Abstract
In addition to their well-known role in transporting cargoes in the cytoplasm, microtubule motors organize their own tracks, microtubules. While this function is mostly studied in the context of cell division, it is essential for microtubule organization and generation of cell polarity in interphase cells. Kinesin-1, the most abundant microtubule motor, plays a role in the initial formation of neurites. This review describes the mechanism of kinesin-1-driven microtubule sliding and discusses its biological significance in neurons. Recent studies describing the interplay between kinesin-1 and cytoplasmic dynein in translocation of microtubules are discussed. In addition, we evaluate recent work exploring the developmental regulation of microtubule sliding during axonal outgrowth and regeneration. Collectively, the discussed works suggest that sliding of interphase microtubules by motors is a novel force-generating mechanism that reorganizes the cytoskeleton and drives shape change and polarization.
Kinesin-1-driven microtubule sliding in neurons and non-neuronal cells
The standard and well-studied function of conventional kinesin (also known as kinesin-1 or KIF5) is to transport cargoes along microtubules towards plus-ends. Recently, several studies, including the work of our group, have uncovered a novel “unconventional” function of kinesin-1, sliding cytoplasmic microtubules against each other. Biochemical studies demonstrated, that in addition to the well-characterized N-terminal motor domain [1, 2], kinesin-1 has another microtubule-binding site at the C-terminus of the heavy chain (kinesin-1 heavy chain, KHC) [3, 4]. This C-terminal microtubule-binding site contains multiple basic amino acids that are highly conserved across many species (Figure 1). This site can bind to an acidic E-hook at the tubulin C-terminus through complementary electrostatic interactions [3, 4], and, unlike the N-terminal motor domain, is ATP-independent. Using the N-terminal motor domain and the C-terminal binding site, kinesin-1 can cross-bridge two microtubules and move them against each other (microtubule sliding, Figure 1). In this case, the microtubule that is bound to the C-terminus of the heavy chain becomes a “cargo” for kinesin-1, while the other microtubule serves as a track (Figure 1). When kinesin-1 motor walks on one microtubule (track), it moves another microtubule (cargo) relative to the track microtubule (Figure 1). The experiments of overexpression of Kinesin-1 C-terminal tail in tissue culture cells showed that C-terminal tail decorates microtubules uniformly [5, 6], indicating that all microtubules identical in their ability to bind kinesin-1 C-terminal tail. Thus kinesin-1 probably binds to two microtubules in a random orientation (statistically 50% upright and 50% upside-down, simplified as two opposite kinesin-1 motors in Figure 2). Therefore, it is likely that every microtubule serves as a cargo and as a track at the same time. In this scenario, kinesin-1 only slides anti-parallel microtubules against each other (Figure 2A). If it cross-bridges parallel microtubules, the forces of kinesins in opposite orientations are subtracted, and no movement can be produced (Figure 2B).
Figure 1. Highly conserved C-terminal microtubule binding site of kinesin-1 heavy chain.
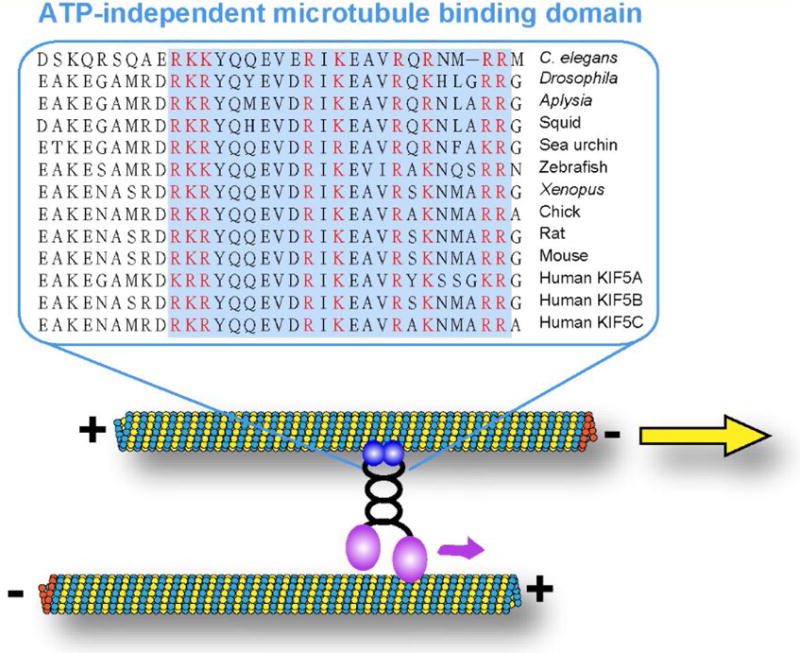
Amino acids of kinesin-1 heavy chain C-terminal region from 11 species are aligned in Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) using the following kinesin heavy chain protein sequences: Caenorhabditis elegans, Drosophila melanogaster, Aplysia californica, Doryteuthis pealeii (squid), Strongylocentrotus purpuratus (sea urchin), Danio rerio (zebrafish), Xenopus tropicalis, Gallus gallus (chick), Rattus norvegicus (rat), Mus musculus (mouse) and Homo sapiens (KIF5A, KIF5B, and KIF5C). ATP-independent microtubule binding site is highlighted in the light blue box, and all basic residues in the microtubule binding site are labeled in red.
