Abstract
IMPORTANCE
Acute kidney injury (AKI) after pediatric cardiac surgery is associated with high short-term morbidity and mortality; however, the long-term kidney outcomes are unclear.
OBJECTIVE
To assess long-term kidney outcomes after pediatric cardiac surgery and to determine if perioperative AKI is associated with worse long-term kidney outcomes.
DESIGN, SETTING, AND PARTICIPANTS
This prospective multicenter cohort study recruited children between ages 1 month to 18 years who underwent cardiopulmonary bypass for cardiac surgery and survived hospitalization from 3 North American pediatric centers between July 2007 and December 2009. Children were followed up with telephone calls and an in-person visit at 5 years after their surgery.
EXPOSURES
Acute kidney injury defined as a postoperative serum creatinine rise from preoperative baseline by 50% or 0.3 mg/dL or more during hospitalization for cardiac surgery.
MAIN OUTCOMES AND MEASURES
Hypertension (blood pressure ≥95th percentile for height, age, sex, or self-reported hypertension), microalbuminuria (urine albumin to creatinine ratio >30 mg/g), and chronic kidney disease (serum creatinine estimated glomerular filtration rate [eGFR] <90 mL/min/1.73 m2 or microalbuminuria).
RESULTS
Overall, 131 children (median [interquartile range] age, 7.7 [5.9–9.9] years) participated in the 5-year in-person follow-up visit; 68 children (52%) were male. Fifty-seven of 131 children (44%) had postoperative AKI. At follow-up, 22 children (17%) had hypertension (10 times higher than the published general pediatric population prevalence), while 9 (8%), 13 (13%), and 1 (1%) had microalbuminuria, an eGFR less than 90 mL/min/1.73 m2, and an eGFR less than 60 mL/min/1.73 m2, respectively. Twenty-one children (18%) had chronic kidney disease. Only 5 children (4%) had been seen by a nephrologist during follow-up. There was no significant difference in renal outcomes between children with and without postoperative AKI.
CONCLUSIONS AND RELEVANCE
Chronic kidney disease and hypertension are common 5 years after pediatric cardiac surgery. Perioperative AKI is not associated with these complications. Longer follow-up is needed to ascertain resolution or worsening of chronic kidney disease and hypertension.
Pediatric acute kidney injury (AKI) is a significant health concern because it is associated with inpatient mortality and length of hospital stay.1,2 Acute kidney injury is estimated to occur in 25% to 60% of children undergoing cardiac surgery.3,4 The mechanism of AKI after cardiac surgery is multifactorial, including systemic inflammatory response to cardiopulmonary bypass, renal ischemia, oxidative stress, microemboli, and reperfusion injury.5
It is currently unknown whether an episode of AKI in any pediatric population is associated with permanent renal damage and progressive decline in kidney function. Previous studies in children have reported on long-term outcomes in patients with AKI without any suitable controls.6 Therefore, the relative risk of long-term outcomes after pediatric AKI remains unknown. There is a robust association between AKI and chronic kidney disease (CKD) in adults.7 Recent evidence from a large prospective cohort study in adults also supports the association of AKI with a long-term risk of hypertension.8 This potential association of AKI with hypertension and CKD in children is crucial to elucidate because they are important and potentially treatable cardiovascular risk factors. Moreover, current AKI guidelines in children do not provide recommendations for long-term follow-up, primarily due to a lack of studies with reliable evidence.
The long-term risk of renal complications is increasingly important as the outcomes of children after cardiac surgery have improved dramatically and more children are surviving to adulthood.9,10 An excess burden of renal disease has been reported in adults with congenital heart disease but has not been elucidated in children.11,12 The goal of the present study was to assess long-term renal outcomes after cardiac surgery and determine if perioperative AKI is associated with these outcomes.
Methods
Study Cohort
This is a prospective longitudinal cohort study of children with congenital cardiac defects who were enrolled between July 2007 and December 2009. Children who were 1 month to 18 years old undergoing cardiac surgery with cardiopulmonary bypass at Cincinnati Children’s Hospital, Montreal Children’s Hospital, and Yale New Haven Children’s Hospital, were enrolled in the Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) study.3,13,14 Children were excluded if they had a history of renal transplantation or dialysis. Details around patient recruitment before the cardiac surgery were published previously.3,13,14 Before discharge from the cardiac surgery hospitalization, children were invited to participate in follow-up studies along with permission for future contact. At a median of 3 years after discharge from the index hospitalization, the children who consented to future contact were invited to participate in the current study. Informed consent was obtained from all parents or legal guardians, along with assent, when appropriate, from children. This study was approved by the institutional review board of each participating institution.
