Abstract
Objective:
The contents of transforming growth factor-β and insulin-like growth factor-1 in disc of diabetic rats were measured at three different periods after injected with 1,25-Dihydroxyvitamin D3, and compared with that in normal rats. The significance of content changes was also discussed.
Methods:
Fourty-five Sprague-Dawley (SD) rats were divided into three groups, namely the experimental group (STZ+calcitriol), control group (STZ+citrate buffer), and normal group (citrate buffer). Complete lumbar discs in these groups were obtained at the second, fourth, sixth week, respectively. After paraffin-embedded sections and HE staining, the structure and morphology changes of disc were observed. The content of transforming growth factor-β and insulin-like growth factor-1 was measured by immunohistochemical method, and the expression of transforming growth factor-β and insulin-like growth factor-1 was detected by Western Blot.
Results:
In hematoxylin–eosin staining, degenerative changes were observed in disc of experimental and control group at three different periods, and there were no changes in disc in normal group. Immunohistochemical method indicated the content of transforming growth factor-β and insulin-like growth factor-1 in experimental and control group was significantly lower than normal group at three different periods (P < 0.05). And there were significant differences between experimental and control group at three different periods (P < 0.05).
Conclusion:
Vitamin D can protect the degeneration of intervertebral disc and improve the content of transforming growth factor-β and insulin-like growth factor-1 in the intervertebral disc, which provides a new idea for the prevention and treatment of degenerative changes of the intervertebral disc in diabetic patients.
Impact statement
No researchers reported Vitamin D could protect degeneration of intervertebral disc. That is to say, we found a new method to prevent and treat degenerative changes of the intervertebral disc in diabetic patients. And Vitamin D prevented the discs by improving the content of TGF-β and IGF-1.
Keywords: Vitamin D, transforming growth factor-β, insulin-like growth factor-1, STZ, calcitriol, diabetes
Introduction
Diabetes is characterized by hyperglycemia, and accompanied by retinopathy, kidney disease, myocardial disease and other complications. Pathophysiological basis of organ damage in diabetic patients includes the thickening of capillary basement membranes, microvascular barrier dysfunction and other microangiopathy.1 Ziv et al.2 has proved that intervertebral disc tissue would be affected by microangiopathy. There were no blood vessels in intervertebral disc tissue, so nutritions of the nucleus pulposus are mainly from the proliferation of nutrients in the vertebral blood vessels.3 Microangiopathy of diabetes includes destruction of capillary sprout in endplate, stenosis of vascular lumen, and reduction in the number of vascular lumen, which lead to insufficient nutrition supply and accumulation of the metabolic toxin in intervertebral disc, and thus cause disc degeneration.4,5 In addition, changes of cytokines in disc of diabetic patients will affect on the intervertebral disc. TGF-β and IGF-1 are cytokines that regulate the synthesis of extracellular matrix in intervertebral disc and prevents apoptosis of intervertebral disc cells. The content of TGF-β and IGF-1 can also indirectly reflect the degree of disc degeneration.
Yi et al.6 have proved that 1,25-Dihydroxyvitamin D3 can be used for the treatment of diabetic complication. Calcium is a second messenger associated with the process of insulin secretion, and vitamin D is essential in the process of calcium metabolism; 1,25-Dihydroxyvitamin D3, as the most important active form of vitamin D, in addition to maintain stable calcium environment in the body, can regulate proliferation and differentiation of various cells. It can inhibit the secretion of renin, reduce oxidative stress, thus improving urinary protein of diabetic rats. What’s more, it can inhibit inflammatory reaction, treat diabetic cardiomyopathy, promote the recovery of bone mineral density, and increase the sensitivity of insulin. However, whether 1,25-Dihydroxyvitamin D3 can relieve disc degeneration by affecting the content of TGF-β and IGF-1 is still unknown to us.
In this study, the contents of TGF-β and IGF-1 in three groups at different time points were measured. We analyzed these data and discussed the effect of Vitamin D on TGF-β and IGF-1 contents in disc of diabetic rats and its significance in disc in diabetic rats.
Materials and methods
Animals
This animal experiment was approved by Ethics Review Committees of Animal Research Institution of Hebei Medical University. Sixty-five adult male SD rats (weight: 180–200 g) were raised in a clean environment in Animal Experimental Center of Hebei Medical University.
