Abstract
Balanced anesthesia allows for a reduced dosage of each component, while inducing general anesthesia of sufficient depth with potentially fewer side effects. Here, we compare two anesthetic protocols combining sevoflurane anesthesia with pre-medication (ketamine [K] or fentanyl-midazolam [FM]) to a sevoflurane monoanesthesia (S) concerning their ability to provide reliable anesthesia suitable for moderate surgery in laboratory mice. Twenty-one female C57BL/6J mice assigned randomly to one of three protocols underwent a 50-min anesthesia and a sham embryo transfer. Heart rate and core body temperature were continuously recorded by telemetry intra-operatively and for three days pre- and three days post-surgery. Intra-operative respiratory rate was determined by counting thorax movements. Body weight, food, and water intake were measured daily for three days pre- and three days post-surgery. The heart rate in the KS group remained at baseline level throughout the 50-min of anesthesia and surgery. FMS caused a lower heart rate and S alone caused a higher heart rate compared to baseline values. Intra-operative body temperature was at baseline levels in all groups. A decreased respiratory rate was observed in all groups compared to baseline values obtained from resting mice of the same strain, sex and age-distribution. Surgical stimuli induced no significant changes in heart rate and respiratory rate in the KS or FMS group but significant respiratory alteration in the S group compared to baseline values obtained 10 s before applying the stimulus. Post-operative heart rate was above baseline values in all groups; with a significant deviation in the S group. There were no changes in body weight, food, and water intake. In summary, FMS was superior to KS and S for moderate surgery in laboratory mice resulting in less inter-individual variability in response to painful stimuli. Fentanyl and midazolam reduced the depressant effect of sevoflurane on the respiratory rate and the negative post-anesthetic effects on the heart rate.
Impact statement
With approximately 65 million animals used per year mice are still the most prevalent laboratory mammal species worldwide. In course of biomedical research projects approximately 40% of mice will undergo one or more short or long-term anesthesia. Sufficient anesthetic depth, cardiovascular stability, adequate analgesia, and short recovery times are essential requirements of anesthetic protocols to meet animal welfare. Anesthesia in mice and rats are only to be performed by personnel with appropriate basic training and experience. However, more and more adapted and advanced anesthetic protocols, required to answer very specific scientific questions, often exceed the skills acquired through basic training and present a major challenge to researchers. It is therefore of great importance to further develop and evaluate safe and reliable anesthetic protocols as presented in this study to provide new perspectives on this challenging problem.
Keywords: Balanced anesthesia, surgery, mice, fentanyl-midazolam, sevoflurane, refinement
Introduction
Safe and reliable anesthesia in laboratory mice and rats has long been an important concern in biomedical research. Sufficient anesthetic depth, cardiorespiratory stability, and short recovery times are essential requirements of anesthetic protocols; optimizing these factors attenuates the mortality associated with rodent anesthesia by minimizing hypothermia and hypoxia.1 Anesthetics can be delivered to laboratory rodents by different routes: injection, inhalation, or as a combination of both. Modern commercial inhalation anesthetics, such as isoflurane and sevoflurane, have been shown to be well controllable in terms of anesthetic depth due to their low blood:gas partition coefficients, and also have the advantages of providing rapid induction of anesthesia, being short-acting and easily removed from the body with minimal metabolism, biotransformation and excretion.2,3 Therefore, volatile anesthetics might have fewer confounding effects on scientific read-out from experiments.4 Furthermore, simple adjustment and titration of the dose diminishes issues with strain-, age- or sex-related variability in minimal alveolar concentration,5,6 and allows a rapid response to the situational needs of individual animals.4 However, inhalation delivery of anesthetics is often attended by negative effects on the cardiovascular system, and respiratory depression caused by halogenated volatile anesthetics;7,8 another factor to consider is the need for specific equipment and its associated costs.9 In contrast, injecting anesthetics seems easy, requiring minimal operator skill, and incurs minimal costs and equipment.9 Completely antagonisable anesthesia protocols allow to overcome the often described limitation of injection anesthesia, namely a prolonged recovery period accompanied by hypothermia and compromised physiologic functions.10,11 Nonetheless, total intravenous anesthesia (TIVA), which is often used in humans and larger animals due to the advantage of using short-acting drugs (e.g. propofol), is hard to master in small animals such as mice. The intraperitoneal or subcutaneous application route is therefore normally chosen in this species.12,13 The course and safety of anesthesia following intraperitoneal or subcutaneous injection of an initial dose is often unpredictable, and safety margins are often very narrow. High intra- and inter-strain, as well as sex-dependent, dose variability regularly hamper the attainment of the desired depth of anesthesia.13,14
The concept of combining different anesthetic drugs—independent of delivery route—is often referred to as balanced anesthesia. This approach was introduced as early as 1926 by John S Lundy, who argued successfully that the simultaneous administration of various anesthetic agents lowered the risk of overdose of one single anesthetic agent.15 Reduced doses of each individual agent can be used due to their synergistic and additive effects. Balanced anesthetic protocols not only led to unconsciousness but also ensure muscle relaxation, analgesia, and stable cardiorespiratory function.16
Two anesthetic protocols in laboratory mice describing a combination of sevoflurane (S) anesthesia with pre-medication of either ketamine (K) or fentanyl-midazolam (FM) were previously published.17 In this published setting of anesthetizing mice without subjecting them to surgery, pre-medicating with FM was superior to pre-medicating with K or no pre-medication at all in regard to its marked MAC sparing effect, a decrease of induction time and an improvement of induction quality. Additionally, during the first 12 h post anesthesia mice pre-medicated with FM showed less of an increase of heart rate compared to the other groups. However, during anesthesia a decrease of core body temperature and heart rate as well as marked respiratory depression leading to hypoxia, hypercapnia and acidosis were evident in all groups. Both pre-medications seemed to intensify these effects. Some mice pre-medicated with K even showed apnea, tachypnea and cardiac arrhythmia, and two mice in this group died.17 Based on this study the questions arose whether the lack of surgical stimulation may have exacerbated undesired effects associated with duration of anesthesia and whether any of the described protocols would be suitable for moderate surgical interventions in laboratory mice.
The goal of the present study was therefore to apply the same anesthesia protocols (i.e. sevoflurane either as monoanesthesia [S] or after pre-medication with S-ketamine [KS] or a mixture of fentanyl and midazolam [FMS]) this time for a moderate surgical intervention in laboratory mice. The aim was to evaluate and compare them in regard to their ability to provide a balanced anesthesia with sufficient analgesia for a 50-min surgery. Motor reflex responses were assessed at the beginning of anesthesia and surgery to ensure a level of surgical plane. During anesthesia and surgery (sham embryo transfer), heart rate, core body temperature, and respiratory rate were monitored. The long-term effects of the three protocols on recovery from anesthesia and surgery were investigated through telemetric measurements of heart rate and core body temperature, as well as food and water intake for the three days following anesthesia and surgery.
Materials and methods
Ethics statement
This study was approved by the Cantonal Veterinary Office (Zurich, Switzerland) under license number 111/2007. Animal housing and all experimental procedures were in accordance with Swiss animal welfare protection law, and conform to European Directive 2010/63/EU of the European Parliament and the Council on the Protection of Animals used for Scientific Purposes, and to the Guide for the Care and Use of Laboratory Animals.18
Housing and husbandry
Health status was monitored throughout the experiment using a surveillance program according to FELASA recommendations; all mice were free of all viral, bacterial, and parasitic pathogens listed therein.19
Mice were housed in Eurostandard type III open-top plastic cages (Techniplast, Indulab; Gams, Switzerland) interspersed with autoclaved aspen bedding (80–90 g/cage) (LTE E-001; Abbed, Indulab).
