Abstract
Incorporating effective smoking cessation interventions into lung cancer screening (LCS) programs will be essential to realizing the full benefit of screening. We conducted a pilot randomized trial to determine the feasibility and efficacy of a telephone-counseling (TC) smoking cessation intervention vs. usual care (UC) in the LCS setting. In collaboration with 3 geographically diverse LCS programs, we enrolled current smokers (61.5% participation rate) who were: registered to undergo LCS, 50–77 years old, and had a 20+ pack-year smoking history. Eligibility was not based on readiness to quit. Participants completed pre-LCS (T0) and post-LCS (T1) telephone assessments, were randomized to TC (N=46) vs. UC (N=46), and completed a final 3-month telephone assessment (T2). Both study arms received a list of evidence-based cessation resources. TC participants also received up to 6 brief counseling calls with a trained cessation counselor. Counseling calls incorporated motivational interviewing and utilized the screening result as a motivator for quitting. The outcome was biochemically verified 7-day point prevalence cessation at 3-months post-randomization. Participants (56.5% female) were 60.2 (SD=5.4) years old and reported 47.1 (SD=22.2) pack years; 30% were ready to stop smoking in the next 30 days. TC participants completed an average of 4.4 (SD=2.3) sessions. Using intent-to-treat analyses, biochemically verified quit rates were 17.4% (TC) vs. 4.3% (UC), p<.05. This study provides preliminary evidence that telephone-based cessation counseling is feasible and efficacious in the LCS setting. As millions of current smokers are now eligible for lung cancer screening, this setting represents an important opportunity to exert a large public health impact on cessation among smokers who are at very high risk for multiple tobacco-related diseases. If this evidence-based, brief, and scalable intervention is replicated, TC could help to improve the overall cost-effectiveness of LCS.
Keywords: lung cancer screening, smoking cessation, telephone counseling, randomized trial
1. Introduction
The National Lung Screening Trial (NLST) reported a 20% lung cancer mortality reduction following low-dose computed tomography (LDCT) screening.1 As a result, LDCT is recommended for individuals at high-risk for lung cancer.2 If widely adopted, LDCT screening is estimated to prevent 12,000 U.S. lung cancer deaths annually.3 To maximize the health benefit from LDCT screening, the Centers for Medicare and Medicaid Services (CMS) mandated that all smokers undergoing screening must receive cessation assistance.4 Although there are multiple cessation interventions with proven effectiveness,5 presently none have demonstrated efficacy in the lung cancer screening (LCS) setting.6
Providing cessation interventions in conjunction with LCS may capitalize on the ‘teachable moment,’ when smokers may be especially amenable to considering quitting.7,8 The goal is to leverage increased motivation that may be provided by an abnormal screening result and to counteract the potential for reduced motivation following a normal result.9 This setting provides a unique opportunity to motivate smokers to quit by incorporating the LDCT result.
There have been four randomized cessation trials conducted within LCS programs, each reporting promising cessation rates, but with null findings.10–13 Building on our prior work,9,14–16 we evaluated a scalable telephone counseling (TC) cessation intervention to provide a personalized, intensive intervention in which the LCS result is leveraged to enhance motivation. TC has demonstrated effectiveness among older smokers,5,17–21 smokers who are not ready to quit,22–32 and non-treatment seeking smokers,29,33 making it an important intervention to test in this setting. In a randomized clinical trial, we hypothesized that TC would yield higher quit rates than usual care.
2. Material and Methods
2.1 Participants
Based on the National Comprehensive Cancer Network’s (NCCN) screening criteria,34 eligible screening participants were 50–74 years old with a 20+ pack-year smoking history. Current smokers were registered for screening at three sites (Table). Neither readiness to quit nor number of cigarettes per day (CPD) were eligibility criteria.
Table.
