whole blood of healthy volunteers. Detection of each receptor chain (IL-20RA, IL-22RB, and IL-20RB) by flow cytometry revealed expression of IL-22RA on a proportion of neutrophils that varied between donors (Figure 1A). In contrast, there was little to no IL-20RA and IL-20RB detected on peripheral blood neutrophils (Figure 1A).
Figure 1. Human neutrophils express IL-20RB when activated.
(A) Detection of indicated IL-20 receptor chains on live neutrophils isolated from peripheral whole blood. Flow cytometry histograms from a representative donor (left) and summary data from multiple donors of percentage of neutrophils staining positive for each receptor chain (right) are shown. (B) Detection of IL-20R receptor chains on live neutrophils that extravasated from circulation into suction blisters induced on healthy volunteers. The suction blisters contained saline or killed S. aureus (SA1 or SA2, two distinct clinical isolates). Flow cytometry dot plots from a representative blister subject (left) and summary data from multiple subjects (right, each line connects data from a unique subject, n=6) are shown. (C–E) Healthy donor-derived peripheral blood neutrophils were assessed for IL-20R chain expression after exposure to indicated conditions in vitro. In (C), neutrophils were stained directly after isolation (peripheral blood), after migration across transwell inserts to uninfected or S. aureus-infected BEAS2b cells, or after adherence and exposure to supernatant from S. aureus-infected BEAS2b cells (adherent+sup). In (D), neutrophils were stained directly after isolation (peripheral blood) or after migration across transwell inserts into wells containing IL-8 (100 ng/ml), S. aureus (2×106 CFU/well), or both. In (E), neutrophils were incubated in media (unprimed) or primed with TNFα (10 ng/ml, 30 minutes) and then incubated for 1 hour in the absence or presence of S. aureus (Sa, 1×106 CFU/well=MOI 1). Data show mean frequency of live CD66b+ cells that express indicated receptor chains from three healthy volunteers (C–D), or fold-change in frequency of receptor-positive cells relative to unprimed neutrophils in 6 healthy volunteers (E). Error bars reflect SEM. *p< 0.05, **p<0.01, ***p<0.001 compared to peripheral blood (C–D) or unprimed (E) neutrophils.
Since neutrophils harbor receptors in their granules and alter cell surface expression of receptors upon migration to a site of inflammation or infection (27, 28), we extended our evaluation to include neutrophils that had extravasated from circulation in vivo in response to an inflammatory stimulus. To do this, we harvested neutrophils recruited into suction blister chambers on healthy volunteers that contained saline or lethally irradiated strains of S. aureus. These extravasated neutrophils displayed altered expression of IL-20 receptor chains compared to those in peripheral blood. Compared to each donor’s own peripheral blood neutrophils, a lower frequency of their blister neutrophils displayed IL-22RA and a higher frequency displayed IL-20RB (Figure 1B). Extravasation into the saline-containing blister chamber accounted for most of the alteration in receptor expression, but exposure to S. aureus seemed to further alter expression in some donors (Figure 1B).
To determine whether migration of neutrophils toward infected epithelial cells in vitro could mimic the IL-20R expression pattern seen in the in vivo blister studies, we infected human bronchial epithelial (BEAS2b) cells with S. aureus. Neutrophils were then allowed to migrate for three hours across a semi-permeable transwell membrane into the well containing the infected epithelial cells. The migrated neutrophils had significantly upregulated IL-20RB and downregulated IL-22RA compared to peripheral blood neutrophils (Figure 1C). Low levels of migration to uninfected cells occurred, but did not significantly alter receptor expression (Figure 1C). Migration toward infected cells appeared to be a key trigger for this pattern of receptor expression, as incubating adherent neutrophils with sterile filtered supernatant from infected cells similarly reduced IL-22RA expression but did not alter IL-20RB expression (Figure 1C).
Neutrophils encounter and respond to cytokines released at sites of inflammation. IL-8 is an important neutrophil chemoattractant, and incubation with TNFα is known to ‘prime’ neutrophils and enhance their subsequent functionality (8, 13). To mimic migration induced by IL-8 and determine whether this induced changes in IL-20 receptor chain expression, neutrophils were allowed to migrate across a semi-permeable transwell insert into wells containing either recombinant human (rh) IL-8 alone or rhIL-8 with S. aureus. Migration to rhIL-8 caused a significant reduction in IL-22RA expression, but no significant change in IL-20RB. Migration across the transwell membrane toward S. aureus induced both a significant increase in IL-20RB expression and a decrease in IL-22RA, and this was not further altered by concurrent administration of rhIL-8 (Figure 1D). Priming of neutrophils by direct incubation with TNFα similarly reduced the frequency of IL-22RA+ cells and increased the frequency of IL-20RB+ cells (Figure 1E). Direct incubation with S. aureus did not alter IL-20RB surface expression in unprimed neutrophils, but further augmented it in those primed with TNFα (Figure 1E). IL-20RA expression was examined separately since detection antibodies for IL-20RA and IL-22RA were both generated in rabbits and could not be used together. IL-20RA was detected in a low frequency of peripheral blood neutrophils (Figure 1), and was further downregulated by IL-8 or TNFα (Supplemental Figure 1). Taken together, these results indicate that human neutrophils modulate their expression of the IL-20 receptor chains IL-22RA and IL-20RB. Downregulation of IL-22RA seems to be a common response to migration, inflammatory cytokines, and bacteria. In contrast, induction of IL-20RB appears to be part of a more stringently controlled neutrophil response to infection, as it was stimulated only by S. aureus-induced migration or infection of TNFα-primed neutrophils.