Mice were kept in pairs; a non-implanted mouse of the same age and strain thus accompanied each transmitter-implanted mouse. Nesting material was provided in the form of autoclaved hay (8–12 g/cage; Winzeler, Affoltern am Albis, Switzerland) and two cotton nesting pads (5 x 5 cm; Nestlets®, Indulab). A standard cardboard house (Ketchum Manufacturing, Brockville, Canada) served as a shelter. A pelleted mouse diet (3431, Provimi Kliba, Kaiseraugst, Switzerland) was fed ad libitum. Unrestricted access to drinking water through water bottles was ensured.
A 12:12 h light:dark (1500–0300 light phase) cycle was maintained with artificial lights. Animals were adapted to this light cycle for several months. To minimize interfering influences on data recording, all necessary husbandry procedures were performed one day before the start of the telemetric recording (3 d prior to anesthesia and surgery) and were resumed after the telemetric recording was completed (3 d after anesthesia and surgery). Health checks were performed daily, simultaneous to body weight assessment and food and water weighing procedures.
Experimental set-up
Twenty-one female C57BL/6J mice (aged 16–36 weeks) had been equipped earlier with commercially available telemetric transmitters (TA10ETA-F20, Data Sciences International, St Paul, MN) as described previously in detail.20 After a recovery period of at least four weeks, mice were assigned randomly to one of the three anesthesia protocols described in detail below.
Anesthesia
The first anesthetic protocol represented a sevoflurane monoanesthesia (S) (5%) (Sevorane®, Abott; Baar, Switzerland). The other two protocols comprised combinations of injectable pre-medication and the inhalant agent sevoflurane for anesthetic maintenance. The combinations used were either S-Ketamine (30 mg/kg SC; S-Keta®, Graeub AG; Bern, Switzerland) and Sevoflurane (5%) (KS), or Fentanyl (0.04 mg/kg SC; Kantonsapotheke Zürich, Zurich, Switzerland), Midazolam (4 mg/kg SC; Dormicum®, RochePharma, Schweiz AG, Reinach, Switzerland) and Sevoflurane (3.3%) (FMS). Applied volume percentage of sevoflurane was based on vaporizer settings and corresponded to those previously described (i.e. 3.3% sevoflurane in FMS and 5% sevoflurane in the KS and S groups).17
To avoid any influence of circadian rhythm, all experiments and weighing procedures were done between 1500 and 1800 (beginning of the light phase). Anesthesia and surgery were performed in a separate operating area within the animal room to avoid transportation of the mice, and to ensure stable conditions of humidity, air pressure, room temperature, and ventilation.
All injectable agents were diluted in PBS immediately before injection in such a manner that dosing could be achieved by application of an injection volume of 2 µL/g body weight. Injectable agents were administered subcutaneously 8–10 min before sevoflurane anesthesia was induced. A commercially available rodent anesthesia device (Provet; Lyssach, Switzerland) equipped with a sevoflurane vaporizer (Ohmeda Sevotec 5, Abbott, Baar, Switzerland) was used for delivery of inhalation anesthesia. Pressurized fresh oxygen was used as a carrier gas at a constant flow of 600 mL/min. A pump-driven filter system ensured the continuous removal of waste anesthetic gas during anesthesia maintenance. Sevoflurane was introduced into the induction chamber at a concentration of 8%. Immediately after loss of the righting reflex (at ≤ 3 min of induction in the chamber), animals were transferred from the induction chamber to the nose mask, where anesthesia was maintained by spontaneous breathing of an oxygen-sevoflurane mixture. During the 50-min of anesthesia and surgery animals were in sternal recumbence on a water-filled heating pad (TP500, Gaymar, Orchard Park, NY) set at 39 ± 1℃.
Motor reflexes
After transferring animals from the induction chamber to the nose mask, animals were placed initially in dorsal recumbence. Tail pinch, pedal withdrawal, and abdominal skin pinch were used for a one-time assessment of surgical tolerance before placing animals in ventral recumbence for the surgical procedure. Blunt forceps with a spacer between the arms was used to allow uniform application of pressure. The reflex test was registered as positive or negative.
Surgical procedure
A one-side sham embryo transfer was performed under aseptic conditions. In detail, the hair around the surgical site was clipped, and the area disinfected with ethanol (80%). An incision of approximately 0.5 cm was made in the flank. The ovary was retracted for 30 s and then repositioned in the abdomen. The abdominal wall was sutured with Vicryl 6-0 (Ethicon; Norderstedt, Germany). For skin closure, single use surgical staples (Precise, 3 M Health Care, St. Paul, MN) were utilized. Five minutes before the end of anesthesia, animals were injected with an analgesic in the form of a single dose of the nonsteroidal-anti-inflammatory drug carprofen (5 mg/kg SC; Rimadyl®, Zoetis GmbH, Zurich, Switzerland).
The timing of all procedural steps was aligned as follows: 0–3 min (induction), 4–8 min (equilibration time), 10–12 min (skin incision [SI]), 15–17 min (abdominal wall incision [AI]), 20–22 min (manipulation of the ovary [O]), 30–32 min (suturing of the abdominal wall [AS]), 40–42 min (skin stapling [ST]), 45 min (injection of analgesic [I]). The procedure and time required for anesthesia amounted to a total of 50 min. After 50 min, sevoflurane anesthesia was stopped. Mice were left to recover for 10 min on the heating-pad, breathing oxygen through the nose mask, before being placed back into their home cages.
Telemetric data acquisition of heart rate and core body temperature
Throughout the experiment, the telemetric transmitters allowed continuous measurements of heart rate and core body temperature. Telemetric measurements were processed using the Dataquest LabPRO program, version 3.11 (Data Sciences International). The telemetric transmitter was switched on by touching the animal with a magnet; signals were detected by a receiver plate placed under the animal’s cage. Telemetric data acquisition was started three days prior to the experimental procedure and continued for three days afterwards. To establish baseline values, and to investigate post-anesthetic effects, heart rate was measured for 30 s every 5 min, and core body temperature was measured for 10 s every 5 min. Baseline values represent means from the three days prior to the experimental procedure.
To assess the acute effect after pre-medication, and during anesthesia and surgery, heart rate and core body temperature recording was performed continuously, and a mean value was calculated every 10 s (6 data points per minute). From these data, mean values of heart rate and core body temperature were calculated for each minute for each mouse.
Visual acquisition of respiratory rate
During anesthesia, the respiratory rate was recorded visually by counting movements of the thorax. Respiratory rate progression was compared to baseline values obtained from resting mice of the same strain, sex and age-distribution (150 ± 10 breaths per minute [brpm]) assessed in our lab. To determine respiratory rate alteration caused by surgical stimuli, the respiratory rate was recorded 10 s prior to, and directly after, the respective surgical stimulus. The measurement prior to the stimulus served as the baseline.
Body weight and food and water intake
Body weight was measured once daily for three days prior to, and three days after, surgical intervention. Data were obtained using a precision balance (PR 2003 Delta Range, Mettler-Toledo AG, Greifensee, Switzerland) that was adjusted specifically for use with moving animals. Body weights were corrected to account for the weight of the implanted telemetry transmitter (3.6 g). The last body weight measurement prior to the experimental procedure served as baseline to determine post-surgical body weight progression.
Food and water were weighed once daily for three days prior to, and three days after, surgical intervention using the same precision balance used for body weight assessment. Since animals were housed in pairs, daily intake was estimated by measuring the total food and water consumption for both mice divided by two. Baseline of food and water intake was calculated from the mean daily values calculated from the three days prior to the experimental procedure.
Statistics
Power calculation for group size determination was performed with G*Power 3.1 (alpha = 0.05; power 80%). Statistical analyses were performed with GraphPad Prism software version 5.04 (GraphPad Software, Inc.; La Jolla, CA). All data were tested for normal distribution and homogeneity of variance (Kolmogorov–Smirnov test and D’Agostino and Pearson omnibus normality test).
