Abstract
The purpose of this study was to determine whether obesity would reduce the capacity of peripheral blood mononuclear cells (PBMCs) to produce the anti-inflammatory protein pentraxin 3 (PTX3) in response to ex vivo stimulation with lipopolysaccharide (LPS), and if acute aerobic exercise would enhance this PTX3 production capacity. In addition, the inter-relationships of LPS-induced PTX3 with the inflammatory cytokines (interleukin 6 [IL-6], IL-10, and tumor necrosis factor alpha) were examined. Twenty-one healthy subjects (10 obese and 11 normal-weight) performed an acute bout of aerobic exercise at 75% VO2max. The capacity of PBMCs to produce PTX3 ex vivo following LPS stimulation was the same in obese and normal-weight subjects at rest, and decreased equally in both subject groups following acute aerobic exercise. This is in contrast to plasma PTX3, which is lower in obese subjects at rest and increased equally in both obese and normal-weight subjects following exercise. In addition, ex vivo PTX3 production was positively associated with IL-6 and IL-10 in response to acute aerobic exercise (r = 0.686, P = 0.020; r = 0.744, P = 0.009, respectively) in normal-weight, but not in obese individuals (r = 0.429, P = 0.249; r = 0.453, P = 0.189, respectively). These findings indicate that concentrations of PTX3 observed in plasma are relatively independent of those produced by PBMCs ex vivo and the mechanisms associated with PTX3-mediated anti-inflammatory signaling may differ during obesity.
Impact statement
Our laboratory has previously demonstrated that obese individuals present with lower plasma concentrations of the anti-inflammatory protein pentraxin 3 (PTX3), whereas acute aerobic exercise increases plasma PTX3 levels similarly compared to normal-weight individuals. As a follow-up, the present study demonstrates that PBMCs isolated from obese and normal-weight individuals produce comparable amounts of PTX3 ex vivo in response to lipopolysaccharide (LPS). Furthermore, given that acute aerobic exercise reduced the ex vivo production of PTX3 in both groups, our results clearly indicate that plasma PTX3 levels are relatively independent of those produced by PBMCs ex vivo. In addition, our findings suggest that the mechanisms associated with PTX3-mediated production of the anti-inflammatory cytokine interleukin 10 may be impaired in obese individuals, and thus provides a key finding necessary for the elucidation of PTX3’s role in the mediation of anti-inflammatory profiles and the subsequent amelioration of inflammatory disease during obesity.
Keywords: Pentraxin 3, aerobic exercise, cardiovascular disease, inflammatory cytokines, monocytes, obesity
Introduction
Pentraxin 3 (PTX3), an acute phase response protein, plays an anti-inflammatory role in obesity-related inflammation.1 Rapid increases in plasma PTX3 are reported in endotoxemia.2 It has also been demonstrated that plasma lipopolysaccharide (LPS) endotoxin levels originated from the gut gram-negative bacteria are increased in obesity,3 and as a result, LPS generally induces pro-inflammatory mediators as well as the production of the anti-inflammatory protein PTX3 by peripheral blood mononuclear cells (PBMCs).4,5 More specifically, PTX3 is expressed and released from adipocytes, monocytes, and monocyte-derived macrophages upon cellular activation with LPS and the pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α).1,4,5 During obesity, expression of PTX3 mRNA is increased in visceral adipose tissue.1,6 To the contrary, circulating concentrations of PTX3 are lower in obese compared to normal-weight individuals,7,8 indicating that plasma PTX3 levels are distinct from those produced at local inflammatory sites in obesity.
PTX3 synthesis and production following cellular activation by LPS is mediated by the transcription factors nuclear factor (NF)-κB and activator protein 1 (AP-1).9–11 Consequently, PTX3 has been shown to serve as a counter-regulatory protein which inhibits pro-inflammatory signaling,5 protects the hosts from inflammatory damage of the heart (i.e. reduced lesion size within the coronary artery and aorta),12,13 and improves survival from toxic shock caused by LPS in vivo.14 In addition, the anti-inflammatory cytokine interleukin 10 (IL-10) produced by PTX3-stimulated PBMCs ex vivo is considered a key anti-inflammatory mechanism of PTX3.15 Therefore, increased PTX3 levels may be important to protect against obesity-associated inflammatory diseases, such as cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM).
Importantly, aerobic exercise training has been shown to promote cardiorespiratory fitness and reduce the risk of obesity-associated inflammatory diseases by conferring an anti-inflammatory effect.16,17 For example, resting levels of plasma PTX3 are elevated in aerobically trained men and women,18,19 and in obese individuals following aerobic exercise intervention.20 We have recently demonstrated that acute aerobic exercise enhances plasma PTX3 levels in obese and normal-weight subjects, but obese PTX3 levels are still less than 50% of the normal-weight subject levels.8 Therefore, the purpose of this study was to determine whether or not obesity would reduce the capacity of PBMCs to produce PTX3 in response to ex vivo stimulation with LPS, and if acute aerobic exercise would enhance this PTX3 production capacity. In addition, the inter-relationships of LPS-induced PTX3 with the inflammatory cytokines (IL-6, IL-10 and TNF-α) were examined.
