Abstract
Oxidative phosphorylation enables cells to generate the large amounts of ATP required for development and maintenance of multicellular organisms. However, under conditions of reduced O2 availability, electron transport becomes less efficient, leading to increased generation of superoxide anions. Hypoxia-inducible factors switch cells from oxidative to glycolytic metabolism, to reduce mitochondrial superoxide generation, and increase the synthesis of NADPH and glutathione, in order to maintain redox homeostasis under hypoxic conditions.
1. Oxygen and reactive oxygen species
All human cells require a constant supply of O2 for continued survival. O2 is utilized by electron transport chain (ETC) complex IV (cytochrome c oxidase) as the final electron acceptor in the process of mitochondrial oxidative phosphorylation. O2 levels in cells are tightly regulated to balance benefit (generation of ATP) and risk (generation of reactive oxygen species [ROS]). Under physiological PO2 levels, ETC complex IV transfers electrons to O2 to form water. However, when PO2 levels deviate from the physiological range, electrons escape from the ETC at an increased rate and react with O2 prior to complex IV, resulting in the formation of superoxide anion radical (O2•-), which can subsequently be converted to hydrogen peroxide (H2O2) and other reactive molecules, including hydroxyl radical (OH•), singlet oxygen (1O2) and ozone (O3) [2], [3]. At the plasma membrane, ROS are generated through the activity of NADPH oxidase (NOX) enzymes [1]. ROS are generated by many other enzymatic reactions and are also produced when cells encounter external agents such as pollutants, tobacco smoke or radiation [4].
The consequences of ROS generation in cells are dependent on context and concentration. Physiological ROS signaling involves H2O2-mediated oxidation of cysteine residues within proteins [5]. At nanomolar concentrations, H2O2 oxidizes the thiolate anion form of cysteine residues (Cys-S-) to the sulfenic form (Cys-SOH) [6]. This modification may cause allosteric changes to proteins that have functional consequences but is reversible. At higher H2O2 concentrations, the thiolate anion oxidizes to sulfinic (SO2H) or sulfonic (SO3H) species, which is reversed by sulfiredoxin [7].
2. Redox signaling and its biological consequences
Redox signaling is important for the maintenance of physiological functions, such as cellular differentiation and tissue regeneration, whereas loss of redox homeostasis is associated with disease states, such as cancer and autoimmunity. The innate and adaptive immune systems are critical for pathogen-specific defense, immunological memory and tissue repair. ROS signaling plays a critical role in cells of the innate and adaptive immune systems [8], [9]. Physiological levels of ROS maintain a healthy immune system, whereas decreased ROS levels inhibit activation of the immune system, leading to immunosuppression, and increased ROS levels induce hyperactivation of immune system through release of inflammatory cytokines, leading to autoimmunity [10]. Redox signaling also plays an important role in tissue regeneration. Physiological ROS levels are required for stem cell maintenance, whereas high levels of ROS lead to stem cell exhaustion [11]. The same may hold true for certain cancers. At lower concentrations, ROS are important signaling molecules involved in cellular proliferation, migration, and metabolic adaptation. However, at higher concentrations, they cause damage due to irreversible oxidation of proteins, lipids and DNA, leading to cell death [12]. Several treatment approaches in cancer, including chemotherapy and radiation therapy, generate ROS at high levels, which are toxic to cancer cells. In this review, we will discuss how breast cancer cells maintain ROS homeostasis under hypoxic conditions in the tumor microenvironment.
