Abstract
Objective
Chronic, persistent infections complicate otologic procedures utilizing implantable devices such as cochlear implants or tympanostomy tubes. These infections are thought to be due to the establishment of microbial biofilms on implant surfaces. To address this issue, we hypothesized that surface charge modification may inhibit the formation of Pseudomonas aeruginosa biofilms on implant surfaces in vitro and in vivo.
Study Design
We evaluated the effect of surface charge modification on bacterial biofilm formation by assessing the effect of the surface charge on bacterial adhesion in vitro and bacterial persistence in vivo.
Methods
To study the effect of surface charge in vitro, the surface wells in culture plates were modified using a layer-by-layer polyelectrolyte assembly method. Bacterial adherence was measured at 30, 60 and 120-minute intervals. To study the effect of surface charge modification in vivo, the surface of titanium micro-screws was similarly modified and then surgically implanted into the dorsal calvaria of adult rats and inoculated with bacteria. Two weeks after implantation and inoculation, the number of bacteria remaining in vivo was evaluated.
Results
Surface charge modification results in a significant decrease in adherence of bacteria in vitro. Surface charge modification of titanium micro-screw implants also resulted in a significant decrease in P. aeruginosa recovered two weeks after surgical implantation.
Conclusion
Charge modification decreases the number of bacteria adherent to a surface in vitro and also decreases the risk and severity of implant infection in an in vivo rat infection model. These results have promising biomedical applications.
Keywords: Surface modification, biofilm, P. aeruginosa, infection, persistence
Introduction
Persistent and recalcitrant infections involving biofilms on otologic implants can cause significant morbidity. For example, cochlear implants can become infected by a variety of bacterial species, most commonly, Staphylococcus aureus but also gram negative species including Pseudomonas aeruinosa1-6. Likewise, biofilms on tympanostomy tubes have been associated with post-tympanostomy tube otorrhea and plugging7. Persistent infections on implanted devices are not unique to otologic implants. There are about 2 million nosocomial infections per year in the USA, which cost roughly $11 billion8,9 and about half of these case are associated with indwelling devices10,11. The morbidity associated with surgical implant infection is great, often requiring repeated surgical procedures, eventual explantation, and reimplantation11. The number of implant associated infections and the economic impact of these infections are bound to increase as life expectancy continues to rise worldwide and as the use of biomedical implants grow12.
Persistent colonization of surgical implants by bacteria often requires formation of a biofilm11,13. Biofilms are communities of microorganisms, adhered to a surface that are encased in a complex exopolysaccharide matrix14. In biofilm form, bacteria are particularly resistant to eradication with antibiotics and are protected from the host immune system15,16,17. Bacterial adhesion is the first step in biofilm formation as it signals the production of extracellular polymeric substances (EPS) that assemble and form a biofilm18-21. Immediately after implantation there is a critical time period, typically the first six hours, during which successful bacterial colonization can occur22,23. At this time, the introduced pathogens are often metabolically active and susceptible to the host immune system. However, if adhesion occurs, biofilms can form and become highly resistant12. Thus, prevention of adhesion has been a popular area of study and regarded as the most critical step in preventing implant associated infections12.
For this reason, numerous studies have tested the ability of surface modifications to prevent adherence of bacteria in vitro. Many surface properties have been reported to affect adhesion, including surface hydrophobicity, hydrophilicity, roughness, charge, and potential24,25,26-31. This has led to testing the effect of surface abrasions25, chemical coatings32 and chemical graftings26-29,33,34. One method to modify surface charge is polyelectrolyte layer-by-layer (LbL) assembly, which involves the alternate adsorption of oppositely charged polyelectrolytes. LbL assembly is practical due to its simplicity and repeatability35, and offers excellent control over the thickness of the polyelectrolyte (PEM) down to ~1 nm36.
In this study, we tested the effect of surface charge modification on adhesion to simulate the colonization of bacteria on biomedical implants. Employing LbL assembly, positively charged poly(allylamine hydrochloride) (PAH) and negatively charged poly(styrenesulfonate) (PSS) were used to alter the surface charge. We studied the effect of surface charge modification on two commonly studied strains of P. aeruginosa, PAO1 and PA14, as well as three otopathogenic strains of P. aeruginosa, OPPA 8, 13 and 1537. We followed this with an in vivo study of bacterial adherence and persistence using charge modified titanium screws in a rat model.