Baseline Demographic, Tobacco, and Lung Screening Characteristics
|
Usual Care (N = 46) |
Telephone Counseling (N = 46) |
|
|---|---|---|
| Demographic Characteristics | ||
| Site | ||
| Georgetown University Med Ctr | 7 (15.2%) | 7 (15.2%) |
| Lahey Hospital and Med Ctr | 33 (71.7%) | 35 (76.1%) |
| Hackensack University Med Ctr | 6 (13.0%) | 4 (8.7%) |
| Gender | ||
| Female (N, %) | 27 (58.7%) | 25 (54.3%) |
| Age (Mean, SD) | 60.1 (5.7) | 60.4 (5.1) |
| Median (Range) | 59.5 (50–70) | 60.0 (51–73) |
| Marital Status | ||
| Married/Marriage-like relationship (N, %) | 20 (43.5%) | 19 (41.3%) |
| Race | ||
| White | 43 (93.5%) | 43 (93.5%) |
| African-American | 3 (6.5%) | 2 (4.3%) |
| Native American | 0 (0%) | 1 (2.2%) |
| Education | ||
| ≤ HS graduate | 12 (26.1%) | 19 (41.3%) |
| Some college | 20 (43.5%) | 14 (30.4%) |
| ≥ College Grad | 14 (30.4%) | 13 (28.3%) |
| Employment | ||
| Not employed | 8 (17.4%) | 5 (10.9%) |
| Full-time/Part-time | 18 (39.1%) | 23 (50.0%) |
| Retired | 14 (30.4%) | 13 (28.3%) |
| Other (disability) | 6 (13.0%) | 5 (10.9%) |
| Tobacco-Related Comorbidities | ||
| 0 | 10 (21.7%) | 16 (34.8%) |
| 1 | 18 (39.1%) | 17 (37.0%) |
| 2+ | 18 (39.1%) | 13 (28.3%) |
| Health Insurance Status N (% Yes) | 46 (100%) | 45 (97.8%) |
| Personal History of Caa N (%Yes) | 12 (26.7%)b | 12 (26.7%)b |
| Family History of Lung Ca N (% Yes) | 16 (34.8%) | 20 (44.4%)b |
| Alcohol Use | ||
| Non-drinker | 15 (34.1%)c | 13 (28.9%)b |
| Monthly or less | 6 (13.6%) | 6 (13.3%) |
| 2–4 times a month | 7 (15.9%) | 7 (15.6%) |
| 2–3 times a week | 9 (20.5%) | 10 (22.2%) |
| 4+ times a week | 7 (15.9%) | 9 (20.0%) |
| Tobacco Use Characteristics | ||
| Pack Years (Mean, SD) | 50.3 (20.4) | 43.8 (23.7) |
| Median (Range) | 45.0 (26–100) | 40.0 (23–165) |
| Nicotine Dependenced,e (M, SD) | 4.6 (2.0) | 4.1 (1.9) |
| Cigarettes per Daye | ||
| ≤ 10 | 10 (22.7%) | 12 (27.9%) |
| 11–19 | 10 (22.7%) | 14 (32.6%) |
| 20 | 14 (31.8%) | 11 (25.6%) |
| ≥ 21 | 10 (22.7%) | 6 (14.0%) |
| Past 30 days–other tobacco products | ||
| Pipe, Tiparillos, Smokeless Tob.e | 0 (0%) | 0 (0%) |
| Cigarse | 2 (4.5%) | 2 (4.7%) |
| Electronic Cigarettesf | 7 (17.1%) | 2 (4.8%) |
| Readiness to Quite | ||
| Not Ready to Quit | 22 (50.0%) | 25 (58.1%) |
| Ready to Quit-next 6 mos | 9 (20.5%) | 5 (11.6%) |
| Ready to Quit-next 30 days | 13 (29.5%) | 13 (30.2%) |
| Lung Screening Characteristics | ||
| Screening History (% Yes) | 22 (47.8%) | 18 (39.1%) |
| Screening Resultg | ||
| Normal | 21 (45.7%) | 24 (52.2%) |
| Minor abnormality/Not susp for LC | 16 (34.8%) | 13 (28.3%) |
| Suspicious for lung cancer | 9 (19.6%) | 9 (19.6%) |
Cancers: breast, skin, prostate, bladder, colorectal, Hodgkin’s lymphoma, kidney, thyroid, cervical, liver, testicular, throat;
Missing: N = 1
Missing: N = 2
Fagerstrom Test for Nicotine Dependence35
Missing N = 5
Missing N = 9
Screening result categories are based on the NLST categories, with categories 2 and 3 collapsed due to small sample sizes in the ‘minor abnormality’ group.