Preparation for diabetic rat model
SD rats were randomly divided into experimental group (n = 20), control group (n = 20), and normal group (n = 15). In experimental and control group, SD rats were injected with streptozocin (STZ) solution (Cayman, Ann Arbor, MI) dissolved in citrate buffer (Solarbio, Beijing) for three times in every three days with a maximum dose of 30 mg/kg to maintain hyperglycemia and induce diabetic rats. Rapid glucose meter (Rightest GM550, Hua guang sheng ji, Taiwan) was used for measuring fasting blood glucose in tail vein of rats. If the concentration of blood glucose was ≥ 200 mg/dL, the rat was identified as diabetic rat. Pre-test results showed the establish rate of success diabetic rat model was about 80%. Two rats were excluded because of complication of diabetes. Once the diabetic rats were diagnosed, the rats were divided into control group (n = 15) and experimental group (n = 15) again. Then, rats in experimental group were injected intraperitoneally with 1 mg/kg calcitriol (Cayman, Ann Arbor, MI) dissolved in edible oil, and rats in control and normal groups were injected intraperitoneally with 1 mg/kg citrate buffer. Finally, each group had 15 SD rats.
Treatment for specimens
Five rats were taken from each group at the second, fourth, sixth week, respectively, and killed by 1% pentobarbital sodium via intraperitoneal injection. Fresh lumbar disc tissues were obtained and weighted, rinsed with 0.02 mol/L phosphate-buffered saline (PBS). Tissue blocks less than 0.5 cm × 0.5 cm × 0.1 cm were fixed with 4% paraformaldehyde and dehydrated using graded ethanol. Then, paraffin tissue blocks were embedded by copper mold. Intervertebral disc tissue was cut into pieces, grinded, and centrifuged. The supernatant was collected to determine the concentration of protein.
Hematoxylin–eosin staining
Paraffin section was dewaxed by xylene, dehydrated using graded ethanol, stained with hematoxylin for 5 min, rinsed with tap water, differentiated in differentiation solution for 30 s, soaked in tap water for 15 min, stained with 2 min eosin, rinsed with tap water, dehydrated using graded ethanol, transparent with xylene, and mounting with neutral resins, and observed under microscope.
Immunohistochemical method
Paraffin sections were cut into 5 um tissue sections and attached to a polylysine-coated slide. Tissue sections were baked for 4.5 h at 65℃. After dewaxing and putting into PBS, wax was streaked. Primary antibody was dropped into section at 4℃ overnight. After 10–20 min at room temperature, sections were put into PBS. Biotinylated second antibody was dropped into section at 37℃ for 20 min. Sections were put into PBS. Finally, streptavidin horseradish peroxidase conjugate (SA-HRP) was dropped into section at 37℃ for 20 min. Sections were put into PBS. 3,3′-diaminobenzidine (DAB) color was used. Nucleus was redyed by hematoxylin. After dehydration using graded ethanol, transparent with xylene, and mounting with neutral resins, the section was observed under microscope. Histomorphology images captured under a microscope were analyzed by Image-Pro Plus system (version 5.1, Media Cybernetics). Five visual fields with the strongest positive expression were calculated, and the scores of cytoplasmic staining and positive cell rates were: 0, no staining; 1, light yellow; 2, brown-yellow; 3, brown; 0, a proportion <5%; 1, a proportion of 5%–25%; 2, a proportion of 26%–50%; 3, a proportion of 51%–75%; 4, a proportion > 75%. The two kinds of scores were added. Scores < 2, negative expression (−); scores of 2–3, slightly positive expression (±); scores of 4–5, positive expression (+); scores of 6–7, strong positive expression (++).7
Western Blot
Protein (10 ug) was separated on a 15% SDS-PAGE gel and electrophoretically transferred to a cellulose acetate membrane. The membrane was blocked for 3 h, then TGF-β and IGF-1 antibody were added and incubated at 4℃ overnight. Membrane was rinsed with TBST for three times. Secondary antibody was incubated for 2 h at room temperature, the membranes were subsequently washed and the blots were visualized using a Bio-Rad Gel Doc™ XR + Imaging system. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was set as internal reference, and the ratio of the expression of target proteins to the expression of internal reference protein was the relative expression of target proteins. The experiment was performed in triplicate.