IL-20 inhibits killing of S. aureus by TNFα-primed neutrophils in vitro
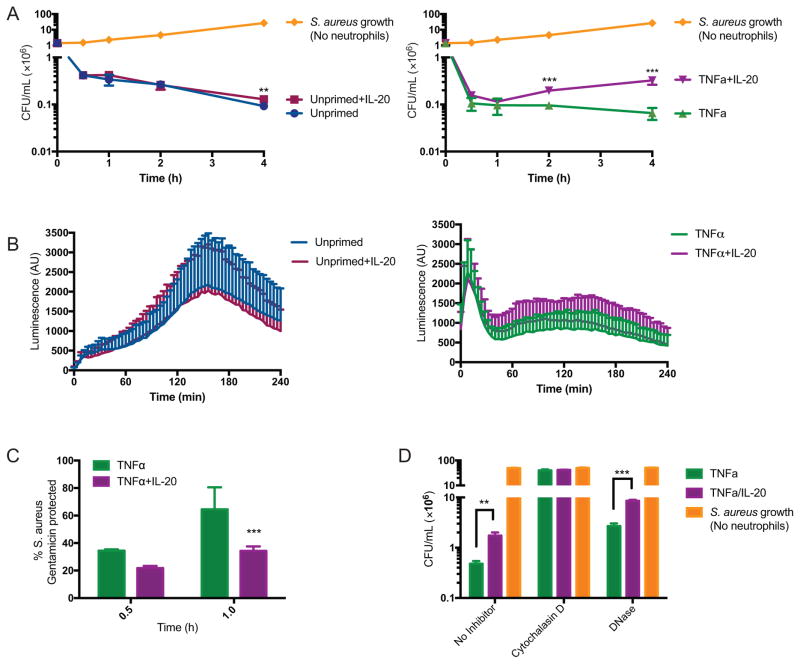
Since priming of neutrophils with TNFα modulated their IL-20 receptor expression, we evaluated whether this altered neutrophil responsiveness to IL-20. TNFα is known to enhance multiple bactericidal mechanisms in neutrophils (8, 13). Consistent with this, fewer colony forming units (CFU) of S. aureus were recovered in the presence of TNFα-primed neutrophils (Figure 2A, right panel) compared to unprimed neutrophils (Figure 2A, left panel). Addition of IL-20 significantly increased CFU recovered from TNFα-primed neutrophils at 2 and 4 hours post-infection (Figure 2A, right panel). Production of reactive oxygen species (ROS) is a major mechanism utilized by neutrophils to kill staphylococci (2). Total ROS production was evaluated using luminol, which generates luminescence when reacting with ROS. Measurements were taken every four minutes for four hours and showed no significant difference in ROS production mediated by IL-20 in unprimed or TNFα-primed neutrophils (Figure 2B).
Figure 2. IL-20 inhibits killing of S. aureus by TNFα-primed neutrophils in vitro.
(A) Bacterial CFU recovered from adherent peripheral blood neutrophils that were unprimed (left panel) or primed with TNFα (10 ng/mL, 30 min, right panel) and then incubated with S. aureus (MOI 1) in the presence or absence of IL-20 (50 ng/mL) for the indicated time. Data shown are the mean of results from seven donors with each donor assayed in quadruplicate. (B) ROS detected by luminescence of luminol (1 mM) added to wells with neutrophils that were treated as described in (A). Conditions were performed in triplicate and measurements were taken every 4 minutes; data shown as mean +/− SEM of results from four donors. (C) Neutrophils were incubated with S. aureus +/− IL-20 after priming with TNFα as described in (A). Gentamicin was added at the indicated time points after infection to kill extracellular bacteria and CFU were enumerated. Data shown as percent of S. aureus remaining compared to average of total S. aureus present in duplicate gentamicin-untreated wells and reflect mean +/− SEM of results from four donors. (D) Neutrophils primed with TNFα and then incubated with S. aureus (MOI 1) +/− IL-20 as described in (A), in the absence or presence of cytochalasin D (10 μg/mL) or DNase (10 U/mL). CFU was determined in quadruplicate for each sample at 4 hours post-infection. Data shown are mean +/− SEM of results from three donors. For (A) and (C–D): **p<0.01, ***p<0.001 compared to unprimed (A, left) or TNFα-primed (A, right; C–D) neutrophils.
To investigate the potential involvement of pathways other than ROS production on inhibition of bactericidal activity by IL-20, we next assessed the effect of IL-20 on phagocytosis of S. aureus by neutrophils, an actin-dependent process essential for intracellular killing (2, 11). We used a gentamicin protection assay to assess phagocytosis. In this assay, extracellular bacteria are killed by gentamicin treatment and the detectable CFU reflect phagocytosed intracellular bacteria. By one hour after infection, IL-20 exposure lowered the percentage of intracellular S. aureus recovered from TNFα-primed neutrophils (Figure 2C), suggesting impairment of phagocytosis by IL-20. Inhibition of phagocytosis, using the actin polymerization inhibitor cytochalasin D (12), abrogated killing activity of TNFα-primed neutrophils whether or not IL-20 was present (Figure 2D). Addition of DNase to inhibit formation of neutrophil extracellular traps (NETs) (29), an extracellular mechanism of killing, partially inhibited killing by TNFα-primed neutrophils, but a significant difference in killing attributable to IL-20 remained (Figure 2D). Taken together, these results suggest that IL-20 does not directly inhibit ROS- and NET-based killing mechanisms but may inhibit phagocytosis-mediated uptake needed for killing of bacteria by neutrophils.