3. Hypoxia and HIFs
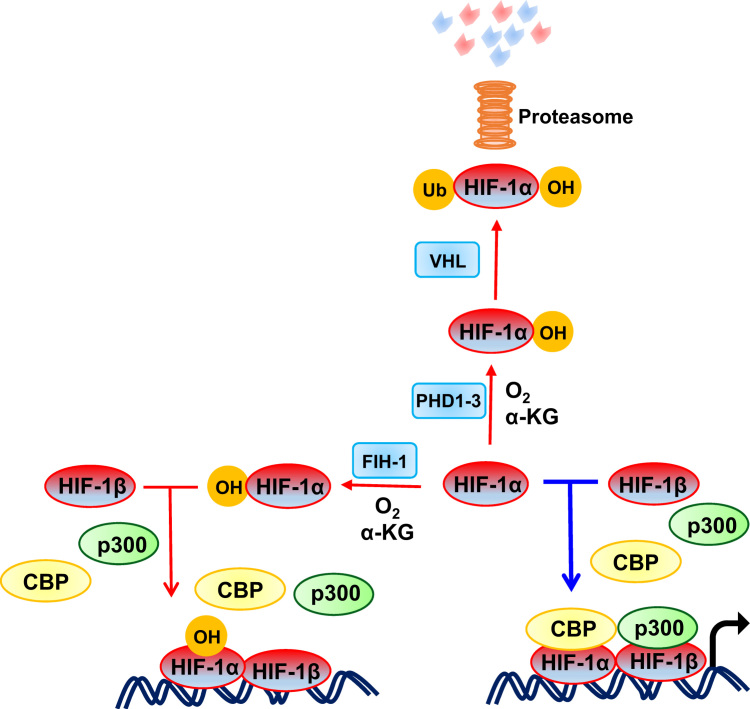
Hypoxia-inducible factors (HIFs) are transcriptional activators that serve as master regulators of oxygen homeostasis [13]. Many solid tumors have regions of low oxygenation (PO2 < 10 mmHg) due to an imbalance between O2 supply and demand. HIFs are heterodimeric transcription factors composed of an O2-regulated HIF-1α, HIF-2α or HIF-3α subunit and a constitutively expressed HIF-1β subunit. HIF-1α, HIF-2α or both are required for the transcriptional activation of a large battery of genes, whose protein products are required for discrete steps in cancer invasion and metastasis [14]. Under normoxic conditions, HIF-α subunits are hydroxylated at two proline residues (Pro-402 and Pro-564 in human HIF-1α). This post-translational modification is mediated by three HIF prolyl hydroxylase domain proteins (PHD1-3), which are α-ketoglutarate – dependent dioxygenases, with PHD2 playing a particularly important role [15], [16]. The von Hippel-Lindau (VHL) ubiquitin ligase complex recognizes prolyl hydroxylated HIF-α subunits and marks them for proteasomal degradation (Fig. 1). The PHDs are believed to have a relatively high KM value (Michaelis-Menten constant) for O2, such that their hydroxylase activity falls when oxygenation is inadequate [16].
Fig. 1.
Oxygen-regulated activity of HIF-1. Under normoxic conditions, prolyl hydroxylase domain (PHD) proteins use O2 and α-ketoglutarate (αKG) to hydroxylate human HIF-1α at Pro-402 and Pro-564. Hydroxylated HIF-1α is bound by VHL, ubiquitylated, and degraded by the proteasome. Under normoxic conditions, factor inhibiting HIF-1 (FIH-1) hydroxylates Asn-803 in human HIF-1α, which blocks the binding of the coactivators CBP (CREB-binding protein) and p300, and thereby inhibits the transcriptional activity of HIF-1. Under hypoxic conditions, the Pro and Asn hydroxylation reactions are inhibited and HIF-1α accumulates, recruits co-activators, dimerizes with HIF-1β and binds to hypoxia response elements to activate the transcription of hundreds of target genes. Red and blue arrows indicate reactions that are favored under aerobic and hypoxic conditions, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In addition to the O2-dependent negative regulation of protein stability by PHDs, HIF-1α (and HIF-2α) transactivation function is negatively regulated by factor inhibiting HIF-1 (FIH-1) [17], which is also an O2- and α-ketoglutarate-dependent dioxygenase. FIH-1 catalyzes asparaginyl hydroxylation (Asn-803 in human HIF-1α), which blocks binding of the coactivators CBP (CREB-binding protein) and p300 [18], [19], as shown in Fig. 1. In summary, the stability and activity of the HIFs are modulated by hydroxylation, such that changes in O2 availability are transduced to the nucleus as changes in HIF transcriptional activity. In order to maintain redox homeostasis in the presence of intratumoral hypoxia, HIFs activate the transcription of target genes encoding proteins that serve to either decrease production of mitochondrial ROS or increase production of ROS scavengers, as described in detail in the following section.