Study Visits
Once the participant was recruited into this long-term follow-up study, he or she was followed up annually by telephone until a 5-year in-person visit. The telephone calls consisted of detailed questionnaires focused on interim medical history, hospitalizations, diagnosis of hypertension, dialysis requirement, medical visits for a kidney problem, current medications, and interim laboratory testing. Five-year in-person visits were performed by a research nurse at the hospital clinic or at the participant’s home (per family preference). Height, weight, and blood pressure were measured, and blood and urine were collected at the time of the visits scheduled by participant availability. Height was measured with a SECA stadiometer (SECA) and weight was measured with a Precision Health Scale (A&D Engineering). Percentiles and z scores of height, weight, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) were calculated using Centers for Disease Control and Prevention growth charts.15 Blood pressures were measured with an appropriate-size cuff using the Omron HEM-711AC home blood pressure monitor (Omron Healthcare, Inc). The blood pressure monitor was standardized and annually calibrated at each of the participating institutions. The average of 3 blood pressure measurements at each visit were used to calculate the blood pressure percentile. If the average blood pressure was greater than the 95th percentile, the blood pressure was remeasured manually. Blood pressure percentiles were calculated as per The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents.15 During the visit, other details on the medical history, interim hospital admissions, medication history, and laboratory testing since cardiac surgery were also collected. Discharge summaries from hospital admissions and other medical records were obtained for each hospitalization from the respective medical institutions. Full details of the analyte measurements, including sample collection and processing, are included in the eMethods in the Supplement.
Data From the Index Cardiac Surgery Admission
The following detailed index cardiac surgery admission information was available in all participants from our prior in-hospital study: preoperative characteristics, operative details, and postoperative complications using the definitions of the Society of Thoracic Surgeons.16 We used the risk adjustment for congenital heart surgery-1 (RACHS-1) consensus-based scoring system to categorize the complexity of surgery.16 Higher scores signify more complex surgeries. This method of risk stratification is a widely accepted tool for the evaluation of differences in outcomes of surgery for congenital heart disease. Acute kidney injury was defined as the development of at least stage I AKI, defined by Acute Kidney Injury Network (AKIN)17 as at least a 50% or more increase or a 0.3 mg/dL (to convert mg/dL to μmol/L, multiply by 88.4) or more increase from baseline serum creatinine during hospitalization after cardiac surgery. We also analyzed the outcome of stage II AKI, defined as receiving acute dialysis during the hospital stay or a doubling in serum creatinine from baseline. We measured urinary biomarkers of tubular injury (neutrophil gelatinase–associated lipocalin [NGAL], interleukin 18 [IL-18], kidney injury molecule 1 [KIM-1], and liver fatty acid–binding protein [L-FABP]) on the preoperative and first postoperative day and assessed the relationship of these biomarkers with 5-year renal outcomes. Details of the in-hospital study have been previously described.3,13,14
Outcome Definitions
For participants up to age 18 years, hypertension was defined as systolic or diastolic blood pressure greater than or equal to the 95th percentile for age, height, and sex.15 For participants older than 18 years, hypertension was defined as systolic or diastolic blood pressure greater than 140 mm Hg or greater than 90 mm Hg, respectively.18 Prehypertension was defined as a systolic or diastolic blood pressure from the 90th to the 95th percentile in children 18 years or younger. For patients older than 18 years old, prehypertension was defined as systolic or diastolic blood pressure 120 mm Hg to 139 mm Hg or 80 mm Hg to 89 mm Hg, respectively. Additionally, participants were classified as having hypertension if they reported a history of hypertension. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease in Children study (CKiD) serum creatinine–based equation.19 Additionally we calculated cystatin C–based eGFR and combined serum creatinine-cystatin C–based eGFR using the CKiD-derived equations.19,20 Microalbuminuria was defined as urine albumin to creatinine ratio greater than 30 mg/g. Chronic kidney disease was defined as an eGFR less than 90 mL/min/1.73 m2 or microalbuminuria.21
Statistical Analysis
Baseline characteristics of those who completed and did not complete follow-up visits were compared. Of the 131 participants with follow-up visits, all had blood pressure measurements or responded to the questionnaire regarding hypertension, 116 had urine samples taken, and 101 had blood samples taken. For each outcome, only those participants with non-missing values were included in the analysis.
Continuous variables were compared with 2-sample t test or Wilcoxon rank sum test and dichotomous variables with the χ2 test or Fisher exact test. Five-year outcome prevalence confidence intervals were calculated with the Wilson score method. We performed multivariate analyses to examine the association between the baseline covariates of age, sex, race, preoperative GFR, and postoperative serum creatinine rise with the outcomes of hypertension, microalbuminuria, and CKD. SAS version 9.4 (SAS Institute Inc) was used for analyses.
Results
Of the 311 children enrolled in the original TRIBE-AKI pediatric cohort, 305 children survived the index hospitalization; 105 children declined future contact, which left 200 children eligible for participation in the follow-up study. After various exclusions, 131 children, at a mean of 5.4 years after surgery, agreed to in-person follow-up (Figure 1). Baseline characteristics were not significantly different between children that participated in the long-term follow-up vs did not (eTable 1 in the Supplement). Patient baseline characteristics along with their preoperative medications at the time of cardiac surgery by AKI status are shown in Table 1. Several patients were taking medications for optimization of hemodynamics that also lower blood pressure. Children with AKI had a younger median age (P = .05). The median (interquartile range [IQR]) age of the cohort at the 5-year follow-up was 7.7 (5.9–9.9) years, and 68 children (52%) were male. Overall, 57 children (44%) had experienced AKI after their index cardiac surgery. Median height and weight z scores of the cohort were −0.56 and −0.14 at 5 years after cardiac surgery and did not significantly differ by AKI status (Table 2). Median BMI z score was 0.36 and significantly lower in children with AKI compared with children without AKI (P = .01).