Mean and standard deviation were calculated for all parameters. A descriptive coefficient of variation (CV) was calculated for each group to determine the uniformity of effects on the heart rate and respiratory rate during the 50-min of anesthesia and the application of surgical stimuli for each protocol.
Two-tailed t-tests for dependent samples were performed for comparison of baseline and experimental HR and RR of each group.
No tests for changes over time within a group were conducted.
Significance for all statistical tests was established at P ≤ 0.05.
Results
Induction of anesthesia
Few minutes following pre-medication with FM, all mice showed signs of sedation such as reduced locomotion and sleep-like posture. When placed in the sevoflurane induction chamber behaviors observed in the sevoflurane mono-anesthesia group (e.g. defection, urinating and jumping) were completely absent in this group. Mice in the K group showed a less uniform behavior pattern following injection of ketamine. Pre-medication with ketamine lead to reduced locomotion in these mice. Some animals showed effects like tremor, ataxia, and apparent dizziness. When placed in the sevoflurane induction chamber, animals pre-medicated with ketamine less frequently exhibited behaviors observed in the S group.
Motor reflexes
Tail pinch, pedal withdrawal, and abdominal skin pinch reflexes were negative in all animals before positioning of the animal into ventral recumbence and beginning the surgical procedure.
Intra-operative measurements of heart rate and core body temperature
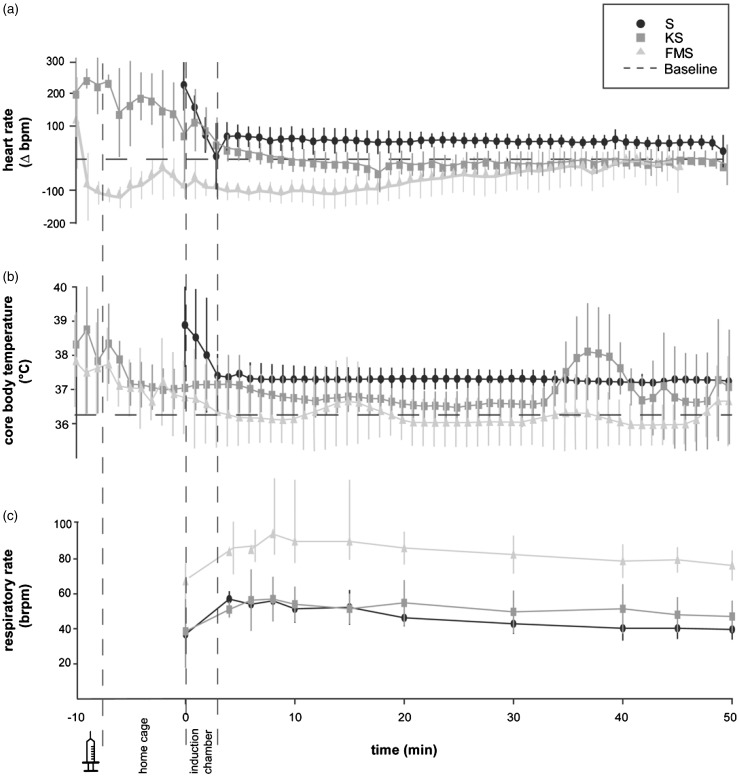
An increased heart rate compared to baseline values was observed at the time of pre-medication with injectable agents in the KS and FMS group and during exposure to sevoflurane in the induction chamber in the KS and S group (Figure 1(a)). Following pre-medication with ketamine and induction with sevoflurane, heart rate in animals in the KS group leveled off at mean baseline values, which were obtained at the corresponding time of day in non-anesthetized mice. Heart rate in the FMS group dropped rapidly after injection of FM to a nadir of 381 bpm (Δ-105 bpm compared to baseline values) at 4 min following pre-medication before increasing steadily and remaining close to but by tendency below baseline values throughout the 50-min of anesthesia and surgery. Animals in the S group showed by tendency an increased heart rate throughout the 50-min of anesthesia and surgery (Figure 1(a)).
Figure 1.
Heart rate, core body temperature and respiratory rate after pre-medication (−10 min), in the home cage (−10 to 0 min), in the induction chamber (0–3 min), and during the surgical procedure (10–50 min). (a) Mean ± SD (n = 7) heart rate deviation in beats per minute (Δbpm) compared to baseline values obtained before anesthesia at the corresponding time of day in conscious mice. (b) Mean ± SD (n = 7) core body temperature in degrees celsius (℃). All animals were in ventral recumbence and placed on a heating mat set at 39℃ throughout the surgical procedure. (c) Mean ± SD (n = 7) respiratory rate in breaths per minute (brpm). Respiratory rate was established by counting movements of the thorax wall
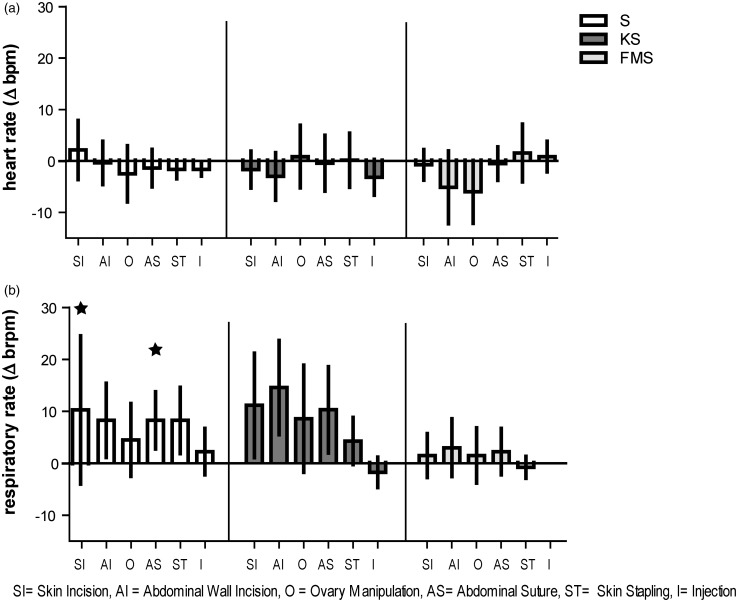
Statistical analyses did not show a significant increase in heart rate after application of surgical stimuli compared to baseline values obtained 10 s prior to each stimulus in any of the groups (Figure 2(a)). Anyhow, in the FMS group there was a significant decrease in heart rate during ovary manipulation (P = 0.028).
Figure 2.
Response of heart rate and respiratory rate to surgical stimuli. Surgical stimuli in chronological order: skin incision (SI), abdominal wall incision (AI), ovary manipulation (O), abdominal wall suture (AS), skin stapling (ST), and injection of analgesic agent (I). (a) Mean ± SD (n = 7) heart rate deviation in beats per minutes (Δbpm) compared to baseline values obtained 10 s prior to applying stimulus. (b) Mean ± SD (n = 7) respiratory rate deviation in breaths per minute (Δ brpm). Asterisks indicate significance (P ≤ 0.05) compared to baseline obtained 10 s prior to applying stimulus by counting movements of the thorax wall
The dispersion of heart rate during the 50-min of anesthesia and surgery was largest in the KS group (CV =9.32%). It was smaller in the other two groups (FMS: CV = 5.14%; S: CV = 4.04%). During the application of surgical stimuli heart rate dispersion was comparable to the dispersion seen without stimulus.
An increased core body temperature was observed at the time of pre-medication in the KS and FMS group and at the time of induction with sevoflurane in the S group (Figure 1(b)) (KS: 38.00 ± 0.76℃; FMS: 37.66 ± 1.17℃; S: 38.66 ± 1.22℃) compared to mean baseline values measured at the corresponding time phase in non-anesthetized mice (KS: 36.20 ± 0.98℃; FMS: 36.23 ± 0.84℃; S: 36.12 ±0.85℃. A decrease of core body temperature was observed in all animals with the onset of unconsciousness and the loss of the righting reflex but leveled off within baseline ranges (Figure 1(b)).