Materials and methods
Subjects
Twenty-one healthy subjects (10 obese [4 males and 6 females] and 11 normal-weight [5 males and 6 females]) 18 to 35 years of age were recruited to participate in the study. Subjects with a body mass index (BMI) above or equal to 30 kg/m2 were classified as obese, and those with a BMI between 18.5 and 24.9 kg/m2 were classified as normal-weight. All subjects provided informed consent and completed a medical history questionnaire prior to data collection. Additionally, a seven-day physical activity record was obtained indicating that all subjects participated in less 150 min of physical activity per week prior to their participation in this research investigation. The study was approved by the University’s Institutional Review Board.
Subjects diagnosed with inflammatory diseases/conditions (e.g. CVD, chronic kidney or liver disease, diabetes), under the current administration of medication known to alter inflammatory and/or metabolic profiles, who were users of tobacco products (cigarettes, cigars, chewing tobacco), and/or consumed an average of 10 or more standard alcoholic beverages per week were excluded. All subjects were instructed to undergo an overnight fast for at least 8 h and to abstain from alcohol, caffeine intake, and intense physical activity for at least 24 h prior to each laboratory visit. Finally, women who were pregnant or nursing also were excluded from the study because of the potential effects on immune responses.21
Procedures
Subjects arrived at the laboratory between 7:00 and 9:00 on the morning of two separate testing sessions separated by a minimum of one week. During session one, subjects provided informed consent and were familiarized with all instruments and procedures. Thereupon, anthropometric measures (BMI and waist-to-hip ratio) were obtained and a maximal oxygen consumption (VO2max) test was administered in gradation on a treadmill beginning with a 3-min warm-up at 3 mph with 0% grade. Speed was increased to elicit 80% ± 5 bpm of the subject’s age predicted maximal heart rate (HR) within the first 2 min (stage one), and allowed to reach a steady-state during the next 2 min (stage two). After 4 min, grade was increased 2% every 2 min while speed remained constant until the subjects reached voluntary exhaustion within 12 to 15 min. VO2 max was determined using ParvoMedics Metabolic Measurement System (ParvoMedics, Sandy, UT). HR and rating of perceived exertion (RPE) were recorded during the final 15 s of every exercise stage. Rates of oxygen consumption (VO2) and carbon dioxide production (VCO2) were assessed and averaged every 15 s to calculate respiratory exchange ratio (RER: VCO2/VO2). Criteria for attaining VO2 max included a plateau in O2 consumption and two of the following secondary criteria: RER ≥ 1.15, HR within 10 bpm of subject’s age-predicted maximum HR (220-age), and an RPE ≥ 19. HR and blood pressure (BP) were assessed by HR monitors (Polar T31, Polar Electro, Kempele, Finland) and sphygmomanometer (752M-Mobile Series, American Diagnostic Corporation, Hauppauge, NY) prior to exercise and during recovery.
The second exercise testing session consisted of 30 min of continuous exercise at 75% VO2max as determined during session one, with HR and BP assessment prior to and immediately post-exercise. A 10 mL whole blood sample was drawn from each subject’s anticubital vein prior to, immediately post, and at 1 and 2 h into recovery (R1h and R2h) using a 21G butterfly needle into a tube containing K2 ethylenediaminetetraacetic acid (K2EDTA) (BD Vacutainer, Franklin Lakes, NJ).
Assessments of LPS-induced PTX3 and inflammatory cytokine secretion
LPS-stimulated PTX3 and inflammatory cytokine production by PBMCs ex vivo in obesity was assessed by the previously published method.22 Whole blood samples were immediately centrifuged at 3000 rpm for 20 min at room temperature. The buffy coat was collected and carefully layered over equal volume of Ficoll-Paque (ρ = 1.077 g/mL; Sigma-Aldrich, St. Louis, MO) in a conical tube and centrifuged at 400 × g for 30 min at room temperature. The mononuclear cell layer was isolated, and washed with saline three times. PBMC cells at 1.0 × 106 cells/mL were cultured in RPMI 1640 media plus 5% fetal bovine serum (FBS) and 1% penicillin and streptomycin in a 96-well cultured plate (Corning Incorporated, Corning, NY). Some cultures were stimulated with LPS (10 ng/mL; Phenol extraction from E. coli, Sigma-Aldrich). After incubation at 37℃ for 24 h, culture supernatant was isolated and analyzed to assess cellular secretion of PTX3, IL-6, IL-10, and TNF-α.