IL-20 inhibits exocytosis of tertiary (gelatinase) granules
Because IL-20 has been previously reported to affect actin polymerization in endothelial cells (30), we hypothesized that IL-20 may affect other actin-mediated processes in neutrophils in addition to the effects on phagocytosis observed above. Neutrophil granule exocytosis in response to bacteria or other activating stimuli requires translocation to the plasma membrane via actin remodeling (12). Primary, secondary, and tertiary granules each contain different compositions of proteases, antimicrobial peptides, and other proteins that contribute to their distinct functional roles in the response to infection (28, 31). Exocytosis of each granule population occurs in a hierarchical manner dependent on differential association with actin (32).
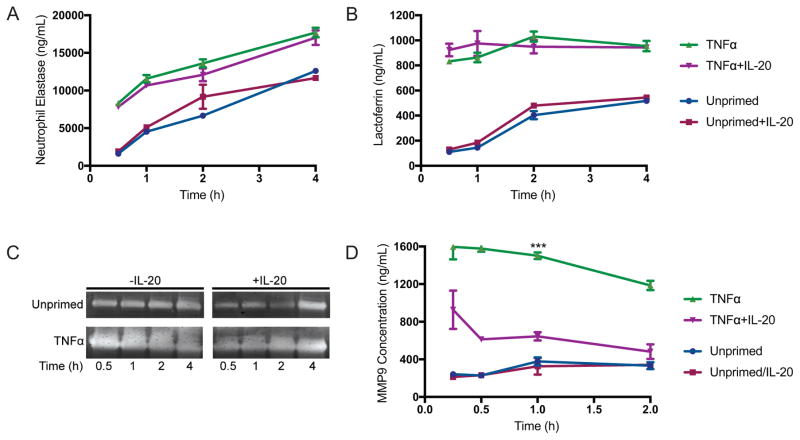
To evaluate whether IL-20 affects this actin-mediated process, we measured the release of protein markers for each granule population in supernatants of neutrophils that were infected with S. aureus in vitro. IL-20 did not affect the concentration of neutrophil elastase and lactoferrin, markers of primary and secondary granules, respectively (Figure 3A–B). Matrix metalloproteinases (MMPs), also called gelatinases, comprise the predominant protein population in tertiary granules (28, 31). We evaluated the activity of gelatinases in neutrophil supernatants by gelatin zymography, where the clearing of gelatin in polyacrylamide gels is visualized by Coomassie stain. IL-20 reduced gelatinase activity in the supernatants of TNFα-primed neutrophils that were infected with S. aureus (Figure 3C). The concentration of the tertiary granule protein MMP9 was also significantly reduced after exposure of infected neutrophils to IL-20 (Figure 3D). The reduced levels of gelatinase activity and MMP9 in supernatants of neutrophils that were treated with IL-20 suggests that the release of tertiary granules is inhibited by IL-20. Since tertiary granules are more highly associated with actin than primary and secondary granule populations, the inhibition of their release implies modulation by IL-20 of actin dynamics in neutrophils during in vitro infection with S. aureus.
Figure 3. IL-20 inhibits exocytosis of tertiary (gelatinase) granules.
(A) Concentration of neutrophil elastase (primary/azurophilic granule marker) in supernatants from human neutrophils, incubated with S. aureus MOI 1 for indicated times, measured by reaction with colorimetric substrate, calculated using standard of known concentration. (B) Lactoferrin concentration, marker for secondary (specific) granules, measured by ELISA of neutrophil supernatants. Data shown in (A) and (B) as mean +/− SEM from three healthy donors. (C) Gelatin zymography of supernatants from neutrophils infected for indicated times with S. aureus (MOI 1). Results shown are representative of neutrophils from four independent healthy donors. (D) Concentration of MMP9, marker for tertiary (gelatinase) granule release, in supernatants of neutrophils infected for indicated times with S. aureus (MOI 1) +/− IL-20. Data shown as mean +/− SEM from four healthy donors. ***p<0.001 compared to TNFα+IL-20-treated neutrophils by Two-way ANOVA.
