Abstract
Chronic subjective tinnitus is an auditory phantom phenomenon characterized by abnormal neuronal synchrony in the central auditory system. As shown computationally, acoustic coordinated reset (CR) neuromodulation causes a long-lasting desynchronization of pathological synchrony by downregulating abnormal synaptic connectivity. In a previous proof of concept study acoustic CR neuromodulation, employing stimulation tone patterns tailored to the dominant tinnitus frequency, was compared to noisy CR-like stimulation, a CR version significantly detuned by sparing the tinnitus-related pitch range and including substantial random variability of the tone spacing on the frequency axis. Both stimulation protocols caused an acute relief as measured with visual analogue scale scores for tinnitus loudness (VAS-L) and annoyance (VAS-A) in the stimulation-ON condition (i.e. 15 min after stimulation onset), but only acoustic CR neuromodulation had sustained long-lasting therapeutic effects after 12 weeks of treatment as assessed with VAS-L, VAS-A scores and a tinnitus questionnaire (TQ) in the stimulation-OFF condition (i.e. with patients being off stimulation for at least 2.5 h). To understand the source of the long-lasting therapeutic effects, we here study whether acoustic CR neuromodulation has different electrophysiological effects on oscillatory brain activity as compared to noisy CR-like stimulation under stimulation-ON conditions and immediately after cessation of stimulation. To this end, we used a single-blind, single application, cross over design in 18 patients with chronic tonal subjective tinnitus and administered three different 16-minute stimulation protocols: acoustic CR neuromodulation, noisy CR-like stimulation and low frequency range (LFR) stimulation, a CR type stimulation with deliberately detuned pitch and repetition rate of stimulation tones, as control stimulation. We measured VAS-L and VAS-A scores together with spontaneous EEG activity pre-, during- and post-stimulation. Under stimulation-ON conditions acoustic CR neuromodulation and noisy CR-like stimulation had similar effects: a reduction of VAS-L and VAS-A scores together with a decrease of auditory delta power and an increase of auditory alpha and gamma power, without significant differences. In contrast, LFR stimulation had significantly weaker EEG effects and no significant clinical effects under stimulation-ON conditions. The distinguishing feature between acoustic CR neuromodulation and noisy CR-like stimulation were the electrophysiological after-effects.
Acoustic CR neuromodulation caused the longest significant reduction of delta and gamma and increase of alpha power in the auditory cortex region. Noisy CR-like stimulation had weaker and LFR stimulation hardly any electrophysiological after-effects. This qualitative difference further supports the assertion that long-term effects of acoustic CR neuromodulation on tinnitus are mediated by a specific disruption of synchronous neural activity. Furthermore, our results indicate that acute electrophysiological after-effects might serve as a marker to further improve desynchronizing sound stimulation.
Keywords: Alpha band activity, Coordinated reset neuromodulation, Delta band activity, Electroencephalography, Gamma band activity
Highlights
-
•
Acute effects and acute after-effects of dedicated tinnitus sound therapy are studied with EEG.
-
•
Sound therapy causing long-lasting, sustained effects causes pronounced acute after-effects.
-
•
Acute EEG after-effects might serve as marker to develop desynchronizing sound therapy.
1. Introduction
A widely accepted consensus guideline (Tunkel et al., 2014) provides current tinnitus definitions. Secondary (objective) tinnitus is defined as tinnitus associated with an identifiable organic condition other than sensorineural hearing loss. In contrast, primary (subjective) tinnitus is an idiopathic symptom that may or may not be associated with sensorineural hearing loss. However, primary tinnitus is typically initiated by damage to the peripheral hearing system (Eggermont and Roberts, 2004, Irvine et al., 2001, Lockwood et al., 2002, Norena et al., 2002, Weisz et al., 2006) that leads to a sequence of structural and functional changes in the central hearing system (Eggermont, 2007, Eggermont and Roberts, 2004, Lockwood et al., 2002, Moller, 2003). The latter give rise to a phantom auditory perception, i.e. a conscious awareness of an internally generated sensory percept when no corresponding auditory stimulus is present (Eggermont and Roberts, 2004, Snow, 2004). In recent years, there is a growing body of evidence for a critical role of pathological neuronal synchronization in the auditory system in tinnitus pathophysiology (Eggermont and Tass, 2015, Shore et al., 2016). An increase of neuronal synchrony has been described both in animal studies after noise trauma (Norena and Eggermont, 2003, Ochi and Eggermont, 1997, Seki and Eggermont, 2003) and in tinnitus patients (Norena and Eggermont, 2003, Ochi and Eggermont, 1997, Seki and Eggermont, 2003, Weisz et al., 2005, Weisz et al., 2007b). Magnetoencephalography (MEG) and electroencephalography (EEG) studies revealed specific alterations of oscillatory power in particular frequency bands (Llinas et al., 1999, Weisz et al., 2005, Weisz et al., 2007b) in patients with chronic subjective tinnitus. Specifically, increase of the oscillatory power in the delta, theta, and gamma frequency ranges as well as reduction of alpha power in the auditory cortex region were associated with the presence of tinnitus and its intensity (Adamchic et al., 2014a, Adjamian et al., 2012, De Ridder et al., 2011, De Ridder et al., 2014, De Ridder et al., 2015, Elgoyhen et al., 2015, Llinas et al., 2005, Llinas et al., 1999, Tass et al., 2012a, Van der Loo et al., 2009, Weisz et al., 2005, Weisz et al., 2007b). An increase of oscillatory EEG power is typically interpreted as an increase in neuronal synchronization in terms of coincident firing within neuronal populations (Hamalainen et al., 1993, Klass and Daly, 1979, Niedermeyer and Da Silva, 1999, Nunez, 1981).
Studies in cortical primary sensory areas have revealed that neuronal plasticity is not restricted to periods early in life, but is present and can be reactivated in the mature brain, too [for review see (Hübener and Bonhoeffer, 2014)]. The neuronal timing pattern plays a key role in shaping synaptic connectivity (Hebb, 1949, Bliss and Lomo, 1973). Neurons adapt the strength of their synapses to the relative timing of their action potentials according to the fundamental mechanism of spike timing-dependent plasticity (STDP) (Gerstner et al., 1996, Markram, 1997). Computationally it was shown that in networks with STDP stable synchronized states with up-regulated strength of synaptic connectivity and stable desynchronized states with down-regulated synaptic connectivity may generically coexist (Tass and Majtanik, 2006, Tass and Hauptmann, 2006, Tass and Hauptmann, 2007, Tass and Hauptmann, 2009, Hauptmann and Tass, 2007). Along the lines of a computational approach (Tass, 1999, Tass, 2002) coordinated reset (CR) stimulation (Tass, 2003a, Tass, 2003b) was developed to specifically counteract abnormal neuronal synchrony by desynchronization. To this end different neuronal subpopulations of a target population are stimulated through different stimulation sites sequentially at different times in order to reset the phases of the different subpopulations equidistantly in time (Tass, 2003a, Tass, 2003b). CR stimulation causes a desynchronization and, hence, a reduction of the strength of the synaptic connections, which ultimately results in an anti-kindling, i.e. an unlearning of abnormally up-regulated synaptic connectivity and neural synchrony (Tass and Majtanik, 2006, Tass and Hauptmann, 2006, Tass and Hauptmann, 2007, Tass and Hauptmann, 2009, Hauptmann and Tass, 2007, Hauptmann and Tass, 2009). The network is shifted from a pathological model state with abnormally strong synapses to a desynchronized state with weaker synapses, and the stimulation effect outlasts the cessation of stimulation (Tass and Majtanik, 2006, Tass and Hauptmann, 2006, Tass and Hauptmann, 2007, Tass and Hauptmann, 2009, Hauptmann and Tass, 2007, Hauptmann and Tass, 2009).
CR stimulation was first developed computationally for electrical deep brain stimulation (DBS) for the treatment of Parkinson's disease (Tass, 2003a, Tass, 2003b), computationally studied in networks with (Tass and Majtanik, 2006, Tass and Hauptmann, 2006, Tass and Hauptmann, 2007, Tass and Hauptmann, 2009, Hauptmann and Tass, 2007, Hauptmann and Tass, 2009) and without (Lysyansky et al., 2011, Lysyansky et al., 2013) STDP and later on successfully applied in pre-clinical (Tass et al., 2012b, Wang et al., 2016) and clinical (Adamchic et al., 2014b) proof of concept studies. In addition, the CR concept was extended to sensory stimulation (Popovych and Tass, 2012), especially acoustic CR stimulation for the treatment of chronic primary tinnitus (Tass and Popovych, 2012, Tass et al., 2012a). In a prospective, randomized, single blind, placebo-controlled proof of concept study in 63 patients acute and long lasting clinical effects of a 12-week treatment with CR neuromodulation and noisy CR-like stimulation were assessed with visual analogue scale (VAS) scores for loudness (VAS-L) and annoyance (VAS-A) as well as with the TF score (Tass et al., 2012a). The TF (“Tinnitus-Fragebogen”) (Goebel and Hiller, 1993) is the German adaptation of the tinnitus questionnaire (TQ) (Hallam et al., 1984). Acoustic CR neuromodulation and noisy CR-like stimulation share the basic rhythmic CR pattern and employ tones that are tailored to the patient's dominant tinnitus frequency. Acoustic CR neuromodulation uses a template of four stimulation tones with fixed frequency ratios with respect to the tinnitus frequency. In contrast, noisy CR-like stimulation is characterized by randomly selecting the actual four stimulation tones during each stimulation cycle from a larger set of tones, sparing the tinnitus-related pitch range and including substantial random variability of the tone spacing on the frequency axis where all stimulation tones were defined by frequency ratios with the tinnitus frequency (Tass et al., 2012a). In the proof of concept study CR stimulation turned out to be safe and well-tolerated and led to a significant decrease of tinnitus symptoms as assessed by VAS and TF scores (Tass et al., 2012a). EEG recordings performed before and after 12 weeks of treatment with acoustic CR neuromodulation revealed a significant reduction of pathologically elevated delta and gamma activity together with an increase of pathologically reduced alpha activity in a network of brain areas comprising auditory as well as non-auditory cortices (Adamchic et al., 2014a, Silchenko et al., 2013, Tass et al., 2012a).
The starting point of this paper is the significant difference of the clinical effects of the two stimulation protocols: Acoustic CR neuromodulation and noisy CR-like stimulation both had acute effects (with respect to baseline) as assessed with VAS-L and VAS-A scores in the stimulation-ON condition (i.e. 15 min after turning on stimulation), but only acoustic CR neuromodulation had sustained long-lasting effects as assessed in the stimulation-OFF condition (i.e. after having turned off stimulation for at least 2.5 h) after 12 weeks of treatment (Tass et al., 2012a). As yet, electrophysiological effects during and shortly after cessation of acoustic CR neuromodulation have not been studied. Accordingly, we here set out to study acute effects and acute after-effects of both stimulation protocols with VAS scores and, in particular, with EEG recordings. This is to elucidate acute electrophysiological stimulation responses and mechanisms that might lead to therapeutic sustained long-lasting effects. Specifically, based on computational studies (Tass and Majtanik, 2006, Tass and Popovych, 2012, Tass et al., 2012b) we hypothesize that acoustic CR neuromodulation causes a desynchronization of delta oscillations followed by a desynchronizing after-effect, provided the stimulation duration is sufficient. Since our computational predictions are based on qualitative rather than quantitative models, it remains to be tested, whether the selected 16 min stimulation duration is appropriate. We have selected a 16 min duration, since in a previous proof of concept study (Tass et al., 2012b) this dose was sufficient to at least induce acute clinical CR effects. Note, based on previous clinical (Tass et al., 2012b) and computational (Tass and Majtanik, 2006, Tass and Popovych, 2012, Tass et al., 2012b) findings, we would hypothesize acutely delivered acoustic CR neuromodulation and noisy CR stimulation to differ with respect to EEG parameters rather than clinical scores. Objective, EEG-based features underlying acute effects and after-effects of acoustic CR neuromodulation might enable to further optimize treatment parameters and delivery.
In addition, we want to study whether the observed EEG effects are specific to appropriately calibrated acoustic CR neuromodulation. Accordingly, as additional stimulation control condition we use a systematically and deliberately detuned acoustic CR neuromodulation protocol that we hypothesize to be ineffective – both clinically and electrophysiologically. To this end, we use CR with tones in the low frequency range (pitch) and at significantly reduced stimulus repetition rate with respect to the target delta rhythm, in order to stimulate the tonotopically organized auditory system spatially off-target and at the wrong pace. If proven to be ineffective under acute conditions, such a detuned CR protocol, in what follows denoted as low frequency range stimulation, might be a candidate for a sham/placebo stimulation for a controlled trial.
2. Materials and methods
2.1. Participants
Twenty participants with primary chronic tonal tinnitus participated in the study. Two patients were excluded from the analysis because of excessive muscle and movement artifacts in the EEG recordings. Subjects with pulsatile, ringing, buzzing, roaring, or hissing tinnitus and subjects with a history of auditory hallucinations, Méniere's disease, middle ear disorders and diagnosed neurological or mental disorders as well as subjects taking CNS-acting medication or using hearing aids were not included. Data of eighteen participants with subjective bilateral chronic tonal tinnitus (14 males and 4 females) was analyzed. Regarding laterality of tinnitus loudness, 10 reported bilaterally equal, 3 subjects reported left dominant and 5 right dominant tinnitus. The mean age was 45.89 (12.97; standard deviation, SD), and the mean tinnitus duration was 9.83 (7.08) years. Otoscopic examination was performed in all participants. The extent of the hearing loss was investigated in a soundproof and electromagnetically shielded chamber (Desone Modulare Akustik, Berlin, Germany) by determining pure-tone air-conduction hearing thresholds from 0.125 kHz to 16 kHz (Supplementary Fig. 1) using Type AT900 audiometer with HDA200 (Sennheiser GmbH, Wedemark, Germany) and B71 headphones (Auritec Medizindiagnostische GmbH, Hamburg, Germany). A pure tone matching procedure was used to determine the best matching tinnitus pitch (from 0.5 to 13 kHz). Subjects were instructed to match the frequency of a pure tone to the perceived pitch of their tinnitus. During this procedure intensity and frequency of the matching tone were controlled by the participant using a custom-made tone generator that allowed continuous frequency adjustment. The tinnitus matching started either well below or well above the subject's tinnitus frequency. The participant had to adjust the matching tone to his/her tinnitus. The matching tone was repeatedly interrupted to facilitate the comparison between matching tone and tinnitus. The tinnitus pitch matching was performed until patient confirmed the best matching pitch twice (difference between two matched tones < 100 Hz). All participants were comprehensively informed about the scope, aim, benefits and risks of the study participation, and a written informed consent was obtained from all subjects according to the Declaration of Helsinki and Good Clinical Practice. The study was approved by the relevant ethics committee (Ethics Committee of Cologne University's Faculty of Medicine).