2.2 Procedure
Between November 2013–March 2016, each screening site invited smokers to learn more about this study when scheduling their LDCT appointment (Figure). Georgetown University Medical Center (GUMC) interviewers called to describe the study to eligible individuals, obtain verbal consent, and conduct the baseline interview (T0) prior to screening. Each site’s IRB required a mailed information sheet explaining study procedures, participant rights, and potential risks, but did not require signed consent forms.
CONSORT Figure.
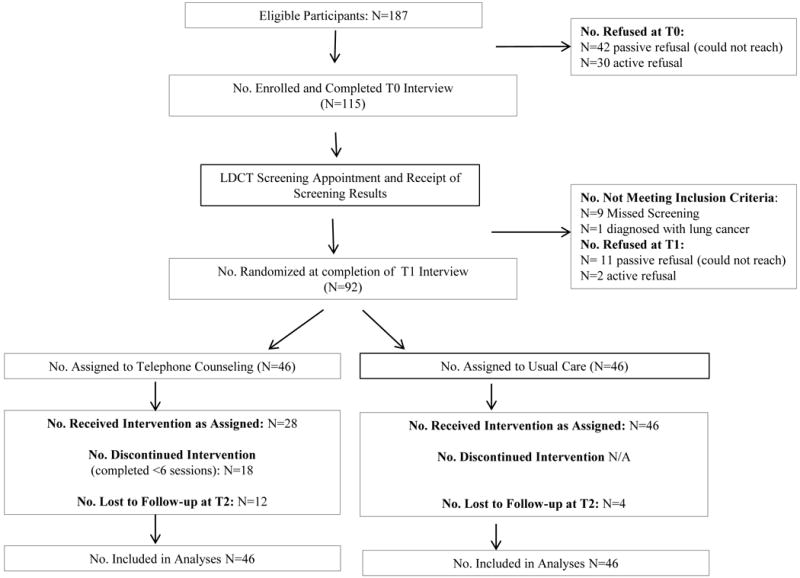
Following participants’ receipt of their screening results, interviewers conducted the T1 telephone interview and random assignment. During the T1 interview, participants read the letter describing their screening results to the interviewer. The final telephone interview (T2) was conducted 3-months post-randomization.
2.3 Measures
2.3.1 Background Characteristics
We assessed demographic and clinical characteristics (Table).
2.3.2. Tobacco Use
We assessed smoking history, CPD, non-cigarette tobacco use, nicotine dependence,35 and readiness to quit,36–40 (i.e., those ready within the next 30 days/next six months were “ready to quit” vs. “not ready to quit”).
2.3.3. Lung Cancer Screening
We assessed LCS history and current LCS results. Based on the NLST classification,1 self-reported screening results were categorized as “normal” (no nodules/abnormalities), “minor/other abnormality” (benign or noncalcified nodules <4mm or clinically significant abnormality), or “suspicious for lung cancer” (noncalcified nodule ≥ 4mm). One participant diagnosed with lung cancer was excluded pre-randomization.
2.3.4. Biochemical Verification
Participants reporting abstinence at T2 were mailed a NicAlert saliva strip test kit with instructions to complete and return by mail. Nicotine replacement therapy (NRT) users completed CO monitoring at the screening site. The standard abstinence cutoffs were: ≤1 for NicAlert and <6ppm for CO.41 Those completing verification received a $30 gift card for a national retailer.
2.4. Randomization Procedures
Following the T1 interview, randomization was conducted in blocks of 4, stratified by site, readiness to quit at T1 (ready/not ready), and screening result (normal/abnormal). The interviewer accessed the computerized randomization system, entered the stratification characteristics, and received the randomization assignment.