PTX3 (R&D Systems, Minneapolis, MN) and cytokine concentrations (IL-6, IL-10, and TNF-α; Biolegend, Inc. San Diego, CA) were quantified in duplicate by ELISA methods according to manufacturer’s instructions. PTX3 and cytokine levels from PBMC cultures without LPS were lower than minimum detection levels, and therefore not included in the analysis.
Statistical analyses
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS version 22.0). Independent t-tests were conducted to compare anthropometric and cardiorespiratory profiles, as well as LPS-stimulated PTX3, IL-6, IL-10, and TNF-α concentrations between obese and normal-weight subjects at rest. A two group (obese and normal-weight) by four time point (pre, post, R1h, R2h) repeated measures analysis of variance (ANOVA) was used to examine the effect of acute aerobic exercise on LPS-stimulated PTX3, IL-6, IL-10, and TNF-α responses. If the Mauchly's test indicated violation of the sphericity assumption, the degrees of freedom were corrected by using Greenhouse-Geisser estimates. Furthermore, the percent change (pre to immediately post-exercise) in PTX3, IL-6, IL-10, and TNF-α concentrations were calculated to examine the relationship among these variables and with BMI, waist-to-hip ratio, and relative VO2max by Pearson’s correlations. Finally, data on plasma PTX3 concentrations from our current subjects (9 obese and 9 normal-weight only) have been previously published.8 Thus, this study also examined the relationship of plasma and LPS-stimulated ex vivo production of PTX3 with LPS-stimulated ex vivo IL-6, IL-10, and TNF-α production at rest and in response to acute exercise. All data are presented as means ± SEM unless otherwise stated with statistical significance being defined as a P value ≤0.05.
Results
Participants’ anthropometric and cardiovascular characteristics
Table 1 demonstrates anthropometric characteristics of obese and normal-weight subjects. Obese subjects exhibited significantly greater measures of body weight (t [19] = 5.51, P < 0.001), BMI (t [11.42] = 9.98, P < 0.001), waist and hip circumferences (t [19] = 7.11; t [11.98] = 7.20, P < 0.001, respectively), waist-to-hip ratio (t [19] = 3.32, P = 0.004), and resting systolic and diastolic blood pressures (t [19] = 4.10, P = 0.001; t [19] = 4.21, P < 0.001, respectively). Cardiorespiratory fitness levels (relative VO2max) were significantly lower in obese compared to normal-weight subjects (t [19] = −5.19, P < 0.001). Although male subjects weighed more (t [19] = 2.25, P = 0.037), were taller (t [19] = 2.386, P < 0.001), and presented with greater waist-to-hip ratios (t [19] = 3.45, P = 0.003) compared to female subjects, no differences in any other variables, including BMI or VO2max, were observed between male and female subjects.
Table 1.
Participant descriptive characteristics.a
| Variable | Normal-weight (n = 11: 5M/6F) | Obese (n = 10: 4M/6F) | P value |
|---|---|---|---|
| Age (y) | 23.27 ± 0.68 | 22.8 ± 1.50 | 0.77 |
| Weight (kg) | 63.42 ± 3.74 | 100.92 ± 5.84 | < 0.001* |
| Height (m) | 1.69 ± 0.04 | 1.67 ± 0.03 | 0.59 |
| BMI | 21.89 ± 0.49 | 36.08 ± 1.33 | <0.001* |
| Waist (cm) | 71.36 ± 2.17 | 99.50 ± 3.41 | <0.001* |
| Hip (cm) | 94.64 ± 1.26 | 118.44 ± 3.06 | <0.001* |
| Waist-to-hip ratio | 0.75 ± 0.02 | 0.84 ± 0.02 | 0.004* |
| Resting HR (bpm) | 68.36 ± 2.19 | 74.2 ± 3.04 | 0.131 |
| Resting SBP (mmHg) | 110.36 ± 3.04 | 127.0 ± 2.64 | 0.001* |
| Resting DBP (mmHg) | 72.00 ± 2.02 | 82.60 ± 1.43 | <0.001* |
| Relative VO2max (mL/kg/min−1) | 46.14 ± 2.28 | 30.61 ± 1.88 | <0.001* |
BMI: body mass index; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; VO2max: maximal oxygen uptake.
Data are presented as means ± SEM.
A significant difference between normal-weight and obese groups at baseline (P < 0.05).