Inhibition of phagocytosis and tertiary granule exocytosis by IL-20 is mediated by ERK1/2
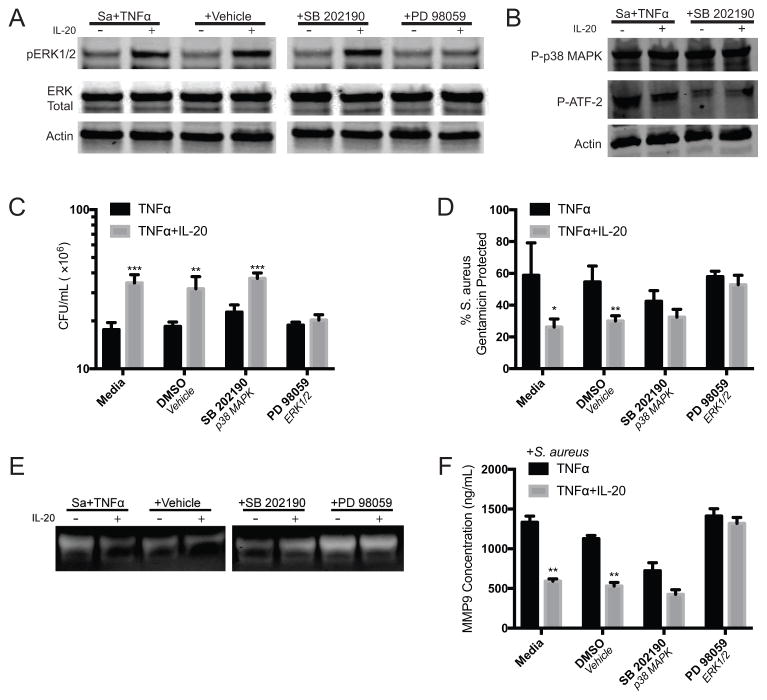
IL-20 has been reported to activate STAT3, p38 MAPK, or ERK 1/2 in different cell types (20, 22, 24, 30, 33). After exposure of neutrophils to IL-20, we found STAT3 phosphorylation to be minimal and inconsistent (not shown). Treatment of neutrophils with signaling inhibitors for p38 MAPK (SB 202190) or ERK1/2 (PD 98059) was used to identify if either of these were involved in IL-20-mediated inhibition of bacterial killing or granule exocytosis in TNFα-primed neutrophils. Western blot of lysates obtained from neutrophils that were incubated with S. aureus and TNFα for 10 minutes showed phosphorylation of ERK 1/2 that was augmented by IL-20, and this phosphorylation was inhibited by pretreatment with PD 98059 (Figure 4A). Phosphorylation of p38 MAPK by TNFα was detected, and was not affected by IL-20 (Figure 4B). The inhibitor SB 202190 does not inhibit p38 MAPK phosphorylation directly, but inhibits activation of its downstream targets, including ATF (34); accordingly, this inhibition was confirmed by Western blot (Figure 4B).
Figure 4. Inhibition of phagocytosis and tertiary granule exocytosis by IL-20 is mediated by ERK1/2.
(A–B) Western blot of lysates of neutrophils that were treated with the indicated inhibitors and infected/stimulated with S. aureus and TNFα +/− IL-20 for 10 minutes. Results shown are representative of neutrophils from 3 healthy donors. (C) S. aureus CFU recovered from adherent peripheral blood neutrophils that were treated with the indicated inhibitors and then incubated with S. aureus (MOI 1) and TNFα (10 ng/mL) in the presence or absence of IL-20 (50 ng/mL) for four hours. (D) Neutrophils were treated with indicated inhibitors and incubated with S. aureus and TNFα +/− IL-20 as described in (B). Gentamicin was added at the indicated time points after infection to kill extracellular bacteria and CFU were enumerated. Data shown as percent of S. aureus remaining compared to average of total S. aureus present in duplicate gentamicin-untreated wells. (E) Gelatin zymography of cell free supernatants from neutrophils incubated with for one hour with S. aureus (MOI 1) and TNFα +/− IL-20 after treatment with indicated inhibitors. Results shown are representative of neutrophils from three healthy donors. (F) Concentration of MMP9 in supernatants of neutrophils infected for one hour with S. aureus (MOI 1) +/− IL-20 after priming under indicated conditions. For (C), (D), and (F), data shown reflect mean +/− SEM of results from three donors, and *p<0.05, **p<0.01, ***p<0.001 by t test.
To determine whether ERK1/2 activation by IL-20 was required for the observed inhibitory effects of this cytokine, bacterial killing and phagocytosis were evaluated as before using neutrophils treated with SB 202190 or PD 98059. Inhibition of ERK1/2 by PD 98059 abrogated the IL-20-mediated differences in bacterial killing and phagocytic uptake by TNFα-primed neutrophils (Figure 4C and 4D). Additionally, when ERK1/2 was inhibited, IL-20 treated neutrophils displayed similar levels of tertiary granule release, as indicated by gelatin zymography and measurement of MMP9 in supernatants of TNFα-primed neutrophils that were infected with S. aureus in vitro (Figure 4E and 4F). Taken together, the enhanced phosphorylation of ERK1/2 observed in the lysates of IL-20 treated neutrophils and restoration of phagocytosis and tertiary granule release in PD 98059-treated neutrophils indicates that the inhibitory effects of IL-20 are dependent on ERK1/2 activation.
IL-20 inhibits neutrophil migration in vitro
Neutrophils have a hierarchical response to multiple signals that allows them to reach a site of infection and arrest movement at the point of threat, be it wound or infection (44, 47). Actin dynamics that establish polarization and pseudopod extension and other morphological changes are a critical component of neutrophil migration (37, 38). To determine whether IL-20 affects this actin-mediated process, neutrophils were exposed to various stimuli in transwell migration assays.