2.5. Intervention Arms
2.5.1. Usual Care
Following the T0 interview, all participants received the following cessation resources: Legacy’s BecomeAnEx booklet42,43 and website,42,43 contact information for local cessation resources, a text messaging link,44 and the LIVESTRONG My Quit App link.45
2.5.2. Telephone Counseling
TC participants received the same resource list and were offered six weekly, proactive (counselor-initiated) counseling calls (15–20 minutes each) that began 1–2 days post-randomization. The TC protocol46 included validated cessation techniques:5,47 motivational interviewing,48,49 identifying and coping with smoking triggers, and encouragement to consider NRT and to speak with their doctors about varenicline and bupropion. Discussion of an abnormal LCS result was designed to increase risk perceptions and emotional reactions to the result, and challenge one’s self-concept as a smoker. Discussion of a normal LCS result provided education that this was not a permanent ‘clean bill of health’ and that older adults who quit can still add years to their lives,50 challenging thoughts that minimized the consequences of smoking.
2.6. Statistical Analyses
In intent-to-treat analyses (non-responders were classified as current smokers), we assessed three-month self-reported and biochemically-verified seven-day point prevalence abstinence using chi-square tests (two-sided). The chi-square test (and not Fisher’s Exact test) is appropropriate as there were no expected cell counts <5. This pilot study was designed to evaluate feasibility and provide preliminary data for a subsequent multicenter trial. Analyses were performed using SPSS Version 23.0.
3. Results
The baseline participation rate was 61.5% (115/187; Figure). Compared to decliners, participants did not differ on age (p>.80) or gender (p>.10), but reported more pack years (p=.05). At 3-months (T2), compared to dropouts, those retained reported more pack years (p<.05) and were more likely to be in the UC arm (p<.05). There were no other significant differences. The majority of participants were from the Lahey site due to their higher volume of screening. The baseline demographic, tobacco-related, and screening-related variables are presented in the Table.
3.1. Three-Month Tobacco-Related Outcomes
In intent-to-treat analyses, there was no significant group difference on 7-day point prevalence self-reported abstinence (UC: 19.6% (N=9) vs. TC: 21.7% (N=10), p=.80). There was a significant group difference on biochemically verified abstinence (UC: 4.3% (N=2) vs. TC: 17.4% (N=8), p=.04). (See Supplementary Material).
Given the small number of UC participants with biochemically verified abstinence, we were unable to conduct moderation analyses. Instead, we provide descriptive data on two variables of interest: screening result and readiness to quit among those with verified abstinence. In each arm, 19.6% (N=9) had results suspicious for lung cancer (Table). At the T2 assessment, no UC participants (0/9) who had results suspicious for lung cancer had quit, vs. 22.2% (2/9) of TC participants. Similarly, regarding baseline readiness to quit, 30% (N=13) in each arm were ready to quit in the next 30 days (Table). Of those, 7.7% (1/13) of UC participants had verified abstinence, compared to 46.2% (6/13) of TC participants.
3.2. Intervention Process Outcomes
An average of 4.4 (SD=2.3) TC sessions were completed, and 60.9% of TC participants completed all six sessions. At 3-months, 55.6% of TC participants reported liking phone-based counseling, 27.6% preferred in-person counseling but still liked the phone counseling, and 14.7% preferred in-person counseling. Further, 75% said that six was an appropriate number of counseling calls, while 25% reported it was too few. Among self-reported quitters, the groups (TC vs UC) did not differ on use of NRT (60% vs 55.6%), varenicline (0% in both groups), or bupropion (11.1% vs. 20%).
4. Discussion
This study provides preliminary evidence that TC is feasible and efficacious in the LCS setting. Relative to UC, TC resulted in significantly greater abstinence at three months post-randomization. The 17% cessation rate is comparable to other studies including smokers not ready to quit,51 as well as prior interventions in the LCS setting.10–13
TC is at the intersection of scalability and intensity, both of which are necessary to impact cessation among smokers eligible for LCS. If it is shown to be effective in subsequent studies, TC could improve the cost-effectiveness of LCS,52 via its implementation in state and national quitlines, for use by LCS participants nationwide.
The inclusion of smokers who are not ready to quit is particularly important given the potential for the intervention, the screening setting, and the screening result to have a positive effect on motivation to quit. LCS participants represent an important group of smokers with whom to intervene, given the increased life expectancy among older smokers who quit.50,53 The LCS setting represents an opportunity to exert a large public health impact among smokers who are at very high risk for tobacco-related diseases.