Materials/Methods
In Vitro Studies
Bacterial Strains
We used two common laboratory strains of P. aeruginosa, PAO1 and PA14, as well as several otopathogenic strains of P. aeruginosa (OPPA 8, 13, and 15) obtained from surgically resected cholesteatomas37. PAO1 was obtained from Dr. Colin Manoil (University of Washington) and PA14 was obtained from Dr. Stephen Lory (Harvard University). The strains were maintained in 80% glycerol at 80°C, inoculated into LB broth and grown to log phase at 37°C, 225 rpm for 16-18 hours. The absorbance of the cultures was measured at an optical density of 600 nm and adjusted to an OD of 3.0 at 600 nm.
Surface Charge Modification of 24 well plates
A 24-well cell culture plate was charge modified (CellStar 24W suspension multiwall plate, Greiner Bio One, Austria) using LbL assembly36. The culture plates were immersed in 10 mM PSS in 0.1 M NaCl aqueous solution for 15 minutes followed by rinsing with nanopure water for 30 sec and rinsing with 0.1 M NaCl solution for an additional 30 seconds. Then the plates were immersed in a solution of 10mM PAH in 0.1 M NaCl for 15 minutes followed by the rinsing procedure described above thus creating the first polyelectrolyte bilayer. The thickness of each polyelectrolyte bilayer is estimated to be ~2nm36. Three bilayers were assembled for surface charge modification in this study38 and successful charge modification was confirmed by binding the plates with negatively charged gold nanorods with UV-vis extinction spectra resonance wavelength between 509-514 nm36,39. The UV-vis spectra of these gold nanorods was then measured using a Shimadzu UV-1800 UV-vis spectrometer (Kyoto, Japan).
Adhesion Assay40
Bacterial cultures were diluted 1:1000 in sterile M63 minimal media supplemented with MgCl2 (1mM), D-glucose (0.2%) and Casamino acids (0.5%)41. One milliliter of the bacterial suspension was aliquoted into each well of an unmodified, positively modified, and negatively modified 24-well polystyrene cell culture plate and incubated at 30°C for 30 min, 1 hour and 2 hours. The plates were washed with water to remove non-adherent cells, dried overnight and stained with 0.1% crystal violet in 12% ethanol. Adherent bacteria were imaged under phase contrast microscopy (Nikon Eclipse TE2000-U, Tokyo, Japan) at 20x magnification and images were taken (analySIS® Soft Imaging System GmbH, Munster, Germany). For imaging, a paper stencil was made for each well containing 52 numbered viewing spots. A random set of 30 numbers from 1-52 was generated and a total of 60 images were obtained for each bacterial strain at each time point. The images were uploaded into ImageJ® and bacterial counts were obtained40.
In vitro biofilm assay of PAO1 on Unmodified and Charge Modified Surfaces41
PAO1 bacterial cultures were diluted 1:1000 fold in M63 media41. One milliliter of this bacterial suspension was aliquoted into unmodified, positively and negatively charged wells of a 24-well cell culture plate. The plates were covered with a diffusion membrane (Diversified Biotech, Dedham, MA) and incubated at 30°C for 48 hours. After 48 hours, the plates were washed, dried overnight and stained with 0.1% crystal violet in 12% ethanol for 10 minutes. The crystal violet was then solubilized in 33% glacial acetic acid and the absorbance at 595 nm measured using a SynergyHT Multi-Detection microplate reader (Bio-Tec Instruments, Inc, Winooski, VT, USA).
In Vivo Studies
Animal Design and Rationale
Twenty-four six week old, male Sprague Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN). Rats were chosen as a model because of the thickness of their dorsal calvarium, which measures 0.71 ± 0.03 mm42, allowing for stable implantation of the screw and retention of the implant. The animals weighed 164 g ± 10 g. Eight animals were designated into three groups and received an unmodified, positively or negatively modified implant. Upon implantation, 3.8 × 106 bacteria in 10 microliters were spotted onto each implant surface to establish an infection. This study was approved by the Washington University Animal Studies Committee.
Bacteria Preparation for Implant Infection
PAO1 bacterial cultures were diluted 1:1000 in M63 minimal media41. One hundred microliters of this prepared bacterial suspension was aliquoted into wells of a 96-well plate (Corning® 96 well clear flat bottom polyvinyl chloride 96 well plate, Corning Incorporated, Corning, NY). The plate was covered with a diffusion membrane and incubated at 30°C for 48 hours. Following incubation, the biofilms were serially diluted in PBS and plated on LB-agar to determine CFUs. The bacteria were diluted 1:100 fold in PBS and spotted onto the implant surface during sterile surgical implantation of titanium implants.