Supplementary Fig. 1.
2.2. Paradigm and recordings
Participants were seated in an upright position in a comfortable chair. EEG recordings were obtained in a soundproof and electromagnetically shielded chamber. EEG data were collected from 128 surface electrodes (HydroCel Geodesic Sensor Net, Electrical Geodesis Inc., Eugene, USA). All electrodes were referenced to Cz. The EEG signals were amplified with a Net Amps 200 amplifier (Electrical Geodesis Inc., Eugene, USA), digitized at 1 kHz and band-pass filtered from 0.1 to 400 Hz. Photogrammetry was performed for all subjects using the Geodesis Photogrammetry system (Electrical Geodesis Inc., Eugene, USA) and the individual head shape was modeled for each subject and EEG session.
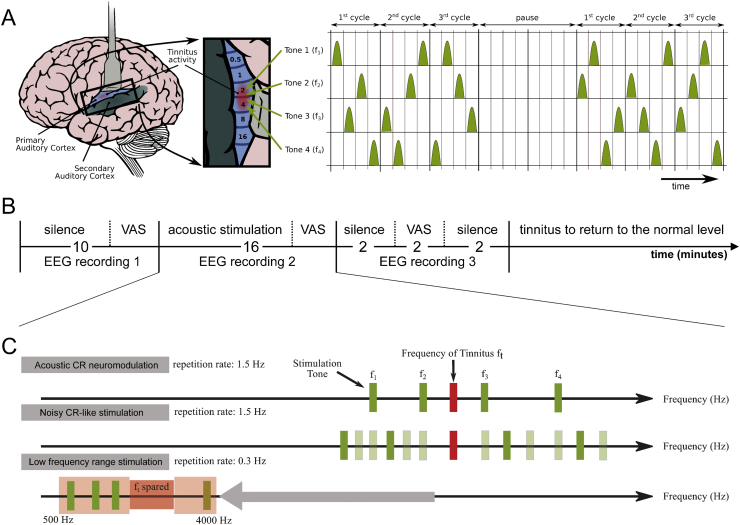
The first part of each experimental session consisted of a 10 min period of silence (Fig. 1A). The participants sat still and listened to their tinnitus (baseline period; during this period recordings were performed in alternating two minute intervals with eyes closed and with eyes open as orally instructed, and the eyes closed EEG data was selected for further analysis; the alternating two minute intervals were selected to prevent excessive drowsiness as well as to prevent participants from falling asleep). At the end of this resting period, the participants were asked to indicate the mean tinnitus loudness and annoyance during the baseline resting period on a 100 mm long visual analogue scale verbally anchored at the endpoints (Adamchic et al., 2012). VAS for loudness (VAS-L; tinnitus is not audible = 0, tinnitus is extremely loud = 100) and annoyance (VAS-A; tinnitus is not annoying = 0, tinnitus is extremely annoying = 100) were obtained. After the rating, acoustic CR neuromodulation, the noisy CR-like stimulation or the LFR stimulation were presented for exactly 16 min (during this period recordings were performed in alternating two minute intervals with eyes closed and with eyes open as orally instructed, and the eyes closed EEG data was selected for further analysis). At the end of this stimulation period, the participants were asked to indicate the mean tinnitus loudness and annoyance during the stimulation period on the VAS-L and VAS-A. The stimulus presentation was followed by 2 min long resting period (eyes closed), VAS-L and VAS-A rating between 2 and 4 min after the stimulation followed by another 2 min (eyes closed) of rest EEG recording. Subjects were asked to listen to their tinnitus. Transitions between eyes open and eyes closed conditions were guided by oral instruction. Acoustic CR neuromodulation, the noisy CR-like stimulation and the LFR stimulation were each presented one time in one of the three experimental sessions. The order of the experimental sessions was pseudorandomly counterbalanced across subjects. The sequence of events in a typical experimental session is illustrated in Fig. 1A.
Fig. 1.
Scheme of the experimental paradigm and the three types of acoustic stimulation. (A) CR stimulation pattern: for acoustic CR neuromodulation, we employ the tonotopic organization of the primary auditory cortex (left panel, brain adapted from (Chittka and Brockmann, 2005) with kind permission of the authors) and deliver brief sinusoidal tones of different frequencies (pitch) f1, …, f4 equidistantly in time at a cycle repetition rate of 1.5 Hz (Tass et al., 2012a). Three CR cycles, each comprising a randomized sequence of four tones (right panel), were followed by two silent cycles without stimuli (“pause”). The 3 cycles stim ON-2 cycles stim OFF pattern was repeated periodically (Tass and Majtanik, 2006, Tass, 2003a, Tass, 2003b, Lysyansky et al., 2011). Right panel from (Tass et al., 2012a) with kind permission by the authors. Copyright by Forschungszentrum Jülich GmbH. (B) Experimental session: during the first 10 min of silence, the baseline EEG was recorded and the baseline VAS-L and VAS-A scores were obtained. Thereafter one of the three stimulation paradigms, i.e. acoustic CR neuromodulation, noisy CR-like stimulation or LFR stimulation, was performed for 16 min. VAS-L and VAS-A were obtained during stimulation at the end of this stimulation period. During the next period of silence EEG recording was obtained for 2 min followed by the VAS-L and VAS-A evaluation (2 min) and another 2 min of rest EEG recording. After each session participants received a pause during which tinnitus returned to the normal level. Thereafter the next session was started. (C) Stimulus tones and repetition rates of acoustic CR neuromodulation, noisy CR-like stimulation and low frequency range stimulation (explanation see text). Panel partly redrawn from (Tass et al., 2012a) with kind permission by the authors.
Copyright by Forschungszentrum Jülich GmbH.
2.3. Acoustic stimulation
We used three different acoustic stimulation protocols (Fig. 1). All protocols used similar cyclic patterns of sequentially delivered pure tones (see Fig. 1A), but differed in the selection of tones and/or the cycle repetition rate. The basic rhythmic pattern, consisting of repeated sequences of phase resetting stimuli (Fig. 1A), was developed computationally and aims at a desynchronization of abnormally synchronized neuronal activity. For the dynamic principles we refer to (Tass, 2003a, Tass, 2003b, Tass and Majtanik, 2006, Lysyansky et al., 2011, Popovych and Tass, 2012, Tass and Popovych, 2012). The main parameters of the stimulation protocols are loudness of tones, selection of tones, tone duration, cycle repetition rate, and cyclic stimulation/pause pattern.
2.3.1. Loudness of tones
For all three stimulation protocols stimulation tones were equally loud and just super-threshold. The loudness of the stimulation tones was set using the following procedure: First the loudness of the stimulation tone with the lowest frequency was set at hearing level (at this particular frequency) + 20 dB. Loudness of the other stimulation tones was set so that they were perceived by the participant as equally loud to the first tone. The acoustic signals were generated using custom written scripts in MATLAB 2012a, amplified using Cambridge Audio DacMagic Plus digital/analog converter (Cambridge Audio, London, United Kingdom) and delivered through a customized open fit earphone (Adaptive Neuromodulation (ANM) GmbH, Cologne, Germany). Participants who were not able to hear all stimulation tones were excluded from the study.
2.3.2. Selection of tones
CR neuromodulation aims at desynchronizing a synchronized population of neurons by selectively resetting the rhythmic activity of different subpopulations at different times, in this way disrupting the overall phase coherence (Tass, 2003a, Tass, 2003b). Accordingly, using the tonotopic organization of the central auditory system, for acoustic CR neuromodulation we used tones of different frequencies centered around the matched tinnitus frequency (ft), see Fig. 1B (Tass and Popovych, 2012, Tass et al., 2012b). Using four different stimulation sites (i.e. stimulation tones) was appropriate, since CR effects do not simply increase with an increase of numbers of stimulation sites (i.e. stimulation tones), see (Lysyansky et al., 2013). However, the overlap of the volume of neurons activated by the different stimulation sites (i.e. tones) should only be moderate (Tass, 2003a, Tass, 2003b, Lysyansky et al., 2013). Accordingly, based on the logarithmic tonotopic organization of the auditory cortex and in accordance with the initial choice in a proof of concept study (Tass et al., 2012b) for acoustic CR neuromodulation the four stimulation tones f1 , … , f4 were adapted to the patient's tinnitus frequency ft and placed within the approx. one octave-wide interval [0.766·ft, 1.4·ft]: f1 = 0.766 · ft, f2 = 0.9 · ft , f3 = 1.1 · ft, f4 = 1.4 · ft. For the noisy CR-like stimulation prior to each cycle 4 frequencies f1 , … , f4 were randomly chosen out of a set of frequencies g1 to g12 with equal probability (see Fig. 1B) in the following way: f1 was chosen from the set S1 = {g1, g2, g3}, f2 was chosen from S2 = {g4, g5, g6}, f3 was chosen from S3 = {g7, g8, g9}, and f4 was chosen from S4 = {g10, g11, g12}, where gj = djft and d1 = 0.690, d2 = 0.728, d3 = 0.766, d4 = 0.810, d5 = 0.855, d6 = 0.900, d7 = 1.100, d8 = 1.182, d9 = 1.265, d10 = 1.400, d11 = 1.505, d12 = 1.610. Denoting the mean frequencies of the four sets S1 , … , S4 by , , , , we obtain , , , . For both acoustic CR neuromodulation and noisy CR-like stimulation the spacing characteristics depend on the tinnitus frequency in the following way:
Span between stimulation tones with highest and lowest pitch: The span for acoustic CR neuromodulation reads f4 − f1 = 0.634 ft. For noisy CR-like stimulation the span ranges from g12 − g1 = 0.920 ft to g10 − g3 = 0.634 ft with an average (over stimulation cycles) of = 0.777 ft. The average span of noisy CR-like stimulation is approx. 23% greater than that of acoustic CR neuromodulation.
Distance between stimulation tones and tinnitus frequency ft: For acoustic CR neuromodulation the distance between tones 1,…,4 and the tinnitus frequency ft read ft − f1 = 0.234 ft, ft − f2 = 0.1 ft, f3 − ft = 0.1 ft, f4 − ft = 0.4 ft. In contrast, for noisy CR-like stimulation the average distance between the four sets S1 , … , S4 and the tinnitus frequency ft is = 0.272 ft, = 0.145 ft, = 0.182 ft, = 0.505 ft. On average, the stimulation tones of the noisy CR-like stimulation are further away from the tinnitus frequency ft. In particular, the distance between the lower and higher inner tones of the noisy CR-like stimulation and the tinnitus frequency ft are on average 45% and 82% greater than the corresponding distance of the lower and higher inner tones of the acoustic CR neuromodulation. Hence, the tinnitus frequency ft is spared by noisy CR-like stimulation compared to acoustic CR neuromodulation.
Mutual distance between stimulation tones: For acoustic CR neuromodulation the distance between adjacent tones reads f2 − f1 = 0.134 ft, f3 − f2 = 0.2 ft, f4 − f3 = 0.3 ft. For noisy CR-like stimulation the average distance between adjacent sets of tones is given by = 0.127 ft, = 0.327 ft, = 0.323 ft, and the minimum/maximum difference between adjacent sets S1 and S2 reads 0.044 ft/0.210 ft, between S2 and S3 it is 0.200 ft/0.455 ft, and between S3 and S4 it reads 0.135 ft/0.510 ft. While in the case of acoustic CR neuromodulation the gap between adjacent tones increases from lower to higher tones, for noisy CR-like stimulation the gap of the inner sets of tones is greatest. Furthermore, the gap between the inner sets of tones of the noisy CR-like stimulation is on average approx. 64% greater than the gap between the inner CR tones f2 and f3. Accordingly, acoustic CR neuromodulation is more focused on the tinnitus frequency ft. In addition, in case of the noisy CR-like stimulation the distance between adjacent tones varies considerably, in particular for the outer pairs of tones. For instance, the ratio between the maximum difference between the sets S1 and S2 and the minimum difference between the sets S1 and S2 amounts to 4.77.
The LFR stimulation tones included frequencies in the 0.5 to 4 kHz range, excluding frequencies around the tinnitus pitch (by sparing a one octave-wide interval in case of a low-frequency tinnitus) in order to minimize the acoustic input into the auditory cortex region corresponding to tinnitus pitch. This was done under the assumption that tones not targeting the direct tinnitus frequency range will have minimal or no effect on the tinnitus intensity or on oscillatory brain activity.
2.3.3. Tone duration
Tone duration was 150 ms for all three stimulation protocols.
2.3.4. Cycle repetition rate
Cycle repetition rate should optimally be adapted to the targeted abnormal synchronized oscillation. Hence, cycle repetition rate was 1.5 Hz (in the delta range) for acoustic CR neuromodulation and the noisy CR-like stimulation whereas for the LFR stimulation the repetition rate was deliberately detuned by setting it to 0.3 Hz. For acoustic CR neuromodulation a cycle repetition rate of 1.5 Hz, i.e. in the lower delta frequency range was selected because the primary target for desynchronization was the pathological delta band activity. Cycle repetition rate of the noisy CR-like neuromodulation was matched to the cycle repetition rate of the acoustic CR neuromodulation. The 4 tones per cycle were played in a random order. For the acoustic CR neuromodulation and noisy CR-like stimulation one cycle consisted of two tones below and two tones above the tinnitus frequency. For the LFR stimulation one cycle consisted of four tones in the 0.5 to 4 kHz range. Each tone of the cycle was presented separate from the other tones with interstimulus interval 0.167 s for the acoustic CR neuromodulation and noisy CR-like stimulation. Interstimulus interval for the LFR stimulation was 0.83 s.
In summary, The LFR stimulation was used as an acoustic placebo/sham that was expected to have minimal or no effect on the tinnitus intensity or on oscillatory brain activity. Firstly, it did not overlap with the assumed synchronized tinnitus focus (see Selection of tones). Secondly, its repetition rate was significantly detuned so that, based on computational studies (Tass, 2003a, Tass, 2003b) we expected the desynchronizing effect of CR neuromodulation to be significantly reduced. Thirdly, the low repetition rate significantly reduced the acoustic input and accordingly decreased the possibility of effects caused by forward inhibition.