Study limitations include the limited sample size, brief follow-up period, and self-reported LCS results, each of which is common among pilot studies. Further, the classification of non-respondents as current smokers can be problematic.54 Strengths include the use of biochemical verification, the 60% uptake of the intervention, and the cessation rate in the TC arm, particularly given that 50% were not ready to quit at baseline. These results suggest that similar trials should consider enrolling all smokers, and not only those who are ready to quit. Further, our results provide preliminary support for the TC intervention among those with a positive screening result and among those who were ready to quit at baseline, compared to those undergoing screening without a cessation intervention.55
In sum, TC has the potential to improve cessation in a setting that reaches a large number of hard-to-reach, long-term smokers who are at high risk for multiple tobacco-related diseases. Importantly, verifying quit rates in LCS-based intervention studies, as well as in other medical settings, is clearly warranted.56,57 Larger studies are needed to address the scalability and adoption of cessation interventions within the LCS setting.
Supplementary Material
Highlights.
Telephone-based smoking cessation intervention trial was conducted in the lung screening setting
Verified quit rates were significantly higher in the telephone counseling arm vs. usual care
Preliminary evidence that telephone counseling is feasible and efficacious in this setting
This is an opportunity to have a large public health impact among high risk smokers
Acknowledgments
Sources of funding: This work was supported by the Prevent Cancer Foundation and the Lombardi Comprehensive Cancer Center’s NCI Cancer Center Support Grant P30 CA051008.
Role of the sponsors: The sponsors had no role in the conduct of this study, the data analysis, interpretation of the data, or the manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: NCT02267096, https://clinicaltrials.gov
The authors appreciate the contributions of Scott Kelly, Ph.D., Riley Zinar, B.A., Daniel Leigh, B.S., and Susan Marx, B.A.
References
- 1.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S.Preventive Services Task Force. Final Update Summary: Lung Cancer: Screening. 2015 Jul; http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lung-cancer-screening. Accessed June 27, 2016.
- 3.Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381–1385. doi: 10.1002/cncr.27813. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Medicare & Medicaid Services (CMS) Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N) 2015 http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed June 27, 2016.
- 5.Fiore MC, Jaen CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Accessed June 27, 2016. [Google Scholar]
- 6.Fucito LM, Czabafy S, Hendricks PS, Kotsen C, Richardson D, Toll BA. Pairing smoking-cessation services with lung cancer screening: A clinical guideline from the Association for the Treatment of Tobacco Use and Dependence and the Society for Research on Nicotine and Tobacco. Cancer. 2016;122(8):1150–1159. doi: 10.1002/cncr.29926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- 8.Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. preventive services task force. Ann Am Thorac Soc. 2014;11(4):619–627. doi: 10.1513/AnnalsATS.201312-460OC. [DOI] [PubMed] [Google Scholar]
- 9.Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56(1):125–134. doi: 10.1016/j.lungcan.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Clark MM, Cox LS, Jett JR, et al. Effectiveness of smoking cessation self-help materials in a lung cancer screening population. Lung Cancer. 2004;44(1):13–21. doi: 10.1016/j.lungcan.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Ferketich AK, Otterson GA, King M, Hall N, Browning KK, Wewers ME. A pilot test of a combined tobacco dependence treatment and lung cancer screening program. Lung Cancer. 2012;76(2):211–215. doi: 10.1016/j.lungcan.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall HM, Courtney DA, Passmore LH, et al. Brief tailored smoking cessation counseling in a lung cancer screening population is feasible: a pilot randomized controlled trial. Nicotine Tob Res. 2016 Feb 1; doi: 10.1093/ntr/ntw010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.van der Aalst CM, de Koning HJ, van den Bergh KA, Willemsen MC, van Klaveren RJ. The effectiveness of a computer-tailored smoking cessation intervention for participants in lung cancer screening: a randomised controlled trial. Lung Cancer. 2012;76(2):204–210. doi: 10.1016/j.lungcan.