Statistical analysis
The data of scores were analyzed by Kruskal–Wallis H test. The data of Western blot were expressed as The differences among multiple groups were compared using one-way ANOVA. P < 0.05 was considered statistically significance.
Result
Blood glucose in rats
The concentrations of blood glucose of diabetic rats in STZ-treated groups, namely the experimental and control groups, were significantly higher than normal group at the second, fourth, and sixth week (P < 0.05). There were no significant differences between experimental and control groups (P > 0.05) (Table 1).
Table 1.
Comparison of blood glucose in rats at different periods mmol/L
| Groups | Concentration of blood glucose |
|||
|---|---|---|---|---|
| Initial | The second week | The fourth week | The sixth week | |
| Experimental group | 24.61 ± 5.15a | 20.98 ± 4.89a | 20.89 ± 6.23a | 23.47 ± 5.55a |
| Control group | 24.77 ± 5.34b | 26.22 ± 5.18b | 23.76 ± 4.46b | 25.53 ± 6.52b |
| Normal group | 4.94 ± 0.65 | 5.01 ± 0.66 | 4.89 ± 0.82 | 4.63 ± 0.58 |
Level of significance with P < 0.05 compare with normal group.
Level of significance with P < 0.05 compare with normal group.
Degeneration in disc of diabetic rats
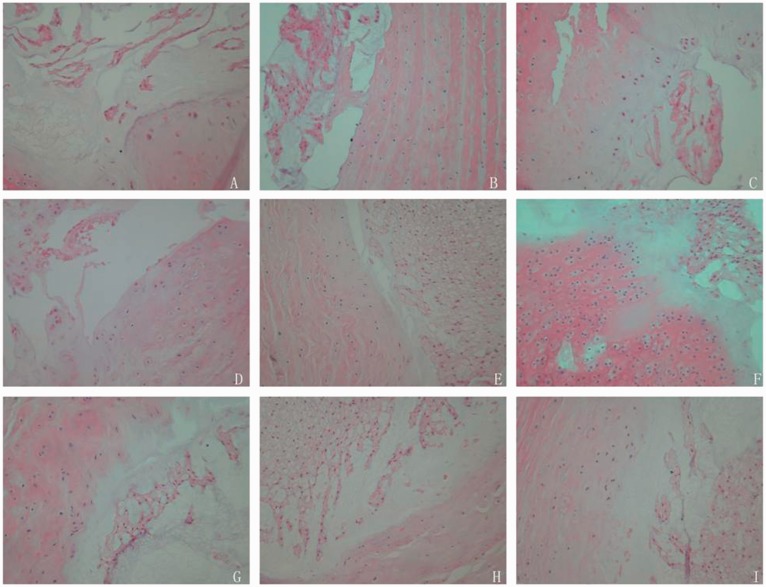
In control group, HE staining showed different degrees of degeneration changes in disc of diabetic rats. Endplate calcification occurred and chondrocytic cells appeared. Notochordal cells were decreased. Collagen fiber hyperplasia appeared, and annulus fibrosus meanderingly arranged. Nucleus pulposus matrix shrinked, decreased and disordered arranged. Nucleus pulposus and annulus fibrosus bulged outward, and part of the nucleus pulposus cells arranged in clusters. In normal group, the structure of disc was normal. Annulus fibrosus had well-regulated layered structure, and collagen bundles were regular arranged which were mainly consisted of fibroblasts. Endplate was consisted by a layer of hyaline cartilage. There were abundant notochordal cells which were strip-shaped distributed, and the boundary of nucleus pulposus and annulus fibrosus was clear. And there were abundant cells in matrix. In experimental group, the structure of disc of diabetic rats in experimental group was normal. There were no calcification and chondrocytic cells in endplate cartilage. The boundary of annulus fibrosus and nucleus pulposus was clear, and no significant shrink of matrix appeared, which was clear arranged. Few nucleus pulposus cells were disorderly arranged. There was no significant disc degeneration (Figure 1).
Figure 1.