Figure 1. TRIBE-AKI Study Population.
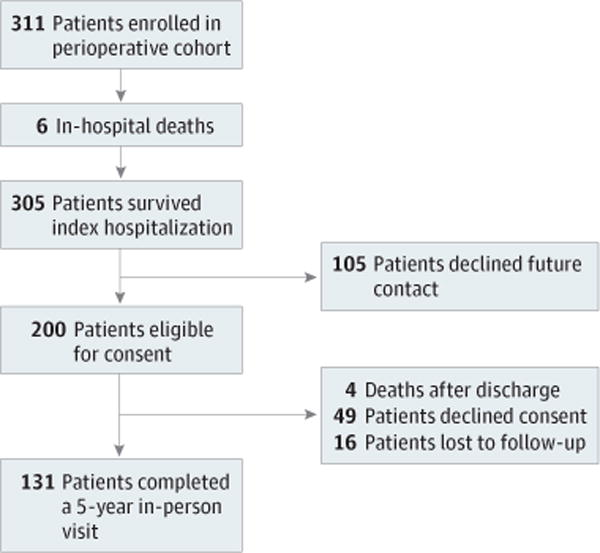
Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) long-term follow-up cohort selection process.
Table 1.
Baseline and Postoperative Characteristics of Study Cohort by AKI Status
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| Overall (n = 131) |
AKI Group (n = 57) |
Non-AKI Group (n = 74) |
||
| Preoperative/baseline | ||||
| Age, median (IQR), mo | 31.9 (6.0–58.6) | 13 (5.8–48.7) | 38 (7.4–68.9) | .05 |
| Male sex | 68 (52) | 32 (56) | 36 (49) | .39 |
| Nonwhite | 20 (15) | 10 (18) | 10 (14) | .52 |
| Preoperative estimated SCr-GFR, median (IQR) | 78 (93–110) | 97 (78–126) | 89 (81–101) | .08 |
| Preoperative SCr-eGFR, mg/dL, abnormal for agea | 26 (20) | 5 (9) | 21 (29) | .005 |
| Preoperative urine albumin to creatinine ratio, mg/g | 23.6 (10.9–75.5) | 23.6 (11.9–88.5) | 24.0 (10.5–72.1) | .14 |
| Preoperative microalbuminuriab | 26 (20) | 11 (19) | 15 (20) | .56 |
| Preoperative CKDc | 48 (37) | 16 (28) | 32 (43) | .07 |
| RACHS-1 surgical category | ||||
| 1 | 7 (5) | 0 | 7 (9) | <.001 |
| 2 | 66 (51) | 26 (46) | 40 (54) | |
| 3 | 52 (40) | 25 (45) | 27 (36) | |
| 4 | 5 (4) | 5 (9) | 0 | |
| Type of surgery | ||||
| Septal defect repair | 44 (36) | 18 (33) | 26 (38) | .41 |
| Inflow/outflow tract or valve procedure | 23 (19) | 8 (15) | 15 (22) | |
| Combined procedure | 56 (46) | 28 (52) | 28 (41) | |
| Index hospitalization | ||||
| Peak postoperative SCr rise from baseline, mg/dL, median (IQR) | 0.2 (0.3–0.7) | 0.7 (0.5–1) | 0.2 (0, 0.3) | <.001 |
| Renal replacement | 3 (2) | 3 (5) | 0 | .04 |
| CRRT, No. (%) | 1 (33) | 1 (33) | 0 | |
| Peritoneal dialysis, No. (%) | 2 (67) | 2 (67) | 0 | |
Abbreviations: AKI, acute kidney injury; CRRT, continuous renal replacement therapy; GFR, glomerular filtration rate; IQR, interquartile range; RACHS-1, risk adjustment for congenital heart surgery; SCr, serum creatinine.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 88.4.
Defined as age-based GFR thresholds: 2 years or older, less than 90 mL/min/1.73 m2; 1.5 to 2.0 years, less than 76 mL/min/1.73 m2; 1 to 1.5 years, less than 74 mL/min/1.73 m2; younger than 1 year, less than 65 mL/min/1.73 m2; 3 months to 8 months old, less than 58 mL/min/1.73 m2.
Preoperative microalbuminuria defined by age-based albumin to creatinine ratio thresholds: older than 2 years, more than 30 mg/g; 6 months to 2 years, more than 75 mg/g.
Preoperative CKD defined as eGFR less than than age-based thresholds or microalbuminuria more than age-based thresholds.
Table 2.