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Barry SA, Tammemagi MC, Penek S, et al. Predictors of adverse smoking outcomes in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Cancer Inst. 2012;104(21):1647–1659. doi: 10.1093/jnci/djs398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagerman CJ, Tomko CA, Stanton CA, et al. Incorporating a smoking cessation intervention into lung cancer screening programs: preliminary studies. J Psychosoc Oncol. 2015;33(6):703–723. doi: 10.1080/07347332.2015.1082171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tammemagi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106(6):dju084. doi: 10.1093/jnci/dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joyce GF, Niaura R, Maglione M, et al. The effectiveness of covering smoking cessation services for medicare beneficiaries. Health Serv Res. 2008;43(6):2106–2123. doi: 10.1111/j.1475-6773.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan GD, Noll EL, Orleans CT, Rimer BK, Amfoh K, Bonney G. Reaching midlife and older smokers: tailored interventions for routine medical care. Prev Med. 1996;25(3):346–354. doi: 10.1006/pmed.1996.0065. [DOI] [PubMed] [Google Scholar]
- 19.Ossip-Klein DJ, Carosella AM, Krusch DA. Self-help interventions for older smokers. Tob Control. 1997;6(3):188–193. doi: 10.1136/tc.6.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimer BK, Orleans CT. Tailoring smoking cessation for older adults. Cancer. 1994;74(7 Suppl):2051–2054. doi: 10.1002/1097-0142(19941001)74:7+<2051::aid-cncr2820741711>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Tait RJ, Hulse GK, Waterreus A, et al. Effectiveness of a smoking cessation intervention in older adults. Addiction. 2007;102(1):148–155. doi: 10.1111/j.1360-0443.2006.01647.x. [DOI] [PubMed] [Google Scholar]
- 22.Britt J, Curry SJ, McBride C, Grothaus L, Louie D. Implementation and acceptance of outreach telephone counseling for smoking cessation with nonvolunteer smokers. Health Educ Q. 1994;21(1):55–68. doi: 10.1177/109019819402100107. [DOI] [PubMed] [Google Scholar]
- 23.Curry SJ, McBride C, Grothaus LC, Louie D, Wagner EH. A randomized trial of self-help materials, personalized feedback, and telephone counseling with nonvolunteer smokers. J Consult Clin Psychol. 1995;63(6):1005–1014. doi: 10.1037//0022-006x.63.6.1005. [DOI] [PubMed] [Google Scholar]
- 24.Ellerbeck EF, Mahnken JD, Cupertino AP, et al. Effect of varying levels of disease management on smoking cessation: a randomized trial. Ann Intern Med. 2009;150(7):437–446. doi: 10.7326/0003-4819-150-7-200904070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emmons KM, Puleo E, Park E, et al. Peer-delivered smoking counseling for childhood cancer survivors increases rate of cessation: the partnership for health study. J Clin Oncol. 2005;23(27):6516–6523. doi: 10.1200/JCO.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein E, Glasgow RE, Lando HA, Ossip-Klein DJ, Boles SM. Telephone counseling for smoking cessation: rationales and meta-analytic review of evidence. Health Educ Res. 1996;11(2):243–257. doi: 10.1093/her/11.2.243. [DOI] [PubMed] [Google Scholar]
- 27.McBride CM, Scholes D, Grothaus LC, Curry SJ, Ludman E, Albright J. Evaluation of a minimal self-help smoking cessation intervention following cervical cancer screening. Prev Med. 1999;29(2):133–138. doi: 10.1006/pmed.1999.0514. [DOI] [PubMed] [Google Scholar]
- 28.Orleans CT, Schoenbach VJ, Wagner EH, et al. Self-help quit smoking interventions: effects of self-help materials, social support instructions, and telephone counseling. J Consult Clin Psychol. 1991;59(3):439–448. doi: 10.1037//0022-006x.59.3.439. [DOI] [PubMed] [Google Scholar]
- 29.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2013;8:CD002850. doi: 10.1002/14651858.CD002850.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Tzelepis F, Paul CL, Walsh RA, McElduff P, Knight J. Proactive telephone counseling for smoking cessation: meta-analyses by recruitment channel and methodological quality. J Natl Cancer Inst. 2011;103(12):922–941. doi: 10.1093/jnci/djr169. [DOI] [PubMed] [Google Scholar]
- 31.Yong HH, Borland R, Balmford J, et al. Heaviness of smoking predicts smoking relapse only in the first weeks of a quit attempt: findings from the International Tobacco Control Four-Country Survey. Nicotine Tob Res. 2014;16(4):423–429. doi: 10.1093/ntr/ntt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu SH, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: effects of single-session and multiple-session interventions. J Consult Clin Psychol. 1996;64(1):202–211. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]
- 33.Emmons KM, Puleo E, Mertens A, Gritz ER, Diller L, Li FP. Long-term smoking cessation outcomes among childhood cancer survivors in the Partnership for Health Study. J Clin Oncol. 2009;27(1):52–60. doi: 10.1200/JCO.2007.13.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Lung Cancer Screening Version 2.2016. https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf. Accessed June 27, 2016.