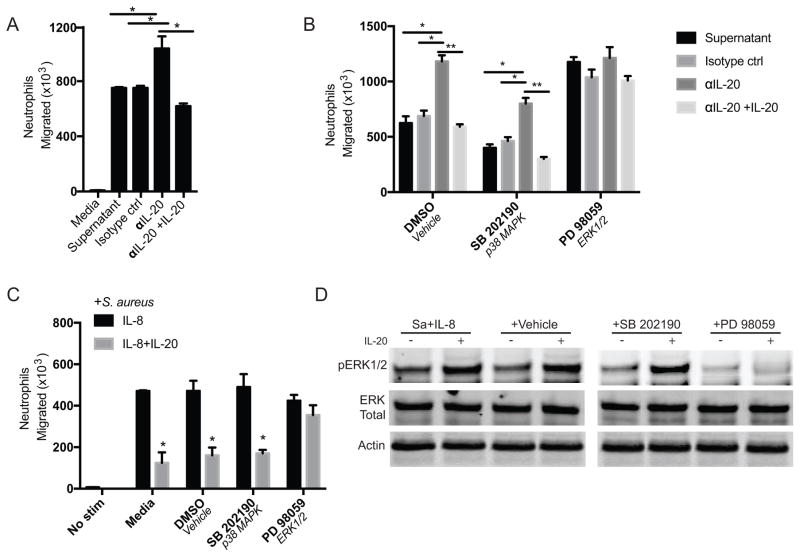
The aforementioned BEAS2b cells produce IL-20 and IL-8 in response to infection with S. aureus (Supplemental Figure 2). Supernatants from these cells were obtained 4 hours post-infection and sterile filtered. The supernatants were then depleted of IL-20 by immunoprecipitation. Migration through a semi-permeable transwell membrane to the IL-20-depleted supernatant was significantly increased relative to untreated or isotype control-treated supernatant, and addition of IL-20 to the depleted supernatant returned migration to the lower levels seen with intact supernatant (Figure 5A). In this system, inhibition of ERK1/2 but not p38 increased migration to levels similar to that seen with IL-20 depletion and this was not further affected by IL-20 depletion or repletion (Figure 5B). Addition of IL-20 also inhibited migration of neutrophils toward recombinant IL-8 (Figure 5C). Inhibition of ERK1/2 did not affect migration induced by IL-8, but abrogated the inhibition of migration by IL-20 (Figure 5C). Specific activity of the ERK1/2 inhibitor was verified in infected neutrophils treated with IL-8 +/− IL-20 (Figure 5D).
Figure 5. IL-20 inhibits neutrophil migration in vitro.
(A) 1.5×106 peripheral blood neutrophils were placed in the upper chamber of transwell plates. After 3 hours, the number of cells that migrated across the transwell insert to lower chambers was counted using a hemacytometer. The lower chambers contained media or supernatants from human bronchial epithelial (BEAS2b) cells that had been infected with S. aureus (MOI 2) for 4 hours, sterile-filtered, and then subjected to immunoprecipitation with isotype control or IL-20 antibody. Under some conditions (αIL-20+IL-20), recombinant IL-20 (10 ng/mL) was added back to supernatant after IL-20 immunoprecipitation. (B) Neutrophils were treated with indicated inhibitors and stimulated to migrate across a transwell chamber as in (A). (C) Neutrophils untreated or treated with indicated inhibitors and stimulated to migrate across transwell inserts for 1 hour to wells containing S. aureus (2×106 CFU/well) + IL-8 (100 ng/mL) alone or with IL-20 (10 ng/mL). (D) Western blot of lysates from neutrophils, untreated or treated with indicated inhibitors, infected with S. aureus (MOI 1) and stimulated with IL-8 alone or with IL-20 for 10 minutes. For A–C, data shown reflect mean +/− SEM of results from three donors, and *p<0.05, **p<0.01 by t test.
The effects of IL-20 on migration were confirmed using time lapse microscopy of neutrophils seeded onto coverglass slides in chambers that were infected with S. aureus in media alone or media supplemented with IL-8 or IL-8+IL-20. Neutrophils in the presence of IL-20 began to arrest movement at approximately 15 minutes, while the neutrophils in the other chambers continued their movement (Supplemental Videos 1–3). The transwell migration assays and time lapse microscopy show that IL-20 inhibits neutrophil migration, and this inhibition is dependent on activation of ERK1/2.