LPS-induced PTX3 and inflammatory cytokine secretion following acute aerobic exercise
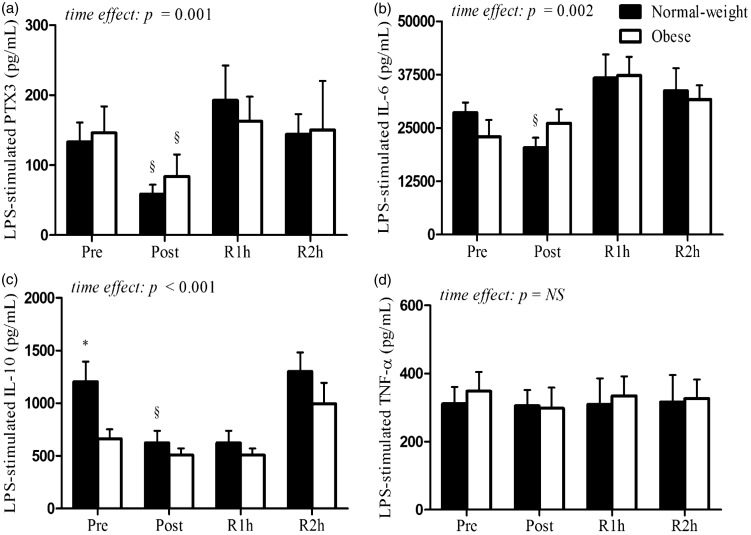
Resting levels of LPS-stimulated PTX3, IL-6, IL-10, and TNF-α are shown in Figure 1. Obese subjects showed an attenuated production of LPS-stimulated IL-10 concentrations compared to normal-weight subjects (t [14.18] = − 2.59, P = 0.021), while no differences were observed in PTX3, IL-6, and TNF-α concentrations between two groups. In response to acute aerobic exercise, repeated measures ANOVA revealed that LPS-stimulated cellular secretion of PTX3 (F [3, 57] = 6.298, P = 0.001), IL-6 (F [2.248, 40.462] = 7.062, P = 0.002), and IL-10 (F [3, 57] = 9.185, P < 0.001), but not TNF-α, was lower in both obese and normal-weight subjects (Figure 1(a)–(d)).
Figure 1.
LPS-stimulated PTX3, IL-6, IL-10, and TNF-α concentrations produced ex vivo from isolated PBMCs of normal-weight and obese subjects at rest and following acute aerobic exercise. Although no difference in LPS-stimulated PTX3, IL-6, and TNF-α concentrations were observed between obese and normal-weight subjects at rest (panels a, b, and d), LPS-stimulated IL-10 concentrations were attenuated in obese compared to normal-weight subjects (panel c). A significant time effect for LPS-stimulated PTX3, IL-6, and IL-10 (panels a–c), but not TNF-α (panel d), was observed in obese and normal-weight subjects. The * indicates a significant difference between normal-weight and obese groups at rest and following exercise. The § indicates a significant difference compared to resting concentrations (P < 0.050). Data are presented as means ± SEM. Pre, prior to exercise; Post, immediately post-exercise; R1h, 1 h into recovery from exercise; R2h, 2 h into recovery from exercise
Correlations among variables at rest and following acute aerobic exercise
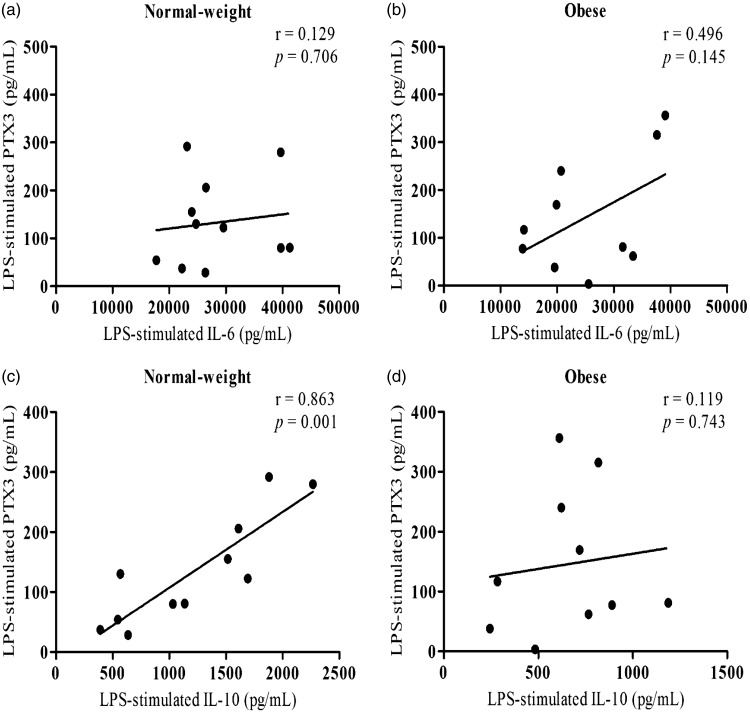
At rest, LPS-stimulated PTX3 was positively correlated with IL-10 (r = 0.863, P = 0.001), but not IL-6 or TNF-α in normal-weight, whereas no associations were observed with IL-6, IL-10, or TNF-α following LPS stimulation in obese subjects (Figure 2(a)–(d)). Additionally, waist-to-hip ratio was negatively correlated with resting levels of LPS-stimulated PTX3 and IL-10 (r = −0.429 P = 0.050; r = −0.475, P = 0.029, respectively), while BMI was negatively correlated with LPS-stimulated IL-10 (r = −0.526, P = 0.014) in all subjects.