HE staining of experimental, control and normal groups at different periods. There was significant disc degeneration in control group (P < 0.05). There was no significant different in disc degeneration between control group and experimental group (P > 0.05). (a–c) corresponded to the second, fourth, and sixth weeks in normal group. (d–f) corresponded to the second, fourth, and sixth weeks in control group. (g–i) corresponded to the second, fourth, and sixth weeks in experimental group. (A color version of this figure is available in the online journal.)
Changes of the contents of TGF-β and IGF-1
There was no significant difference between TGF-β and IGF-1 level in normal group at three different periods (P > 0.05) (Tables 2 and 3). The situations in control and experimental groups were the same (Tables 2 and 3).
Table 2.
Comparison of TGF-β level in disc of rats in normal, control and experimental groups at three different periods
| Groups | Weeks | N | Rank mean of TGF-β | df | P |
|---|---|---|---|---|---|
| Normal | 2 | 5 | 7.15 | 2 | 0.375 |
| 4 | 5 | 8.69 | |||
| 6 | 5 | 8. 69 | |||
| Control | 2 | 5 | 6.73 | 2 | 0.325 |
| 4 | 5 | 8.21 | |||
| 6 | 5 | 9.66 | |||
| Experimental | 2 | 5 | 6.83 | 2 | 0.251 |
| 4 | 5 | 8.21 | |||
| 6 | 5 | 9.66 |
Table 3.
Comparison of IGF-1 level in disc of rats in normal, control and experimental groups at three different periods
| Groups | Weeks | N | Rank mean of IGF-1 | df | P |
|---|---|---|---|---|---|
| Normal | 2 | 5 | 7.15 | 2 | 0.375 |
| 4 | 5 | 8.69 | |||
| 6 | 5 | 8. 69 | |||
| Control | 2 | 5 | 7.15 | 2 | 0.731 |
| 4 | 5 | 8.69 | |||
| 6 | 5 | 8.69 | |||
| Experimental | 2 | 5 | 6.83 | 2 | 0.251 |
| 4 | 5 | 8.21 | |||
| 6 | 5 | 9.66 |
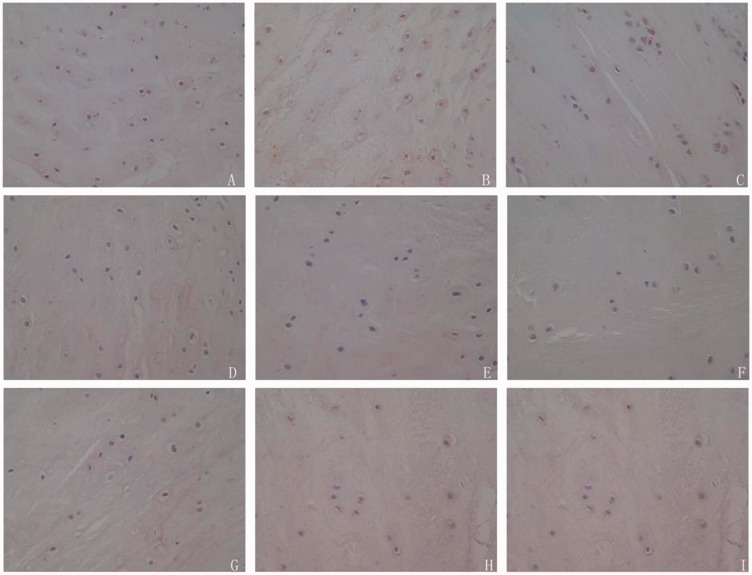
The positive rate of TGF-β and IGF-1 in disc cells of diabetic rats in experimental group was significantly lower than that of normal group, and higher than that of control group at three different periods (P < 0.05) (Figures 2 and 3; Tables 4 and 5).
Figure 2.
Immunohistochemistry of TGF-β in disc cells from experimental, control and normal groups at different periods. The positive rate of TGF-β in disc cells of diabetic rats in experimental group was significantly lower than that of normal group, and higher than that of control group at three different periods (P < 0.05). (a–c) corresponded to the second, fourth, and sixth weeks in normal group. (d–f) corresponded to the second, fourth, and sixth weeks in control group. (g–i) corresponded to the second, fourth, and sixth weeks in experimental group. (A color version of this figure is available in the online journal.)
Figure 3.