Intra-operative measurement of respiratory rate
In all anesthetic protocols a marked decrease in respiratory rate was observed, with a mean of 46.9 ± 7.02 brpm in the S group, 50.85 ± 4.95 brpm in the KS group, and 83.03 ± 7.17 brpm in the FMS group compared to baseline values obtained from resting mice of the same strain, sex, and age-distribution (150 ± 10 brpm) assessed in our lab (Figure 1(c)).
Statistical analyses showed no significant increase in respiratory rate in the KS and FMS group as response to any of the surgical stimuli applied. Several of the surgical stimuli applied led to a significant increase in respiratory rate in the S group compared to baseline values obtained 10 s prior to each stimulus (Figure 2(b)), namely skin incision (P = 0.024) and abdominal wall suture (P = 0.030). Abdominal incision as well as skin stapling caused a non-significant increase in respiratory rate in the S group.
The dispersion of respiratory rate during the 50-min of anesthesia was largest in the KS group (CV = 19.64%). During the application of surgical stimuli the dispersion of respiratory rate in the KS increased by tendency (CV = 25.80%). In the FMS group the dispersion was by tendency the smallest during the 50-min of anesthesia (CV = 11.97%) as well as during the application of surgical stimuli (CV = 11.70%). In the S group the CV increased by tendency from 12.49% during the 50-min of anesthesia to 15.53% during the application of surgical stimuli.
Post-operative measurement of heart rate and core body temperature
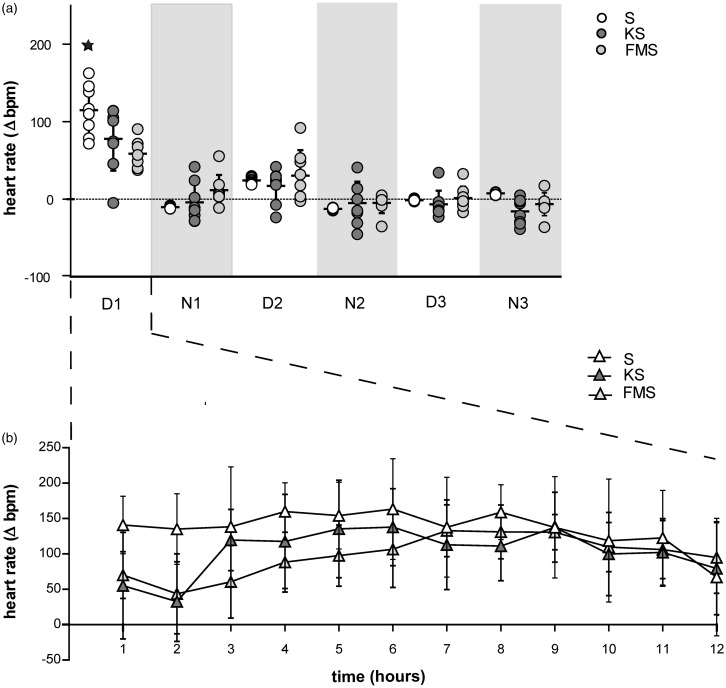
Heart rate was increased during the first 12 h (light phase) after anesthesia and surgery in all groups. Heart rate elevation was evident, although not significant, with a mean deviation of Δ77.88 ± 41.09 bpm in the KS group and Δ 58.87 ± 17.49 bpm in the FMS group compared to baseline values obtained during the three days prior to anesthesia and surgery at the corresponding time of day. Heart rate elevation was significant in the S group compared to baseline values (P = 0.0317), with a mean of Δ115.10 ± 32.47 bpm (Figure 3(a),(b)).
Figure 3.
Heart rate during immediate post-operative recovery phase (12 h) and for three light–dark cycles following anesthesia and surgery. (a) Mean ± SD (n = 7) of heart rate deviation in beats per minute (Δbpm) for three light–dark cycles following the experimental procedure. Circles show the mean results of individual animals. Asterisks indicate significance (P ≤ 0.05) compared to baseline and between groups. Dark phase is indicated by the light grey background. (b) Mean ± SD (n = 7) heart rate deviation during immediate post-operative recovery phase (12 h [light phase] following experimental procedure) in beats per minute (Δbpm) compared to baseline values obtained before the experimental procedure at the corresponding time of day in conscious mice
All groups showed a non-significant re-increase of heart rate on day 2 (light phase) post anesthesia and surgery.
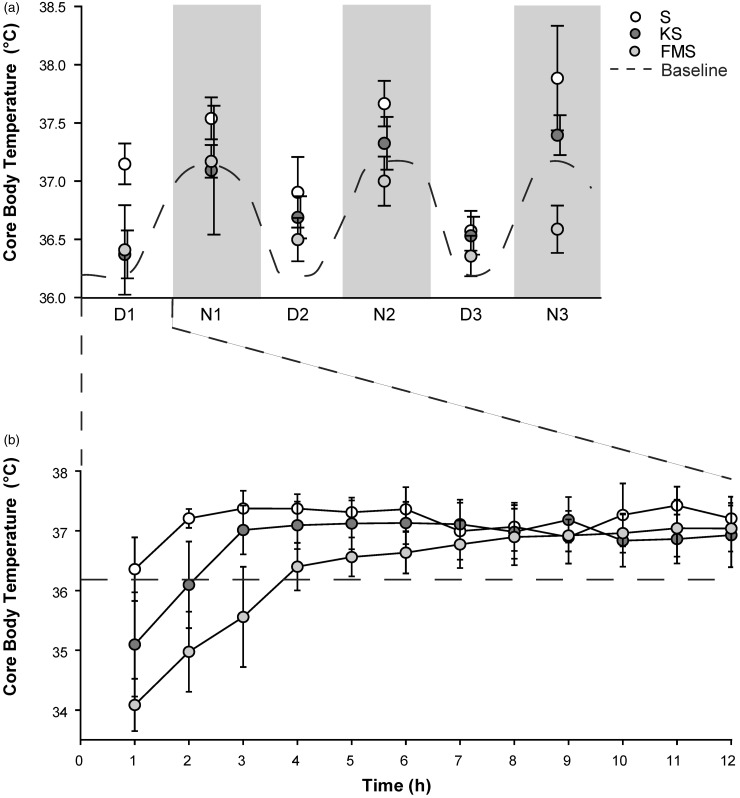
A drop in core body temperature below intra-operative measured values was observed in both pre-medicated groups during the first hour following anesthesia and surgery, with means of 34.56 ± 1.26℃ in the KS group and 34.08 ± 0.4℃ in the FMS group. Animals in the S group maintained a stable core body temperature of 36.35 ±0.45℃. Core body temperature in the KS group increased more rapidly, reaching baseline levels 2–3 h following anesthesia and surgery compared to animals in the FMS group, which reached baseline levels at 8 h post anesthesia and surgery (Figure 4(b)). Core body temperature resembled normal circadian fluctuation over the next three light–dark cycles.
Figure 4.
Core body temperature during immediate post-operative recovery phase (12 h) and for three light–dark cycles following anesthesia and surgery. (a) Mean ± SD (n = 7) core body temperature in degree celsius (℃) during 3 light–dark cycles following experimental procedure. Dark phase is indicated by the light grey background. (b) Mean ± SD (n = 7) core body temperature progression during immediate post-operative recovery phase (12 h [light phase] following the experimental procedure) in degree celsius (℃)
Body weight, and food and water intake
Body weight
Mice weighed on average 27 ± 2.17 g (S), 30.58 ± 2.63 g (KS), and 30.2 ± 0.94 g (FMS) at baseline. Anesthesia and surgery did not have an effect on body weight progression in any group (Table 1).