2.3.5. Cyclic stimulation/pause pattern
During acoustic CR neuromodulation and the noisy CR-like stimulation three stimulation cycles were followed by two silent cycles (Tass et al., 2012a, Lysyansky et al., 2011). During the LFR stimulation one stimulation cycle was followed by one silent cycle. The 3:2 ON:OFF cycles stimulation/pause pattern for acoustic CR neuromodulation and the noisy CR-like stimulation is favorable according to computational studies (Lysyansky et al., 2011) and was successfully applied in a number of pre-clinical and clinical studies (Tass et al., 2012a, Tass et al., 2012b, Adamchic et al., 2014b, Wang et al., 2016). In contrast, the 1:1 ON:OFF cycles stimulation/pause pattern for LFR stimulation might, in principle, be effective according to computational studies (Lysyansky et al., 2011). However, the 1:1 ratio further reduces the active stimulation time and might, hence, further reduce LFR's efficacy.
2.4. Data processing
The scalp EEG, recorded under the eyes closed condition, was re-referenced to average reference. We selected the eyes closed data for further analysis as they were less affected by artifacts. Signals were additionally digitally filtered with a 0.8 (48 dB slope)–130 (12 dB slope) Hz digital filter. Noisy channels were interpolated in BESA using the spherical spline interpolation. Each EEG recording was corrected for blink, eye movement, muscle and other artifacts using EEGLAB (http://sccn.ucsd.edu/eeglab). First, signal periods containing large non-neural artifacts (e.g., large EMG artifacts, large movement artifacts) were removed. Then independent component analysis decomposition was performed on the sensor level EEG data (Delorme et al., 2007). Components containing blink or eye movement artifacts were removed. Myogenic components, that is, components containing EMG activity in the absence of any identifiable neurogenic activity, were selected and removed based on all of the following criteria: (i) high broad peaks around either 30–40 Hz or higher, (ii) a moderately small and clustered distribution on the topographic maps that mimicked the underlying scalp musculature, (iii) periods of high-frequency activation in the time-domain, (iv) equivalent dipole(s) of the identified components located outside the brain volume and having a residual variance of 15% or less. Dipole locations were modeled using DIPFIT plug-in in EEGLAB. Artifact component selection procedure was performed twice with inter-rater reliability assessed using Krippendorff's alpha (a = 0.95).
Overview of the different inverse EEG analysis techniques used in this study (and explained below in detail):
-
•
We analyzed spectral power of auditory cortex activity by means of a BESA source montage approach (Scherg et al., 2002) (i) during stimulation compared to baseline as well as (ii) after cessation of stimulation in consecutive 10 s windows to assess time course and duration of the after-effects (compared to baseline).
-
•
Although there is a considerable variance in the reported duration of the residual inhibition after masking sound, many studies report the duration of residual inhibition in the order of tens of seconds (Roberts et al., 2008, Terry et al., 1983). Accordingly, in order to try to avoid or reduce the electrophysiological effects due to residual inhibition we analyzed changes of cortical spectral power compared to baseline with sLORETA, both whole head and especially focused on Brodman Areas (BAs) 41 and 42, in the time window 60–120 s after the end of the stimulation (directly preceding the VAS testing).
Auditory cortex source activity: The surface EEG was transformed into brain source activity, using a BESA source montage approach (Scherg et al., 2002). The source montage consisted of ten regional sources distributed over the brain volume and represented activity from all major brain regions. Sources were placed in the left and right auditory cortex regions, the left and right dorsolateral prefrontal areas, left and right orbitofrontal areas, in the left and right parietal regions, one in the middle posterior and one in the middle anterior regions. The strength of the source montages approach is that one can obtain time courses of brain activities from distinct brain regions (Scherg et al., 2002). The locations of the regional sources were predefined and were the same for all participants and recordings. As tinnitus related oscillatory brain activity abnormalities are especially pronounced in the auditory cortices (Adamchic et al., 2014a, Adamchic et al., 2014b, Van der Loo et al., 2009, Weisz et al., 2005) we focused on changes of oscillatory brain activity in the auditory cortex region.
The mean EEG length available for the analysis after artifact rejection was before stimulation/during stimulation/after stimulation [mean(standard deviation)]: (i) for acoustic CR neuromodulation 320.5(27.5)/415.1(33.6)/211.4(20.2) s; (ii) for noisy CR-like stimulation 301.3(34.5)/441.3(30.1)/230.1(16.1) s; (iii) for LFR stimulation 317.5(26.3)/420.8(31.5)/219.8(17.5) s. No significant differences were observed between the lengths of the EEG recording available for analysis between the three stimulation types.
Fast Fourier transform was performed on the artifact-free source waveforms after windowing the signal with a 4096 ms wide cosine squared (cos2) window with 50% overlap. This resulted in a frequency resolution of 0.244 Hz. After calculation of spectral power of the spontaneous brain activity, frequency bands were defined as delta (1.0–4.0 Hz), alpha (8.0–13.0 Hz), gamma (30.0–48.0 Hz). The mean of the spectral power changes from the left and right temporal sources (left and right auditory cortex regions) was calculated. Power changes during and after stimulation were defined as the percentage power increase or power decrease in relation to the 10 minute long baseline period that preceded each stimulation session was chosen as the baseline period (only the eyes closed EEG data was selected). The measurement interval containing stimulation was divided into four epochs and the EEG power was averaged over time intervals ending at 4, 8, 12 and 16 min. BESA analysis of time courses of after-effects on auditory cortex source activity: To investigate the time course of the electrophysiological after-effects, the spectral power of the brain source activity was calculated with the BESA source montage approach (Scherg et al., 2002) as explained above and averaged over 10 s long time intervals to provide appropriate time resolution.
Current source density: With sLORETA, we computed a three-dimensional linear inverse solution to the EEG inverse problem with a three-shell spherical head model registered to the Talairach human brain atlas digitized at the Brain Imaging Center of the Montreal Neurological Institute (Pascual-Marqui, 2002). The solution space was constrained to the gray matter voxels that belonged to cortical and hippocampal regions (a total of 6430 voxels at a 5-mm spatial resolution). Analysis of whole brain after-effects with sLORETA: The localization of the differences in current density power between baseline and the time period 60–120 s after cessation of the acoustic stimulation was assessed by voxel-by-voxel t-tests of the sLORETA images. This time period (i.e., 60–120 s after the end of the stimulation, preceding the VAS evaluation time window) was selected because we were especially interested in the electrophysiological after-effects of the tested acoustic stimulation paradigms and also in order to avoid transient after-effects of the used acoustic stimulations. In the resulting statistical three-dimensional maps, a nonparametric approach was used to identify statistically significant differences of cortical voxels (Nichols and Holmes, 2002). The voxels with p values of < 0.05 were colored in a MRI template.
Additionally, the current source density (CSD) for the auditory cortex regions was calculated using sLORETA by including all voxels located within Brodman Areas (BAs) 41 and 42 into auditory region of interest (ROI). The CSD for the auditory ROI was calculated for the baseline and for the time period 60–120 s after the end of the stimulation and averaged over both hemispheres for statistical analysis.
Baseline calculation: BESA and sLORETA baseline analysis was performed on the EEG data obtained during the two minute intervals with eyes closed during the baseline period (see above).
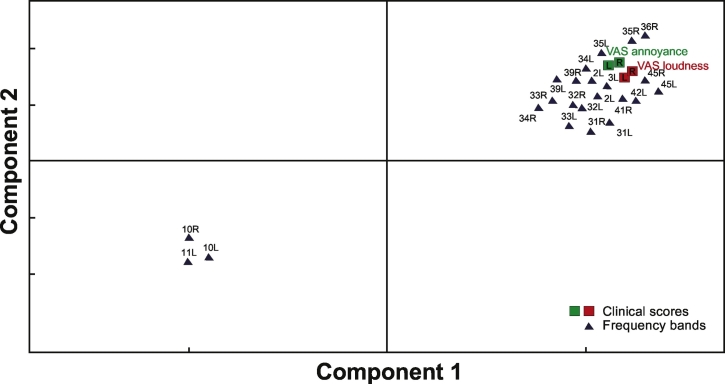
To determine the relationship between power spectra changes (calculated from BESA auditory cortex ROIs) and changes in clinical scores, we applied the PLS analysis (see Supplementary material).
2.5. Statistical analysis
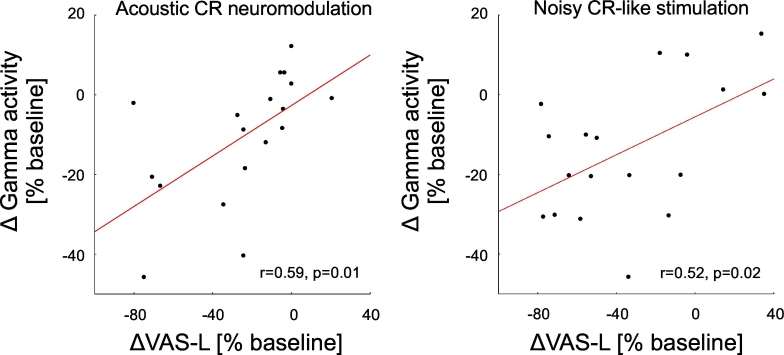
Analysis of variance was performed to analyze differences between baseline VAS, spectral power of auditory cortex source activity (BESA) and current source density (sLORETA). Repeated-measures analysis of variance (rmANOVA) was performed with factors time ([1] baseline, [2] during stimulation and [3] after stimulation), stimulation type ([1] acoustic CR neuromodulation, [2] noisy CR-like stimulation, [3] LFR stimulation) and the frequency band of interest ([1] delta, [2] alpha, [3] gamma). To allow the use of rmANOVA we first performed log transformation and then tested the data using Shapiro–Wilk test. Wilcoxon matched pairs signed-rank test was used to confirm post-stimulation changes of oscillatory auditory cortex source activity (obtained with BESA) and of the VAS scores. Results of the statistical tests were corrected for the number of tests conducted using the false discovery rate method (FDR) (Benjamini and Hochberg, 1995). Pearson correlation was performed between relative changes of power of sLORETA current source density in the auditory cortex ROI (i.e., difference between sLORETA power at baseline and 60–120 s after the end of the acoustic stimulation) and the corresponding relative change of the subjective tinnitus loudness (Fig. 8). Data in the text are presented as mean (standard deviation, SD).
Fig. 8.
Correlation between changes of the gamma current source density (CSD) after the end of acoustic stimulation and changes of the subjective tinnitus loudness. Significant negative correlation between relative changes of gamma power of current source density (CSD) in the auditory cortex ROI (BAs 41 and 42; sLORETA) (for the time period 60–120 s after the end of the stimulation as compared to baseline) and the relative change of the subjective tinnitus loudness after acoustic CR neuromodulation (r = 0.59, p = 0.01; A) and the noisy CR-like stimulation (r = 0.52, p = 0.02; B).
3. Results
3.1. Clinical data
The mean (SD) matched tinnitus frequency was 7.09 (2.56) kHz. The mean VAS-L/VAS-A at the beginning of each stimulation block were: (i) before acoustic CR neuromodulation 42.8 (26.6)/37.1(28.0); (ii) before noisy CR-like stimulation 42.9(22.2)/39.8(25.1); (iii) before LFR stimulation 39.9(23.8)/37.4(25.8). No significant baseline VAS-L/VAS-A differences were observed between the three stimulation types at baseline: VAS-L (F = 0.93, p = 0.91), VAS-A (F = 0.06, p = 0.95).
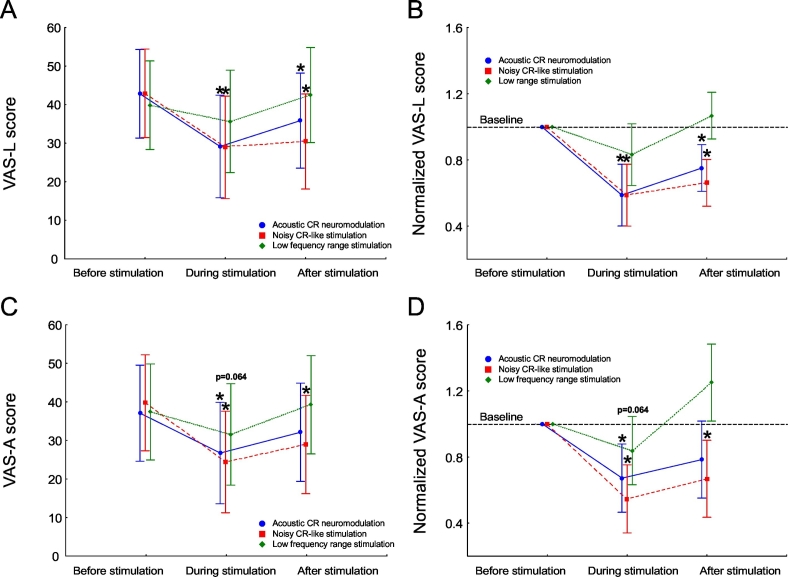
A stimulation (acoustic CR neuromodulation, noisy CR-like stimulation, LFR stimulation) × time (baseline, during stimulation, post stimulation) interaction was found to be significant for the VAS-L (F = 3.47, p = 0.012). Planned comparisons revealed that subjective tinnitus loudness was significantly reduced, as compared to baseline, during both acoustic CR neuromodulation (p = 0.002) and the noisy CR-like stimulation (p = 0.003; Fig. 2A, B). Tinnitus loudness was also significantly reduced, as compared to baseline, 2 min after cessation of acoustic CR neuromodulation (p = 0.048) and of noisy CR-like stimulation (p = 0.006). Reduction of the VAS-L was not significant during (p = 0.122) and after (p = 0.222) the LFR stimulation (Fig. 2A, B). However, the amount of reduction of the VAS-L during stimulation was significantly different between the noisy CR-like stimulation and the LFR stimulation (p = 0.037) as well as between acoustic CR neuromodulation and the LFR stimulation (p = 0.048). The amount of the tinnitus loudness reduction 2 min after the end of stimulation was significantly different between: (i) acoustic CR neuromodulation and the LFR stimulation (p = 0.01) and (ii) between the noisy CR-like stimulation and LFR stimulation (p < 0.001). There were no significant differences between acoustic CR neuromodulation and the noisy CR-like stimulation with respect to the amount of the tinnitus loudness reduction during and after the end of stimulation.
Fig. 2.