4. HIF-1 inhibits ROS production by regulating acetyl-CoA synthesis
Reduced O2 availability has profound effects on cellular metabolism. Glucose and glutamine are considered primary metabolic substrates for many cancer cells. Glucose is converted to pyruvate through the Embden-Meyerhof (glycolytic) pathway (EMP). Under aerobic conditions, pyruvate dehydrogenase (PDH) converts pyruvate to acetyl-CoA for oxidation in the tricarboxylic acid (TCA) cycle. Glutamine is converted to glutamate and then to α-ketoglutarate, which also enters the TCA cycle. A third major energy substrate for cancer cells is fatty acids, which are oxidized to generate acetyl-CoA [20], as described in greater detail below. The further oxidation of acetyl-CoA in the TCA cycle generates reducing equivalents (NADH and FADH2), which are donated to the ETC to generate a proton gradient that is used to generate ATP. Thus, the flux of acetyl-CoA metabolized by the TCA cycle determines the flux of electrons delivered to the ETC.
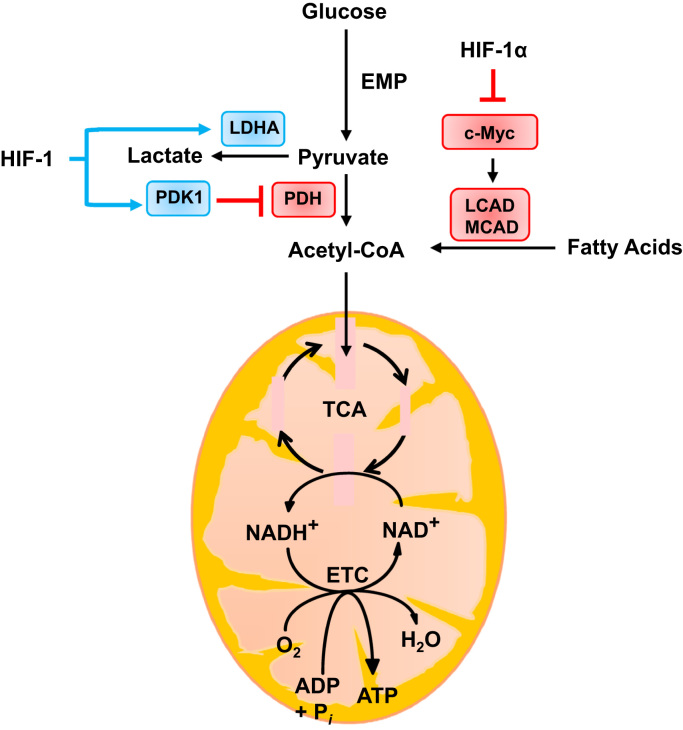
Previously, it was thought that cells subjected to hypoxic conditions (e.g. transfer of tissue culture cells from 20% to 1% O2 ambient environment) utilized glycolysis to generate ATP because O2 becomes limiting for respiration. Studies of wild-type (WT) and HIF-1α–deficient (Hif1α-/-) mouse embryo fibroblasts (MEFs) revealed that WT MEFs switched from oxidative to glycolytic metabolism when the cells were exposed to 1% O2 or less for 48 h or more, leading to decreased ATP levels, because, compared to respiration, glycolysis generates much less ATP per mole of glucose consumed. Hif1α-/- MEFs did not switch from oxidative to glycolytic metabolism and maintained higher ATP levels at 1% O2 than did WT MEFs at 20% O2, demonstrating that 1% O2 is not limiting for oxidative phosphorylation. However, the Hif1α-/- MEFs died due to excessive ROS production [21], [22]. HIF-1 determines the balance between oxidative and glycolytic metabolism of glucose by regulating the expression of LDHA and PDK1, which promote conversion of pyruvate to lactate, relative to PDH, which converts pyruvate to acetyl-CoA [22], [23] as shown in Fig. 2.