Preparation of Surface Modified Titanium Implants
Sterile titanium screws measuring 1/16 inch (Antrin Miniature Specialties Inc., Fallbrook, CA) were charge modified using the LbL assembly method. A total of six polyelectrolyte bilayers were assembled. To confirm charge, negatively charged Alexafluor® 350 hydrazide sodium salt dissolved in 200mM NaCl to a final concentration of 1mM (Molecular Proves, Eugene, OR, USA) was bound to unmodified, positively modified and negatively modified screws followed by rinsing with water. The screws were imaged on a Leica optical microscope (DM4000M) (Leica Camera, Wetzlar, Germany) using a filter with excitation range 340-380 nm and emission wavelength of 425 nm.
In vivo Implantation
Following induction and maintenance of anesthesia by continuous 0.5-4% isoflurane administration, hair was removed from the dorsal scalp and 70% ethanol was applied followed by application of a povidone-iodine topical solution (Betadine Purdue Products, Stamford, CT, USA) at the surgical site. A 7.5 mm vertical incision was made in the scalp between the ears and a flap was raised on either side of the incision to expose the periosteum. The periosteum was excised and a hole was made anterolateral to the confluence of the transverse and superior sagittal sinuses using a Healthco Dental Engine drill (Healthco, Inc, Model NCL-35SH/H, Tualatin, OR) fitted with a 1.18 mm diamond burr (SS White, Lakewood, NJ). A sterile titanium screw (unmodified, positively or negatively modified) was then screwed into the hole. Ten microliters containing 3.8 × 106 CFUs of PAO1 in sterile PBS was spotted onto the surface of the implant. The bacteria were allowed to adhere for 1 minute and then the wound was closed. Two weeks following implantation, the rats were euthanized, the implants were recovered, and the tissue overlying the implants was harvested and homogenized in 1ml of sterile PBS using a Virtis 278077 Cyclone Virtishear Tissue Homogenizer Mixer (Virtis, Chicago, IL). The homogenized sample was serially diluted, plated onto LB agar plates, incubated at 37°C for 18 hours and CFUs were determined.
Statistics
For the in vitro cell counts, means were calculated and analysis of variance (ANOVA) was completed in Excel for significance and variation among means. The analysis of persistent bacteria (CFUs) in the in vivo study was performed using the Wilcoxon rank sum test to compare the unmodified and charge modified groups (MATLAB, Mathworks, Natick, MA, USA).
Results
Surface Charge Modification of 24 Well Plates and Titanium Screws
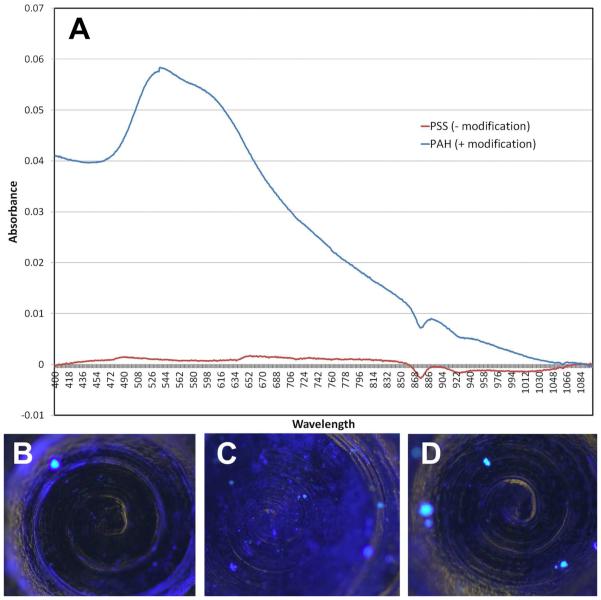
To study the effect of surface charge modification on bacterial adherence and biofilm formation, a layer-by-layer assembly method was used to modify the surface charge of polystyrene plates and titanium screws (see Materials/Methods). Successful charge modification of the polystyrene plate surface was confirmed using a gold nanorod (AuNR) assay where only a positively modified surface binds negatively charged AuNRs (Figure 1A). Successful charge modification of the titanium screws was confirmed by immersing the modified screws into a solution of negatively charged Alexafluor® 350 hydrazide sodium salt. Increased binding was observed on a positive surface (Figure 1C) compared to a non-modified screw (Figure 1B) or a negatively charged screw (Figure 1D).