Tinnitus loudness and annoyance pre-, during- and post-stimulation. Mean intensity rating of tinnitus (0 = tinnitus is inaudible, 100 = tinnitus is extremely loud/annoying) for all 18 subjects. Significant changes of the tinnitus loudness and tinnitus annoyance as compared to baseline are marked with asterisk. Error bars indicate the 2 × SEM.
No significant stimulation (acoustic CR neuromodulation, noisy CR-like stimulation, LFR stimulation) × time (baseline, during stimulation, post stimulation) interaction was found for the VAS-A (F = 1.87, p = 0.122). However, significant main effects for time were found for acoustic CR neuromodulation (F = 3.87, p = 0.031), the noisy CR-like stimulation (F = 8.6, p < 0.001), and the LFR stimulation (F = 4.60, p = 0.017). Planned comparisons revealed that tinnitus annoyance was significantly reduced, as compared to baseline, during both acoustic CR neuromodulation (p = 0.029) and the noisy CR-like stimulation (p = 0.002). During the LFR stimulation the reduction of tinnitus annoyance approached significance (p = 0.064; Fig. 2C, D). Tinnitus annoyance was significantly reduced, as compared to the baseline, 2 min after the end of the noisy CR-like stimulation (p = 0.006). No significant changes of the VAS-A were found 2 min after the end of acoustic CR neuromodulation (p = 0.081) and after the LFR stimulation (p = 0.619; Fig. 2). The significant main effect for time for the LFR stimulation resulted from the significant difference between the VAS-A values during and 2 min after the LFR stimulation (p = 0.006).
3.2. Changes of auditory cortex source activity (BESA) during acoustic stimulation
No significant differences of the power of the baseline auditory cortex source activity (calculated with BESA) were observed before the three stimulation types in any of the three tested bands: delta band (F = 0.11, p = 0.90), alpha band (F = 1.13, p = 0.33), gamma band (F = 0.65, p = 0.53). Repeated measures ANOVA demonstrated a significant interaction effect of stimulation × time (baseline and during stimulation) × frequency band (F = 8.9, p < 0.001). In each of the investigated frequency bands, significant interactions of stimulation type and time (baseline and during stimulation) were found: delta band (F = 19.9, p < 0.001), alpha band (F = 14.8, p < 0.001), gamma band (F = 2.0, p < 0.048, Fig. 3). Significant changes of power during stimulation, as compared to baseline, were observed during all of the stimulation types and in all frequency bands (one-way rmANOVA; Fig. 3), that is, during acoustic CR neuromodulation, the noisy CR-like stimulation and the LFR stimulation (see Table S1). However, there was no significant main effect for time (one-way rmANOVA) for any of the stimulation types and frequency bands when only the four measurements during stimulation (after 4, 8, 12, and 16 min of stimulation) were included into the analysis.
Fig. 3.
Changes of spectral power of auditory cortex source activity (BESA) during acoustic stimulation. Time course of the mean delta (1–4 Hz), alpha (8–12 Hz) and gamma (30–48 Hz) auditory cortex power during acoustic CR neuromodulation (blue), noisy CR-like stimulation (red) and low frequency range stimulation (green) expressed as a percentage change from baseline activity. There was a significant change in the auditory cortex power during all types of acoustic stimulation in all frequency bands. Significant changes of the auditory cortex power during acoustic CR neuromodulation and the noisy CR-like stimulation as compared to the auditory cortex power changes during LFR stimulation are marked with asterisk. Error bars indicate the 2 × SEM.
A significant reduction of the delta power was observed during all stimulation types and was strongest during acoustic CR neuromodulation and the noisy CR-like stimulation (Fig. 3A). The mean reduction of the delta power was 17.39 (5.73) % (during acoustic CR neuromodulation), 16.54 (5.70) % (during noisy CR-like stimulation), and 5.61 (10.14) % (during LFR stimulation). The mean reduction of the delta power during acoustic CR neuromodulation and the noisy CR-like stimulation were significantly greater than during the LFR stimulation, p < 0.001 and p = 0.001, respectively. However, the average reduction of the delta power during both acoustic CR neuromodulation and the noisy CR-like stimulation were not significantly different (p = 0.689).
A significant reduction of the alpha power, as compared to baseline, was observed during all stimulation types and was strongest during acoustic CR neuromodulation and the noisy CR-like stimulation (Fig. 3B). The mean reduction of the alpha power was 7.55 (5.90) % (during acoustic CR neuromodulation), 7.63 (4.95) % (during noisy CR-like stimulation), and 2.76 (3.21) % (during LFR stimulation). The average reduction of the alpha power during acoustic CR neuromodulation and the noisy CR-like stimulation were significantly greater than during LFR stimulation, p < 0.001 and p < 0.001, respectively. However, the average reduction of the alpha power during both acoustic CR neuromodulation and noisy CR-like stimulation were not significantly different (p = 0.986).
A significant increase of the gamma power was observed during all stimulation types (Fig. 3C). The mean increase of the gamma power was 19.69 (8.66) % (during acoustic CR neuromodulation), 20.67 (10.00) % (during noisy CR-like stimulation), and 16.65 (7.14) % (during LFR stimulation). However, differences between the increases of the gamma power averaged over the duration of the stimulation did not reach significance between any of the stimulation types (Fig. 3C).
3.3. Changes of auditory cortex source activity (BESA) and current source density (sLORETA) after acoustic stimulation
For the auditory cortex source activity (calculated with BESA) the stimulation × time (baseline and after stimulation) × frequency band interaction was significant (F = 25.7, p < 0.001). In each of the investigated frequency bands, an interaction of stimulation and time was also found significant (Table S2a). Additionally, a significant main effect for time could be observed for all of the three frequency bands and all of the stimulation types, that is, acoustic CR neuromodulation, noisy CR-like stimulation and LFR stimulation (Table S2b). This confirms the assumption that stimulation changed the patterns of spontaneous brain activity in all three frequency bands. We followed up these findings by planned comparisons between power of the auditory cortex source activity (BESA) as well as current source density (sLORETA) at baseline and after cessation of stimulation in all of the three frequency bands. Planned comparisons were performed separately for each of the stimulation types and frequency bands (Fig. 4, Fig. 5, Fig. 6, Fig. 7).
Fig. 4.
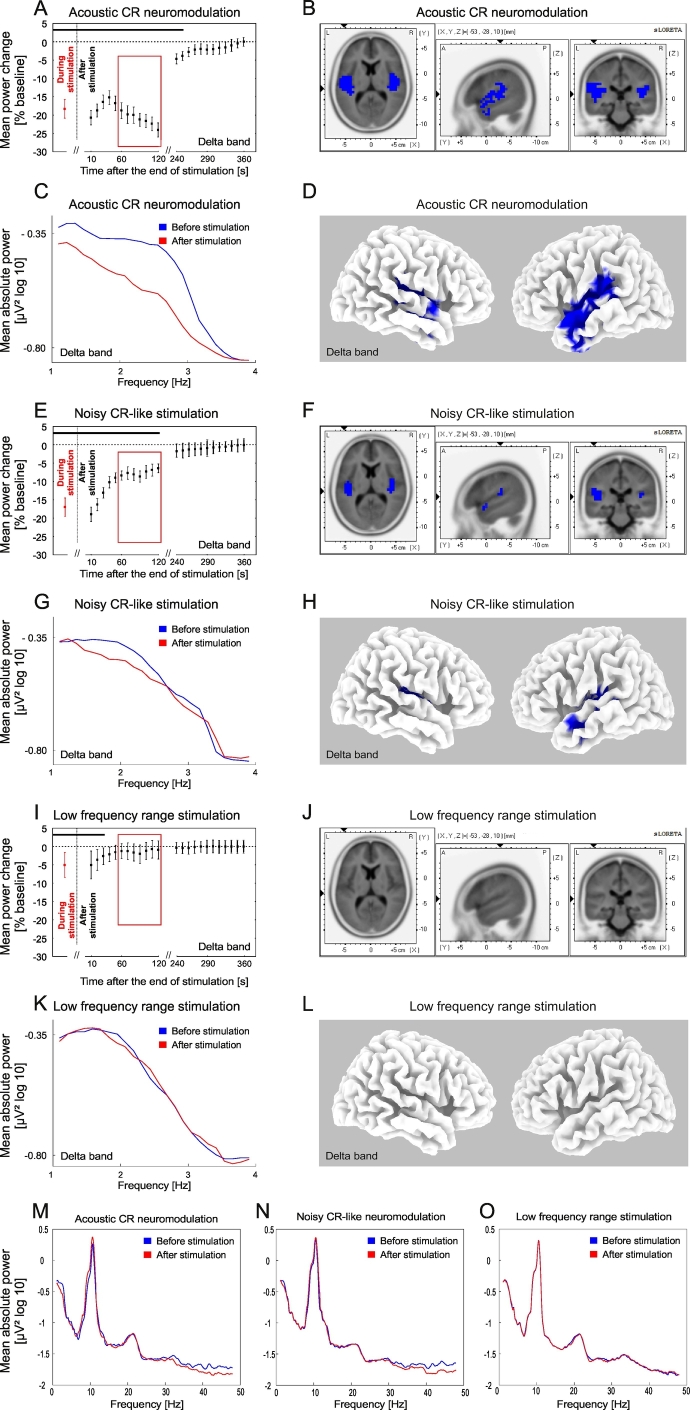
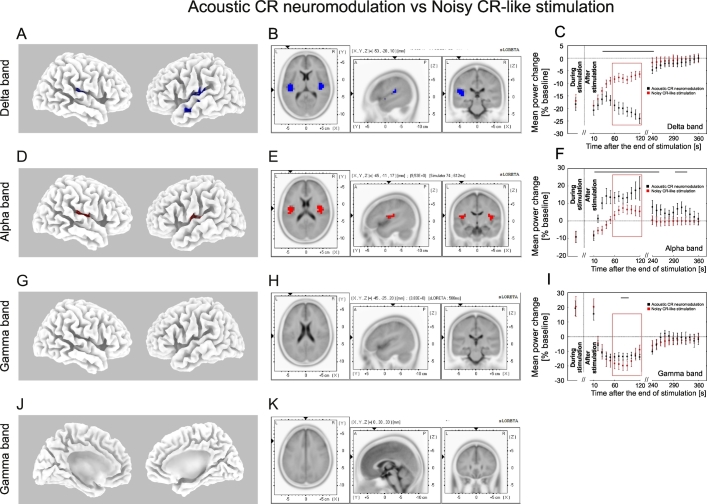
Changes of the delta power of auditory cortex source activity (BESA) and current source density (sLORETA) after the end of acoustic stimulation. Time course of the mean delta (1–4 Hz) power of the auditory source activity (determined with BESA) during (averaged over the whole stimulation period, red) and after the end (black) of acoustic CR neuromodulation (A), the noisy CR-like stimulation (E) and the LFR stimulation (I) expressed as a percentage change from the baseline activity. Significant changes with respect to baseline are marked with the horizontal black line (A, E, I). As there was no significant main effect for time during stimulation for any of the stimulation types and frequency bands (see Fig. 3), power spectra of the auditory source activity (BESA) during stimulation were averaged over the whole stimulation period (A, E, I). Error bars indicate the 2 × SEM. Power spectra for the delta frequency range of the auditory source activity (BESA) at baseline and for the time period 60–120 s (inside the red window in A, E, I) after cessation of the acoustic CR neuromodulation (C), the noisy CR-like stimulation (G) and the LFR stimulation (K). The effect of acoustic CR neuromodulation (B, D), the noisy CR-like stimulation (F, H) and the LFR stimulation (J, L) on the mean current source density were analyzed by sLORETA (results are presented for the time period 60–120 s after the end of the stimulation; inside the red window). The strongest decrease (indicated by blue voxels) compared to baseline was localized in the left transverse temporal gyrus after acoustic CR neuromodulation (xyz − 51, − 19, 11; BA 41). Power spectra of the auditory source activity (BESA) at baseline and for the time period 60–120 s for acoustic CR neuromodulation (M), the noisy CR-like stimulation (N) and the LFR stimulation (O).
Fig. 5.
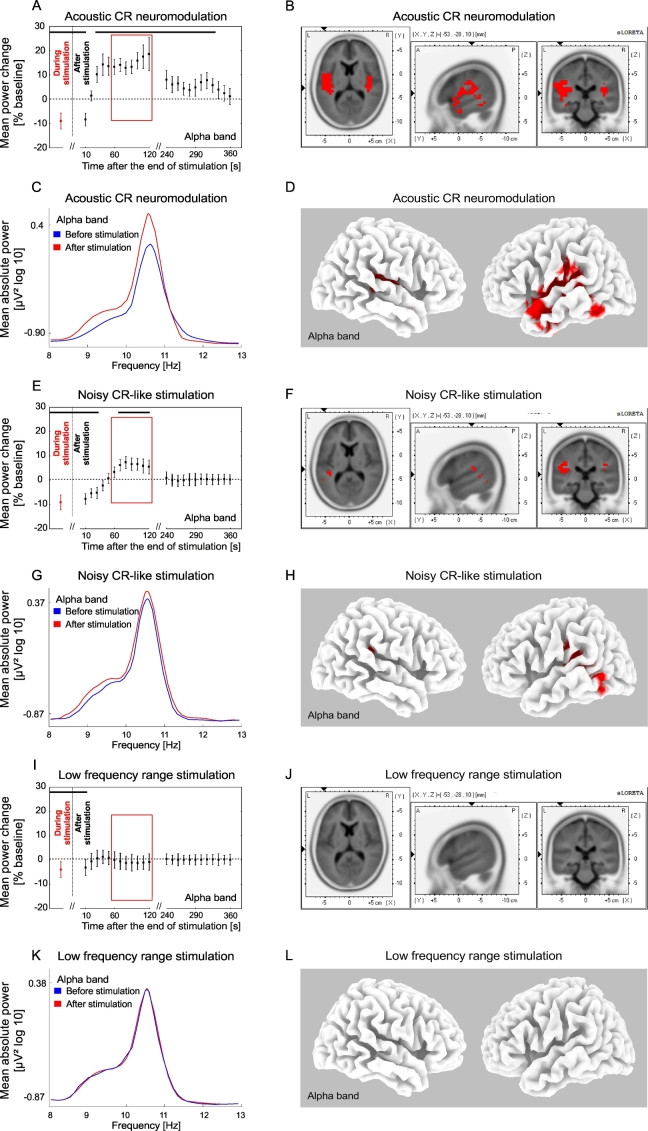
Changes of the alpha power after the end of acoustic stimulation. Time course of the mean alpha (8–13 Hz) power of the auditory BESA source activity during (averaged over the whole stimulation period, red) and after the end (black) of acoustic CR neuromodulation (A), the noisy CR-like stimulation (E) and the LFR stimulation (I) expressed as a percentage change from the baseline activity. Significant changes are marked with the horizontal black line (A, E, I). Format as in Fig. 4. Power spectra for the alpha frequency range at baseline and for the time period 60–120 s (inside the red window in A, E, I) after end of the acoustic CR neuromodulation (C), the noisy CR-like stimulation (G) and the LFR stimulation (K). The effect of acoustic CR neuromodulation (B, D), the noisy CR-like stimulation (F, H) and the LFR stimulation (J, L) on the mean current source density analyzed by sLORETA (format as in Fig. 4). The strongest increase compared to baseline (indicated by red voxels) was localized in the left and right transverse temporal gyrus after acoustic CR neuromodulation (xyz − 40, − 29, 9; BA 41).