Figure 2.
Associations of LPS-stimulated PTX3 with IL-6 and IL-10 concentrations in obese and normal-weight subjects at rest. Although LPS-stimulated PTX3 concentrations were not associated with IL-6 (panel a), a positive association was observed with IL-10 in normal-weight subjects (panel c). LPS-stimulated PTX3 concentrations were not associated with IL-6 or IL-10 in obese subjects at rest (panels b and d) (P ≤ 0.050).
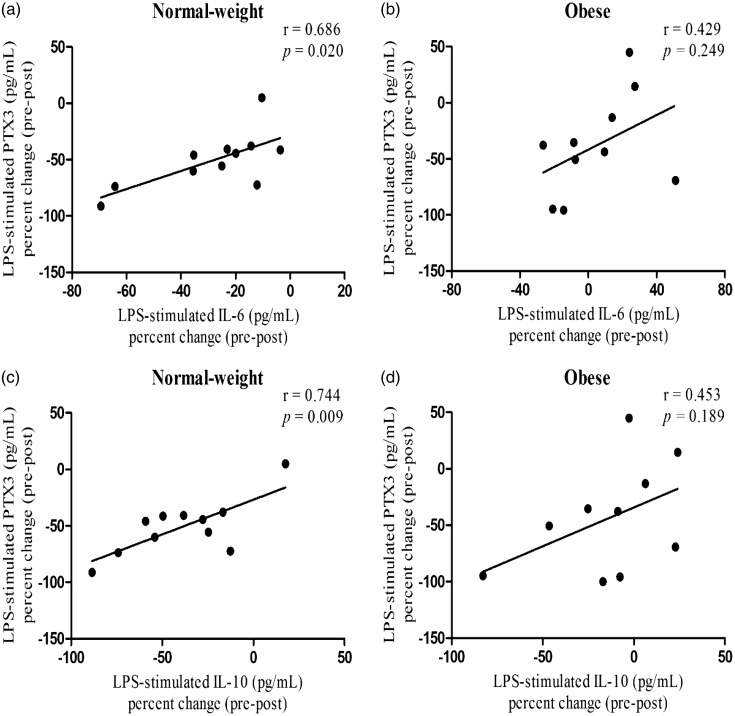
In response to acute aerobic exercise, LPS-stimulated PTX3 percent change (pre to immediately post-exercise) was positively correlated with IL-6 and IL-10 percent change (r = 0.686, P = 0.020; r = 0.744, P = 0.009; Figure 3(a) and (c)) in normal-weight, but not obese (r = 0.429, P = 0.249; r = 0.453, P = 0.189; Figure 3(b) and (d)). However, no association was observed between LPS-stimulated TNF-α and other variables. Furthermore, LPS-induced PTX3 concentrations at rest and in response to exercise were neither associated with BMI and relative VO2max. Finally, no correlations were found in plasma and LPS-stimulated ex vivo production of PTX3 with LPS-stimulated ex vivo IL-6, IL-10, and TNF-α production at rest and in response to exercise.
Figure 3.
Associations of LPS-stimulated PTX3 percent change (pre–post) with IL-6 and IL-10 percent change following exercise in obese and normal-weight subjects. The LPS-stimulated PTX3 percent change was positively associated with IL-6 and IL-10 percent change in normal-weight (panels a and c), but not obese subjects (panels b and d) (P ≤ 0.050).