Five-Year Outcomes by AKI Status
| Characteristics | Overall (n = 131) |
AKI Group (n = 57) |
Non-AKI Group (n = 74) |
P Value |
|---|---|---|---|---|
| Height z score, median (IQR) | −0.56 (−1.6 to 0.5) | −0.58 (−1.43 to −0.18) | −0.52 (−1.74 to −0.74) | .90 |
| Weight z score, median (IQR) | −0.14 (−1.01 to 0.61) | −0.33 (−1.29 to 0.43) | −0.08 (−0.73 to 0.74) | .16 |
| BMI z score, median (IQR) | 0.36 (−0.45 to 1.05) | 0.03 (−0.92 to 0.66) | 0.49 (−0.14 to 1.14) | <.001 |
| Patients enrolled in school, No. (%) | 117 (90) | 48 (86) | 69 (93) | .16 |
| Patients enrolled in special education, No. (%) | 42 (36) | 20 (42) | 22 (32) | .28 |
| Any form of dialysis | 0 | 0 | 0 | NA |
| Nephrologist follow-up, No. (%) | 5 (4) | 2 (4) | 3 (4) | .86 |
| Family history of renal disease, No. (%) | 61 (47) | 25 (44) | 36 (49) | .56 |
| At least 1 hospital/ED readmission, No. (%) | 32 (24) | 12 (21) | 20 (27) | .43 |
| GFRa | ||||
| eGFR, median (IQR) | 101 (112–126) | 118 (105–132) | 108 (93–121) | .01 |
| eGFR<90 mL/min/1.73 m2, No. (%) | 13 (13) | 0 | 13 (22) | <.001 |
| eGFR<60 mL/min/1.73 m2, No. (%) | 1 (1) | 0 | 1 (2) | .39 |
| Albuminuria, No. (%) | ||||
| Proteinuriab | ||||
| Negative | 109 (90) | 44 (86) | 65 (93) | |
| Trace | 5 (4) | 3 (6) | 2 (3) | .49 |
| Small | 7 (6) | 4 (8) | 3 (4) | |
| Large | 0 | 0 | 0 | |
| Albumin to creatinine ratio, mg/g, median (IQR) | 5.1 (2.39–12.13) | 5.92 (2.76–10.43) | 4.88 (1.96–13.78) | .87 |
| Microalbuminuriac | 9 (8) | 3 (6) | 6 (9) | .54 |
| CKD,d No. (%) | 21 (18) | 3 (6) | 18 (27) | <.001 |
| Hypertension | ||||
| Systolic BP z score, median (IQR) | 0.41 (−0.21 to 1.03) | 0.45 (−0.31 to 1.01) | 0.41 (−0.17 to 1.1) | .62 |
| Systolic pre-HTN, No. (%) | 12 (9) | 3 (5) | 9 (12) | .29 |
| Systolic HTN, No. (%) | 14 (11) | 5 (9) | 9 (12) | .29 |
| Diastolic BP z score, median (IQR) | −0.01 (−0.27 to 0.35) | −0.1 (−0.32 to 0.26) | 0.03 (−0.25 to 0.46) | .15 |
| Diastolic pre-HTN, No. (%) | 8 (6) | 2 (4) | 6 (8) | .11 |
| Diastolic HTN, No. (%) | 4 (3) | 0 | 4 (5) | .11 |
| Pre-HTN, No. (%) | 12 (9) | 3 (5) | 9 (12) | .29 |
| HTN,e No. (%) | 22 (17) | 6 (11) | 16 (22) | .09 |
Abbreviations: AKI, acute kidney injury; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; CKD, chronic kidney disease; ED, emergency department; eGFR, estimated GFR; GFR, glomerular filtration rate; HTN, hypertension; IQR, interquartile range; NA, not available; SCr, serum creatinine.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 88.4.
Estimated GFR calculated with SCr–based Chronic Kidney Disease in Children Study equation.19
Proteinuria measured by urine dipstick.
Microalbuminuria defined as more than 30 mg/g.
Chronic kidney disease defined as the composite of estimated GFR less than 90 mL/min/1.73 m2 or microalbuminuria.
Hypertension defined by BP or self-report.
Five-Year Renal Outcomes
Glomerular Filtration Rate
Blood samples were available on 101 of 131 children to assess eGFR. At follow-up, median (IQR) eGFR in children with and without AKI was not significantly different: 118 (105–132) mL/min/1.73 m2 and 108 (93–121) mL/min/1.73 m2, respectively, using the serum creatinine–based CKiD equation (P = .09) (Table 2). Estimated GFR less than 90 mL/min/1.73 m2 was identified in 13 children (13%), and an eGFR less than 60 mL/min/1.73 m2 in 1 child (1%). All 13 children with an eGFR less than 90 mL/min/1.73 m2 were in the non-AKI group. Table 3 displays eGFR results using other estimating equations. Using a cystatin C–based eGFR equation, we identified 17 participants with AKI and 24 participants without AKI with an eGFR between 60 mL/min/1.73 m2 and 90 mL/min/1.73 m2.