Immunohistochemistry of IGF-1 in disc cells from experimental, control and normal groups at different periods. The positive rate of IGF-1 in disc cells of diabetic rats in experimental group was significantly lower than that of normal group, and higher than that of control group at three different periods (P < 0.05). (a–c) corresponded to the second, fourth, and sixth weeks in normal group. (d–f) corresponded to the second, fourth, and sixth weeks in control group. (g–i) corresponded to the second, fourth, and sixth weeks in experimental group. (A color version of this figure is available in the online journal.)
Table 4.
Comparison of TGF-β level in disc among normal, control group and experimental groups
| Group | TGF-β level |
Rank mean | df | P | ||||
|---|---|---|---|---|---|---|---|---|
| Negative (−) | Slightly positive (±) | Positive (+) | Strong positive (++) | Total | ||||
| Normal group | 0 | 0 | 1 | 14 | 15 | 36.53 | 2 | 0.000 |
| Control group | 4 | 11 | 0 | 0 | 15 | 8.40a | ||
| Experimental group | 0 | 1 | 13 | 1 | 15 | 24.07a,b | ||
| Total | 4 | 12 | 14 | 15 | 45 | – | – | – |
Level of significance with P < 0.05 compare with normal group.
Level of significance with P < 0.05 compare with control group.
Table 5.
Comparison of IGF-1 level in disc among normal, control group and experimental groups
| Group | IGF-1 level |
Rank mean | df | P | ||||
|---|---|---|---|---|---|---|---|---|
| − | ± | + | ++ | Total | ||||
| Normal group | 0 | 0 | 1 | 14 | 15 | 37.03 | 2 | 0.000 |
| Control group | 4 | 11 | 0 | 0 | 15 | 8.37a | ||
| Experimental group | 0 | 1 | 13 | 1 | 15 | 23.06a,b | ||
| Total | 4 | 12 | 14 | 15 | 45 | – | – | – |
Level of significance with P < 0.05 compare with normal group.
Level of significance with P < 0.05 compare with control group.
Expression of TGF-β and IGF-1 proteins in disc cells
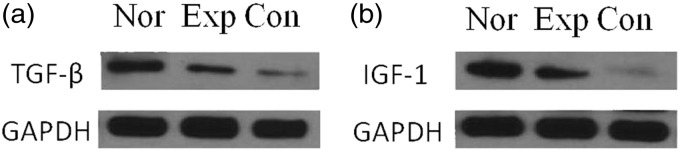
The expressions of TGF-β and IGF-1 proteins in disc cells of experimental group were lower than that of normal group, and higher than that of control group (P < 0.05) (Figure 4, Table 6).
Figure 4.
Expression of TGF-β and IGF-1 proteins in disc cells. (a) Expression of TGF-β proteins in disc cells. (b) Expression of IGF-1 proteins in disc cells
Table 6.
Comparison of the expression of TGF-β and IGF-1 proteins in discs of among normal, control group and experimental groups
| Group | TGF-β | IGF-1 |
|---|---|---|
| Normal group | 0.58 ± 0.06 | 0.75 ± 0.03 |
| Experimental group | 0.38 ± 0.04a | 0.56 ± 0.03a |
| Control group | 0.11 ± 0.02a,b | 0.08 ± 0.05a,b |
Level of significance with P < 0.05 compare with normal group.
Level of significance with P < 0.05 compare with control group.