Fig. 2.
Oxygen-dependent regulation of ROS production by HIF-1. In well-oxygenated cells, glucose is metabolized to pyruvate, which is converted to acetyl-CoA by pyruvate dehydrogenase (PDH) for entry into the tricarboxylic acid cycle (TCA). However under hypoxic conditions, HIF-1 decreases glucose oxidation by activating the transcription of PDK1, which encodes a kinase that phosphorylates and inactivates PDH; and LDHA, which encodes the enzyme that converts pyruvate to lactate. HIF-1 also decreases fatty acid oxidation by repressing the c-Myc-dependent expression of MCAD and LCAD. Blue rectangles indicate proteins that are products of HIF-1 target genes. Blue arrows and red blocked arrows indicate induction and inhibition, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Besides glucose, fatty acid oxidation (FAO) is an additional source of acetyl–CoA. FAO generates one molecule of acetyl CoA in each oxidation cycle and two molecules in the last cycle. Transcription of the MCAD and LCAD genes encoding medium-chain and long-chain acyl-CoA dehydrogenases, which catalyze the first step of FAO in the mitochondria, was repressed when hepatocellular carcinoma cells were subjected to hypoxic conditions [24]. Under hypoxia, HIF-1α suppressed the expression of C-MYC, which is a transcriptional activator of the gene encoding PGC-1β, which is in turn a coactivator of MCAD and LCAD transcription. The decrease in FAO decreases mitochondrial ROS generation, as shown in Fig. 2.
5. HIF-1 inhibits mitochondrial ROS production in hypoxic cells by regulating mitochondrial proteins and autophagy
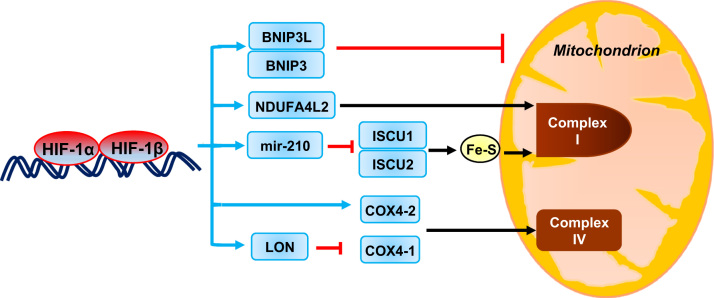
HIF-1-dependent expression of BNIP3 in MEFs [21] and BNIP3L in cancer cells [25] was shown to stimulate mitochondrial-selective autophagy. The reduction in mitochondrial mass suppresses the oxidation of both glucose and fatty acids and thus decreases mitochondrial ROS production under hypoxic conditions, as shown in Fig. 3.
Fig. 3.
Oxygen-dependent regulation of mitochondrial function by HIF-1. Under hypoxic conditions HIF-1 induces the expression of: (a) BNIP3 and BNIP3L, which trigger mitochondrial selective autophagy; (b) NDUFA4L2, which inhibits complex I activity; (c) miR-210, which represses ISCU, a protein that is required for ETC assembly; and (d) COX4-2 and LON, which orchestrate a subunit switch in complex IV that increases electron transfer efficiency. The expression of each of these HIF- regulated genes serves to decrease mitochondrial ROS production. Blue rectangles indicate mRNAs and microRNA that are products of HIF-1 target genes. Blue arrows indicate increased expression and blocked red arrows denote destruction of mitochondria and COX4-1 by autophagy and LON protease, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Major sites of ROS production in mammalian mitochondria are the ubiquinone reduction site of complex I and the outer quinone-binding site of the Q cycle in complex III [26]. Hypoxia modulates respiratory chain function in cancer cells by orchestrating a subunit switch (from COX4-1 to COX4-2) in cytochrome c oxidase (COX). COX catalyzes the terminal transfer of electrons to O2. In hypoxic cells, HIF-1 activates the transcription of the COX4l2 and LON genes. LON is a mitochondrial protease that is required for COX4-1 degradation. The subunit switch from COX4-1 to COX4-2 may increase the efficiency of electron transfer to O2 and allow continued respiration without increased ROS levels when O2 availability is modestly reduced [27].