Fig. 6.
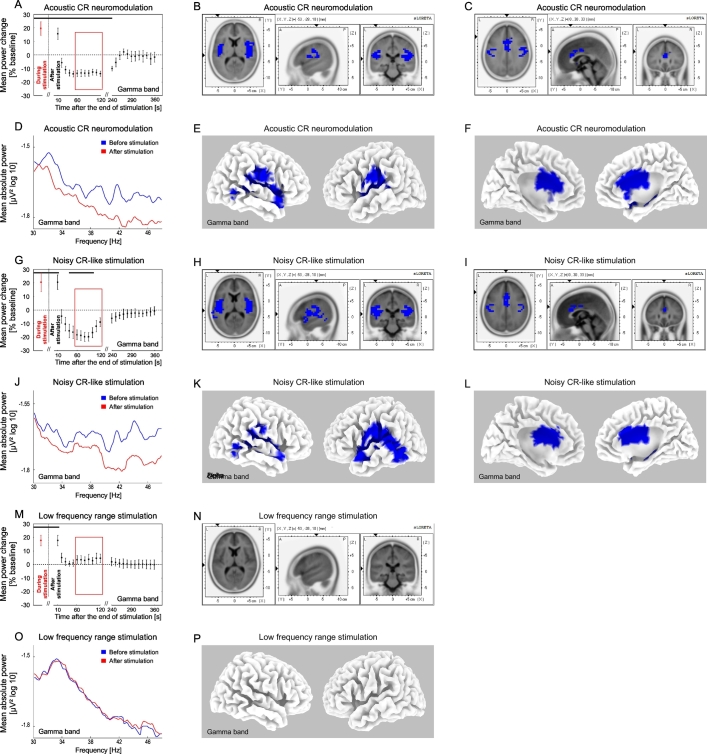
Changes of the gamma power after the end of acoustic stimulation. Time course of the mean gamma (30–48 Hz) power of the auditory BESA source activity during (averaged over the whole stimulation period, red) and after the end (black) of acoustic CR neuromodulation (A), the noisy CR-like stimulation (G) and the LFR stimulation (M) expressed as a percentage change from the baseline activity. Significant changes are marked with the horizontal black line (A, G, M). Format as in Fig. 4. Power spectra for the gamma frequency range at baseline and for the time period 60–120 s (inside the red window in A, G, M) after end of the acoustic CR neuromodulation (D), the noisy CR-like stimulation (J) and the LFR stimulation (O). The effect of acoustic CR neuromodulation (B, C, E, F), noisy CR-like stimulation (H, I, K, L) and the LFR stimulation (N, P) on the mean current source density analyzed by sLORETA (format as in Fig. 4). The strongest decrease compared to baseline (indicated by blue voxels) was localized in the left and right superior temporal gyrus after the noisy CR-like stimulation (xyz 37, − 28, 9; BA 41).
Fig. 7.
Effects of acoustic CR neuromodulation, the noisy CR-like stimulation and the LFR stimulation on the mean current source density in the auditory cortex region. The current source density (CSD) changes in the auditory cortex ROI (BAs 41 and 42; sLORETA) for the time period 60–120 s after the end of the stimulation as compared to baseline averaged over both hemispheres. Error bars indicate the 2 × SEM.
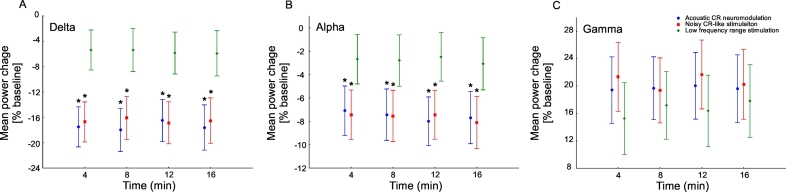
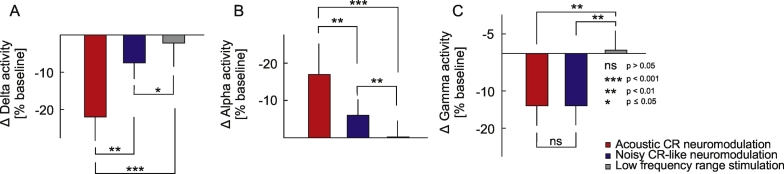
A significant reduction of delta power of the auditory cortex source activity (calculated with BESA) was observed after all stimulation types and was longest after acoustic CR neuromodulation (0–250 s) and shortest after the LFR stimulation (0–30 s; Fig. 4A, E, I). After noisy CR-like stimulation delta band power decreased for 120 s (0–120 s; Fig. 4E). sLORETA analysis, performed in the time window 60–120 s after cessation of stimulation, showed significant power reduction, as compared to baseline, in the delta frequency band after acoustic CR neuromodulation and after noisy CR-like neuromodulation (Fig. 4B, D, F, H). Spatial peaks (i.e., local maxima) of delta power reduction of the CSD were localized in the left temporal cortex after both acoustic CR neuromodulation (Brodman area [BA] 41, t = 6.52) and noisy CR-like stimulation (BA 41, t = 5.32; Table 1). Reduction of the current source density (CSD) in the delta band in the auditory cortex ROIs (i.e., BAs 41 and 42) was significantly greater after acoustic CR neuromodulation as compared to both noisy CR-like stimulation and LFR stimulation (Fig. 7A).
A short term significant decrease of alpha power of the auditory cortex source activity (BESA) was observed after all stimulation types (Fig. 5A, E, I); 0–10 s after acoustic CR neuromodulation, 0–40 s after the noisy CR-like stimulation and 0–10 s after the LFR stimulation. This decrease was followed by a significant increase of alpha power after acoustic CR neuromodulation (30–330 s) and after noisy CR-like stimulation (70–120 s). sLORETA whole brain analysis showed significant power increase, as compared to baseline, in the alpha frequency band after acoustic CR neuromodulation and noisy CR-like neuromodulation (Fig. 5B, D, F, H; Table 1). Spatial maxima of the alpha power increase of the CSD were localized in the left temporal cortex after both acoustic CR neuromodulation (BA 41, t = 6.51) and noisy CR-like stimulation (BA 41, t = 6.43; Table 1). Increase of the CSD in the alpha band in the auditory cortex ROIs was significantly greater after acoustic CR neuromodulation as compared to both noisy CR-like stimulation and LFR stimulation (Fig. 7B).
In the gamma frequency band of the auditory cortex source activity (BESA) we observed a transient significant increase of power for 10 s (0–10 s) after all stimulation types, followed by a significant reduction after acoustic CR neuromodulation and noisy CR-like stimulation (Fig. 6A, G, M). A significant reduction of gamma power of the auditory cortex source activity (BESA) was observed after acoustic CR neuromodulation (20–240 s) and after noisy CR-like stimulation (40–100 s). In the time window 60–120 s after cessation of stimulation sLORETA whole brain analysis revealed a significant power reduction, as compared to baseline, in the gamma frequency band after acoustic CR neuromodulation and noisy CR-like neuromodulation (Fig. 6B, C, E, F, H, I, K, L; Table 1). Spatial maxima of the gamma power reduction of the CSD were localized in the left temporal cortex after acoustic CR neuromodulation (BA 41, t = 6.91) and in the right temporal cortex after noisy CR-like stimulation (BA 41, t = 6.95; Table 1).
Reduction of the CSD in the gamma band in the auditory cortex ROIs was similar after both acoustic CR neuromodulation and noisy CR-like stimulation (Fig. 7C). Thus, changes in the delta, alpha and gamma frequency bands were specific for each of the acoustic stimulation types. No significant changes were observed in the theta and beta frequency ranges.
3.4. Differences of auditory cortex source activity (BESA) and current source density (sLORETA) between groups
Delta power reduction of the auditory cortex source activity (BESA) was significantly greater after acoustic CR neuromodulation as compared to after noisy CR-like stimulation between 40 and 240 s (Fig. 9C). After acoustic CR neuromodulation the increase of alpha band power of the auditory cortex source activity (BESA) was significantly greater than after noisy CR-like stimulation between 20 and 250 s as well as between 300 and 330 s (Fig. 9F). Gamma power reduction was significantly greater after noisy CR-like stimulation as compared to acoustic CR neuromodulation within the time window between 80 and 90 s (Fig. 9I). sLORETA analysis, applied to the time window 60–120 s after stimulation offset, showed significantly greater reduction of CSD in the delta band after acoustic CR neuromodulation as compared to the noisy CR-like stimulation (Fig. 9A, B). Spatial peaks of CSD delta band differences between acoustic CR neuromodulation and the noisy CR-like stimulation were localized in the temporal cortex (BA 41). Increase of the CSD in the alpha band in the auditory cortex was significantly greater after acoustic CR neuromodulation as compared to the noisy CR-like stimulation (Fig. 9D, E). Spatial peaks of alpha band CSD differences between acoustic CR neuromodulation and the noisy CR-like stimulation were localized in the temporal cortex (BA 41). No CSD differences were found between effects of acoustic CR neuromodulation and the noisy CR-like stimulation in the theta, beta or gamma bands (Fig. 9G, H, J, K).
Fig. 9.
Differential changes of EEG power for the acoustic CR neuromodulation and noisy CR-like stimulation. The effect of acoustic CR neuromodulation on the time-averaged mean current source density analyzed by sLORETA as compared to the noisy CR-like stimulation (results are presented for the time window 60–120 s after the end of the stimulation; inside the red window (C, F, I)): Delta decrease (indicated by blue voxels) was significantly greater after acoustic CR neuromodulation as compared to the noisy CR-like stimulation (A, B). Alpha increase (indicated by red voxels) was significantly greater after acoustic CR neuromodulation as compared to the noisy CR-like stimulation (D, E). There was no significant differences in the gamma band power change between acoustic CR neuromodulation and the noisy CR-like stimulation (G, H, J, K). Time course of the mean delta (C), alpha (F) and gamma (I) power during (averaged over the whole stimulation period) and after the end of acoustic CR neuromodulation (black) and the noisy CR-like stimulation (red) expressed as a percentage change from the baseline activity. Significant differences in power between acoustic CR neuromodulation and the noisy CR-like stimulation are marked with the horizontal black line on the top of the plot (C, F, I).
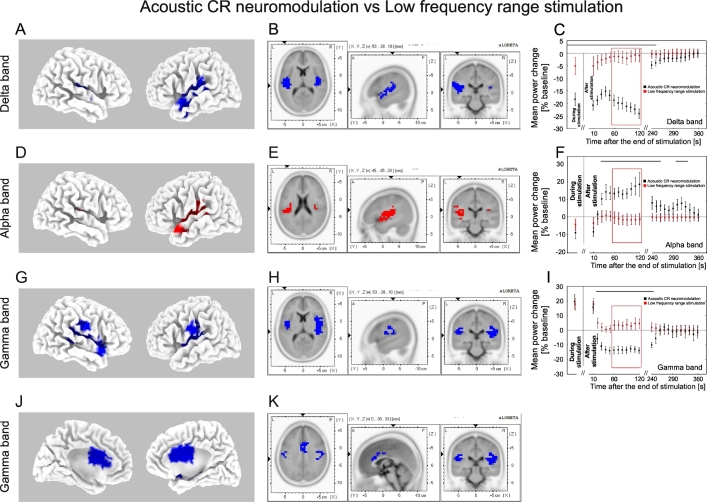
Delta power reduction of the auditory cortex source activity (BESA) was significantly greater after acoustic CR neuromodulation as compared to the LFR stimulation between 0 and 250 s (Fig. 10C). After LFR stimulation alpha band power of the auditory cortex source activity (BESA) increased significantly less between 30 and 260 s as well as between 310 and 330 s (Fig. 10F) as compared to acoustic CR neuromodulation. Gamma power reduction of the auditory cortex source activity (BESA) was significantly greater after acoustic CR neuromodulation as compared to the LFR stimulation between 20 and 240 s (Fig. 10H). sLORETA analysis showed significantly greater reduction of CSD in the delta and gamma bands after acoustic CR neuromodulation as compared to LFR stimulation (Fig. 10A, B, G, H, J, K). Spatial peaks of CSD differences between acoustic CR neuromodulation and LFR stimulation were localized in the temporal cortex for both delta and gamma bands (BA 41 for delta and gamma bands). Increase of the CSD in the alpha band in the auditory cortex was significantly greater after acoustic CR neuromodulation as compared to LFR stimulation (Fig. 10D, E). Spatial peaks of alpha band CSD differences between acoustic CR neuromodulation and LFR stimulation were localized in the temporal cortex (BA 41).
Fig. 10.
Differential changes of EEG power for the acoustic CR neuromodulation and LFR-stimulation. The effect of acoustic CR neuromodulation on the sLORETA mean current source density as compared to the LFR-stimulation (format as in Fig. 9). Delta decrease (indicated by blue voxels) was significantly greater after acoustic CR neuromodulation as compared to the LFR-stimulation (A, B). Alpha increase (indicated by red voxels) was significantly greater after acoustic CR neuromodulation as compared to the LFR-stimulation (D, E). Gamma decrease (indicated by blue voxels) was significantly greater after acoustic CR neuromodulation as compared to the LFR-stimulation (G, H, J, K). Time course of the mean delta (C), alpha (F) and gamma (I) power during (averaged over the whole stimulation period) and after the end of acoustic CR neuromodulation (black) and the LFR-stimulation (red) expressed as a percentage change from the baseline activity. Significant differences in power between acoustic CR neuromodulation and the LFR-stimulation are marked with the horizontal black line on the top of the plot (C, F, I).
No significant changes were observed between acoustic CR neuromodulation and noisy CR-like stimulation as well as between acoustic CR neuromodulation and the LFR stimulation in both theta and beta frequency ranges.