Discussion
Diabetes has become one of the major diseases in China,8 which has different degrees of effect on the connective tissues of the body, including lumbar disc tissues. There are many factors that can cause disc degeneration in diabetic patients. The distribution of nutrition of intervertebral disc is mainly through the cartilage endplate to the nucleus pulposus tissue, and harmful metabolites disperse through the original way out of the end plate.9 While the concentration gradient within the original intervertebral disc is destroyed by hyperglycemia because of diabetes, thus affecting transfer power of nutrition of intervertebral disc, which leads to the reduction of nutrition of intervertebral disc and deposition of metabolites. These will cause damage of the nucleus pulposus cells, lead to the reduction of water content of intervertebral disc matrix and resistance to compression of the intervertebral disc tissue, and finally result in degenerative performances in intervertebral disc, such as biomechanical changes and decrease of stability. For vascular bud tissues that deep into cartilage endplate and feed nucleus pulposus cells, hyperglycemia will cause dysfunction and progressive occlusion of the barrier of vascular bud tissues,10 eventually lead to the reduction of vascular number, lumen thinning, and abnormal changes of PH, oxygen partial pressure, and osmotic pressure in intervertebral disc.11
Vitamin D shows its activity mainly through the form of 1,25(OH)2D3 in the body. 1,25(OH)2D3, also called calcitriol, is a kind of fat-soluble vitamins and belongs to steroid compound, which plays its role by combining to intracellular specific receptor VDR.12 van Halteren et al.13 found the complex of 1,25(OH)2D3 and VDR had comprehensive physiological and pathological effects on kidney and cardiovascular system. When combined with the VDR of kidney, it could inhibit the secretion of rennin, prevent the infiltration of macrophage in renal tissue, and reduce the oxidative stress response and the progress of renal fibrosis. Adorini et al.14 proved that 1,25(OH)2D3 could reduce urinary protein and injury of the nephritis animal model, so as to play its role in the protection of the kidney of diabetic rats. What’s more, it could inhibit and reduce inflammatory reaction, and induce Bcl-2 expression, thus maintaining cell autophagy in an appropriate scope and avoiding autophagic cell death. For islet β-cells, vitamin D can reduce the antigen presenting capacities of macrophages, reduce the content of inflammatory factor IL-2, increase the sensitivity of insulin, and thus playing a protective role in islet β-cells in diabetes and anti-inflammatory effect.15 However, the deficiency of Vitamin D is common in diabetic patients, and lacking 1,25 -(OH)2D3 is more pronounced than 25 -(OH)D3.16 When the deficiency of Vitamin D occurs, calcium channel is closed, which can directly affect the synthesis and secretion of insulin, interfere with insulin signaling pathway and increase the incidence of insulin resistance by causing blocking of insulin receptor substrate phosphorylation.17 In the five year follow-up study, Mitri et al.18 found the deficiency of Vitamin D could increase the risk of diabetes, and the supplement of Vitamin D could reduce the risk of type 2 diabetes. The function of islet B cell could be improved through short-term supplementation of calcitriol in adult patients with type 2 diabetes, and the content of glycosylated hemoglobin could be reduced, thus alleviating the condition of diabetic patients.19 In this study, the blood glucose in experimental group was lower than control group, which suggested that Vitamin D could decrease the blood glucose in diabetic rats.
TGF-β and IGF-1 are two kinds of cytokines that can change extracellular matrix of the intervertebral disc and prevent apoptosis of the intervertebral disc, and the contents decrease at different levels in the degenerated intervertebral discs. TGF-β is a kind of dimeric polypeptide and plays important role in the development, growth and maintenance of spine and intervertebral disc tissues,20 which plays a special role in promoting synthesis of cartilage matrix, maintaining dynamic equilibrium of decomposition and anabolism of cartilage matrix, inhibiting expression and secretion of extracellular matrix degrading enzyme in intervertebral disc, and reducing the degradation of extracellular matrix through non-Smad-dependent signaling pathway.21 In the diabetic state, the decrease of TGF-β causes the decrease of proteoglycan. Proteoglycan, as one of the important extracellular matrix, can combine with collagen, fibronectin, and elastin in extracellular matrix, which constitutes characteristic extracellular matrix. Moreover, it contains large amounts of water, so the nucleus pulposus cells have sufficient flexibility and can undertake sufficient pressure,22 forming the essential components to maintain the normal disc structure, metabolism and physiological function. In this study, generally, TGF-β levels in control and experimental groups were lower than normal group, indicating TGF-β was reduced in diabetic rats. Hence, the proteoglycan in diabetic rats might be decreased.