Table 1.
Body weight progression, and food and water intake.a
| S (n = 7) | KS (n = 7) | FMS (n = 7) | |
|---|---|---|---|
| Baseline body weight (g) | 27 ± 2.17 | 30.58 ± 2.63 | 30.2 ± 0.94 |
| Post-surgical body weight progression (g) | |||
| Day 1 | 27.03 ± 2.05 | 31.95 ± 2.06 | 30.0 ± 1.07 |
| Day 2 | 27.47 ± 1.99 | 31.08 ± 2.23 | 29.6 ± 0.21 |
| Day 3 | 27.85 ± 1.85 | 30.83 ± 2.53 | 29.9 ± 1.24 |
| Baseline food intake (g/day) | 3.68 ± 0.41 | 3.24 ± 0.68 | 3.48 ± 0.48 |
| Post-surgical food intake (g/day) | |||
| Day 1 | 3.66 ± 0.45 | 3.11 ± 0.78 | 3.42 ± 0.24 |
| Day 2 | 3.88 ± 0.49 | 3.19 ± 0.93 | 3.35 ± 0.67 |
| Day 3 | 3.34 ± 0.31 | 3.35 ± 0.83 | 3.63 ± 0.30 |
| Baseline water consumption (g/day) | 3.63 ± 0.53 | 4.53 ± 1.75 | 4.29 ± 0.80 |
| Post-surgical water consumption (g/day) | |||
| Day 1 | 4.38 ± 1.24 | 3.94 ± 0.62 | 4.35 ± 0.66 |
| Day 2 | 3.32 ± 0.64 | 3.65 ± 0.75 | 4.23 ± 0.82 |
| Day 3 | 3.45 ± 0.71 | 3.57 ± 0.37 | 3.85 ± 0.38 |
Mean ± SD (n = 7) of baseline measurements of body weight in grams (g), food and water intake in grams per day (g/day). Mean ± SD (n = 7) of body weight progression in grams (g), food and water intake in grams per day (g/day) for 3 days following experimental procedure.
Food and water intake
Prior to anesthesia and surgery, mice ate an average of 3.68 ± 0.41 g (S), 3.24 ± 0.68 g (KS), and 3.48 ± 0.48 g (FMS) of food pellets a day. The pre-anesthetic water intake was 3.63 ± 0.53 g (S), 4.53 ± 1.75 g (KS), and 4.29 ± 0.80 g (FMS) of water a day. Mice did not decrease their water or food intake after anesthesia and surgery (Table 1).
Discussion
This study used intra-operatively acquired physiological parameters such as heart rate, core body temperature and respiratory rate as well as post-operatively acquired parameters such as heart rate, core body temperature, body weight, and food and water consumption to evaluate three different anesthesia protocols with regard to their suitability for moderate surgical interventions in laboratory mice. All three protocols provided a reliable 50-min anesthesia with all motor reflex responses to noxious stimuli suppressed prior to the beginning of the surgical procedure. Aside from motor reflexes, physiological parameters, such as heart rate and respiratory rate, have shown to be valuable indicators of sufficient anesthetic depth and absence of nociception.21–23 However, aberrations in cardiorespiratory values can also be due to various other causes ranging from elevated corticosterone levels stemming from pre-anesthetic handling stress,24 over cardiovascular side-effects caused by the anesthetic agents applied25,26 to metabolic or respiratory acid–base abnormalities.27 In this study, in animals in the KS and FMS group a short-term increase in heart rate and core body temperature was observed at the time point of pre-medication. This short-term increase of heart rate and core body temperature was also seen in the S group when exposed to sevoflurane in the induction chamber. It is most likely due to stress caused by handling and restraining of the animal, and the exposure of the animals to a new environment (induction chamber).17
After onset of unconsciousness and the loss of the righting reflex, and throughout the 50-min of anesthesia and surgery, heart rate stabilized at distinct levels for all groups. As no fatal abnormalities occurred in any of the groups, heart rate variability was not assessed in this study. Sevoflurane alone caused the heart rate to remain by tendency increased throughout the 50-min of anesthesia and surgery compared to baseline values obtained at the corresponding time of day in non-anesthetized mice, most likely due to the known effects of sevoflurane on the cardiovascular system.28,29 Although sevoflurane has, in contrast to isoflurane, not been primarily associated with tachycardia in humans,30 higher concentrations of sevoflurane applied in the S and KS protocol compared to the FMS protocol can potentially lead to a progressive decrease in blood pressure, and a subsequent increase in heart rate.31 Ketamine has shown to annul these hypotensive effects of sevoflurane in children.32 However, as blood pressure measurement was not performed in this study, no conclusion can be made whether the elevated heart rate observed in the S group is blood pressure related. In the FMS group, a decreased heart rate was observed during the 50-min of anesthesia and surgery. A decrease in heart rate is often seen as a side-effect of fentanyl and midazolam due to their vago-mimetic properties.33
A respiratory rate decrease, compared to baseline values obtained from resting mice of the same strain, sex, and age-distribution (150 ± 10 brpm) assessed in our lab, was observed to be the most prominent side-effect in all three anesthetic protocols. However, none of the protocols led to side-effects like apnea which had previously been described for the KS protocol.17 Although opioids reportedly tend to decrease minute ventilation (=respiratory rate [RR] × tidal volume [VT]), the respiratory rate decrease was by tendency least pronounced in the FMS group. VT was not measured and gas exchange through arterial blood gases was not assessed. Cesarovic et al.17 using the same anesthetic protocols in the same strain of mice without them undergoing surgery found that pCO2 increased the most with KS, to lesser extend in S and FMS, while pO2 declined similarly in all groups. Based on these findings a declined minute ventilation (caused by a reduced VT) can therefore also be assumed in the FMS group.
What stands out in the data presented here, and can be considered to be of importance, is the great inter-individual variability, expressed through the standard deviation and the CV, which by tendency is larger in the KS and S protocols compared to the FMS protocol. A larger CV suggests a marked individual response to the applied protocol, which may lead to an unpredictable and inadequate anesthetic depth. Inter-individual variability in anesthetic depth must be minimized, not only because it is a crucial aspect of refinement of experimental methods, but also because of the potentially adverse effects on experimental results.34
Surgical stimuli, such as SI and abdominal wall suture, altered the respiratory rate significantly in the S group compared to baseline values obtained 10 s prior to applying the respective stimulus. Other surgical stimuli resulted in a non-significant increase of respiratory rate in the S group as well as in the KS group, which, although not statistically significant, are physiologically relevant. No significant changes were seen in the FMS group. With no analgesic effects reported for sevoflurane,35 alteration in physiological parameters as a response to surgical stimuli was expected in the S group in contrast to the other protocols. However, while an increase in heart rate of more than 20% can be interpreted as insufficient depth of anesthesia,9 no such thresholds can be found in the literature for an increase in respiratory rate. As pain is thought to modulate the sympathetic nervous system,36 one would expect a change in respiratory rate and simultaneously in heart rate as well. Changes in respiratory patterns and frequency can be a sign of pain/nociception or insufficient depth of anesthesia22,23 however, the correlation between pain intensity and respiratory rate has been described as not predictable.23 It therefore remains speculative whether the isolated increase in respiratory rate partially observed in this study during the application of a surgical stimulus is indicative enough for an insufficient depth of anesthesia resulting in pain/nociception. Other mechanisms, such as phrenic and hypoglossal motor outputs,37 which could explain isolated respiratory changes observed during noxious stimuli in the KS and the S group, have to be considered. There is evidence in the literature that respiratory depression in humans caused by volatile anesthetics may be antagonized partially by applying a surgical stimulus.38 This phenomenon is thought to be caused by extensive activation of brainstem reticular neurons leading to an increase in VT and respiratory rate.39,40 Whether this activation in turn is indicative of pain is still unclear.39
The negative side-effects of ketamine as pre-medication described in the study of Cesarovic et al.17 resulted in the recommendation of further combining ketamine with a minor tranquilizer. Nonetheless, combining ketamine pre-medication alone with a sevoflurane maintenance anesthesia remained of interest to us, as ketamine has been shown to provide profound analgesic effects when administered in sub-anesthetic doses in humans41 and is known for its sympathomimetic properties acting positively on the cardiorespiratory system.42 Ketamine is known to interact on a wide range of different receptors in the nervous system. Among others its interaction with the mu-opioid receptor has shown to induce dose-dependent anti-nociception in C57BL/6 mice.43 However, latency to react to a thermal stimulus as applied in common test for nociception in C57BL/6 mice was not altered until a dose of 50 mg/kg of ketamine.43 Additionally, Maxwell et al. claimed the half-life of ketamine for inbred mouse strains (such as C3H/HeHsd, C57BL/6Hsd, FVB/Hsd, and DBA/2Hsd) to be as short as ∼13 min,44 indicating that a single shot of ketamine in the dosage used in this study (30 mg/kg) might be insufficient as an analgesic for a 50-min surgical procedure of moderate impact.