Figure 1. Charge modification of 24 well plates and titanium screws.
24 well polystyrene plates and titanium microscrews were modified using the layer-by-layer polyelectrolyte assembly method. To confirm a charge on plates, they were exposed to negatively charged gold nanorods and examined by UV-vis spectrophotometry. To confirm a charge on the titanium screws, they were exposed to negatively charged hydrazide salt, which appears blue when bound. A) UV-vis absorption spectra of plates exposed to negatively charged gold nanorods. Results for negatively charged plates are indicated by the red line and positively charged plates by the blue line. The gold nanorods have an absorption spectra with peak wavelength between 509-550 nm and will exhibit this on spectrometry. B) An unmodified titanium screw treated with negatively charged hydrazide salt has a low baseline level of binding. C) A positively charged titanium screw bound with negatively charged hydrazide salt (blue) exhibits binding. D) A negatively charged titanium screw treated with negatively charged hydrazide salt has a minimal level of binding.
Adhesion on Charge Modified Surfaces
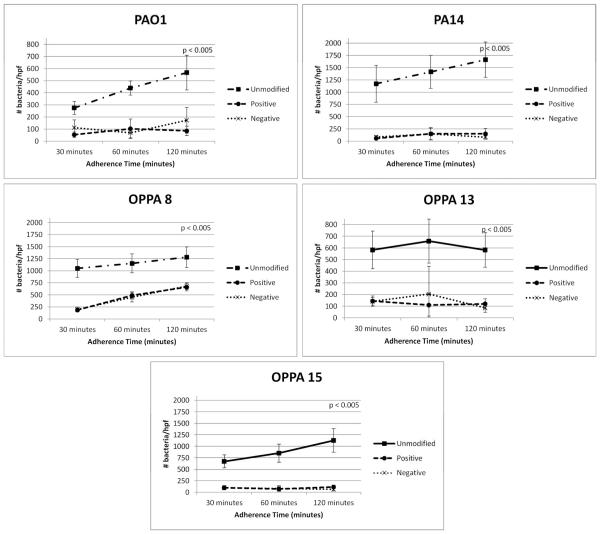
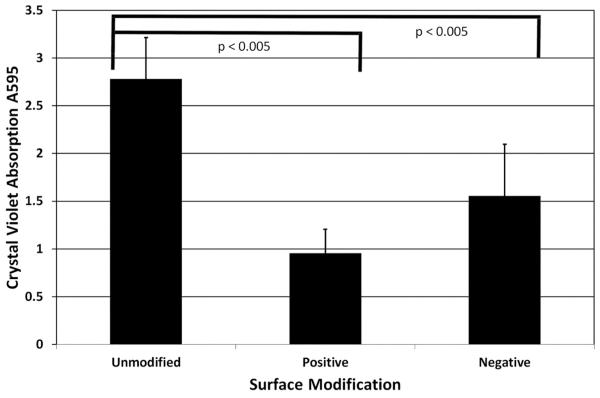
We began our in vitro studies by investigating adhesion of the wild type P. aeruginosa strains PAO1 and PA14 to charge modified polystyrene plates. As previously observed, each strain exhibited increased adhesion over time on an unmodified surface (Figure 2). Both positive and negative charge modification resulted in decreased adherence of both PAO1 and PA14 when compared to unmodified plates (p<0.005). Since P. aeruginosa is also an important pathogen in otologic infections5,37, we also tested adhesion of several otopathogenic strains (OPPA) of P. aeruginosa. Similar to PAO1 and PA14, charge modification of the plates decreased adherence of these strains (p<0.005, Figure 2). We also compared biofilm formation of PAO1 on unmodified versus charge-modified surfaces. Interestingly, PAO1 biofilm formation decreased significantly on a positively (66%, p<0.005) and negatively charged surfaces (44%, p<0.005) (Figure 3).
Figure 2. Adherence of P. aeruginosa strains to unmodified and charge modified surfaces.
Wild type strains of Pseudomonas aeruginosa (PAO1 and PA14) and otopathogenic strains of P. aeruginosa (OPPA 8, 13, 15) were bound to unmodified, positive and negative charge modified plates. The plates were incubated for 30, 60, and 120 minutes, washed and the bacteria were enumerated by microscopy (bacteria/hpf). P-values represent statistical difference between unmodified and modified samples.