IL-20 modulates actin polymerization in neutrophils
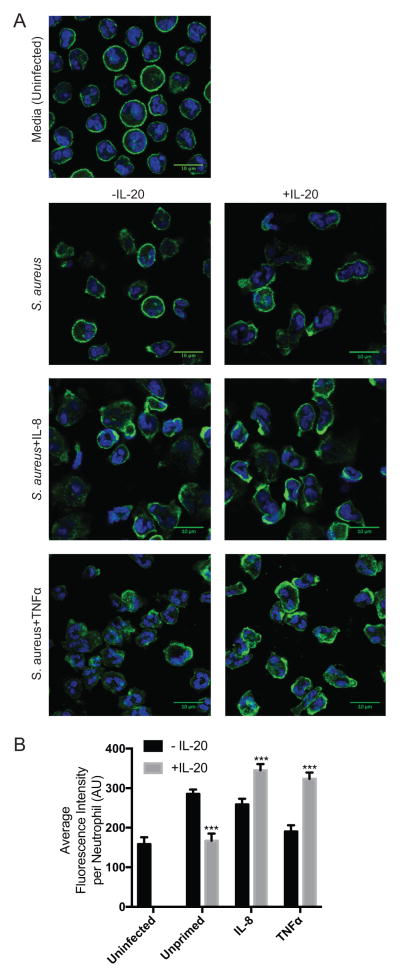
Changes to the actin cytoskeleton drive the neutrophil processes affected by IL-20 in the studies above (phagocytosis, granule exocytosis, and migration). To directly assess the effect of IL-20 on the actin cytoskeleton in neutrophils, polymerized actin (F-actin) was visualized by phalloidin staining and confocal microscopy of infected neutrophils treated with IL-8, TNFα, or IL-20 for 1 minute. Morphological changes with increased peripheral polarization of F-actin attributable to IL-20 was observed in neutrophils treated with either IL-8 or TNFα and was especially prominent in those treated with TNFα (Figure 6A). For each condition, an automated tiling method was used to unbiasedly collect 25 fields of view that were then used for fluorescence quantification to compare levels of F-actin. IL-8- and TNFα-primed neutrophils infected with S. aureus had significantly increased F-actin content when treated with IL-20, while those infected with S. aureus alone responded to IL-20 with significantly reduced levels of F-actin (Figure 6B). These results identify that IL-20 modifies F-actin content and organization in S. aureus-infected human neutrophils, consistent with the observed effects of IL-20 on actin-mediated processes such as phagocytosis, granule exocytosis, and migration.
Figure 6. IL-20 modulates actin polymerization in neutrophils.
(A) Representative confocal microscopy images of neutrophils incubated with indicated stimulation for 1 minute (Sa, S. aureus 2×106 CFU/well; IL-8, 100 ng/mL; TNFα, 10 ng/ml). Green, Alexa Fluor 488-phalloidin; blue, DAPI. (B) Quantification of Alexa Fluor 488-phalloidin fluorescence intensity per cell from analysis of 25 fields of view per condition that were generated by an automated tiling method and normalized to the total number of cells across all images with standard deviation. Data is representative of results from three different donors. ***p<0.001 by t test.
Discussion
Neutrophil function is modulated by the cytokines and chemokines encountered during infection. Prior work by our lab in a model of S. aureus skin infection identified anti-inflammatory effects of IL-20 receptor signaling that included diminished recruitment of neutrophils to infected lesions in mice (19). IL-20 has been reported to similarly inhibit neutrophil recruitment in a mouse model of corneal wound healing (48). These studies focused on the impact of IL-20 on neutrophil recruitment by epithelial cells, recognized targets of this cytokine in models of psoriasis and wound healing (1, 7–8, 17–18). However, analyses of synovial fluid neutrophils from patients with rheumatoid arthritis have suggested neutrophils may directly respond to this cytokine (23, 24). Our current study identifies the ability of inflammatory and infectious stimuli to induce receptor expression on human neutrophils in a manner that allows IL-20 to directly target these cells and influence their anti-bacterial and inflammatory properties.
Two IL-20 receptors have been reported, comprised of a common IL-20RB chain that forms a heterodimer with either IL-20RA (Type I) or IL-22RA (Type II) (20, 21). Both receptors have been found on epithelial cells (20, 22), but their expression on leukocytes has been less well characterized. RT-PCR data has shown expression of IL-20RB alone on peripheral blood mononuclear cells, B cells, T cells, and NK cells (23, 24). IL-20RB expression has not been reported on neutrophils, but IL-20RA and IL-22RA receptor chains have been detected by flow cytometry on synovial membranes and synovial fluid cells, including neutrophils, from patients with rheumatoid arthritis (23, 24), suggesting that the expression of these receptors may be regulated by environmental cues.
In the present study, freshly isolated peripheral blood neutrophils expressed detectable IL-22RA on their surface but did not express IL-20RA or IL-20RB. We found that expression of IL-20RB, common to both Type I and Type II IL-20 receptors, was upregulated in response to migration from circulation in vivo or stimulation with S. aureus and pro-inflammatory host factors in vitro. These same conditions caused a decrease in IL-22RA expression, indicating a possible balance between IL-20 receptor and IL-22 receptor signaling since IL-22RA is used by both. Because detection of both IL-20RA and IL-22RA depended on rabbit antibody reagents, we were limited in our ability to determine co-expression of IL-20RA but found that its expression on neutrophils was low and was further decreased on infected neutrophils after priming with TNFα. Expression of IL-20RB correlated with responsiveness of neutrophils to IL-20, suggesting that IL-20RB imparted its function by pairing with low levels of IL-22RA or IL-20RA. However, we cannot rule out the possibility that IL-20 signals through a non-canonical receptor in neutrophils.
Consistent with utilization of JAK-STAT signaling pathways by the Class II cytokine receptor family (16, 42–43), IL-20 receptor signaling has been described to involve STAT3 in epithelial cells (19–20, 22). Involvement of p38 MAPK and ERK1/2 has also been reported in dendritic cells and endothelial cells, respectively (30, 33). Furthermore, IL-20 has been shown to activate ERK1/2 in rheumatoid arthritis synovial fibroblasts (24). We detected inconsistent and minimal phosphorylation of STAT3 in neutrophils in response to IL-20, and show that activation of ERK1/2 occurs in neutrophils and is required for the functional effects of IL-20 observed during infection in vitro.