- 35.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 36.Apodaca TR, Abrantes AM, Strong DR, Ramsey SE, Brown RA. Readiness to change smoking behavior in adolescents with psychiatric disorders. Addict Behav. 2007;32(6):1119–1130. doi: 10.1016/j.addbeh.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 38.Burgess DJ, van RM, Noorbaloochi S, et al. Smoking cessation among African American and white smokers in the Veterans Affairs health care system. Am J Public Health. 2014;104(Suppl 4):S580–S587. doi: 10.2105/AJPH.2014.302023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sachs R, Wild TC, Thomas L, Hammal F, Finegan BA. Smoking cessation interventions in the pre-admission clinic: assessing two approaches. Can J Anaesth. 2012;59(7):662–669. doi: 10.1007/s12630-012-9716-6. [DOI] [PubMed] [Google Scholar]
- 40.Webb HM, Baker EA, McNutt MD. Racial/Ethnic differences among smokers: revisited and expanded to help seekers. Nicotine Tob Res. 2014;16(5):621–625. doi: 10.1093/ntr/ntt206. [DOI] [PubMed] [Google Scholar]
- 41.Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100(2):159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- 42.American Legacy Foundation. Re-Learn Life Without Cigarettes. EX™. www.becomeanex.org/docs/becomeanEXbook.pdf. Accessed June 27, 2016.
- 43.American Legacy Foundation. BecomeAnEx.org. www.becomeanex.org/. Accessed June 27, 2016.
- 44.Smokefree.gov, National Cancer Institute. SmokefreeTXT. http://smokefree.gov/smokefreetxt. Accessed June 27, 2016.
- 45.Demand Media I. LIVESTRONG MyQuit Coach - Dare to Quit Smoking. https://itunes.apple.com/us/app/livestrong-myquit-coach-dare/id383122255?mt=8. Accessed June 27, 2016.
- 46.Graham AL, Papandonatos GD, DePue JD, et al. Lifetime characteristics of participants and non-participants in a smoking cessation trial: implications for external validity and public health impact. Ann Behav Med. 2008;35(3):295–307. doi: 10.1007/s12160-008-9031-1. [DOI] [PubMed] [Google Scholar]
- 47.Stead LF, Lancaster T. Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2012;12:CD009670. doi: 10.1002/14651858.CD009670.pub2. [DOI] [PubMed] [Google Scholar]
- 48.Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behaviors. 2nd. New York, NY: Guilford Press; 2002. [Google Scholar]
- 49.Soria R, Legido A, Escolano C, Lopez YA, Montoya J. A randomised controlled trial of motivational interviewing for smoking cessation. Br J Gen Pract. 2006;56(531):768–774. [PMC free article] [PubMed] [Google Scholar]
- 50.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 51.Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav. 2011;36(7):764–768. doi: 10.1016/j.addbeh.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One. 2013;8(8):e71379. doi: 10.1371/journal.pone.0071379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor DH, Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92(6):990–996. doi: 10.2105/ajph.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102(10):1564–1573. doi: 10.1111/j.1360-0443.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- 55.Ashraf H, Saghir Z, Dirksen A, et al. Smoking habits in the randomised Danish Lung Cancer Screening Trial with low-dose CT: final results after a 5-year screening programme. Thorax. 2014;69(6):574–579. doi: 10.1136/thoraxjnl-2013-203849. [DOI] [PubMed] [Google Scholar]
- 56.Klesges RC, Krukowski RA, Klosky JL, et al. Efficacy of a tobacco quitline among adult survivors of childhood cancer. Nicotine Tob Res. 2015;17(6):710–718. doi: 10.1093/ntr/ntu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morales NA, Romano MA, Michael CK, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control. 2013;24(6):1223–1230. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