The approach of combining fentanyl with midazolam has been adapted from a protocol often applied in human medicine as part of preemptive analgesia. Preemptive analgesia regimes aim to prevent sensitization of the nervous system to subsequent stimuli that could amplify pain, which can imprint themselves indelibly on the nervous system and cause hyperalgesia and allodynia.45 Fentanyl is a highly potent synthetic opioid analgesic with a rapid onset after 7–8 min following intra-muscular injection, a serum half-life of approximately 10 min, and usually requiring repeated injections or continuous infusion.35 This rapid onset is a result of fast redistribution of fentanyl to the brain.46 Fentanyl brain concentration appear to remain higher than assumed by the short half-life measured in serum.46 From a pharmacokinetic view point midazolam is described to have a half-life of 30-40 min in mice.9,47,48 The benzodiazepine-opioid interactions for analgesia are described to be synergistic (supra-additive).49 Therefore, this synergistic effect might have led to the absence of response to surgical stimulus in heart and respiratory rate in the FMS group compared to the other two groups. However, physiological parameters need to be examined carefully when evaluating anesthetic depth and pain management using opioids, especially in high-dose, due to the fact that these compounds alter the cardiovascular response.50
In the immediate recovery time after anesthesia and surgery, all mice were left on the water-filled heating pad and substituted with oxygen for 10 min before being transferred back to their home cage. Based on the literature, ketamine and fentanyl should be completely, and midazolam mostly, eliminated by this time point.9,35,44 Nonetheless, core body temperature dropped in both pre-medicated groups during the first hour following anesthesia and surgery, indicating that further warming measurements (e.g. warming cabinet) are needed when using either of these two protocols. Based on the work of Cesarovic et al.,17 similar immediate recovery times and unchanged locomotor activity were expected for all groups regardless of the anesthesia protocol applied. The observed hypothermia may therefore be ascribed rather to other factors such as the wide distribution of body weight and age of the animals used for this study as older and heavier animals have been shown to have an impaired thermoregulation in comparison to younger animals.51,52
It has been shown that, among other factors, measuring heart rate is also useful in assessing post-operative pain in laboratory mice, especially at a level of pain that cannot be detected easily by observation.21 In the present study, heart rate was increased during the first 36 h after anesthesia and surgery for all groups compared to baseline values obtained prior to anesthesia and surgery at the corresponding time of day. This heart rate elevation is most likely to be attributed to the well-described stress-response to surgery found in humans characterized by enhanced secretion of pituitary hormones and an activation of the sympathetic nervous system.53,54 The stimuli for the stress response to surgery are thought to arise from visceral and peritoneal afferent nerve fibers as well as from the abdominal wall and can be attenuated by the application of local anesthesia prior to trauma.53 Opioids are also known to suppress hypothalamic and pituitary hormone secretion,55 which might be a possible explanation for the diminished post-anesthetic tachycardia seen in the FMS group compared to the other two protocols. All animals received the same analgesic regime (one-time application of carprofen 5 mg/kg BW s.c.) just shortly before the end of the anesthesia to avoid interference with the study design. Carprofen reaches its peak plasma concentration at 3–4 h after subcutaneous injection56 and should therefore, in conjunction with the anesthetic protocols evaluated in this study, be applied prior to surgical intervention to ensure incessant analgesia. Carprofen has a duration of action of 12–24 h in mice.57–59 Available data based on standardized pain evaluation criteria suggests that rodents should receive at least 7 h of analgesia after abdominal surgery, with treatment extension for more invasive models or strains known to be pain-sensitive.60 The effectiveness of pain management in animals is often further assessed through physiologic (e.g. body weight) or behavioral (e.g. food and water intake) indices.61,62 Animals in pain are suggested to have a depressed food and water intake.63,64 However, weight loss and reduced appetite may not always reliably reflect a behavioral response to postsurgical pain but could result from various other factors such as effects of anesthesia and analgesia, and adaptations to post-surgical handling.61,62 Especially the often used analgesic buprenorphine (alone or in combination with NSAIDs) has shown to have a suppressing effect on the appetite,61,65,66 occasionally leading to a more pronounced reduction in food intake than in mice not receiving any pain treatment post-surgically.61 These effects are described as less pronounced and of shorter duration when mice are treated with NSAIDs alone, such as carprofen or meloxicam.61,62 None of the anesthetic protocols in this study appeared to have adverse post-surgical effect on body weight, or on food and water intake leading to the assumption that the overall influence of our applied protocols for the selected procedure to be of only short duration. However, since animals were housed in pairs and the daily intake of food and water was estimated by measuring the total food and water consumption for both mice divided by two, small changes in the individual animal might have been missed. The evident re-increase of heart rate observed in all groups on the second day (light phase) after anesthesia and surgery, may still indicate an underestimation of pain associated with the procedure performed, and further re-evaluation of the analgesic protocol applied may be required.
Conclusion
Safe and reliable anesthesia protocols remain a major concern in biomedical research. The suitability of an anesthetic protocol for laboratory mice depends on not only strain-, age-, and sex-related variability but also correlates closely with the expected painfulness of the intervention, the duration of the procedure, and the operational set-up (e.g. positioning of the animal). Provided appropriate drugs are combined, a balanced anesthetic protocol should be chosen over a mono-anesthetic protocol whenever possible as it is meant to lead to better achievement of the desired effects, namely unconsciousness with muscle relaxation, analgesia, and stable cardiorespiratory function. Additive and synergistic effects allow for reduced doses of each individual agent, thereby minimizing undesired side effects.
Ketamine has some beneficial properties such analgesic effects in sub-anesthetic doses and sympathomimetic properties acting positively on the cardiorespiratory system.42 Nonetheless, based on this study together with the findings of Cesarovic et al.,17 ketamine as pre-medication in combination with a sevoflurane maintenance anesthesia is insufficient to achieve a 50-min balanced and safe anesthesia in female C57BL/6J mice.
Combining FM as pre-medication with a sevoflurane maintenance anesthesia on the other hand was found to be beneficial on the reduction of stress during the induction phase and to lead to a faster achievement of unconsciousness, as well as a marked gas saving effect in female C57BL/6J mice.17 In the present study, we also found mice pre-medicated with FM to show a more reliable anesthesia with less inter-individual variability, based on responses of heart rate and respiratory rate to painful stimuli, compared to the other two protocols. Furthermore, fentanyl and midazolam reduce the depressant effect of sevoflurane on the respiratory rate during anesthesia and reduce the negative post-anesthetic effects on the heart rate.