Under hypoxic conditions, HIF-1 activates transcription of the NDUFA4L2 gene, which encodes NADH dehydrogenase [ubiquinone] 1α subcomplex, 4-like 2. ROS levels were increased in hypoxic cells lacking NDUFA4L2, which may act by inhibiting complex I activity [28]. Thus, NDUFAL2 gene expression represents another adaptive mechanism that reduces flux through the ETC, thereby reducing ROS production, as shown in Fig. 3.
HIFs also transactivate genes encoding microRNAs (miRs), which are small RNAs that bind to mRNAs in a sequence-specific manner to either inhibit their translation or induce their degradation [29]. Hypoxia induces miR-210 expression in many cell types in a HIF-dependent manner [30]. miR-210 represses ISCU which encodes iron-sulfur cluster assembly factors that are required for the biogenesis of iron-sulfur-dependent enzyme complexes including complex I, complex III, and aconitase, which are critical for electron transport [31]. Inhibition of ISCU1/2 expression reduces mitochondrial ROS generation.
Finally, hypoxia also induces the transformation of complex I from the active to ‘deactive’ form in endothelial cells and brain tissue, which involves a switch from NADH-ubiquinone oxidoreductase activity to Na+/H+ antiporter activity, which suppresses ROS production [32].
6. HIF regulates antioxidant defenses in hypoxic cells
Cellular redox homeostasis represents a balance between oxidants (principally, ROS) and antioxidants (principally, reduced glutathione). Antioxidant defense is dependent on the generation of NADPH, which is used to maintain glutathione in a reduced form, and also on the de novo synthesis of glutathione. In human breast cancer cells, HIFs function as master regulators that orchestrate the generation of NADPH and glutathione under hypoxic conditions.
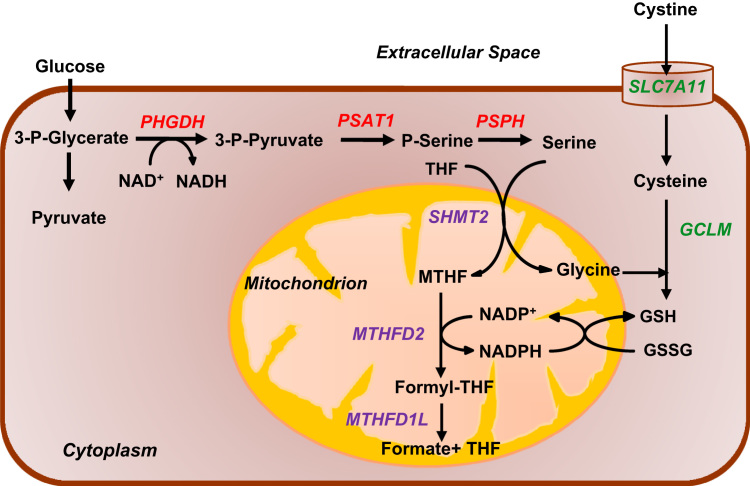
There are two major pathways in the cell that generate NADPH. One is the pentose phosphate pathway (PPP), which generates NADPH in the cytoplasm. In the first step of the PPP, glucose-6-phosphate dehydrogenase (G6PD) converts the glycolytic intermediate glucose-6-phosphate and NADP+ to 6-phosphogluconolactone and NADPH. The other major pathway for NADPH production is one-carbon metabolism (1CM; also known as the folate cycle). Serine is the major donor of one-carbon units to the folate cycle. The serine synthesis pathway (SSP) provides a mechanism for glucose-derived carbons to be diverted from glycolysis for de novo serine synthesis [33]. Serine is required for a number of biosynthetic and signaling pathways, including synthesis of amino acids, such as glycine and cysteine, both of which are required for the synthesis of glutathione. As shown in Fig. 4, in the SSP, phosphoglycerate dehydrogenase (PHGDH) converts the glycolytic intermediate 3-phosphoglycerate (3-PG) to 3-phosphopyruvate (3-PHP). The next enzyme in the SSP, phosphoserine aminotransferase (PSAT1), converts 3-PHP into phosphoserine (P-Ser) and, finally, phosphoserine phosphatase (PSPH) converts P-Ser into serine. Serine and NADP+ are then utilized for 1CM, either in the cytosol or mitochondria, which generates glycine and NADPH. In the mitochondria, 1CM is catalyzed by serine hydroxymethyltransferase 2 (SHMT2), methylene tetrahydrofolate dehydrogenase 2 (MTHFD2) or MTHFD2-like (MTHFD2L), and MTHFD1L. In the cytosol, SHMT1 and MTHFD1 carry out the same reactions (MTHFD1 catalyzes the reactions performed by both MTHFD2/MTHFD2L and MTHFD1L) [33].