We found that IL-20 affected multiple actin-mediated functions in activated neutrophils. Although it did not affect ROS production or NET-mediated killing of S. aureus, IL-20 did inhibit phagocytic uptake of staphylococci and tertiary granule exocytosis by TNFα-primed neutrophils. It also inhibited neutrophil migration in response to the chemotactic factor IL-8, as well as the supernatant from S. aureus-infected epithelial cells. Our observed inhibitory effects on neutrophil migration are consistent with decreased neutrophil accumulation reported in in vivo models (19, 48), but contrast with the reported ability of IL-20 to directly induce neutrophil chemotaxis in vitro (24). These discrepancies may reflect differential neutrophil activation states that are determined by environmental cues. We suggest that the inhibition of neutrophil function seen in our in vitro models mimicking infection with S. aureus is linked to the altered polymerization of actin seen by confocal microscopy of neutrophils after exposure to IL-20, since phagocytosis (44), tertiary granule exocytosis (12, 28), and migration (10) all require dynamic actin rearrangement.
The observed inhibitory effects of IL-20 on the neutrophil responses to infection are consistent with the anti-inflammatory properties of this cytokine on epithelial cells during cutaneous staphylococcal infection (19). Further verification in appropriate infectious and inflammatory models will be needed to confirm the inhibitory nature of IL-20 activity on neutrophil function during infection in vivo. This may be complicated because of differences between mouse and human neutrophils (45–46), and the preferential expression of IL-20 by human cells compared to the tendency of murine cells to secrete IL-19 and IL-24 instead (19). However, taken together with previous reports of IL-20 function, our results support an overall homeostatic role for IL-20 that promotes epithelial proliferation and function while potentially dampening inflammatory tissue destruction by activated neutrophils.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program at NIH, NIAID.
We thank the staff from the Biological Imaging Section in the Research Technology Branch at NIAID for their help with the microscopy studies. We also thank Katrin Mayer-Barber, Inka Sastalla and Hatice Karauzum for valuable input and technical advice, and Ian Myles for providing exudate cells from the blister model.
References
- 1.Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spaan AN, Surewaard BGJ, Nijland R, van Strijp JAG. Neutrophils Versus Staphylococcus aureus: A Biological Tug of War. Annu Rev Microbiol. 2013;67:627–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 4.Winkelstein JA, Marino MC, Johnston RB, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. Chronic Granulomatous Diseases. Medicine. 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lowy FD. Staphylococcus aureus Infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 6.Foster TJ. Immune Evasion by Staphylococci. Nat Rev Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 7.Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-α play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus induced experimental brain abscess. J Neuropathol Exp Neurol. 2004;63:381–396. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- 8.Tufano MA, Cipollaro de I’Ero G, Iannello R, Galdiero M, Galdiero F. Protein A and other surface components of Staphylococcus aureus stimulate the production of IL-1 alpha, IL-4, IL-6, TNF, and IFN-gamma. Eur Cytokine Netw. 1991;2(5):361–366. [PubMed] [Google Scholar]
- 9.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016;273:180–193. doi: 10.1111/imr.12447. [DOI] [PubMed] [Google Scholar]
- 10.Weiner OD, Servant G, Welch MD, Mitchison TJ, Sedat JW, Bourne HR. Spatial control of actin polymerization during neutrophil chemotaxis. Nat Cell Biol. 1999;1:75–81. doi: 10.1038/10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellano F, Chavrier P, Caron E. Actin dynamics during phagocytosis. Semin Immunol. 2001;13:347–355. doi: 10.1006/smim.2001.0331. [DOI] [PubMed] [Google Scholar]
- 12.Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR. The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol. 2007;292:C1690–C1700. doi: 10.1152/ajpcell.00384.2006. [DOI] [PubMed] [Google Scholar]
- 13.Ferrante A, Martin AJ, Bates EJ, Goh DHB, Harvey DP, Parsons D, Rathjen DA, Russ G, Dayer JM. Killing of Staphylcoccus aureus by Tumor Necrosis Factor-α-Activated Neutrophils. J Immunol. 1993;151:4821–4828. [PubMed] [Google Scholar]
- 14.Smith RJ, Sam LM, Leach KL, Justen JM. Postreceptor events associated with human neutrophil activation by interleukin-8. J Leuko Biol. 1992;52:17–26. doi: 10.1002/jlb.52.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Di Cioccio V, Strippoli R, Bizzarri C, Troiani G, Cervellera MN, Gloaguen I, Colagrande A, Cattozzo EM, Paglieli S, Santoni A, Colotta F, Mainiero F, Bertini R. Key role of proline-rich tyrosine kinase 2 in interleukin-8 (CXCL8/IL-8)-mediated human neutrophil chemotaxis. Immunology. 2004;111:407–415. doi: 10.1111/j.1365-2567.2004.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 17.Otkjaer K, Kragballe K, Funding AT, Clausen JT, Noerby PL, Steiniche T, Iversen L. The dynamics of gene expression of interleukin-19 and interleukin-20 and their receptors in psoriasis. Br J Dermatol. 2005;153:911–918. doi: 10.1111/j.1365-2133.2005.06800.x. [DOI] [PubMed] [Google Scholar]