Finally, combining different agents undeniably requires further considerations also concerning potential interference with the experimental design. The appropriate drug combination and dosages should therefore be considered carefully, and chosen according to not only the requirements of animal welfare but also the scientific question asked.
Acknowledgments
This work was sponsored by the ECLAM and ESLAV Foundation. The authors would like to thank Robin Schneider, Hugo Battaglia, and the staff of the Central Biological Laboratory for their valuable support in housing mice.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data, and review of the manuscript: ML conducted data analysis and evaluation and wrote the manuscript; MA and PJ participated in the design of the study and provided critical revision of the manuscript; NC participated in the design and realization of the study and conducted the experiment.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Green C. Laboratory animal handbooks 8: animal anaesthesia, London: Laboratory Animals Ltd, 1979. [Google Scholar]
- 2.Young CJ, Apfelbaum JL. Inhalational anesthetics: desflurane and sevoflurane. J Clin Anesth 1995; 7: 564–77. [DOI] [PubMed] [Google Scholar]
- 3.Jones RM. Desflurane and sevoflurane: inhalation anaesthetics for this decade? Br J Anaesth 1990; 65: 527–36. [DOI] [PubMed] [Google Scholar]
- 4.Heavner JE. Anesthesia update: agents, definitions, and strategies. Comp Med 2001; 51: 500–3. [PubMed] [Google Scholar]
- 5.Sonner JM, Gong D, Li J, Eger EI, 2nd, Laster MJ. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg 1999; 89: 1030–4. [DOI] [PubMed] [Google Scholar]
- 6.Eckel B, Richtsfeld M, Starker L, Blobner M. Transgenic Alzheimer mice have a larger minimum alveolar anesthetic concentration of isoflurane than their nontransgenic littermates. Anesth Analg 2010; 110: 438–41. [DOI] [PubMed] [Google Scholar]
- 7.Groeben H, Meier S, Tankersley CG, Mitzner W, Brown RH. Heritable differences in respiratory drive and breathing pattern in mice during anaesthesia and emergence. Br J Anaesth 2003; 91: 541–5. [DOI] [PubMed] [Google Scholar]
- 8.Groeben H, Meier S, Tankersley CG, Mitzner W, Brown RH. Influence of volatile anaesthetics on hypercapnoeic ventilatory responses in mice with blunted respiratory drive. Br J Anaesth 2004; 92: 697–703. [DOI] [PubMed] [Google Scholar]
- 9.Fish RB, Marilyn, Danneman P, Karas A. Anesthesia and analgesia in laboratory animals., 2nd ed San Diego, CA: Elsevier Inc, 2008. [Google Scholar]
- 10.Fleischmann T, Jirkof P, Henke J, Arras M, Cesarovic N. Injection anaesthesia with fentanyl-midazolam-medetomidine in adult female mice: importance of antagonization and perioperative care. Lab Anim 2016; 50: 264–74. [DOI] [PubMed] [Google Scholar]
- 11.Henke J, Roberts U, Otto K, Lendl C, Matis U, Brill T, Erhardt W. [Clinical investigations of an i.m. combination anesthesia with fentanylclimazolam/xylazine and postoperative i.v. antagonism with naloxone/sarmazenil/yohimbine in guinea pigs]. Tierarztl Prax 1996; 24: 85–7. [PubMed] [Google Scholar]
- 12.Hacker SO, White CE, Black IH. A comparison of target-controlled infusion versus volatile inhalant anesthesia for heart rate, respiratory rate, and recovery time in a rat model. Contemp Top Lab Anim Sci 2005; 44: 7–12. [PubMed] [Google Scholar]
- 13.Arras M, Autenried P, Rettich A, Spaeni D, Rulicke T. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med 2001; 51: 443–56. [PubMed] [Google Scholar]
- 14.Hedrich HJ, Bullock G. The laboratory mouse. San Diego, CA: Elsevier Ltd., 2004.
- 15.Lundy J. Balanced anesthesia. Minn Med 1926; 9: 399–404. [Google Scholar]
- 16.Ellis TA, 2nd, Narr BJ, Bacon DR. Developing a specialty: J.S. Lundy's three major contributions to anesthesiology. J Clin Anesth 2004; 16: 226–9. [DOI] [PubMed] [Google Scholar]
- 17.Cesarovic N, Jirkof P, Rettich A, Nicholls F, Arras M. Combining sevoflurane anesthesia with fentanyl-midazolam or s-ketamine in laboratory mice. J Am Assoc Lab Anim Sci 2012; 51: 209–18. [PMC free article] [PubMed] [Google Scholar]
- 18.Worlein JM, Baker K, Bloomsmith M, Coleman K, Koban TL. The eighth edition of the guide for the care and use of laboratory animals (2011); implications for behavioral management. Am J Primatol 2011; 73: 98–98. [Google Scholar]
- 19.Mahler M, Berard M, Feinstein R, Gallagher A, Illgen-Wilcke B, Pritchett-Corning K, Raspa M. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units (vol 48, pg 178, 2014). Lab Anim Uk 2015; 49: 88–88. [DOI] [PubMed] [Google Scholar]
- 20.Cesarovic N, Jirkof P, Rettich A, Arras M. Implantation of radiotelemetry transmitters yielding data on ECG, heart rate, core body temperature and activity in free-moving laboratory mice. J Vis Exp 2011; 57: pii: 3260–pii: 3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arras M, Rettich A, Cinelli P, Kasermann HP, Burki K. Assessment of post-laparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res 2007; 3: 16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stomberg MW, Sjostrom B, Haljamae H. Assessing pain responses during general anesthesia. AANA J 2001; 69: 218–22. [PubMed] [Google Scholar]
- 23.Grimm KA, Lamont LA, Tranquilli WJ, Greene SA, Robertson SA. Veterinary anesthesia and analgesia. 5th ed. Ames, IA: Wiley-Blackwell, 2015.
- 24.Nohara M, Tohei A, Sato T, Amao H. Evaluation of response to restraint stress by salivary corticosterone levels in adult male mice. J Vet Med Sci 2016; 78: 775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Constantinides C, Mean R, Janssen BJ. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J 2011; 52: e21–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Zuurbier CJ, Emons VM, Ince C. Hemodynamics of anesthetized ventilated mouse models: aspects of anesthetics, fluid support, and strain. Am J Physiol Heart Circ Physiol 2002; 282: H2099–105. [DOI] [PubMed] [Google Scholar]
- 27.Campen MJ, Tagaito Y, Jenkins TP, Balbir A, O'Donnell CP. Heart rate variability responses to hypoxic and hypercapnic exposures in different mouse strains. J Appl Physiol (1985) 2005; 99: 807–13. [DOI] [PubMed] [Google Scholar]
- 28.Ebert TJ, Harkin CP, Muzi M. Cardiovascular responses to sevoflurane: a review. Anesth Analg 1995; 81: S11–22. [DOI] [PubMed] [Google Scholar]
- 29.Ebert TJ. Cardiovascular and autonomic effects of sevoflurane. Acta Anaesthesiol Belg 1996; 47: 15–21. [PubMed] [Google Scholar]
- 30.Rolf N, Van Aken H. [Cardiovascular effects of sevoflurane]. Anaesthesist 1998; 47: S11–8. [DOI] [PubMed] [Google Scholar]
- 31.Hikasa Y, Okuyama K, Kakuta T, Takase K, Ogasawara S. Anesthetic potency and cardiopulmonary effects of sevoflurane in goats: comparison with isoflurane and halothane. Can J Vet Res 1998; 62: 299–306. [PMC free article] [PubMed] [Google Scholar]
- 32.Blumberg D, Congdon N, Jampel H, Gilbert D, Elliott R, Rivers R, Munoz B, Quigley H. The effects of sevoflurane and ketamine on intraocular pressure in children during examination under anesthesia. Am J Ophthalmol 2007; 143: 494–9. [DOI] [PubMed] [Google Scholar]
- 33.Gravlee GP, Ramsey FM, Roy RC, Angert KC, Rogers AT, Pauca AL. Rapid administration of a narcotic and neuromuscular blocker: a hemodynamic comparison of fentanyl, sufentanil, pancuronium, and vecuronium. Anesth Analg 1988; 67: 39–47. [PubMed] [Google Scholar]
- 34.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J 2012; 53: E55–69. [DOI] [PubMed] [Google Scholar]
- 35.Alexandra D. Veterinary anaesthesia: principles to practice. Ames, IA: Wiley-Blackwell, 2010.