4. Discussion
To our knowledge, this is the first study that investigated both subjective changes of tinnitus intensity and changes of the oscillatory brain activity in participants with chronic tonal subjective tinnitus during and immediately after the application of three different auditory stimulation paradigms. Two of the investigated paradigms were expected to have a similar masking effect on the tinnitus sensation but only for one of them, specifically, acoustic CR neuromodulation, a longer lasting desynchronizing effect on the pathologic synchronous activity in the auditory cortex was expected. Effects of acoustic CR neuromodulation and noisy CR-like stimulation were compared to a control stimulation that was expected to produce no or only minimal longer-lasting changes of oscillatory brain activity and of tinnitus loudness/annoyance. The behavioral measures were analyzed in terms of the ability of acoustic CR neuromodulation, noisy CR-like stimulation and LFR stimulation to induce a reduction in tinnitus loudness and annoyance from pre- to during- to post-stimulation. All three stimulation types induced temporally distinct effects on oscillatory brain activity, tinnitus loudness, and annoyance.
4.1. Differential clinical effects of acoustic CR neuromodulation and noisy CR-like stimulation
A major motivation of this study was to understand possible differences between acoustic CR neuromodulation and noisy CR-like stimulation that might contribute to their differential clinical effects: In a previous proof of concept study (Tass et al., 2012a) both stimulation protocols showed acute effects as subjectively measured with VAS-L and VAS-A scores in the stimulation-ON condition (i.e. 15 min after stimulation onset), but only acoustic CR neuromodulation had sustained long-lasting therapeutic effects after 12 weeks of treatment in the stimulation-OFF condition (i.e. with patients being off stimulation for at least 2.5 h). In accordance with the acute findings in the proof of concept study (Tass et al., 2012a), in the present study acoustic CR neuromodulation as well as noisy CR-like stimulation caused a significant decrease of both VAS-L and VAS-A scores with respect to baseline (Fig. 2). We found no significant differences between acoustic CR neuromodulation and noisy CR-like stimulation concerning the VAS-L reduction during and after the end of stimulation. Unlike acoustic CR neuromodulation, only noisy CR-like stimulation caused a reduction of VAS-A after the end of stimulation that was significant compared to baseline. The significant reduction of VAS-A after the end of the noisy CR-like stimulation was the only observed significantly different clinical effect between the two stimulation protocols. Note, a 16 min-epoch of acoustic CR neuromodulation (with currently used settings and parameters) is not sufficient to induce long-lasting effects, e.g., persisting through a 4-week pre-planned treatment pause as observed after 12 weeks of CR therapy with 4–6 h stimulation/day (Tass et al., 2012a).
4.2. Differential electrophysiological acute effects of acoustic CR neuromodulation and noisy CR-like stimulation
Acoustic CR neuromodulation and noisy CR-like stimulation did not differ with respect to their acute effects on the auditory cortex source activity (determined with BESA). During stimulation both stimulation protocols caused a reduction of the delta and alpha band activity together with an increase of the gamma band activity, without significant difference between the two stimulation protocols, respectively (Fig. 3). The significant reduction of delta oscillatory activity observed during acoustic CR neuromodulation and noisy CR-like stimulation is in accordance with results obtained by Adjamian et al. (2012) in subjects with tinnitus, demonstrating that slow-wave activity in the auditory cortex region is reduced during periods with attenuated or masked tinnitus.
4.3. Differential electrophysiological after-effects of acoustic CR neuromodulation and noisy CR-like stimulation
Acoustic CR neuromodulation caused a longer (compared to baseline, Fig. 4A,E) and more pronounced (Fig. 9C) decrease of delta band auditory cortex activity after stimulation offset compared to noisy CR-like stimulation, as evaluated with BESA in a time-resolved manner with 10 s windows. By the same token, a time-averaged CSD analysis with sLORETA, applied to the time window 60–120 s after stimulation offset, showed a significantly greater reduction of CSD in the delta band after acoustic CR neuromodulation compared to noisy CR-like stimulation (Fig. 7), with spatial peak differences in the temporal cortex (BA 41) (Fig. 9A, B). According to Fig. 9 the emergence of the difference in delta power is delayed, i.e. does not occur immediately after cessation of stimulation. This is in accordance with computational studies comparing effective CR stimulation and CR stimulation rendered ineffective by selecting inappropriately high stimulation amplitudes (and hence disabling a selective stimulation of subpopulations): The difference between effective vs. ineffective CR requires sufficient stimulation duration to show up, see e.g. Suppl. Fig. 2 in (Tass et al., 2012b). 16 min are not sufficient to induce immediate and sustained differences in the acute aftereffects of acoustic CR neuromodulation and noisy CR stimulation. In addition, acoustic CR neuromodulation caused a longer (Fig. 4A,E) and stronger (Fig. 9F) increase in alpha band auditory cortex activity as revealed with the time resolved BESA analysis. Accordingly, the time-averaged alpha band CSD, determined with sLORETA in the 60–120 s after stimulation offset, was significantly greater after acoustic CR neuromodulation as compared to the noisy CR-like stimulation, with spatial peak differences in the temporal cortex (BA 41) (Fig. 9E). Gamma power of the auditory cortex source activity (BESA) was significantly reduced compared to baseline after acoustic CR neuromodulation in a longer post-stim time window (20–240 s) as opposed to after noisy CR-like stimulation (40–100 s) (Fig. 4A,G). In a relatively short time window, between 80 and 90 s after stimulation offset, noisy CR-like stimulation caused a significantly greater reduction of auditory cortex gamma power compared to acoustic CR neuromodulation (Fig. 9I). This short-duration difference did not show up in the comparative time-averaged sLORETA current source density analysis, covering the time period 60–120 s after stimulation offset (Fig. 9G, H, J, K). Finally, in the theta and beta band no sLORETA CSD related differences were found between acoustic CR neuromodulation and noisy CR-like stimulation bands.
In summary, we found no significant differences between acoustic CR neuromodulation and noisy CR-like stimulation with respect to the acute clinical and the acute electrophysiological effects. Accordingly, our current study replicates the results of a previous proof of concept study in that both stimulations have similar acute effects on tinnitus loudness and annoyance (Tass et al., 2012a). However, in that proof of concept study acoustic CR neuromodulation caused a long-lasting reduction of both tinnitus loudness and annoyance, as assessed in the off stimulation condition after 12 weeks of treatment as well as after a subsequent, preplanned 4-week treatment pause (Tass et al., 2012a). In contrast, noisy CR-like stimulation did not cause any long-lasting clinical effects (Tass et al., 2012a).
In the present study the two stimulation protocols differed significantly only concerning their acute after-effects. While both stimulation protocols caused a significant after-effect of the VAS-L, only noisy CR-like stimulation led to a significant reduction of VAS-A after the end of the stimulation. However, the acute electrophysiological after-effect was more pronounced after acoustic CR neuromodulation than after noisy CR-like stimulation: Acoustic CR neuromodulation caused (i) a significantly longer and stronger decrease of the delta band power, (ii) a significantly longer and stronger increase of the alpha band power and (iii) a significantly longer decrease of the gamma band power, with spatial peaks in the auditory cortex. Only in a narrow, 10 s time window the gamma band after-effect was significantly greater after noisy CR-like stimulation than after acoustic CR neuromodulation.
Based on the results presented here, acute electrophysiological after-effects, e.g. a significant decrease of the delta band power or combined effects, such as decrease of delta and increase of alpha, might be used as candidate markers for the further optimization of parameters of acoustic CR neuromodulation. Our results are in accordance with a pre-clinical study with CR-DBS in Parkinsonian monkeys where CR-DBS was delivered at optimal and less appropriate intensities (Tass et al., 2012b). In that study it was shown that long and pronounced acute therapeutic after-effects coincide with long-lasting, sustained after-effects. Ultimately, the predictive power of acute electrophysiological after-effects has to be investigated by means of a clinical study comprising EEG recordings and long-term clinical assessment.
Acoustic CR neuromodulation and noisy CR-like stimulation are similar in several respects, but differ in two relevant features that might cause the EEG results reported here: (i) Fixed set vs. random selection of subset of stimulation tones: A fixed set of four tones is used for acoustic CR neuromodulation for all stimulation cycles. In contrast, for noisy CR-like stimulation four stimulation tones are randomly selected from a larger set of 12 tones for each stimulation cycle. In fact, a variant of CR stimulation designed for electrical brain stimulation selects m out of n stimulation contacts for each stimulation cycle, where m ≥ 2 and m < n (Tass, 2006). The motivation behind this “m out of n” CR variant is to avoid stereotypical reverberations of the network's activity (Tass, 2006), see also (Tass and Majtanik, 2006). However, the stimulation sites have to be arranged in a spatially balanced way to ensure stimulation of sufficiently separate neuronal subpopulations (Tass, 2003a, Tass, 2003b, Tass, 2006). For the noisy CR-like protocol m = 4 and n = 12. In fact, the m out of n CR selection mode should not compromise the desynchronizing effect of CR stimulation, provided the different subsets of stimulation sites share the same or at least similar topological characteristics (spatial relation and mutual overlap of stimulated sub-populations) (Tass, 2003a, Tass, 2003b, Tass, 2006). (ii) Spacing of stimulation tones: Compared to acoustic CR neuromodulation the noisy CR-like stimulation avoids stimulation of the frequency range adjacent to the tinnitus frequency ft. In principle, however, the latter is the very target of CR stimulation. In addition, for the noisy CR-like stimulation the distance between adjacent tones varies considerably, especially for the outer pairs of tones, where the spacing variability amounts to a factor of up to 4.77. Based on computational studies (Tass, 2003a, Tass, 2003b, Lysyansky et al., 2011, Lysyansky et al., 2013) and pre-clinical studies (Tass et al., 2012b) in the field of CR-DBS such a spatially unbalanced stimulation will very likely undermine desynchronizing effects of DBS. We used the term noisy CR-like stimulation because of these two major, distinguishing features: avoiding the tinnitus target and high spacing variability of relatively remote stimulation sites. Due to its particular tone arrangement noisy CR-like stimulation noisy CR-like stimulation might predominantly inhibit tinnitus-related activity (Eggermont and Roberts, 2004, Roberts, 2007). In computational studies with different types of neural networks it was shown that blocking and/or inhibitory stimuli typically do not cause long-lasting desynchronizing effects, and excessive neuronal synchrony re-increases more quickly after cessation of blocking/inhibitory stimulation as compared to after desynchronizing stimulation, even in the absence of STDP (Tass and Majtanik, 2006, Hauptmann and Tass, 2007). The quick re-increase of neuronal synchrony may, e.g., be caused by neurons being held close to the same phase during inhibitory or blocking stimulation, see e.g. (Pyragas et al., 2013), in this way enabling a quick restart in synchrony.
The spatial resolution of our EEG study is not sufficient to determine the exact localization of the stimulation-induced changes in oscillatory activity. However, LFR stimulation which differed from CR neuromodulation by the frequency range of the auditory stimuli did not induce any relevant after-effects on oscillatory brain activity. This indicates, that the changes induced by CR neuromodulation depend on the relationship between the tinnitus frequency and the auditory stimulation and may thus occur predominantly in neuronal assemblies coding for the tinnitus frequency.
If CR neuromodulation and noisy CR-like stimulation had similar mechanism of action then similar electrophysiological changes after the two acoustic stimulation types should be observed. However, the reduction of delta band power after the end of acoustic CR neuromodulation lasted markedly longer and was more pronounced than after the end of noisy CR-like stimulation, suggesting that the two stimulation forms exert their tinnitus reducing effect by different mechanisms. The shorter reduction of the delta band power after the end of the noisy CR-like stimulation would be compatible with the notion that tinnitus masking exerts a tinnitus-suppressing effect by feed forward inhibition, acting on the affected frequency regions (Eggermont and Roberts, 2004, Roberts, 2007) and disrupting the abnormal synchronous neural activity that is believed to underlie tinnitus perception (Eggermont and Roberts, 2004, Roberts, 2007, Roberts et al., 2006, Seki and Eggermont, 2003, Weisz et al., 2007a). This disruption of the abnormal synchronous neural activity subsided within 120 s after the end of the noisy CR-like stimulation. Accordingly, the short term reduction of the delta band power after the noisy CR-like stimulation might reflect a short-term suppression of the abnormal synchronous activity, a phenomenon that is thought to underlie the residual inhibition observed after the use of noisers or maskers and that usually extends seconds or minutes beyond the duration of the stimulation (Roberts, 2007, Tass et al., 2012a, Terry et al., 1983). However, as we mentioned above, noisy CR stimulation differs acoustically from the usually used maskers, specifically, sound generators for tinnitus often present a broadband noise containing energy across all frequencies in the spectrum and these are presented continuously. Specifically, the noisy CR-like stimulus consists of the multiple tones that cover tinnitus spectrum around the matched tinnitus pitch. Thus, various implications for the underlying neural effects cannot be ruled out.
The increase of the alpha oscillatory activity was more pronounced and lasted longer (up to 330 s) after acoustic CR neuromodulation as compared to noisy CR-like stimulation (up to 120 s). Our results are in agreement with studies demonstrating that alpha and delta/theta oscillations tend to be reciprocally related to each other (Knyazev, 2007, Knyazev and Slobodskaya, 2003, Knyazev et al., 2003, Robinson, 2001). As was already mentioned above, recent evidence suggests that enhancement of lower frequency (delta) oscillations along the tonotopic axis is caused by deprivation, i.e., by deafferentation due to hearing loss (Eggermont and Roberts, 2004, Llinas et al., 1999, Llinas et al., 2005, Steriade, 2006, Weisz et al., 2006, Weisz et al., 2007b). However, it is currently debated how the increase of delta synchronization is connected to the reduction of alpha rhythms in tinnitus. Different explanations are conceivable: (1) According to the Synchronization-by-Loss-of-Inhibition-Model model, proposed by Weisz and colleagues, input deprivation caused by hearing loss leads to reduced activity and therefore to an enhancement of delta power in the affected regions along the tonotopic axis (Weisz et al., 2007a). As a consequence of deprived input the activity of inhibitory neurons, normally reflected by alpha activity is also suppressed. (2) Other studies suggested that delta and alpha oscillations may be linked to and generated by two different systems with both systems mutually inhibiting each other (Harmony, 2013, Knyazev and Slobodskaya, 2003). A certain degree of incompatibility was also reported between spindles and delta rhythms (Nunez et al., 1992). Therefore, the increased alpha power in the tinnitus free condition may indicate that a more powerful alpha system exerts a stronger inhibition over the delta system (Knyazev and Slobodskaya, 2003). The pathologically enhanced synchronization of low frequencies in tinnitus that may occur as a result of deafferentation or deprivation (Eggermont and Roberts, 2004, Llinas et al., 1999, Weisz et al., 2006) thus may tip the system toward the low frequency (delta/theta) oscillations that will override usually stronger alpha (Knyazev and Slobodskaya, 2003). These explanations require further investigation in longitudinal studies by investigating, e.g., changes of the hierarchical cross-frequency coupling during and after manipulating the tinnitus sensation by application of various stimulation types.