Figure 3. PAO1 biofilm formation on charge modified surfaces.
Wild type P. aeruginosa strain PAO1 biofilms were developed on unmodified and charge modified plates. Plates were incubated for 48 hours, washed, stained with crystal violet and the absorbance at OD595 was measured. P-values comparing unmodified versus modified plates were measured.
In vivo Findings
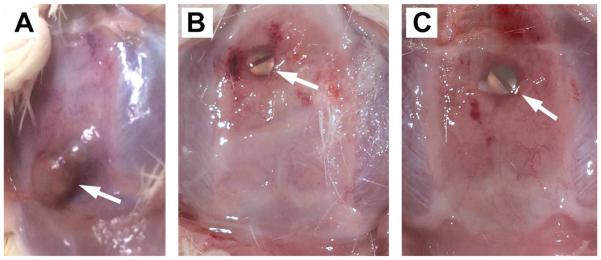
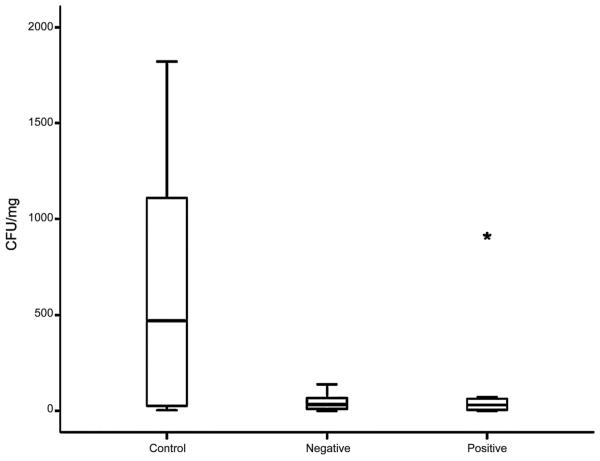
Although our in vitro results were promising, our ultimate goal was to develop an implant modification that could be used clinically to decrease rates of infection. This is critical as most studies examining the effects of modifying implants have only been tested in vitro29,43-45. Our in vivo model consists of implanting a titanium screw into the skull of rats in the presence or absence of P. aeruginosa (see Materials/Methods). All animals survived the implantation and infection without antibiotics and no special care was required. When bacteria were added to the surgical site in the absence of a screw, the infection was cleared indicating that the implant was required for persistent infection. Rat skulls implanted with a screw in the absence of bacteria remained uninfected for the course of the experiment. In contrast, unmodified screws implanted in the presence of P. aeruginosa exhibited noticeable clinical signs of persistent infection two weeks later, including inflammatory changes and the formation of granulation tissue overlying the screw head (Figure 4A). In addition, a significant number of bacteria could be recovered from the tissue overlying the screw (Figure 5). In contrast, we saw a dramatic effect when using charge-modified screws (Figure 4B-C). In those cases, we observed only a translucent, thin layer of tissue overlying the implant consistent with the absence of a persistent infection. In fact, there was a dramatic decrease in the number of bacteria recovered (colony forming units) from the charge-modified groups (Figure 5). Although there was some variability in the distribution of bacteria recovered from the unmodified screws, there was a clear statistical difference in the mean (median) number of bacteria recovered from the unmodified [630 CFU/mg (469 CFU/mg) tissue] versus the negative charged and positive charged groups [45 CFU/mg (32 CFU/mg) tissue and 139 CFU/mg (31 CFU/mg), respectively] (p=0.026, one-tailed Wilcoxon rank sum test). Thus, our in vivo results recapitulated our in vitro results showing a dramatic effect of charge modification on adherence, biofilm formation and persistence during an infection.
Figure 4. Testing of charge modification on titanium screws during an infection in vivo.
Rats were implanted with sterile unmodified or charge modified titanium screws. Prior to closure, a drop of P. aeruginosa was placed on top of the screw, the incision was closed, and the animals were allowed to recover for two weeks. After euthanasia, the skin was removed above the implant and photographs were taken. Photographs are shown for an infected unmodified implant (A), a negatively charged implant (B) and a positively charged implant. The results shown are representative of multiple animals done in three independent experiments.
Figure 5. Distribution of bacteria recovered from unmodified and charge modified implants following infection.
Following 2 weeks of infection, the tissue overlying the screws was harvested, homogenized in Luria Bertani broth, and plated onto LB-agar plates to allow enumeration of colony forming units. Median colony forming units are depicted by the horizontal bar in within each column and error bars indicated the standard deviation. The star around 1000 CFU/mg in the positive group represents an outlier. The results shown are representative of multiple animals done in three independent experiments.