Figure 2. Microtubule sliding by kinesin-1 requires antiparallel orientation.
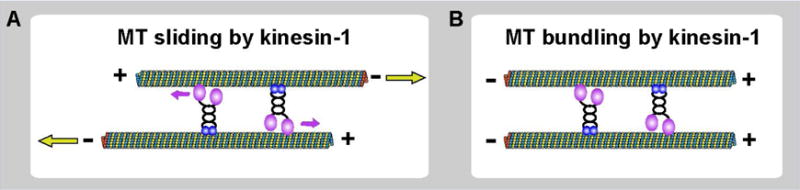
Kinesin-1 non-cooperative binding to two microtubules using its motor domain and C-terminal microtubule binding site leads to sliding of antiparallel microtubules (A) and bundling of parallel microtubules (B).
Microtubule sliding by kinesin-1 was first proposed based on the in vivo observation in a study of a fungus Ustilago maydis. Straube and colleagues demonstrated that kinesin-1 causes microtubule bending and bundling [7]. To directly visualize microtubule sliding, our group fused a photoconvertible protein tag to tubulin and imaged microtubule movement in interphase cells. Local photoconversion of this probe can be used to apply fiduciary marks on microtubules. Following these marks (photoconverted microtubule segments) allows visualization of microtubule movements in live cells (Figure 3). We documented robust microtubule sliding in many types of tissue culture cells, including Drosophila S2 cells, Xenopus fibroblasts, and rat kangaroo epithelial Ptk2 cells [8, 9].
Figure 3. Visualization of microtubule sliding in Drosophila neurons using photoconvertible tubulin.
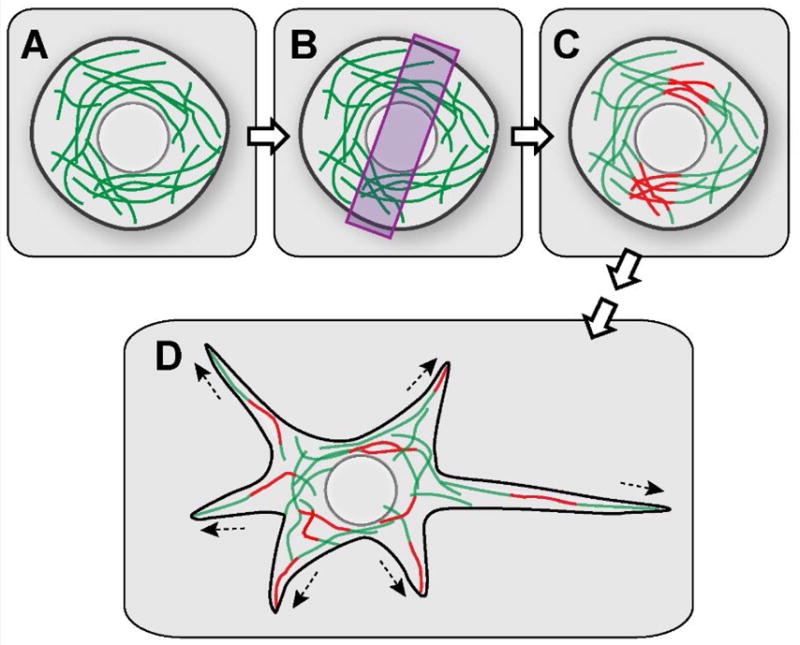
(A) A spherical-shaped young neuron expressing photoconvertible EOS-tagged α-tubulin. (B) 400nm light is applied to a restricted area to photoconvert a subset of microtubules in the young neurons. (C) A subset of microtubules is photoconverted from green to red. (D) Red microtubule fragments are scattered throughout the cell body and in the newly formed neurites by microtubule-microtubule sliding, revealed by time-lapse movies.