Fig. 4.
Regulation of antioxidant production by HIF-1. Under hypoxic conditions, HIFs activate the transcription of PHGDH, PSAT1, and PSPH (red) to increase conversion of glucose to serine (serine synthesis pathway); and SHMT2, MTHFD2, and MTHFD1L (purple) to increase generation of mitochondrial NADPH (by mitochondrial one-carbon metabolism), which is required to convert glutathione from oxidized (GSSG) to reduced (GSH) form to protect against increased ROS generated by the ETC. Under hypoxic conditions, HIFs also activate the transcription of SLC7A11 and GCLM (green) to increase glutathione production. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In breast cancer cells, analysis of gene expression revealed that, irrespective of the estrogen receptor (ER) status, hypoxia induced the expression of the three enzymes of the SSP (PHGDH, PSAT1 and PSPH) and three enzymes required for mitochondrial 1CM (SHMT2, MTHFD2, and MTHFD1L) in a HIF-dependent manner [34]. In contrast, the enzymes required for cytosolic 1CM (SHMT1, MTHFD1) were not hypoxia-induced in most breast cancer cell lines. Additionally, the G6PD gene, was repressed by hypoxia in all breast cancer cell lines analyzed [34]. These results suggest that in hypoxic breast cancer cells, glucose metabolism is reprogrammed to increase flux through the SSP and decrease flux through the PPP. This conclusion was confirmed by metabolomic analysis of cell lysates. Silencing of PHGDH expression by RNA interference in either ER+ or ER- breast cancer cells led to decreased NADPH levels, disturbed mitochondrial redox homeostasis, and increased apoptosis, which abrogated the enrichment of breast cancer stem cells (BCSCs) under hypoxic conditions [34]. These results reveal a novel mechanism by which HIF regulates the cellular antioxidant capacity and thus regulates redox homeostasis, which in turn is required for the maintenance of BCSCs.
Glutathione (γ-L-glutamyl-L-cysteinylglycine) is a small peptide that is generated from the amino acids cysteine, glutamate, and glycine. SLC7A11 encodes the pore-forming subunit of a plasma membrane transporter for cystine uptake. GCLM encodes the regulatory subunit of glutamate cysteine ligase, which catalyzes the first step of glutathione biosynthesis. In breast cancer cells, hypoxia induced SLC7A11 and GCLM expression in a HIF dependent manner [35]. Thus, in response to hypoxia, HIF coordinately activates the transcription of genes encoding enzymes that regulate the production of NADPH and glutathione, which are the major antioxidants responsible for mitochondrial ROS homeostasis. As in the case of PHGDH, loss of SLC7A11 or GCLM expression blocks hypoxia-induced BCSC enrichment. Whereas HIF-1 alone controls expression of LDHA and PDK1, both HIF-1 and HIF-2 regulate SLC7A11, GCLM, and the genes encoding SSP and mitochondrial 1CM enzymes [34], [35].