Discussion
Persistent implant related infections are a challenge to medicine and result in increased healthcare costs and patient morbidity and mortality. Thus, designing an implant modification that can decrease the incidence and prevalence of implant related infections is crucial. Using a surface charge modified representative biomedical implant, we observed a decrease in the number of adherent wild type P. aeruginosa for strains PAO1 and PA14. We extended these observations by demonstrating decreased biofilm formation of PAO1 on a charge-modified surface. In addition, we showed lowered numbers of bacteria could be recovered from charge modified screws two weeks after implant infection in a rat model. From these results, we conclude that positive and negative surface charge modifications may lower the risk of implant infection by P. aeruginosa.
In the past year, there have been a number of reports on the effect of various surface modifications on bacterial adhesion, although the results varied. For example, Zhu et al tested surfaces modified with poly(acrylic acid) and poly-(diallyldimethylammonium chloride)43 and found that neutral and negatively charged surfaces decreased Gram-negative bacterial adhesion, possibly due to the negative potential of the bacterial cell wall. Likewise, Gottenbos and colleagues observed that P. aeruginosa exhibited decreased adhesion to a negatively charged surface, however these authors also observed exponential growth of bacteria on these surfaces28. In contrast, Frueh et al reported contradictory results that polyelectrolyte multilayers resulted in decreased Gram negative adhesion on positively modified surfaces rather than negatively charged surfaces44. Similarly, Rzhepishevska and colleagues demonstrated that P. aeruginosa can sometimes adapt its cell surface to better attach to negatively charged surfaces45. As a result, there is little agreement in the literature for the effect of negative and positive charge modification on Gram-negative bacterial adhesion.
Although variable results have been reported, this may be due to the specific chemicals used to achieve the charge modifications. Our method using PAH and PSS consistently reduced adhesion and biofilm formation in vitro. Moreover, in contrast to the above studies, ours is the first to report an effect in vivo. As the ultimate goal of these experiments is to achieve an in vivo effect on a modified medical implant, we believe our model is not only unique but also promising. In addition, our approach provides a method to study the role of biofilm infection on implants as infected rats in the absence of an implant were able to eliminate the infection while the presence of an implant prevented its clearance.
Conclusion
We have demonstrated two types of surface charge modification result in decreased adhesion and biofilm formation by five different strains of P. aeruginosa. Moreover, the effects translated in vivo, where charge modification decreased colonization of implants by P. aeruginosa. These results exhibit promising biomedical applications as refinement of surface modifications can be applied to biomedical implants and indwelling catheters. As more bacteria are developing multidrug resistance46, the application of charged surfaces will likely be a useful adjuvant to decrease recurrent biofilm related infections on implants.
Acknowledgments
We would like to thank Dr. Srikanth Singamaneni and Dr. Limei Tian for their assistance optimizing our surface charge modification methods.
Supported by Grants from the National Institutes of Health. R01 DC 000263-26(RAC) and P30 DC005209-15(RAC) and 5T32DC000022-28 (KWK)
Footnotes
The authors have no conflicts of interest or financial disclosures to report.