IGF-1 is a kind of active polypeptide, and can inhibit the degeneration of cartilage, promote the proliferation of chondrocyte, and maintain the stability of cartilage collagen in disc through ERK/MAPK signaling pathway.23 It can promote the synthesis of chondrocyte proliferation and cartilage matrix and play a key role in cartilage formation and the self-regulation of cartilage.24 Type II collagen is the main expression product of extracellular matrix in cartilage endplate of the disc.25 IGF-1 can stimulate chondrocyte to synthesize substrate specificity type II collagen, increase the activity of glycosaminoglycans poly enzyme and alkaline phosphatase of osteoblast, so as to protect disc tissue and prevent degeneration and aging. IGF-1 can also stimulate nucleus pulposus cells to secrete extracellular matrix. Seki et al.26 has proved IGF-1 can promote the synthesis of proteoglycan in extracellular matrix and reduce its decomposition. Hence, how to relieve the decrease of TGF-β and IGF-1 in intervertebral disc of diabetic rats plays an important role in preventing the degeneration of intervertebral disc in diabetic rats. In this study, the positive rate of TGF-β and IGF-1 in disc cells of diabetic rats in experimental group was significantly higher than control group at three different periods (P < 0.05), which indicated that Vitamin D could improve the contents of TGF-β and IGF-1 and decrease the risk of diabetes. The expressions of TGF-β and IGF-1 proteins in disc cells of experimental group were higher than control group (P < 0.05), which further supported this founding.
We speculated the changes of HE staining in intervertebral disc were related to the increase of TGF-β and IGF-1 in intervertebral disc, and this result was also confirmed by immunohistochemistry and Western Blot. The deficiency of vitamin D could lead to the decrease of VDR in endplate chondrocytes, while VD3 might increase the content of TGF-β and IGF-1 in the intervertebral disc by combining with VDR, thus promoting the differentiation, proliferation and maturation of chondrocytes, maintaining the stability of cartilage collagen, and playing a critical role in self-regulation of cartilage. In addition, VD3 could also affect the synthesis of proteoglycans through chondrocytes,27 and make a positive feedback with TGF-β and IGF-1. What’s more, Vitamin D could improve the insulin sensitivity, reduce blood glucose concentration in diabetic rats, thereby reducing the intra-disc concentration of blood glucose, improving nutrient transport power in intervertebral disc, and reducing metabolic product deposition, further increasing TGF- Β and IGF-1 contents in diabetic intervertebral disc and alleviating degeneration of intervertebral disc.
Conclusion
Vitamin D can protect the degeneration of intervertebral disc and improve the content of TGF-β and IGF-1 in the intervertebral disc, which provides a new idea for the prevention and treatment of degenerative changes of the intervertebral disc in diabetic patients. In addition, to prevent microangiopathy and actively control blood glucose in diabetic patients, adding vitamin D is also an effective way.
Acknowledgements
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Authors' contributions
WZ conceived of the study, and participated in its design. JZ and LL performed the experiments and collected the data. YS and WD analyzed the data and helped to draft the manuscript. JA interpreted the data and drafted the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Liu YJ, Huang GS, Juan CJ, Yao MS, Ho WP, Chan WP. Intervertebral disk degeneration related to reduced vertebral marrow perfusion at dynamic contrast-enhanced MRI. Am J Roentgenol 2009; 192: 974–9. [DOI] [PubMed] [Google Scholar]
- 2.Ziv I, Moskowitz RW, Kraise I, Adler JH, Maroudas A. Physicochemical properties of the aging and diabetic sand rat intervertebral disc. J Orthop Res 1992; 10: 205–10. [DOI] [PubMed] [Google Scholar]
- 3.Zhao CQ, Zhang YH, Jiang SD, Jiang LS, Dai LY. Both endoplasmic reticulum and mitochondria are involved in disc cell apoptosis and intervertebral disc degeneration in rats. Age 2010; 32: 161–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonow RO, Mitch WE, Nesto RW, O'Gara PT, Becker RC, Clark LT, Hunt S, Jialal I, Lipshultz SE, Loh E. Prevention conference VI: diabetes and cardiovascular disease: writing group V: management of cardiovascular-renal complications. Circulation 2002; 105: 159–64. [DOI] [PubMed] [Google Scholar]