Table 3.
Comparing 5-Year Kidney Function Outcomes Using 3 Pediatric GFR Estimating Equations
| Pediatric Estimating Equations | AKI Group | Non-AKI Group | ||||
|---|---|---|---|---|---|---|
| eGFR | eGFR <90 | eGFR | eGFR <90 | |||
| Renal Measurement | Name | Equation | Median (IQR) | No. (%) | Median (IQR) | No. (%) |
| Creatinine | CKiD19 | 0.413 × ht/Cr | 118 (105–132) | 0 | 108 (93–121) | 13 (22) |
| Cystatin C | CKiD20 | 70.69 × CysC−0.931 | 92 (87–99) | 18 (42) | 89 (83–101) | 30 (52) |
| Creatinine and cystatin C | CKiD19 | 41.6 (ht/Cr)0.599 × (1.8/CysC)0.317 | 101 (95–111) | 2 (5) | 96 (87–105) | 20 (34) |
Abbreviations: AKI, acute kidney injury; CKiD, Chronic Kidney Disease in Children Study; Cr, serum creatinine; CysC, cystatin C; eGFR, estimated glomerular filtration rate (mL/min/1.73 m2); ht, height; IQR, interquartile range.
Albuminuria
Urine samples were available for 116 of 131 children to measure albuminuria. At the 5-year follow-up visit, microalbuminuria was identified in 3 children (6%) with AKI and 6 children (9%) without AKI (P = .54). The median (IQR) albumin to creatinine ratio in children with AKI was 5.92 (2.80–10.40) mg/g and 4.88 (2.00–13.80) mg/g in children without AKI (P = .88). There was no difference in proteinuria by dipstick in children with and without AKI (P = .49).
Chronic Kidney Disease
Chronic kidney disease, defined as the composite of eGFR less than 90 mL/min/1.73 m2 or microalbuminuria, was present in 21 children (18%), 3 children with AKI (6%), and 18 children without AKI (27%) (P = .01). Overall, 37 children (30%) had an eGFR less than 90 mL/min/1.73 m2, microalbuminuria, or hypertension. No children required dialysis since discharge from the index hospitalization. Children with CKD 5 years after cardiac surgery had a lower preoperative eGFR (median [IQR]. 83 [72–89] mL/min/1.73 m2) compared with patients without CKD (median [IQR], 96 [83–115] mL/min/1.73 m2) (P = .02) (eTable 2 in the Supplement). The presence of any AKI or of stage II AKI or worse was not associated with a higher prevalence of 5-year CKD (Table 2) (eTables 3 and 4 in the Supplement).
Hypertension
We determined hypertension status on the entire cohort of 131 children through either blood pressure measurement (18 patients) or self-history (6 patients). Systolic hypertension was found in 5 children (9%) with AKI and 9 children (12%) without AKI. Diastolic hypertension was found in 0 children with AKI and 4 children (5%) without AKI. There was no significant difference in hypertension between children with and without perioperative AKI. Additionally, baseline age, sex, race, preoperative GFR, and postoperative serum creatinine increase were not associated with 5-year hypertension. Overall, hypertension (systolic or diastolic) was identified in 17% of children and prehypertension (systolic or diastolic) was identified in an additional 9% of children (Table 2). Seven children were being treated for hypertension or kidney disease. Only 5 children (4%) were being followed up by a pediatric nephrologist. Figure 2 shows the prevalence of hypertension in 3 other published pediatric cohorts demonstrating that the current cohort had substantially higher hypertension prevalence.22–24 Detailed characteristics of these 3 cohorts are described in eTable 5 in the Supplement.
Figure 2. Hypertension Prevalence Among Pediatric Center Cohorts Included in the TRIBE-AKI Study.
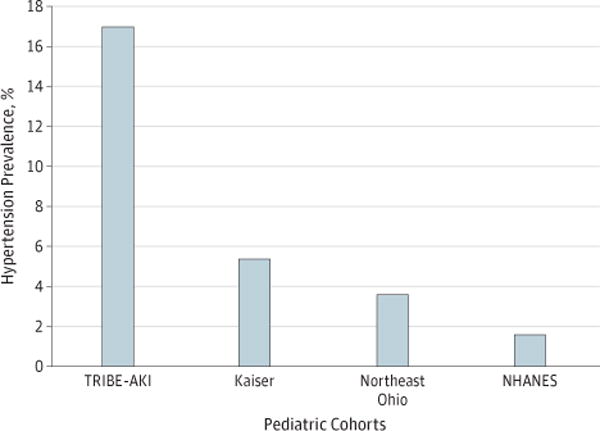
Comparison of hypertension prevalence in 4 pediatric cohorts from the Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) pediatric cohort 5 years after cardiac surgery, the National Health and Nutrition Examination Survey (NHANES), the Kaiser Permanente health care system, and a large urban medical system in Northeast Ohio (eTable 3 in the Supplement). The NHANES, Kaiser, and Northeast Ohio cohorts were identified through a literature review.