Discussion
This is the first study to compare the LPS-stimulated ex vivo production of PTX3 from PBMCs and its association with pro-inflammatory and anti-inflammatory cytokines in obese and normal-weight individuals following acute aerobic exercise. It is speculated that PBMCs, mainly monocytes,23 in obesity would be exposed to higher concentrations of plasma LPS than in normal-weight individuals.3 Thus, although our LPS concentration is higher than actual plasma levels (10 ng/mL vs. ∼0.1–0.2 ng/mL),3 our ex vivo LPS stimulation would mimic such an in vivo peripheral blood condition. Nonetheless, our important findings demonstrate that LPS-induced PTX3 production levels from PBMCs at rest, which are similar in obese and normal-weight individuals, do not appear to contribute to the reduced plasma PTX3 levels that our laboratory has previously observed in obesity.8 These results also indicate that the capacity of PBMCs to produce PTX3 ex vivo as a counter-regulatory protein following inflammatory challenge is intact in obesity. Furthermore, in contrast to our laboratory’s previous finding that acute exercise slightly increases plasma PTX3 levels in both groups,8 the LPS-stimulated ex vivo production of PTX3 in both obese and normal-weight groups was decreased immediately following acute aerobic exercise. Therefore, these results clearly show that the post-exercise plasma PTX3 levels seem to be independent of the reduced ex vivo PTX3 production by PBMCs, and may be more dependent upon other cell types, such as neutrophils, which have been shown to store and release PTX3 into circulating during acute aerobic exercise.24
The other important finding in our study is that the positive associations of PTX3 with IL-6 and IL-10 observed in normal-weight individuals supports the posit that endogenous PTX3 regulates the balance between pro- and anti-inflammation in normal-weight individuals.12–14 However, these associations were not observed in obese individuals. Therefore, despite comparable production levels of PTX3, IL-6, and TNF-α by PBMCs at rest, IL-10 production was significantly diminished, suggesting that the endogenous anti-inflammatory mechanisms of PTX3 which enhance IL-10 expression is impaired in obese PBMCs. This is particularly worrisome given that our laboratory and others have recently began to elucidate the mechanisms by which PTX3 preferentially increases IL-10 production from isolated PBMCs in normal-weight individuals, down-regulates the pro-inflammatory signaling cascade, and protects monocyte-derived macrophages from premature cellular death.5,15,25,26 These findings have provided evidence to suggest that PTX3 aids in the polarization of monocyte-derived macrophages from an M1, pro-inflammatory, to an M2, anti-inflammatory phenotype, and thus, important mechanistic targets that may aid in the prevention in obesity-related pro-inflammatory disease, including CVD and T2DM.
Another possible explanation for the impaired production of IL-10 at rest may be due to the redistribution of monocyte subsets between obese and normal-weight individuals. Monocytes are a heterogeneous population, and the ligation to LPS by the pattern recognition receptor toll-like receptor 4 (TLR4) is facilitated by either classical monocytes (CD14+/CD16−) or pro-inflammatory monocytes (CD14+/CD16+) that results in the elevated cellular secretion of IL-6/IL-10, and TNF-α, respectively.27–29 Although the subset of monocytes responsible for PTX3 production is currently unknown, it is possible that the lower resting levels of LPS-stimulated IL-10 observed in obese subjects is a consequence of reduced proportions of classical relative to pro-inflammatory monocytes in obese individuals.30–32
Furthermore, acute aerobic exercise is purported to induce an anti-inflammatory response by decreasing the capacity of circulating monocytes to produce inflammatory proteins.33–35 While findings from these studies suggest that circulating monocytes may temporarily be in an anergic state (hypo-responsive) that potentially contributes to the reduced capacity of monocytes to produce and secrete PTX3 following LPS stimulation,36 other studies indicate that the exercise exerts an anti-inflammatory response via the downregulation of TLR4 surface expression on CD14+ monocyte.17,37 Interestingly, Hong and Mills demonstrated that 20 min of treadmill running at 65–70% VO2max resulted in the preferential mobilization of pro-inflammatory monocytes, and that while TLR4 expression was reduced on classical monocytes, pro-inflammatory monocytes expressed elevated levels of TLR4.38 Although the percentage of monocytes in the present study are unknown, and therefore the authors cannot verify whether the percentage of monocyte subsets are different among obese and normal-weight individuals at rest or in response to acute aerobic exercise, a recent study has demonstrated that obesity does not influence the distribution of monocyte subsets immediately following acute exercise.32 Therefore, further studies are necessary to examine the phenotypic changes of monocytes in response to acute aerobic exercise as a mechanism to explain the suppressed production of PTX3 and IL-10 that was observed in both obese and normal-weight groups in the present study.
In conclusion, this study demonstrates that PBMCs isolated from obese and normal-weight individuals produce comparable amounts of PTX3 ex vivo in response to LPS. Furthermore, given that acute aerobic exercise reduced the ex vivo production of PTX3 in both groups, our results clearly indicate that plasma PTX3 levels are relatively independent of those produced by PBMCs ex vivo and further suggest that the mechanisms associated with PTX3-mediated anti-inflammatory signaling is impaired in obese individuals. Therefore, additional research focusing on obesity-related mechanistic consequences related to PTX3 signaling may provide comprehensive knowledge necessary for the elucidation of PTX3’s role in the mediation of anti-inflammatory profiles and the subsequent amelioration of inflammatory disease during obesity.39
Authors' contributions
All authors participated in the design, interpretation and analysis of the data, and the review of the manuscript. Conception and design: ALS, MW, C-JH. Data collection: ALS, MW, AM, JBQ, C-JH. Data analysis and interpretation: ALS, YS, C-JH. Manuscript writing: ALS, YS, MW, AM, JMQ, C-JH. Final approval of manuscript: ALS, YS, MW, AM, JMQ, C-JH.