- 5.Jackson AR, Huang C-Y, Gu WY. Effect of endplate calcification and mechanical deformation on the distribution of glucose in intervertebral disc: a 3D finite element study. Comput Meth Biomech Biomed Eng 2011; 14: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi X, Sun J, Li L, Wei Q, Qian Y, Chen X, Ma L. 1,25-Dihydroxyvitamin D3 deficiency is involved in the pathogenesis of diabetic retinopathy in the uygur population of China. IUBMB Life 2016; 68: 445–51. [DOI] [PubMed] [Google Scholar]
- 7.Gu D, Reynolds K, Duan X, Xin X, Chen J, Wu X, Mo J, Whelton PK, He J. Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: international collaborative study of cardiovascular disease in Asia (InterASIA). Diabetologia 2003; 46: 1190–8. [DOI] [PubMed] [Google Scholar]
- 8.Bi Y, Xu Y, Li M, Wang W, Wang T, Wang L, Jiang Y, Xu M, Lu J, Li J. Prevalence and control of diabetes in Chinese adults: the China metabolic risk factor study. Circulation 2013; 12: A11–A11. [Google Scholar]
- 9.Crock HV, Goldwasser M. Anatomic studies of the circulation in the region of the vertebral end-plate in adult Greyhound dogs. Spine 1984; 9: 702–6. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res 1999; 17: 829–35.. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D: a millenium perspective. J Cell Biochem 2003; 88: 296–307. [DOI] [PubMed] [Google Scholar]
- 12.Levi M. Nuclear receptors in renal disease. Biochim Biophys Acta 2011; 1812: 1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Halteren AG, Tysma OM, Van EE, Mathieu C, Roep BO. 1alpha,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J Autoimmun 2004; 23: 233–9. [DOI] [PubMed] [Google Scholar]
- 14.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem 2003; 88: 227–33. [DOI] [PubMed] [Google Scholar]
- 15.Levin A, Le BM, Er L, Andress D, Sigrist MK, Djurdjev O. Incident isolated 1,25(OH)2D3 deficiency is more common than 25(OH)D deficiency in CKD. J Nephrol 2012; 25: 204–10. [DOI] [PubMed] [Google Scholar]
- 16.Pittas AG, Lau J, Hu FB, Dawsonhughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metabol 2007; 92: 2017–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao G, Ford ES, Li C. Associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with surrogate markers of insulin resistance among U.S. adults without physician-diagnosed diabetes: NHANES, 2003-2006. Diab Care 2010; 33: 344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr 2011; 94: 486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin H, Jie S, Wang B, Wang M, Bing S, Di C. TGF-β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett 2011; 585: 1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrana JL. Signaling by the TGFβ superfamily. Cold Spring Harbor Perspect Biol 2013; 5: a011197–a011197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallach CJ, Gilbertson LG, Kang JD. Gene therapy applications for intervertebral disc degeneration. Spine 2003; 28(15 Suppl): 263–70. [DOI] [PubMed] [Google Scholar]
- 22.Yammani RR, Loeser RF. Extracellular nicotinamide phosphoribosyltransferase (NAMPT/visfatin) inhibits insulin-like growth factor-1 signaling and proteoglycan synthesis in human articular chondrocytes. Arth ResTher 2012; 14: R23–R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urano T, Narusawa K, Shiraki M, Usui T, Sasaki N, Hosoi T, Ouchi Y, Nakamura T, Inoue S. Association of a single nucleotide polymorphism in the insulin-like growth factor-1 receptor gene with spinal disc degeneration in postmenopausal Japanese women. Spine 2008; 33: 1256–61. [DOI] [PubMed] [Google Scholar]
- 24.Ahsan R, Tajima N, Chosa E, Sugamata M, Sumida M, Hamada M. Biochemical and morphological changes in herniated human intervertebral disc. J Orthop Sci 2001; 6: 510–8. [DOI] [PubMed] [Google Scholar]
- 25.Alini M, Li W, Markovic P, Aebi M, Spiro RC, Roughley PJ. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine 2003; 28: 446–53. [DOI] [PubMed] [Google Scholar]
- 26.Seki S, Kawaguchi Y, Chiba K, Mikami Y, Kizawa H, Oya T, Mio F, Mori M, Miyamoto Y, Masuda I. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet 2005; 37: 607–12. [DOI] [PubMed] [Google Scholar]
- 27.Askari A, Naghizadeh MM, Homayounfar R, Shahi A, Afsarian MH, Paknahad A, Kennedy D, Ataollahi MR. Increased serum levels of IL-17A and IL-23 are associated with decreased vitamin D3 and increased pain in osteoarthritis. Plos One 2016; 11: e0164757–e0164757. [DOI] [PMC free article] [PubMed] [Google Scholar]