Gamma activity that could be associated with tinnitus perception on the behavioral level (Van der Loo et al., 2009, Weisz et al., 2007b) was significantly decreased after acoustic CR neuromodulation (20–240 s) and noisy CR-like stimulation (40–100 s). The increase of gamma band power during and shortly after stimulation is in line with previous observations of physiological increases of gamma band power during the presence of external sounds (Crone et al., 2001, Joliot et al., 1994). After the end of stimulation a significant reduction of the gamma power persisted longer after acoustic CR neuromodulation (0–260 s) as compared to the noisy CR-like stimulation (0–110 s). However, between 60 and 120 s after the end of both acoustic CR neuromodulation and the noisy CR-like stimulation the amount of the gamma band power reduction was similar. This is an interesting finding as the amount of the alpha power increase (alpha oscillations are assumed to be related to the ongoing functional inhibition that prevents cell assemblies from spontaneous synchronization) between 60 and 120 s was significantly greater after acoustic CR neuromodulation. Since in tinnitus the increased gamma synchronization is assumed to be related to the chronically decreased level of alpha oscillations and thus reduction of inhibition (Eggermont and Roberts, 2004, Lorenz et al., 2009, Weisz et al., 2007a, Weisz et al., 2007b, Weisz et al., 2011), it is conceivable that after more pronounced restoration of the alpha activity (observed after acoustic CR neuromodulation) and accordingly a normalization of cortical excitability the gamma activity will also normalize to a greater degree. However, after a long history of pathological synchronization, it is likely that the synaptic strength between neurons in the affected auditory cortex region that underlies tinnitus percept increases (Gerstner et al., 1996, Markram, 1997, Weisz et al., 2007b) stabilizing to a certain degree the synchronous gamma band activity. The similar reduction of gamma power between 60 and 120 s after the acoustic CR neuromodulation and the noisy CR-like stimulation thus may indicate that a complete propagation of desynchronization into the gamma band might require longer stimulation using acoustic CR neuromodulation than used in this study. The longer lasting reduction of the gamma power after acoustic CR neuromodulation as compared to the noisy CR-like stimulation nevertheless may have resulted from longer and stronger restoration of the functional inhibition exerted by alpha on the synchronous gamma band activity. This important issue has to be addressed in further studies by investigating the cross-frequency interactions between alpha and gamma bands after application of various stimulation types of different duration.
Parallel to the stimulation-induced changes of oscillatory brain activity, described above, acoustic CR neuromodulation and noisy CR-like stimulation significantly reduced tinnitus loudness and annoyance during stimulation and 2 min after cessation of stimulation. No significant changes of the tinnitus intensity were recorded during and 2 min after the end of the LFR stimulation. In this study the reduction of the tinnitus intensity was similar 2 min after the end of both acoustic CR neuromodulation and noisy CR-like stimulation. This finding is consistent with a previous proof of concept study (Tass et al., 2012a) where noisy CR-like stimulation had only a shorter lasting off–stimulation effect resembling the typical short term effects of noisers or maskers (Roberts, 2007, Terry et al., 1983). Reduction of the tinnitus intensity 2 min after acoustic CR neuromodulation and noisy CR-like stimulation is consistent with the notion that an amelioration of the pathological condition, i.e., a disruption of the abnormal synchronous neural activity, that is believed to underlie tinnitus, leads to a normalization of abnormal cortical rhythms in the tinnitus networks diminishing perceptual salience of tinnitus (Adamchic et al., 2014a, Adjamian et al., 2012, De Ridder et al., 2011, De Ridder et al., 2014, Dohrmann et al., 2007, Kahlbrock and Weisz, 2008, Tass et al., 2012a, Weisz et al., 2007a, Weisz et al., 2007b). The findings presented here are also consistent with studies that reported a reduction of initially enhanced delta/theta and gamma activity and enhancement of initially decreased alpha activity after the induced tinnitus reduction (Adamchic et al., 2013; Adjamian et al., 2012, De Ridder et al., 2011, Kahlbrock and Weisz, 2008).
In a previous proof of concept study, no significant (> 2 h) acoustic placebo induced changes of the oscillatory brain activity, tinnitus loudness or annoyance were observed (Tass et al., 2012a). In consistence with these results, no lasting effects of LFR stimulation were observed in this study, even though the placebo stimulation used in Tass et al. (2012a) and LFR stimulation used in the presented study differ. Both of the above mentioned stimulation types, i.e., the acoustic placebo stimulation (Tass et al., 2012a) and the LFR stimulation have in common that the stimulation tones of both stimulation types were shifted away from the center of the assumed synchronized tinnitus focus (Norena et al., 2002, Seki and Eggermont, 2003, Tass et al., 2012a). The LFR stimulation, used in this study, led to a significant reduction of the delta oscillatory activity for up to 30 s after the end of the stimulation. The short-term reduction of the delta band power after the end of the LFR stimulation, observed in our study, can likely be ascribed to some degree of overlap between the spectra of the used LFR stimulation and tinnitus (Eggermont and Roberts, 2004, Norena et al., 2002, Roberts, 2007, Roberts et al., 2006). In this case a certain amount of excitation might have been induced in the affected tonotopic regions of the auditory cortex by the LFR stimulation tones (Eggermont and Roberts, 2004, Roberts, 2007). This disruption of abnormal synchronous neural activity subsided within 30 s after the end of stimulation.
It is plausible to assume that the degree to which frequency specific synchronized neural activity is interrupted by a feed-forward inhibition affecting the tone specific region of the auditory cortex is related to the unit time for which that tone at this specific frequency is presented to the participant. Specifically this may be relevant for the comparison of the noisy CR-like stimulation and acoustic CR neuromodulation as they differ in this dimension. For the noisy CR-like stimulation there is a reduced likelihood that any one specific tone is presented as within each cycle only 4 out of 12 tones are presented. In our study we cannot assess to which degree synchronized neural activity was interrupted at each specific location related to the single stimulation tones, as we assess compound EEG power changes in the selected cortex region. However, taking into account that changes of the EEG power during both stimulation types, i.e., acoustic CR neuromodulation and noisy CR-like stimulation, were almost indistinguishable from each other, it is unlikely that different changes of the EEG power after stimulation were simply a result of the different repetition rate of each specific tone in both stimulation types.
Another relevant aspect of the stimulation protocols refers to the temporal pattern of stimulus delivery. To which extent is the sequential rather than e.g. coincident application of stimulation tones required to induce clinical and/or electrophysiological acute effects, acute after-effects and long-term effects? To address the specificity of the temporal features of the stimulation pattern, in a forthcoming study one should use an appropriate control condition e.g. with the same tones, but different temporal stimulation pattern, such as coincident periodic stimulation, see (Tass et al., 2009).
Based on the results presented here, mechanisms primarily employing lateral inhibition and, hence, a short-term suppression of the tinnitus-related neuronal synchronization cannot be completely ruled out for acoustic CR neuromodulation and noisy CR-like stimulation. However, even if acoustic CR neuromodulation and noisy CR-like stimulation covered similar frequency ranges around the matched tinnitus pitch and induced similar changes of oscillatory brain activity during stimulation, they differed in the electrophysiological changes after the end of stimulation, which almost rules out that these two forms of stimulation act by a common mechanism. The functional changes after noisy CR-like stimulation, which are distinct from those induced by acoustic CR neuromodulation, indicate that the exact spatiotemporal pattern of stimulation tones matters, even if stimulation tones span similar ranges around the matched tinnitus pitch. In this context it is remarkable that changes of the oscillatory brain activity during acoustic CR neuromodulation and the noisy CR-like stimulation did not differ significantly from each other. These findings are in accordance with recent studies in animals and humans (Tass et al., 2009, Tass et al., 2012a, Tass et al., 2012b) and the theoretical studies on which the CR neuromodulation concept is based (Hauptmann et al., 2007, Popovych et al., 2013, Tass, 2003a, Tass, 2003b, Tass and Hauptmann, 2007, Tass and Hauptmann, 2009, Tass and Majtanik, 2006, Tass and Popovych, 2012). Specifically, in computational studies it was shown that CR neuromodulation induces a long-lasting desynchronization mediated by an unlearning of synaptic connectivity (Hauptmann et al., 2007, Tass and Hauptmann, 2009, Tass and Majtanik, 2006, Tass and Popovych, 2012).
4.4. Conclusions
The changes of the oscillatory brain activity induced by noisy CR-like stimulation differed both in strength and in time course from the changes caused by acoustic CR neuromodulation. Thus, similar acute reductions of tinnitus intensity induced by acoustic CR neuromodulation and noisy CR-like stimulation were not associated with similar electrophysiological acute after-effects. These findings suggest that although the tinnitus reduction resulting from acoustic CR neuromodulation and the noisy CR-like stimulation may be subjectively similar, the two stimulations may not be physiologically equivalent. This qualitative difference casts new light on the assertion that CR specific disruption of synchronous neural activity in auditory structures lies at the basis of the long-term effects produced by acoustic CR neuromodulation. With its similar auditory characteristics but the lacking desynchronizing effect both LFR stimulation and noisy CR-like stimulation may represent appropriate control conditions in future clinical studies investigating clinical effects of acoustic CR neuromodulation.
Potential conflict of interest
Ilya Adamchic was an employee at Jülich Research Center during this study. Since then he left Jülich Research Center and is currently working for Vivantes GmbH; Timea Toth was an employee at Jülich Research Center during this study. Since then she left Jülich Research Center and is currently working for HNO-Praxis Langenthal, Switzerland. Berthold Langguth received honoraria and speakers' fee from ANM, Astra Zeneca, Autifony, Lundbeck, Merz, Magventure, Novartis, Pfizer and Servier, research funding from the Tinnitus Research Initiative, the German Research Foundation, the German Bundesministerium für Bildung und Forschung, the American Tinnitus Association, Astra Zeneca and Cerbomed, funding for equipment from Magventure and travel and accommodation payments from Medtronic, Lilly, Servier and Pfizer. Ingrid Klingmann received consulting fees from Jülich Research Center and Actavis Group as well as research funding from two IMI projects. Christian Hauptmann was an employee at Jülich Research Center during this study and the initial writing of the paper. Since then he left Jülich Research Center and is currently an employee of Desyncra Operating GmbH, Bad Neuenahr. Prior to resubmission of this manuscript Peter Alexander Tass was an institute's director at Jülich Research Center, professor at Cologne University and consulting professor at Stanford University. He is now a professor at Stanford University. He is the main inventor of a patent portfolio for acoustic CR neuromodulation owned by Jülich Research Center and Stanford University.
Role of funding source
The study was funded by an institutional grant from the Helmholtz Society (POF). The funding source was not involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
The following are the supplementary data related to this article.
Supplementary Fig. 2.
Supplementary material
Acknowledgements
We are grateful to Natalie Schlothauer for performing the EEG recordings and to Prof. Hartmut Meister for fruitful discussions.
Contributor Information
Martin Walger, Email: martin.walger@uni-koeln.de.
Berthold Langguth, Email: Berthold.Langguth@medbo.de.
Ingrid Klingmann, Email: iklingmann@pharmaplex.be.
Peter Alexander Tass, Email: ptass@stanford.edu.