Declaration of conflicting interests
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
Funding for this study was provided by the Departments of Exercise Science and Health Promotion and Biomedical Science at Florida Atlantic University. The University had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
References
- 1.Osorio-Conles O, Guitart M, Chacón M, Maymo-Masip E, Moreno-Navarrete J, Montori-Grau M, Näf S, Fernandez-Real JM, Vendrell J, Gómez-Foix AM. Plasma PTX3 protein levels inversely correlate with insulin secretion and obesity, whereas visceral adipose tissue PTX3 gene expression is increased in obesity. Am J Physiol Endocrinol Metab 2011; 301: E1254–61. [DOI] [PubMed] [Google Scholar]
- 2.Hill A, Lowes D, Webster N, Sheth C, Gow N, Galley H. Regulation of pentraxin-3 by antioxidants. Br J Anaesth 2009; 103: 833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang H, Hussey SE, Sanchez-Avila A, Tantiwong P, Musi N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PloS One 2013; 8: e63983–e63983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maina V, Cotena A, Doni A, Nebuloni M, Pasqualini F, Milner CM, Day AJ, Mantovani A, Garlanda C. Coregulation in human leukocytes of the long pentraxin PTX3 and TSG-6. J Leukoc Biol 2009; 86: 123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiraki A, Kotooka N, Komoda H, Hirase T, Oyama J-I, Node K. Pentraxin-3 regulates the inflammatory activity of macrophages. Biochem Biophys Rep 2016; 5: 290–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abderrahim-Ferkoun A, Bezy O, Chiellini C, Maffei M, Grimaldi P, Bonino F, Moustaid-Moussa N, Pasqualini F, Mantovani A, Ailhaud G, Amri E-Z. Characterization of the long pentraxin PTX3 as a TNF alpha-induced secreted protein of adipose cells. J Lipid Res 2003; 44: 994–1000. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki K, Kurimura M, Kasai T, Sagara M, Kodama T, Inoue K. Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clin Chem Lab Med 2009; 47: 471–7. [DOI] [PubMed] [Google Scholar]
- 8.Slusher AL, Mock JT, Whitehurst M, Maharaj A, Huang C-J. The impact of obesity on pentraxin 3 and inflammatory milieu to acute aerobic exercise. Metabolism 2015; 64: 323–9. [DOI] [PubMed] [Google Scholar]
- 9.Basile A, Sica A, d'Aniello E, Breviario F, Garrido G, Castellano M, Mantovani A, Introna M. Characterization of the promoter for the human long pentraxin PTX3: role of NF-κB in tumor necrosis factor-α and interleukin-1β regulation. J Biol Chem 1997; 272: 8172–8. [DOI] [PubMed] [Google Scholar]
- 10.Jaillon S, Peri G, Delneste Y, Frémaux I, Doni A, Moalli F, Garlanda C, Romani L, Gascan H, Bellocchio S, Bozza S, Cassatella MA, Jeannin P, Mantovani A. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med 2007; 204: 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paeschke A, Possehl A, Klingel K, Voss M, Voss K, Kespohl M, Sauter M, Overleeft HS, Althof N, Garlanda C, Voigt A. The immunoproteasome controls the availability of the cardioprotective pattern recognition molecule Pentraxin3. Eur J Immunol 2016; 46: 619–33. [DOI] [PubMed] [Google Scholar]
- 12.Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, Pasqualini F, Latini R, Garlanda C, Mantovani A. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 2008; 117: 1055–64. [DOI] [PubMed] [Google Scholar]
- 13.Norata GD, Marchesi P, Venu VKP, Pasqualini F, Anselmo A, Moalli F, Pizzitola I, Garlanda C, Mantovani A, Catapano AL. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation 2009; 120: 699–708. [DOI] [PubMed] [Google Scholar]
- 14.Dias AA, Goodman AR, Dos Santos JL, Gomes RN, Altmeyer A, Bozza PT, Horta MdF, Vilcek J, Reis LFL. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol 2001; 69: 928–36. [PubMed] [Google Scholar]
- 15.Slusher AL, Mischo AB, Acevedo EO. Pentraxin 3 is an anti-inflammatory protein associated with lipid-induced interleukin 10 in vitro. Cytokine 2016; 86: 36–40. [DOI] [PubMed] [Google Scholar]
- 16.Kullo IJ, Khaleghi M, Hensrud DD. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol 2007; 102: 1374–9. [DOI] [PubMed] [Google Scholar]
- 17.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011; 11: 607–15. [DOI] [PubMed] [Google Scholar]
- 18.Miyaki A, Maeda S, Otsuki T, Ajisaka R. Plasma pentraxin 3 concentration increases in endurance-trained men. Med Sci Sports Exerc 2011; 43: 12–7. [DOI] [PubMed] [Google Scholar]