Perioperative Biomarker Results and 5-Year Risk of Renal Outcomes
We examined the relationship between perioperative bio-marker levels and 5-year outcomes. There was no association between preoperative urinary biomarkers of kidney injury (ie, albumin, NGAL, KIM-1, IL-18, L-FABP) and CKD or hypertension at the 5-year follow-up (eTables 6–8 in the Supplement). Additionally, none of the first postoperative (0-hour to 6-hour time point) urinary biomarkers were independently associated with 5-year CKD or hypertension.
Discussion
The present study is the first prospective study that we know of to evaluate long-term renal outcomes after cardiac surgery in children and its association with perioperative AKI. Though the prevalence of hypertension and CKD was high at 5 years after cardiac surgery, we did not detect any increased risk of renal outcomes after exposure to AKI.
The hypertension prevalence in our study population was more than 10 times greater than the prevalence in the general pediatric population.25 This is especially significant and remarkable because our cohort was much younger than those reported elsewhere22–24,26 and would therefore be expected to display a lower prevalence of hypertension. A potential explanation could be the presence of underlying congenital heart disease in our cohort. However, hypertension is not typical in this pediatric population. Long-term risk of hypertension has been described previously after coarctation repair but not after surgery in a diverse group of congenital cardiac conditions.27,28 The hypertension identified in this cohort is likely multifactorial and may have several underlying mechanisms such as an upregulated renin-angiotensin system in an attempt to preserve renal perfusion, a disturbance of cardiac receptors during surgery leading to enhanced sympathetic activity, and the development of either clinical or subclinical CKD.29,30 Chronic kidney disease in our patients may be caused by chronic renal hypoperfusion and neurohormonal activation owing to congenital heart disease and/or the observed hypertension.11 Despite the high prevalence of hypertension and CKD after cardiac surgery, only 7 children were being treated for hypertension or kidney disease. Treatment of hypertension and CKD in childhood is critical because these risk factors in children are associated with increased cardiovascular outcomes in adults.26,31 The impact of CKD and hypertension is especially relevant in children with congenital heart disease because they are a high-risk population for long-term cardiac events.32,33
Prior studies12 have performed cross-sectional analyses examining CKD and hypertension in individuals with congenital heart disease. In a study of 9952 children and adults with congenital heart disease and 29 837 matched controls, congenital heart disease conferred a 1.4-fold higher risk (95% CI, 1.3–1.5) of hypertension and a 3.4-fold higher risk (95% CI, 2.3–5.1) of CKD.12 These findings are relevant to our research but not generalizable because only 25% of children with congenital heart disease require cardiac surgery.34 Our research suggests that the excess burden of CKD and hypertension previously identified in adults with congenital heart disease may indeed develop during childhood.
There are more than 20 studies in adults that have demonstrated an association of AKI with long-term outcomes such as CKD and more recently hypertension.8,35 This association has also been seen in AKI after cardiac bypass surgery in adults.36 We have previously shown that pediatric AKI after cardiac surgery affected short-term outcomes such as length of hospital stay and length of ventilation.3 Thus, the present study was hypothesized to demonstrate that pediatric AKI, like adult AKI, would also affect long-term renal outcomes. Interestingly, we found that children without AKI were more likely to have CKD. We determined that these children with CKD also had an abnormal eGFR prior to cardiac surgery. One potential reason for our lack of association of AKI as a risk factor for long-term outcomes could be the superior renal regenerative potential and greater renal reserve in children compared with older adults with diminished nephron mass.37 Furthermore, children with AKI may develop glomerular hyperfiltration in response to nephron loss, thereby increasing GFR.38
Another possible explanation for the lack of an AKI outcome association is that our duration of follow-up was inadequate. Children may need to be followed up for a longer period of time before there is sufficient nephron loss to cause changes in serum creatinine concentration. This is because serum creatinine starts to rise only after as much as 50% of kidney function has been lost.39 A recent study by Cooper et al40 studied 51 children who underwent cardiopulmonary bypass (33 children with AKI and 18 without) for a mean duration of 7 years. Seven years after cardiopulmonary bypass, there was no difference in eGFR between children with AKI or without. However, at long-term follow-up, patients in the AKI group demonstrated significantly increased levels of the established tubule injury biomarkers IL-18, KIM-1, and L-FABP when compared with the age-matched AKI-negative group. This study suggests the ongoing evolution of subtle subclinical kidney injury and supports the need for long-term follow-up into and throughout adulthood. Nonetheless, adult studies have shown that AKI is a risk factor for CKD with a smaller length of follow-up.41,42 Furthermore, CKD comorbidities may be an important cofactor and play a vital role in the risk for long-term outcomes after AKI. Comorbidities, such as coronary artery disease, diabetes, smoking, and hypertension, are highly prevalent in adults, whereas children are generally free of these comorbidities.