References
- Adamchic I., Langguth B., Hauptmann C., Tass P.A. Psychometric evaluation of visual analog scale for the assessment of chronic tinnitus. Am. J. Audiol. 2012;21(2):215–225. doi: 10.1044/1059-0889(2012/12-0010). [DOI] [PubMed] [Google Scholar]
- Adamchic I., Toth T., Hauptmann C., Tass P.A. Reversing pathologically increased EEG power by acoustic coordinated reset neuromodulation. Hum. Brain Mapp. 2014;35:2099–2118. doi: 10.1002/hbm.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamchic I., Hauptmann C., Barnikol U.B., Pawelcyk N., Popovych O.V., Barnikol T., Silchenko A.N., Volkmann J., Deuschl G., Meissner W., Maarouf M., Sturm V., Freund H.-J., Tass P.A. Coordinated reset has lasting aftereffects in patients with Parkinson's disease. Mov. Disord. 2014;29:1679. doi: 10.1002/mds.25923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjamian P., Sereda M., Zobay O., Hall D.A., Palmer A.R. Neuromagnetic indicators of tinnitus and tinnitus masking in patients with and without hearing loss. J. Assoc. Res. Otolaryngol. 2012;13(5):715–731. doi: 10.1007/s10162-012-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- Bliss T.V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. Jul 1973;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka L., Brockmann A. Perception space — the final frontier. PLoS Biol. 2005;4:564–568. doi: 10.1371/journal.pbio.0030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone N.E., Boatman D., Gordon B., Hao L. Induced electrocorticographic gamma activity during auditory perception. Clin. Neurophysiol. 2001;112(4):565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- De Ridder D., Van der Loo E., Vanneste S., Gais S., Plazier M., Kovacs S., Sunaert S., Menovsky T., Van de Heyning P. Theta-gamma dysrhythmia and auditory phantom perception. J. Neurosurg. 2011;114(4):912–921. doi: 10.3171/2010.11.JNS10335. [DOI] [PubMed] [Google Scholar]
- De Ridder D., Vanneste S., Weisz N., Londero A., Schlee W., Elgoyhen A.B., Langguth B. An integrative model of auditory phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neurosci. Biobehav. Rev. 2014;44:16–32. doi: 10.1016/j.neubiorev.2013.03.021. [DOI] [PubMed] [Google Scholar]
- De Ridder D., Vanneste S., Langguth B., Llinas R. Thalamocortical dysrhythmia: a theoretical update in tinnitus. Front. Neurol. 2015;6:124. doi: 10.3389/fneur.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Sejnowski T., Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage. 2007;34(4):1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrmann K., Elbert T., Schlee W., Weisz N. Tuning the tinnitus percept by modification of synchronous brain activity. Restor. Neurol. Neurosci. 2007;25(3–4):371–378. [PubMed] [Google Scholar]
- Eggermont J.J. Correlated neural activity as the driving force for functional changes in auditory cortex. Hear. Res. 2007;229(1–2):69–80. doi: 10.1016/j.heares.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Eggermont J.J., Roberts L.E. The neuroscience of tinnitus. Trends Neurosci. 2004;27(11):676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Eggermont J.J., Tass P.A. Maladaptive neural synchrony in tinnitus: origin and restoration. Front. Neurol. 2015;6:29. doi: 10.3389/fneur.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen A.B., Langguth B., De Ridder D., Vanneste S. Tinnitus: perspectives from human neuroimaging. Nat. Rev. Neurosci. 2015;16:632–642. doi: 10.1038/nrn4003. [DOI] [PubMed] [Google Scholar]
- Gerstner W., Kempter R., Van Hemmen J.L., Wagner H. A neuronal learning rule for sub-millisecond temporal coding. Nature. 1996;383(6595):76–78. doi: 10.1038/383076a0. [DOI] [PubMed] [Google Scholar]
- Goebel G., Hiller W. Hogrefe; Goettingen: 1993. Tinnitus Fragebogen (TF): Ein Instrument zur Erfassung von Belastung und Schweregrad bei Tinnitus; p. 90. [Google Scholar]
- Hallam R., Rachmann S., Hinchcliffe R. Psychological aspects of tinnitus. In: Rachmann S., editor. Contributions to Medical Psychology. Vol. 3. Pergamon Press; Oxford: 1984. pp. 31–54. [Google Scholar]
- Hamalainen M., Hari R., Ilmoniemi R.J., Knuutila J., Lounasmaa O.V. Magnetoencephalography-theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 1993;65(2):413–497. [Google Scholar]
- Harmony T. The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 2013;7:83. doi: 10.3389/fnint.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann C., Tass P.A. Therapeutic rewiring by means of desynchronizing brain stimulation. Biosystems. 2007;89(1–3):173–181. doi: 10.1016/j.biosystems.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Hauptmann C., Tass P.A. Cumulative and after-effects of short and weak coordinated reset stimulation: a modeling study. J. Neural Eng. 2009;6(1) doi: 10.1088/1741-2560/6/1/016004. (016004) [DOI] [PubMed] [Google Scholar]
- Hauptmann C., Popovych O.V., Tass P.A. Desynchronizing the abnormally synchronized neural activity in the subthalamic nucleus: a modeling study. Expert Rev. Med. Devices. 2007;4(5):633–650. doi: 10.1586/17434440.4.5.633. [DOI] [PubMed] [Google Scholar]
- Hebb D.O. Wiley & Sons; New York: 1949. The Organization of Behavior. [Google Scholar]
- Hübener M., Bonhoeffer T. Neuronal plasticity: beyond the critical period. Cell. 2014;159:727–737. doi: 10.1016/j.cell.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Irvine D.R., Rajan R., Brown M. Injury- and use-related plasticity in adult auditory cortex. Audiol. Neurootol. 2001;6(4):192–195. doi: 10.1159/000046831. [DOI] [PubMed] [Google Scholar]
- Joliot M., Ribary U., Llinas R. Human oscillatory brain activity near 40 Hz coexists with cognitive temporal binding. Proc. Natl. Acad. Sci. U. S. A. 1994;91(24):11748–11751. doi: 10.1073/pnas.91.24.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlbrock N., Weisz N. Transient reduction of tinnitus intensity is marked by concomitant reductions of delta band power. BMC Biol. 2008;6:4. doi: 10.1186/1741-7007-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass D.W., Daly D.D. Raven Press; 1979. Current Practice of Clinical Electroencephalography. [Google Scholar]
- Knyazev G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007;31(3):377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G., Slobodskaya H.R. Personality trait of behavioral inhibition is associated with oscillatory systems reciprocal relationships. Int. J. Psychophysiol. 2003;48(3):247–261. doi: 10.1016/s0167-8760(03)00072-2. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G., Slobodskaya H.R., Safronova M.V., Sorokin O.V., Goodman R., Wilson G.D. Personality, psychopathology and brain oscillations. Personal. Individ. Differ. 2003;35(6):1331–1349. [Google Scholar]
- Llinas R.R., Ribary U., Jeanmonod D., Kronberg E., Mitra P.P. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. U. S. A. 1999;96(26):15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R.R., Urbano F.J., Leznik E., Ramirez R.R., Van Marle H.J. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28(6):325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Lockwood A.H., Salvi R.J., Burkard R.F. Tinnitus. N. Engl. J. Med. 2002;347(12):904–910. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- Lorenz I., Muller N., Schlee W., Hartmann T., Weisz N. Loss of alpha power is related to increased gamma synchronization-a marker of reduced inhibition in tinnitus? Neurosci. Lett. 2009;453(3):225–228. doi: 10.1016/j.neulet.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Lysyansky B., Popovych O.V., Tass P.A. Desynchronizing anti-resonance effect of the m: n ON-OFF coordinated reset stimulation. J. Neural Eng. 2011;8 doi: 10.1088/1741-2560/8/3/036019. [DOI] [PubMed] [Google Scholar]
- Lysyansky B., Popovych O.V., Tass P.A. Optimal number of stimulation contacts for coordinated reset neuromodulation. Front. Neuroeng. 2013;6:5. doi: 10.3389/fneng.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275(5297):213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Moller A.R. Pathophysiology of tinnitus. Otolaryngol. Clin. N. Am. 2003;36(2):249–266. doi: 10.1016/s0030-6665(02)00170-6. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E., Da Silva F.H.L. Williams & Wilkins; 1999. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. [Google Scholar]
- Norena A.J., Eggermont J.J. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear. Res. 2003;183(1–2):137–153. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Norena A.J., Micheyl C., Chery-Croze S., Collet L. Psychoacoustic characterization of the tinnitus spectrum: implications for the underlying mechanisms of tinnitus. Audiol. Neurootol. 2002;7(6):358–369. doi: 10.1159/000066156. [DOI] [PubMed] [Google Scholar]
- Nunez P.L. Oxford University Press; 1981. Electric Fields of the Brain: The Neurophysics of EEG. [Google Scholar]
- Nunez A., Dossi R.C., Contreras D., Steriade M. Intracellular evidence for incompatibility between spindle and delta-oscillations in thalamocortical neurons of cat. Neuroscience. 1992;48(1):75–85. doi: 10.1016/0306-4522(92)90339-4. [DOI] [PubMed] [Google Scholar]
- Ochi K., Eggermont J.J. Effects of quinine on neural activity in cat primary auditory cortex. Hear. Res. 1997;105(1–2):105–118. doi: 10.1016/s0378-5955(96)00201-8. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Popovych O.V., Tass P.A. Desynchronizing electrical and sensory coordinated reset neuromodulation. Front. Hum. Neurosci. 2012;6:58. doi: 10.3389/fnhum.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovych O.V., Yanchuk S., Tass P.A. Self-organized noise resistance of oscillatory neural networks with spike timing-dependent plasticity. Sci. Rep. 2013;3:2926. doi: 10.1038/srep02926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyragas K., Novicenko V., Tass P.A. Mechanism of suppression of spontaneous low-frequency oscillations in high-frequency stimulated neurons. Biol. Cybern. 2013;107:669–684. doi: 10.1007/s00422-013-0567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L.E. Tinnitus: Pathophysiology and Treatment. Vol. 166. 2007. Residual inhibition; pp. 487–495. [Google Scholar]
- Roberts L.E., Moffat G., Bosnyak D.J. Residual inhibition functions in relation to tinnitus spectra and auditory threshold shift. Acta Otolaryngol. 2006;126:27–33. doi: 10.1080/03655230600895358. [DOI] [PubMed] [Google Scholar]
- Roberts L.E., Moffat G., Baumann M., Ward L.M., Bosnyak D.J. Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J. Assoc. Res. Otolaryngol. 2008;9(4):417–435. doi: 10.1007/s10162-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.L. How brain arousal systems determine different temperament types and the major dimensions of personality. Personal. Individ. Differ. 2001;31(8):1233–1259. [Google Scholar]
- Scherg M., Ille N., Bornfleth H., Berg P. Advanced tools for digital EEG review: virtual source montages, whole-head mapping, correlation, and phase analysis. J. Clin. Neurophysiol. 2002;19(2):91–112. doi: 10.1097/00004691-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Seki S., Eggermont J.J. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear. Res. 2003;180(1–2):28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Shore S.E., Roberts L.E., Langguth B. Maladaptive plasticity in tinnitus - triggers, mechanisms and treatment. Nat. Rev. Neurol. 2016;12:150–160. doi: 10.1038/nrneurol.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silchenko A.N., Adamchic I., Hauptmann C., Tass P.A. Impact of acoustic coordinated reset neuromodulation on effective connectivity in a neural network of phantom sound. NeuroImage. 2013;77C:133–147. doi: 10.1016/j.neuroimage.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Snow J.B. BC Decker; 2004. Tinnitus: Theory and Management. [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137(4):1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Tass P.A. Springer-Verlag; Berlin: 1999. Phase Resetting in Medicine and Biology: Stochastic Modelling and Data Analysis. [Google Scholar]
- Tass P.A. Desynchronization of brain rhythms with soft phase-resetting techniques. Biol. Cybern. 2002;87(2):102–115. doi: 10.1007/s00422-002-0322-5. [DOI] [PubMed] [Google Scholar]
- Tass P.A. A model of desynchronizing deep brain stimulation with a demand-controlled coordinated reset of neural subpopulations. Biol. Cybern. 2003;89(2):81–88. doi: 10.1007/s00422-003-0425-7. [DOI] [PubMed] [Google Scholar]
- Tass P.A. Desynchronization by means of a coordinated reset of neural sub-populations. Prog. Theor. Phys. Suppl. 2003;150:281–296. [Google Scholar]
- Tass P.A. 2006. Device for desynchronizing neuronal brain activity. (International patent application WO 2006/063558, EP 1 827 586 B1) [Google Scholar]
- Tass P.A., Hauptmann C. Long-term anti-kindling effects induced by short-term, weak desynchronizing stimulation. Nonl. Phen. Compl. Syst. 2006;9:298–312. [Google Scholar]
- Tass P.A., Hauptmann C. Therapeutic modulation of synaptic connectivity with desynchronizing brain stimulation. Int. J. Psychophysiol. 2007;64(1):53–61. doi: 10.1016/j.ijpsycho.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Tass P.A., Hauptmann C. Anti-kindling achieved by stimulation targeting slow synaptic dynamics. Restor. Neurol. Neurosci. 2009;27(6):589–609. doi: 10.3233/RNN-2009-0484. [DOI] [PubMed] [Google Scholar]
- Tass P.A., Majtanik M. Long-term anti-kindling effects of desynchronizing brain stimulation: a theoretical study. Biol. Cybern. 2006;94(1):58–66. doi: 10.1007/s00422-005-0028-6. [DOI] [PubMed] [Google Scholar]
- Tass P.A., Popovych O.V. Unlearning tinnitus-related cerebral synchrony with acoustic coordinated reset stimulation: theoretical concept and modelling. Biol. Cybern. 2012;106(1):27–36. doi: 10.1007/s00422-012-0479-5. [DOI] [PubMed] [Google Scholar]
- Tass P.A., Silchenko A.N., Hauptmann C., Barnikol U.B., Speckmann E.J. Long-lasting desynchronization in rat hippocampal slice induced by coordinated reset stimulation. Phys. Rev. E. 2009;80(1) doi: 10.1103/PhysRevE.80.011902. [DOI] [PubMed] [Google Scholar]
- Tass P.A., Adamchic I., Freund H.-J., Von Stackelberg T., Hauptmann C. Counteracting tinnitus by acoustic coordinated reset neuromodulation. Restor. Neurol. Neurosci. 2012;30(2):137–159. doi: 10.3233/RNN-2012-110218. [DOI] [PubMed] [Google Scholar]
- Tass P.A., Qin L., Hauptmann C., Dovero S., Bezard E., Boraud T., Meissner W.G. Coordinated reset has sustained after-effects in Parkinsonian monkeys. Ann. Neurol. 2012;72(5):816–820. doi: 10.1002/ana.23663. [DOI] [PubMed] [Google Scholar]
- Terry A.M., Jones D.M., Davis B.R., Slater R. Parametric studies of tinnitus masking and residual inhibition. Br. J. Audiol. 1983;17(4):245–256. doi: 10.3109/03005368309081485. [DOI] [PubMed] [Google Scholar]
- Tunkel D.E., Bauer C.A., Sun G.H., Rosenfeld R.M., Chandrasekhar S.S., Cunningham E.R., Jr., Archer S.M., Blakley B.W., Carter J.M., Granieri E.C., Henry J.A., Hollingsworth D., Khan F.A., Mitchell S., Monfared A., Newman C.W., Omole F.S., Phillips C.D., Robinson S.K., Taw M.B., Tyler R.S., Waguespack R., Whamond E.J. Clinical practice guideline: tinnitus. Otolaryngol. Head Neck Surg. 2014;151(2 Suppl):1–40. doi: 10.1177/0194599814545325. [DOI] [PubMed] [Google Scholar]
- Van der Loo E., Gais S., Congedo M., Vanneste S., Plazier M., Menovsky T., Van de Heyning P., De Ridder D. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One. 2009;4(10) doi: 10.1371/journal.pone.0007396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Nebeck S., Muralidharan J., Vitek J.L., Baker K.B. Coordinated reset deep brain stimulation of subthalamic nucleus produces long-lasting, dose-dependent motor improvements in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine non-human primate model of parkinsonism. Brain Stimul. 2016;9:609–617. doi: 10.1016/j.brs.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N., Moratti S., Meinzer M., Dohrmann K., Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005;2(6) doi: 10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N., Hartmann T., Dohrmann K., Schlee W., Norena A. High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hear. Res. 2006;222(1–2):108–114. doi: 10.1016/j.heares.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Weisz N., Dohrmann K., Elbert T. Tinnitus: Pathophysiology and Treatment. Vol. 166. 2007. The relevance of spontaneous activity for the coding of the tinnitus sensation; pp. 61–70. [DOI] [PubMed] [Google Scholar]
- Weisz N., Muller S., Schlee W., Dohrmann K., Hartmann T., Elbert T. The neural code of auditory phantom perception. J. Neurosci. 2007;27(6):1479–1484. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N., Hartmann T., Muller N., Lorenz I., Obleser J. Alpha rhythms in audition: cognitive and clinical perspectives. Front. Psychol. 2011;2:73. doi: 10.3389/fpsyg.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material