- 36.Bantel C, Trapp S. The role of the autonomic nervous system in acute surgical pain processing – what do we know? Anaesthesia 2011; 66: 541–4. [DOI] [PubMed] [Google Scholar]
- 37.Baker-Herman TL, Strey KA. Similarities and differences in mechanisms of phrenic and hypoglossal motor facilitation. Respir Physiol Neurobiol 2011; 179: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutherland RW, Drummond GB. Effects of surgical skin incision on respiration in patients anaesthetized with enflurane. Br J Anaesth 1996; 76: 777–9. [DOI] [PubMed] [Google Scholar]
- 39.Casey KL. Somatosensory responses of bulboreticular units in awake cat: relation to escape-producing stimuli. Science 1971; 173: 77–80. [DOI] [PubMed] [Google Scholar]
- 40.St John WM. Influence of reticular mechanisms upon hypoglossal, trigeminal and phrenic activities. Respir Physiol 1986; 66: 27–40. [DOI] [PubMed] [Google Scholar]
- 41.Haas DA, Harper DG. Ketamine: a review of its pharmacologic properties and use in ambulatory anesthesia. Anesth Prog 1992; 39: 61–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Timm C, Linstedt U, Weiss T, Zenz M, Maier C. [Sympathomimetic effects of low-dose S(+)-ketamine. Effect of propofol dosage]. Anaesthesist 2008; 57: 338–46. [DOI] [PubMed] [Google Scholar]
- 43.Sarton E, Teppema LJ, Olievier C, Nieuwenhuijs D, Matthes HW, Kieffer BL, Dahan A. The involvement of the mu-opioid receptor in ketamine-induced respiratory depression and antinociception. Anesth Analg 2001; 93: 1495–500,. table of contents. [DOI] [PubMed] [Google Scholar]
- 44.Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther 2006; 316: 315–24. [DOI] [PubMed] [Google Scholar]
- 45.Gottschalk A, Smith DS. New concepts in acute pain therapy: preemptive analgesia. Am Fam Physician 2001; 63: 1979–84. [PubMed] [Google Scholar]
- 46.Kalvass JC, Maurer TS, Pollack GM. Use of plasma and brain unbound fractions to assess the extent of brain distribution of 34 drugs: comparison of unbound concentration ratios to in vivo p-glycoprotein efflux ratios. Drug Metab Dispos 2007; 35: 660–6. [DOI] [PubMed] [Google Scholar]
- 47.van Waterschoot RA, van Herwaarden AE, Lagas JS, Sparidans RW, Wagenaar E, van der Kruijssen CM, Goldstein JA, Zeldin DC, Beijnen JH, Schinkel AH. Midazolam metabolism in cytochrome P450 3A knockout mice can be attributed to up-regulated CYP2C enzymes. Mol Pharmacol 2008; 73: 1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuelsson K, Pickup K, Sarda S, Swales JG, Morikawa Y, Schulz-Utermoehl T, Hutchison M, Wilson ID. Pharmacokinetics and metabolism of midazolam in chimeric mice with humanised livers. Xenobiotica 2012; 42: 1128–37. [DOI] [PubMed] [Google Scholar]
- 49.Miller RD, Eriksson L, Fleisher LA, Wiener-Kronish JP, Young WL. Miller's anesthesia. 7th ed. San Diego, SA: Elsevier Inc., 2011.
- 50.Schwender D, Daunderer M, Klasing S, Mulzer S, Finsterer U, Peter K. [Monitoring intraoperative awareness. Vegetative signs, isolated forearm technique, electroencephalogram, and acute evoked potentials]. Anaesthesist 1996; 45: 708–21. [DOI] [PubMed] [Google Scholar]
- 51.Estler CJ. Efficiency of thermoregulation in acutely cold-exposed young and old mice. Life Sci I 1971; 10: 1291–8. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman-Goetz L, Keir R. Body temperature responses of aged mice to ambient temperature and humidity stress. J Gerontol 1984; 39: 547–51. [DOI] [PubMed] [Google Scholar]
- 53.Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000; 85: 109–17. [DOI] [PubMed] [Google Scholar]
- 54.Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr 2013; 37: 21S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mc Donald R, Evans FT, Weise VK, Patrick RW. Effect of morphine and nalorphine on plasma hydrocortisone levels in man. J Pharmacol Exp Ther 1959; 125: 241–7. [PubMed] [Google Scholar]
- 56.Taylor PM, Delatour P, Landoni FM, Deal C, Pickett C, Shojaee Aliabadi F, Foot R, Lees P. Pharmacodynamics and enantioselective pharmacokinetics of carprofen in the cat. Res Vet Sci 1996; 60: 144–51. [DOI] [PubMed] [Google Scholar]
- 57.Fehr M, Sassenberg L, Zwart P. Krankheiten der Heimtiere. Hannover: Schlütersche Verlagsgesellschaft, 2005.
- 58.Flecknell PA. Analgesia of small mammals. Vet Clin N Am Exot Anim Pract 2001; 4: 47–56, vi. [DOI] [PubMed] [Google Scholar]
- 59.Kendall LV, Hansen RJ, Dorsey K, Kang S, Lunghofer PJ, Gustafson DL. Pharmacokinetics of sustained-release analgesics in mice. J Am Assoc Lab Anim Sci 2014; 53: 478–84. [PMC free article] [PubMed] [Google Scholar]
- 60.Roughan JV, Flecknell PA. Behaviour-based assessment of the duration of laparotomy-induced abdominal pain and the analgesic effects of carprofen and buprenorphine in rats. Behav Pharmacol 2004; 15: 461–72. [DOI] [PubMed] [Google Scholar]
- 61.Adamson TW, Kendall LV, Goss S, Grayson K, Touma C, Palme R, Chen JQ, Borowsky AD. Assessment of carprofen and buprenorphine on recovery of mice after surgical removal of the mammary fat pad. J Am Assoc Lab Anim Sci 2010; 49: 610–6. [PMC free article] [PubMed] [Google Scholar]
- 62.Bourque SL, Adams MA, Nakatsu K, Winterborn A. Comparison of buprenorphine and meloxicam for postsurgical analgesia in rats: effects on body weight, locomotor activity, and hemodynamic parameters. J Am Assoc Lab Anim Sci 2010; 49: 617–22. [PMC free article] [PubMed] [Google Scholar]
- 63.Pain and distress in laboratory rodents and lagomorphs. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Pain and Distress accepted by the FELASA Board of Management November 1992. Lab Anim 1994; 28: 97–112. [DOI] [PubMed] [Google Scholar]
- 64.Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec 1985; 116: 431–6. [DOI] [PubMed] [Google Scholar]
- 65.Goecke JC, Awad H, Lawson JC, Boivin GP. Evaluating postoperative analgesics in mice using telemetry. Comp Med 2005; 55: 37–44. [PubMed] [Google Scholar]
- 66.Sauer M, Fleischmann T, Lipiski M, Arras M, Jirkof P. Buprenorphine via drinking water and combined oral-injection protocols for pain relief in mice. Appl Anim Behav Sci 2016; 185: 103–112. [Google Scholar]