The strengths of this study include its prospective design and 5-year long-term follow-up. This is also the first prospective study, to our knowledge, to assess long-term outcomes after AKI in children and include a representative control group of patients without AKI. Additionally, no children were lost to follow-up after enrollment in the long-term study. The limitations include our sample size, which may have been inadequate. In addition, we had a substantial number of patients who declined to participate in the long-term follow-up. The exclusion of a large number of children may have produced imprecise results, although the baseline characteristics did not differ between participating and nonparticipating children. Finally, we were unable to obtain blood and urine from some children in this cohort owing to child refusal, parent refusal, or technical factors while collecting biospecimens.
Conclusions
We found a high prevalence of hypertension and CKD 5 years after pediatric cardiac surgery, but this risk was not associated with AKI exposure. Despite many children having hypertension and CKD, the majority were not being followed up or treated for these medical problems. Approximately 10 000 infants with congenital heart disease undergo cardiac surgery each year in the United States, and our results bear significant implications for the early detection and prevention of hypertension and CKD in this vulnerable population. These findings will provide data needed to begin making recommendations for renal follow-up after pediatric cardiac surgery. Future studies could consider using ambulatory blood pressure monitoring and echocardiogram to more accurately characterize hypertension risk and cardiac function after cardiac surgery in general, and after cardiac surgery-associated AKI in particular.
Supplementary Material
Key Points.
Question
What are the 5-year kidney outcomes after pediatric cardiac surgery and is perioperative acute kidney injury a risk factor for worse outcomes?
Findings
Hypertension and chronic kidney disease were common 5 years after cardiac surgery. Acute kidney injury was not a risk factor for worse outcomes.
Meaning
These findings will provide data to begin making recommendations for renal follow-up after cardiac surgery in children.
Acknowledgments
Funding/Support: This study was supported by the National Institutes of Health (NIH) (grant R01HL085757 to Dr Parikh) to fund the TRIBE-AKI Consortium to study novel biomarkers of acute kidney injury in cardiac surgery. Dr Greenberg is funded by the T32DK007276-35 training grant to the Yale University School of Medicine Section of Nephrology. Dr Devarajan is supported by the NIH (grant P50DK096418). Dr Garg is supported by Western University’s Dr Adam Linton Chair in Kidney Health Analytics. Dr Parikh is supported by the NIH (grant K24DK090203) and the O'Brien Center (grant P30 DK079310-07). Dr Parikh is also a member of the NIH-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury Consortium (U01DK082185).
Role of the Funder/Sponsor: The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Thiessen-Philbrook and Parikh had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Zappitelli, Devarajan, Krawczeski, Li, Coca, Parikh.
Acquisition, analysis, or interpretation of data: Greenberg, Zappitelli, Devarajan, Thiessen-Philbrook, Krawczeski, Li, Garg, Coca, Parikh.
Drafting of the manuscript: Greenberg, Zappitelli, Devarajan, Parikh.
Critical revision of the manuscript for important intellectual content: Greenberg, Zappitelli, Devarajan, Thiessen-Philbrook, Krawczeski, Li, Garg, Coca, Parikh.
Statistical analysis: Zappitelli, Thiessen-Philbrook. Obtained funding: Zappitelli, Devarajan, Parikh.
Administrative, technical, or material support: Greenberg, Krawczeski, Parikh.
Study supervision: Greenberg, Devarajan, Krawczeski, Parikh.
Conflict of Interest Disclosures: Dr Devarajan reported being a coinventor on the neutrophil gelatinase–associated lipocalin patent. No other conflicts were reported.
Additional Contributions: We thank Gagan Verma, MPH, Program of Applied Translational Research, Yale University School of Medicine, for his assistance with statistical analyses and data processing. Prof Verma was not compensated for his contributions.
References
- 1.Sutherland SM, Ji J, Sheikhi FH, et al. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. 2013;8(10):1661–1669. doi: 10.2215/CJN.00270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devarajan P. Pediatric acute kidney injury: different from acute renal failure but how and why. Curr Pediatr Rep. 2013;1(1):34–40. doi: 10.1007/s40124-012-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Krawczeski CD, Zappitelli M, et al. TRIBE-AKI Consortium Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39(6):1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan CJ, Zappitelli M, Robertson CM, et al. Western Canadian Complex Pediatric Therapies Follow-Up Group Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. 2013;162(1):120–7.e1. doi: 10.1016/j.jpeds.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 5.Kwiatkowski DM, Krawczeski CD. Acute kidney injury after cardiovascular surgery in children. In: Thakar CV, Parikh CR, editors. Perioperative Kidney Injury. New York: Springer; 2015. pp. 99–110. [Google Scholar]
- 6.Greenberg JH, Coca S, Parikh CR. Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol. 2014;15:184. doi: 10.1186/1471-2369-15-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu CY, Hsu RK, Yang J, Ordonez JD, Zheng S, Go AS. Elevated BP after AKI. J Am Soc Nephrol. 2016;27(3):914–923. doi: 10.1681/ASN.2014111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackie AS, Ionescu-Ittu R, Therrien J, Pilote L, Abrahamowicz M, Marelli AJ. Children and adults with congenital heart disease lost to follow-up: who and when? Circulation. 2009;120(4):302–309. doi: 10.1161/CIRCULATIONAHA.108.839464. [DOI] [PubMed] [Google Scholar]
- 10.Williams RG, Pearson GD, Barst RJ, et al. National Heart, Lung, and Blood Institute Working Group on research in adult congenital heart disease Report of the National Heart, Lung, and Blood Institute Working Group on research in adult congenital heart disease. J Am Coll Cardiol. 2006;47(4):701–707. doi: 10.1016/j.jacc.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117(18):2320–2328. doi: 10.1161/CIRCULATIONAHA.107.734921. [DOI] [PubMed] [Google Scholar]
- 12.Billett J, Cowie MR, Gatzoulis MA, Vonder Muhll IF, Majeed A. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart. 2008;94(9):1194–1199. doi: 10.1136/hrt.2007.122671. [DOI] [PubMed] [Google Scholar]
- 13.Parikh CR, Thiessen-Philbrook H, Garg AX, et al. TRIBE-AKI Consortium Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8(7):1079–1088. doi: 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. TRIBE-AKI Consortium Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22(9):1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555–576. (suppl 4th report) [PubMed] [Google Scholar]
- 16.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123(1):110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Society of Nephrology. Kidney Disease Improving Global Outcomes: KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Supplements. http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf. Accessed August 9, 2016.