7. Perspective
ROS act as a double-edged sword: low levels of ROS induce signaling pathways that support normal physiological processes, whereas high levels of ROS are toxic. Redox status might be a potential therapeutic target, but targeting ROS would have to be context dependent. For example, abrogating ROS-mediated signaling might reduce primary tumor growth, but mouse models of breast cancer and melanoma suggest that abrogating oxidative stress promotes metastasis [36], [37]. An alternative therapeutic approach is to inhibit HIFs, which control redox balance. HIF inhibitors block the enrichment of BCSCs that occurs in response to hypoxia [34], [38], [39], [40], [41] or chemotherapy [34], [35], [42] and also block hypoxia-induced expression of many genes that play critical roles in the metastatic process [14].
Sources of funding
Cancer research in the authors’ laboratory is supported in part by grants to G.L.S. from the American Cancer Society (122437-RP-12-090-01-COUN), Armstrong Family Foundation, Cindy Rosencrans Foundation, Department of Defense Breast Cancer Research Program (W81XWH-12-1-0464), and Emerson Collective Cancer Research Fund. G.L.S. is an American Cancer Society Research Professor and the C. Michael Armstrong Professor at the Johns Hopkins University School of Medicine.
Disclosures
None.
References
- 1.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 2.Sosa V., Moliné T., Somoza R., Paciucci R., Kondoh H., LLeonart M.E. Oxidative stress and cancer: an overview. Ageing Res. Rev. 2013;13:376–390. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y., Fiskum G., Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 4.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 5.Rhee S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 6.Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci. Signal. 2012;5(215) doi: 10.1126/scisignal.2002943. (pe10) [DOI] [PubMed] [Google Scholar]
- 7.Winterbourn C.C., Hampton M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 8.West A.P., Shadel G.S., Ghosh S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida L., Lochner M., Berod L., Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Semin. Immunol. 2016;5:514–524. doi: 10.1016/j.smim.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza G.L. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. Eur. Mol. Biol. Organ. J. 2017;36:252–259. doi: 10.15252/embj.201695204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prabhakar N.R., Semenza G.L. Oxygen sensing and homeostasis. Physiology. 2015;30:340–348. doi: 10.1152/physiol.00022.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenza G.L. The hypoxic tumor microenvironment: a driving force for breast cancer progression. Biochim. Biophys. Acta. 2016;1863:382–391. doi: 10.1016/j.bbamcr.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berra E., Roux D., Richard D.E., Pouysségur J. Hypoxia-inducible factor-1α (HIF-1α) escapes O2-driven proteasomal degradation irrespective of its subcellular localization: nucleus or cytoplasm. Eur. Mol. Biol. Organ. Rep. 2001;2:615–620. doi: 10.1093/embo-reports/kve130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaelin W.G., Jr, Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Lando D., Peet D.J., Gorman J.J., Whelan D.A., Whitelaw M.L., Bruick R.K. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dayan F., Roux D., Brahimi-Horn M.C., Pouyssegur J., Mazure N.M. The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1α. Cancer Res. 2006;66:3688–3698. doi: 10.1158/0008-5472.CAN-05-4564. [DOI] [PubMed] [Google Scholar]