- 18.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 19.Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, Ouyang W, Datta SD. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol. 2013;14(8):804–811. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A, Haugen H, Jelinek L, Kelly JD, Madden K, Maurer MF, Parrish-Novak J, Prunkard D, Sexson S, Sprecher C, Waggie K, West J, Whitmore TE, Yao L, Kuechle MK, Dale BA, Chandrasekher YA. Interleukin 20: Discovery, Receptor Identification, and Role in Epidermal Function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 21.Wagenka UM. IL-20: Biological functions mediated through two types of receptor complexes. Cytokine Growth Factor Rev. 2010;21:353–363. doi: 10.1016/j.cytogfr.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Parrish-Novak J, Xu W, Brender T, Yao L, Jones C, West J, Brandt C, Jelinek L, Madden K, McKernan PA, Foster DC, Jaspers S, Chandrasekher YA. Interleukins 19, 20, and 24 Signal through Two Distinct Receptor Complexes: Differences in Receptor-Ligand Interactions Mediate Unique Biological Functions. J Biol Chem. 2002;277:47517–47523. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- 23.Kragstrup TW, Otkjaer K, Holm C, Jørgensen A, Hokland M, Iversen L, Deleuran B. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine. 2007;41:16–23. doi: 10.1016/j.cyto.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Hsu YH, Li HH, Hsieh MY, Liu MF, Huang KY, Chin LS, Chen PC, Cheng HH, Chang MS. Function of Interleukin-20 as a Proinflammatory Molecule in Rheumatoid and Experimental Arthritis. Arthritis & Rheumatism. 2006;54(9):2722–2733. doi: 10.1002/art.22039. [DOI] [PubMed] [Google Scholar]
- 25.Myles IA, Williams KW, Reckhow JD, Jammeh ML, Pincus NB, Sastalla I, Saleem D, Stone KD, Datta SK. Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight. 2016;1(10) doi: 10.1172/jci.insight.86955. pii: 386955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen LA. Immunofluorescence and confocal microscopy of neutrophils. Methods Mol Biol. 2014;1124:251–268. doi: 10.1007/978-1-62703-845-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 28.Borregaard N, Sørensen Theilgaard-Mönch K. Neutrophil Granules: a library of innate immunity proteins. Trends Immunol. 2007;28(8):340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammer T, Tritsaris K, Hübschmann MV, Gibson J, Nisato RE, Pepper MS, Dissing S. IL-20 activates human lymphatic endothelial cells causing cell signaling and tube formation. Microvasc Research. 2009;78:25–32. doi: 10.1016/j.mvr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev. 2016;273:11–28. doi: 10.1111/imr.12440. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell T, Lo A, Logan MR, Lacy P, Eitzen G. Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. Am J Physiol Cell Physiol. 2008;295:C1354–C1365. doi: 10.1152/ajpcell.00239.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bech R, Jalilian B, Agger R, Iversen L, Erlandsen M, Otkjaer K, Johansen C, Paludan SR, Rosenberg CA, Kragballe K, Vorup-Jensen T. Interleukin 20 regulates dendritic cell migration and expression of co-stimulatory molecules. Molecular and Cellular Therapies. 2016;4:1. doi: 10.1186/s40591-016-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu JH, Hong LC, Tsai YY, Chen HW, Chen WX, Wu TS. Mitogen-activated protein kinase (MAPK) signaling pathways in HepG2 cells infected with a virulent strain of Klebsiella pneumoniae. Cell Microbiol. 2006;8(9):1467–74. doi: 10.1111/j.1462-5822.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- 35.Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159(1):91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Ma B, Malik AB, Tang H, Yang T, Sun B, Wang G, Minshall RD, Li Y, Zhao Y, Ye RD, Xu J. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol. 2012;13(5):457–465. doi: 10.1038/ni.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhelev DV, Alteraifi AM, Chodniewicz D. Controlled Pseudopod Extension of Human Neutrophils Stimulated with Different Chemoattractants. Biophysical Journal. 2004;87:688–695. doi: 10.1529/biophysj.103.036699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16:378–391. doi: 10.1038/nri.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 40.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting Edge: Immune Cells as Targets of the IL-10 Family Members? J Immunol. 2002;168(11):5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 41.Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, Asadullah K, Sabat R. Interleukin (IL)-19, IL-20, and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Experimental Dermatology. 2006;15:991–1004. doi: 10.1111/j.1600-0625.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 42.Kotenko SV, Pestka S. Jak-Stat signal transduction pathway through the eyes of cytokine receptor complexes. Oncogene. 2000;19:2557–2565. doi: 10.1038/sj.onc.1203524. [DOI] [PubMed] [Google Scholar]
- 43.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castellano F, Chavrier P, Caron E. Actin dynamics during phagocytosis. Semin Immunol. 2001;13:347–355. doi: 10.1006/smim.2001.0331. [DOI] [PubMed] [Google Scholar]
- 45.Eisenhauer PB, Lehrer RI. Mouse neutrophils lack defensins. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003;188:49–71. doi: 10.1016/s0300-483x(03)00043-x. [DOI] [PubMed] [Google Scholar]
- 47.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil Function: From Mechanisms to Disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, Magadi S, Li Z, Smith CW, Burns AR. IL-20 promotes epithelial healing of the injured mouse cornea. Exp Eye Res. 2016;154:22–29. doi: 10.1016/j.exer.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.