- 22.Xi B, Zhang T, Zhang M, et al. Trends in elevated blood pressure among US Children and adolescents: 1999–2012. Am J Hypertens. 2016;29(2):217–225. doi: 10.1093/ajh/hpv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo JC, Sinaiko A, Chandra M, et al. Prehypertension and hypertension in community-based pediatric practice. Pediatrics. 2013;131(2):e415–e424. doi: 10.1542/peds.2012-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298(8):874–879. doi: 10.1001/jama.298.8.874. [DOI] [PubMed] [Google Scholar]
- 25.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015;169(3):272–279. doi: 10.1001/jamapediatrics.2014.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66(6):1108–1115. doi: 10.1161/HYPERTENSIONAHA.115.05831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bocelli A, Favilli S, Pollini I, et al. Prevalence and long-term predictors of left ventricular hypertrophy, late hypertension, and hypertensive response to exercise after successful aortic coarctation repair. Pediatr Cardiol. 2013;34(3):620–629. doi: 10.1007/s00246-012-0508-0. [DOI] [PubMed] [Google Scholar]
- 28.Clarkson PM, Nicholson MR, Barratt-Boyes BG, Neutze JM, Whitlock RM. Results after repair of coarctation of the aorta beyond infancy: a 10 to 28 year follow-up with particular reference to late systemic hypertension. Am J Cardiol. 1983;51(9):1481–1488. doi: 10.1016/0002-9149(83)90661-6. [DOI] [PubMed] [Google Scholar]
- 29.Wallach R, Karp RB, Reves JG, Oparil S, Smith LR, James TN. Pathogenesis of paroxysmal hypertension developing during and after coronary bypass surgery: a study of hemodynamic and humoral factors. Am J Cardiol. 1980;46(4):559–565. doi: 10.1016/0002-9149(80)90503-2. [DOI] [PubMed] [Google Scholar]
- 30.Cooper TJ, Clutton-Brock TH, Jones SN, Tinker J, Treasure T. Factors relating to the development of hypertension after cardiopulmonary bypass. Br Heart J. 1985;54(1):91–95. doi: 10.1136/hrt.54.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh J, Wunsch R, Turzer M, et al. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106(1):100–105. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 32.Bolger AP, Coats AJ, Gatzoulis MA. Congenital heart disease: the original heart failure syndrome. Eur Heart J. 2003;24(10):970–976. doi: 10.1016/s0195-668x(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez FH, III, Marelli AJ. The epidemiology of heart failure in adults with congenital heart disease. Heart Fail Clin. 2014;10(1):1–7. doi: 10.1016/j.hfc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131(5):e1502–e1508. doi: 10.1542/peds.2012-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim CC, Tan CS, Chia CM, et al. Long-term risk of progressive chronic kidney disease in patients with severe acute kidney injury requiring dialysis after coronary artery bypass surgery. Cardiorenal Med. 2015;5(3):157–163. doi: 10.1159/000381068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein SL. Renal recovery at different ages. Nephron Clin Pract. 2014;127(1–4):21–24. doi: 10.1159/000363679. [DOI] [PubMed] [Google Scholar]
- 38.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49(6):1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 39.Zappitelli M. Epidemiology and diagnosis of acute kidney injury. Semin Nephrol. 2008;28(5):436–446. doi: 10.1016/j.semnephrol.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Cooper DS, Claes D, Goldstein SL, et al. Follow-Up Renal Assessment of Injury Long-Term After Acute Kidney Injury (FRAIL-AKI) Clin J Am Soc Nephrol. 2016;11(1):21–29. doi: 10.2215/CJN.04240415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76(8):893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78(8):803–809. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