Level of Evidence: NA
References
- 1.Vaid N, Vaid S, Manikoth M. Case report - biofilm infection of a cochlear implant. Cochlear Implants Int. 2013;14:117–120. doi: 10.1179/1754762811Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 2.Im GJ, An YS, Choi J, Song JJ, Chae SW, Jung HH. Analysis of Bacterial Biofilms on a Cochlear Implant Following Methicillin-Resistant Staphylococcus Aureus Infection. J Audiol Otol. 2015;19:172–177. doi: 10.7874/jao.2015.19.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinidade A, Rowlands G, Obholzer R, Lavy J. Late skin flap failure following cochlear implantation. Cochlear Implants Int. 2008;9:167–175. doi: 10.1179/cim.2008.9.3.167. [DOI] [PubMed] [Google Scholar]
- 4.Loeffler KA, Johnson TA, Burne RA, Antonelli PJ. Biofilm formation in an in vitro model of cochlear implants with removable magnets. Otolaryngol Head Neck Surg. 2007;136:583–588. doi: 10.1016/j.otohns.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Germiller JA, El-Kashlan HK, Shah UK. Chronic Pseudomonas infections of cochlear implants. Otol Neurotol. 2005;26:196–201. doi: 10.1097/00129492-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Kos MI, Stenz L, Francois P, Guyot JP, Schrenzel J. Immuno-detection of Staphylococcus aureus biofilm on a cochlear implant. Infection. 2009;37:450–454. doi: 10.1007/s15010-008-8335-1. [DOI] [PubMed] [Google Scholar]
- 7.Wang JC, Hamood AN, Saadeh C, Cunningham MJ, Yim MT, Cordero J. Strategies to prevent biofilm-based tympanostomy tube infections. Int J Pediatr Otorhinolaryngol. 2014;78:1433–1438. doi: 10.1016/j.ijporl.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996;17:552–557. doi: 10.1086/647371. [DOI] [PubMed] [Google Scholar]
- 9.Schierholz JM, Beuth J. Implant infections: a haven for opportunistic bacteria. J Hosp Infect. 2001;49:87–93. doi: 10.1053/jhin.2001.1052. [DOI] [PubMed] [Google Scholar]
- 10.Stamm WE. Infections related to medical devices. Ann Intern Med. 1978;89:764–769. doi: 10.7326/0003-4819-89-5-764. [DOI] [PubMed] [Google Scholar]
- 11.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 12.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 13.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 14.Dunne WM., Jr Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev. 2002;15:155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandsberg LF, Ciofu O, Kirkby N, Christiansen LE, Poulsen HE, Hoiby N. Antibiotic resistance in Pseudomonas aeruginosa strains with increased mutation frequency due to inactivation of the DNA oxidative repair system. Antimicrob Agents Chemother. 2009;53:2483–2491. doi: 10.1128/AAC.00428-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyle E, Brenner-Weiss G, Obst U, Prior B, Hansch GM. Immune defense against S. epidermidis biofilms: components of the extracellular polymeric substance activate distinct bactericidal mechanisms of phagocytic cells. Int J Artif Organs. 2012;35:700–712. doi: 10.5301/ijao.5000151. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Martinez JM, Pascual A. Antimicrobial resistance in bacterial biofilms. Rev Med Microbiol. 2006;17:65–75. [Google Scholar]
- 18.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 19.Busscher HJ, van der Mei HC. How do bacteria know they are on a surface and regulate their response to an adhering state? PLoS Pathog. 2012;8:e1002440. doi: 10.1371/journal.ppat.1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher M. Bacterial biofilms and biofouling. Curr Opin Biotechnol. 1994;5:302–306. doi: 10.1016/0958-1669(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 21.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 22.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 23.Poelstra KA, Barekzi NA, Rediske AM, Felts AG, Slunt JB, Grainger DW. Prophylactic treatment of gram-positive and gram-negative abdominal implant infections using locally delivered polyclonal antibodies. J Biomed Mater Res. 2002;60:206–215. doi: 10.1002/jbm.10069. [DOI] [PubMed] [Google Scholar]
- 24.Boks NP, Norde W, van der Mei HC, Busscher HJ. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology. 2008;154:3122–3133. doi: 10.1099/mic.0.2008/018622-0. [DOI] [PubMed] [Google Scholar]
- 25.Morgan TD, Wilson M. The effects of surface roughness and type of denture acrylic on biofilm formation by Streptococcus oralis in a constant depth film fermentor. J Appl Microbiol. 2001;91:47–53. doi: 10.1046/j.1365-2672.2001.01338.x. [DOI] [PubMed] [Google Scholar]