- 19.Semenza G.L., Wang G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carracedo L.C., Cantley L.C., Pandolfi P.P. Cancer metabolism: fatty acid oxidation in the limelight. Nat. Rev. Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H., Bosch-Marce M., Shimoda L.A., Tan Y.S., Baek J.H., Wesley J.B., Gonzalez F.J., Semenza G.L. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Semenza G.L., Jiang B.H., Leung S.W., Passantino R., Concordet J.P., Maire P., Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 24.Huang D., Li T., Li X., Zhang L., Sun L., He X., Zhong X., Jia D., Song L., Semenza G.L. HIF-1-mediated suppression of acyl-CoA dehydrogenases and fatty acid oxidation is critical for cancer progression. Cell Rep. 2014;25:1930–1942. doi: 10.1016/j.celrep.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Bellot G., Garcia-Medina R., Gounon P., Chiche J., Roux D., Pouysségur J., Mazure N.M. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuhrmann D.C., Brüne B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017;12:208–215. doi: 10.1016/j.redox.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuda R., Zhang H., Kim J.W., Shimoda L., Dang C.V., Semenza G.L. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 28.Tello D., Balsa E., Acosta-Iborra B., Fuertes-Yebra E., Elorza A., Ordóñez Á., Corral-Escariz M., Soro I., López-Bernardo E., Perales-Clemente E., Martínez-Ruiz A., Enríquez J.A., Aragonés J., Cadenas S., Landázuri M.O. Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting complex I activity. Cell Metab. 2011;14:768–779. doi: 10.1016/j.cmet.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Ivan M., Harris A.L., Martelli F., Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. J. Cell. Mol. Med. 2008;12:1426–1431. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devlin C., Greco S., Martelli F., Ivan M. miR-210: more than a silent player in hypoxia. Int. Union Biochem. Mol. Biol. Life. 2011;63:94–100. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan S.Y., Zhang Y.Y., Hemann C., Mahoney C.E., Zweier J.L., Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernansanz-Agustína P., Ramosa E., Navarroc E., Paradac E., Sánchez-Lópeza N., Peláez-Aguadod L., Cabrera-Garcíaa J., Telloe D., Buendiac I., Marinad A. Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Redox Biol. 2017 doi: 10.1016/j.redox.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samanta D., Semenza G.L. Serine synthesis helps hypoxic cancer stem cells regulate redox. Cancer Res. 2016;76:6458–6462. doi: 10.1158/0008-5472.CAN-16-1730. [DOI] [PubMed] [Google Scholar]
- 34.Samanta D., Park Y., Andrabi S.A., Shelton L.M., Gilkes D.M., Semenza G.L. PHGDH expression is required for mitochondrial redox homeostasis, breast cancer stem cell maintenance, and lung metastasis. Cancer Res. 2016;76:4430–4442. doi: 10.1158/0008-5472.CAN-16-0530. [DOI] [PubMed] [Google Scholar]
- 35.Lu H., Samanta D., Xiang L., Zhang H., Hu H., Chen I., Bullen J.W., Semenza G.L. Chemotherapy triggers HIF-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc. Natl. Acad. Sci. USA. 2015;112:4600–4609. doi: 10.1073/pnas.1513433112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piskounova E., Agathocleous M., Murphy M.M., Hu Z., Huddlestun S.E., Zhao Z., Leitch A.M., Johnson T.M., DeBerardinis R.J., Morrison S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Gal K., Ibrahim M.X., Wiel C., Sayin V.I., Akula M.K., Karlsson C., Dalin M.G., Akyürek L.M., Lindahl P., Nilsson J., Bergo M.O. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015;7:308re8. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 38.Xiang L., Gilkes D.M., Hu H., Takano N., Luo W., Lu H., Bullen J.W., Samanta D., Liang H., Semenza G.L. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget. 2014;5:12509–12527. doi: 10.18632/oncotarget.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H., Lu H., Xiang L., Bullen J.W., Zhang C., Samanta D., Gilkes D.M., He J., Semenza G.L. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc. Natl. Acad. Sci. USA. 2015;112:6215–6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C., Samanta D., Lu H., Bullen J.W., Zhang H., Chen I., He X., Semenza G.L. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc. Natl. Acad. Sci. USA. 2016;113:2047–2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C., Zhi W.I., Lu H., Samanta D., Chen I., Gabrielson E., Semenza G.L. Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget. 2016;7:64527–64542. doi: 10.18632/oncotarget.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samanta D., Gilkes D.M., Chaturvedi P., Xiang L., Semenza G.L. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc. Natl. Acad. Sci. USA. 2014;111:5429–5438. doi: 10.1073/pnas.1421438111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]