- 19.Miyaki A, Maeda S, Choi Y, Akazawa N, Tanabe Y, Ajisaka R. Habitual aerobic exercise increases plasma pentraxin 3 levels in middle-aged and elderly women. Appl Physiol Nutr Metab 2012; 37: 907–11. [DOI] [PubMed] [Google Scholar]
- 20.Miyaki A, Maeda S, Choi Y, Akazawa N, Tanabe Y, So R, Tnaka K, Ajisaka R. The addition of whole-body vibration to a lifestyle modification on arterial stiffness in overweight and obese women. Artery Res 2012; 6: 85–91. [Google Scholar]
- 21.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 2010; 63: 425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C-J, Acevedo EO, Mari DC, Randazzo C, Shibata Y. Glucocorticoid inhibition of leptin-and lipopolysaccharide-induced interleukin-6 production in obesity. Brain Behav Immun 2014; 35: 163–8. [DOI] [PubMed] [Google Scholar]
- 23.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990; 249: 1431–3. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Kurano M, Hasegawa T, Takano H, Iida H, Yasuda T, Fukuda T, Madarame H, Uno K, Meguro K, Shiga T. Pentraxin 3 and high-sensitivity C-reactive protein are independent inflammatory markers released during high-intensity exercise. Eur J Appl Physiol 2010;110:905–13. [DOI] [PubMed]
- 25.He H, Zhang S, Tighe S, Son J, Tseng SC. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem 2013; 288: 25792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozza S, Campo S, Arseni B, Inforzato A, Ragnar L, Bottazzi B, Mantovani A, Moretti S, Oikonomous V, De Santis R, Carvalho A, Salvatori G, Romani L. PTX3 binds MD-2 and promotes TRIF-dependent immune protection in aspergillosis. J Immunol 2014; 193: 2340–8. [DOI] [PubMed] [Google Scholar]
- 27.Frankenberger M, Sternsdorf T, Pechumer H, Pforte A, Ziegler-Heitbrock H. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood 1996; 87: 373–7. [PubMed] [Google Scholar]
- 28.Belge K-U, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+ CD16+ DR++ monocytes are a major source of TNF. J Immunol 2012; 168: 3536–42. [DOI] [PubMed] [Google Scholar]
- 29.Shalova IN, Kajiji T, Lim JY, Gómez-Piña V, Fernández-Ruíz I, Arnalich F, Iau PT, López-Collazo E, Wong S-C, Biswas SK. CD16 regulates TRIF-dependent TLR4 response in human monocytes and their subsets. J Immunol 2012; 188: 3584–93. [DOI] [PubMed] [Google Scholar]
- 30.Rogacev KS, Ulrich C, Blömer L, Hornof F, Oster K, Ziegelin M, Cremers B, Grenner Y, Geisel J, Schlitt A, Köhler H, Filser D, Girndt M, Heine GH. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur Heart J 2010; 31: 369–76. [DOI] [PubMed] [Google Scholar]
- 31.Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn J-F, Veyrie N, Rizkalla S, Fridman W-H, Sautès-Fridman C, Clément K, Cremer I. CD14dimCD16+ and CD14+ CD16+ monocytes in obesity and during weight loss relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 2011; 31: 2322–30. [DOI] [PubMed] [Google Scholar]
- 32.Wonner R, Wallner S, Orsó E, Schmitz G. Effects of acute exercise on monocyte subpopulations in metabolic syndrome patients. Cytometry B Clin Cytom. Epub ahead of print 10 June 2016. doi: 10.1002/cyto.b.21387. [DOI] [PubMed]
- 33.Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA. Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-α levels after prolonged running. Am J Physiol Cell Physiol 2001; 280: C769–74. [DOI] [PubMed] [Google Scholar]
- 34.Rhind SG, Castellani JW, Brenner IKM, Shephard RJ, Zamecnik J, Montain SJ, Young AJ, Shek PN. Intracellular monocyte and serum cytokine expression is modulated by exhausting exercise and cold exposure. Am J Physiol Regul Integr Comp Physiol 2001; 281: R66–75. [DOI] [PubMed] [Google Scholar]
- 35.Febbraio MA, Starkie RL. Letters to the editor. Am J Physiol Regul Integr Comp Physiol 2002; 282: R1253–57. [DOI] [PubMed] [Google Scholar]
- 36.Cavaillon JM. The nonspecific nature of endotoxin tolerance. Trends Microbiol 1995; 3: 320–3. [DOI] [PubMed] [Google Scholar]
- 37.Flynn MG, McFarlin BK. Toll-like receptor 4: link to the anti-inflammatory effects of exercise? Exerc Sport Sci Rev 2006; 34: 176–81. [DOI] [PubMed] [Google Scholar]
- 38.Hong S, Mills PJ. Effects of an exercise challenge on mobilization and surface marker expression of monocyte subsets in individuals with normal vs. elevated blood pressure. Brain Behav Immun 2008; 22: 590–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das UN. Anti-inflammatory nature of exercise. Nutrition 2004; 20: 323–6. [DOI] [PubMed] [Google Scholar]