- 26.Gottenbos B, Grijpma DW, van der Mei HC, Feijen J, Busscher HJ. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J Antimicrob Chemother. 2001;48:7–13. doi: 10.1093/jac/48.1.7. [DOI] [PubMed] [Google Scholar]
- 27.Gottenbos B, van der Mei HC, Busscher HJ. Initial adhesion and surface growth of Staphylococcus epidermidis and Pseudomonas aeruginosa on biomedical polymers. J Biomed Mater Res. 2000;50:208–214. doi: 10.1002/(sici)1097-4636(200005)50:2<208::aid-jbm16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Gottenbos B, Van Der Mei HC, Busscher HJ, Grijpma DW, Feijen J. Initial adhesion and surface growth of Pseudomonas aeruginosa on negatively and positively charged poly(methacrylates) J Mater Sci Mater Med. 1999;10:853–855. doi: 10.1023/a:1008989416939. [DOI] [PubMed] [Google Scholar]
- 29.Gottenbos B, van der Mei HC, Klatter F, et al. Positively charged biomaterials exert antimicrobial effects on gram-negative bacilli in rats. Biomaterials. 2003;24:2707–2710. doi: 10.1016/s0142-9612(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 30.Hibiya KT, Hirata S. Formation and characteristics of nitrifying biofilm on a membrane modified with positively-charged polymer chains. Colloids Surf B Biointerfaces. 2000;18:105–112. K. [Google Scholar]
- 31.Terada AY, Tsuneda S, Hirata S, Katakai A, Tamada M. Elucidation of dominant effect on initial bacterial adhesion onto polymer surfaces prepared by radiation-induced graft polymerization. Colloids Surf B biointerfaces. 2005;43:99–107. doi: 10.1016/j.colsurfb.2005.03.016. M. [DOI] [PubMed] [Google Scholar]
- 32.Tiller JC, Liao CJ, Lewis K, Klibanov AM. Designing surfaces that kill bacteria on contact. Proc Natl Acad Sci U S A. 2001;98:5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terada A, Yuasa A, Kushimoto T, Tsuneda S, Katakai A, Tamada M. Bacterial adhesion to and viability on positively charged polymer surfaces. Microbiology. 2006;152:3575–3583. doi: 10.1099/mic.0.28881-0. [DOI] [PubMed] [Google Scholar]
- 34.Jansen B, Kohnen W. Prevention of biofilm formation by polymer modification. J Ind Microbiol. 1995;15:391–396. doi: 10.1007/BF01569996. [DOI] [PubMed] [Google Scholar]
- 35.Decher G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science. 1997;227:1232–1237. [Google Scholar]
- 36.Tian L, Chen E, Gandra N, Abbas A, Singamaneni S. Gold nanorods as plasmonic nanotransducers: distance-dependent refractive index sensitivity. Langmuir. 2012;28:17435–17442. doi: 10.1021/la3034534. [DOI] [PubMed] [Google Scholar]
- 37.Wang EW, Jung JY, Pashia ME, Nason R, Scholnick S, Chole RA. Otopathogenic Pseudomonas aeruginosa strains as competent biofilm formers. Arch Otolaryngol Head Neck Surg. 2005;131:983–989. doi: 10.1001/archotol.131.11.983. [DOI] [PubMed] [Google Scholar]
- 38.Kedem O, Tesler AB, Vaskevich A, Rubinstein I. Sensitivity and optimization of localized surface plasmon resonance transducers. ACS Nano. 2011;5:748–760. doi: 10.1021/nn102617d. [DOI] [PubMed] [Google Scholar]
- 39.Tian L, Fei M, Kattumenu R, Abbas A, Singamaneni S. Gold nanorods as nanotransducers to monitor the growth and swelling of ultrathin polymer films. Nanotechnology. 2012;23:255502. doi: 10.1088/0957-4484/23/25/255502. [DOI] [PubMed] [Google Scholar]
- 40.Vesterlund S, Paltta J, Karp M, Ouwehand AC. Measurement of bacterial adhesion-in vitro evaluation of different methods. Journal of microbiological methods. 2005;60:225–233. doi: 10.1016/j.mimet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328:627–629. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Reilly MA, Muller A, Hynynen K. Ultrasound insertion loss of rat parietal bone appears to be proportional to animal mass at submegahertz frequencies. Ultrasound Med Biol. 2011;37:1930–1937. doi: 10.1016/j.ultrasmedbio.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, Janczewski D, Guo S, et al. Polyion multilayers with precise surface charge control for antifouling. ACS applied materials & interfaces. 2015;7:852–861. doi: 10.1021/am507371a. [DOI] [PubMed] [Google Scholar]
- 44.Frueh J, Gai M, Yang Z, He Q. Influence of polyelectrolyte multilayer coating on the degree and type of biofouling in freshwater environment. Journal of nanoscience and nanotechnology. 2014;14:4341–4350. doi: 10.1166/jnn.2014.8226. [DOI] [PubMed] [Google Scholar]
- 45.Rzhepishevska OHS, Ruhal R, Gautrot J, Barbero D, Ramstedt M. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater Sci. 2013;1:589–602. doi: 10.1039/c3bm00197k. [DOI] [PubMed] [Google Scholar]
- 46.Dzidic S, Bedekovic V. Horizontal gene transfer-emerging multidrug resistance in hospital bacteria. Acta Pharmacol Sin. 2003;24:519–526. [PubMed] [Google Scholar]