Experiments in Drosophila S2 cells demonstrated that depolymerization of actin cytoskeleton with Latrunculin B or its fragmentation by Cytochalasin D before plating, results in formation of multiple radial processes filled with microtubules [8, 10–14]. We further demonstrated that this dramatic process formation is driven by kinesin-powered microtubule sliding [8].
We later detected microtubule sliding by kinesin-1 in cultured Drosophila primary neurons, including embryonic neurons [15] and larval motor neurons [16], as well as in sensory neurons in vivo [6]. Furthermore, not only do microtubules slide in differentiating neurons, but, remarkably, their sliding drives initial extension of neurites in cultured neurons, by pushing against the plasma membrane at the tips of the neurites [15–17], consistent with the data that destabilization of actin filaments induced fast axon outgrowth [18]. These data demonstrate that, at least at the initial stages of neurite outgrowth, microtubule sliding by kinesin-1 provides the driving forces for neurite extension [15]. These data are consistent with the previous work in cultured rat hippocampal neurons describing shorter axons after kinesin-1 knock down [19]. Furthermore, kinesin-dependent microtubule sliding is also required for neurite membrane extension during axon regeneration after injury in culture [16]. In a recently published study from our group, we were able to detect kinesin-dependent microtubule sliding in the growing sensory neurons underneath pupal bristles in vivo [6], further indicating that microtubule sliding is important for neuronal development.
Interestingly, another motor that can slide microtubules, cytoplasmic dynein, is dispensable for neurite outgrowth and axonal regeneration [8, 14–16, 20] (see more on the role of dynein in the section of “The interplay between kinesin-1 and dynein in microtubule organization”). In order to experimentally test the kinesin-driven microtubule sliding mechanism presented in Figure 1, we generated a mutant form of kinesin that decreases its microtubule sliding ability without affecting its cargo transporting function. We mutated four highly conserved amino residues at the C-terminal microtubule binding site (914-RKRYQ-918 to 914-AAAYA-918) based on a human kinesin-1 (KIF5B) tail mutant that showed 20-fold decrease in microtubule binding in vitro [4], creating KhcmutA. KhcmutA significantly reduced kinesin-1 tail microtubule binding and thus kinesin-driven microtubule sliding in Drosophila S2 cells, while conventional kinesin-driven cargoes transport was unaffected [6]. This sliding-deficient mutant allows us to experimentally dissociate the defects caused by impaired microtubule sliding from the defects caused by the reduced cargo transport. Based on the S2 work, we generated a genomic knock-in Drosophila line carrying this tail mutant, KhcmutA [6]. Remarkably, KhcmutA displayed reduced in kinesin-driven microtubule sliding in cultured neurons and neurons in vivo. Furthermore, KhcmutA flies showed major defects of neurite outgrowth in culture, and axonal and dendritic patterning in vivo [6]. These defects in the nervous system lead to decreased locomotion, and increased lethality, which indicates that kinesin-driven microtubule sliding is an essential developmental process, besides the well-recognized kinesin-driven cargo transport.
A similar tail mutant in the C-terminal microtubule binding site was generated in C. elegans by the Shen group [21], who showed microtubule polarity in dendrites, but not kinesin-driven mitochondria transport, is affected in this mutant. The Shen group proposed that kinesin-1 slides plus-ends out microtubules out of the dendrites (consistent with the model presented in Figure 2A).
Both the motor domain and the ATP-independent microtubule binding site at the C-terminus of the heavy chain are highly evolutionary conserved [5, 6, 22] (Figure 1). Therefore, we propose that microtubule sliding can serve as a universal and essential mechanism driving reorganization of the microtubule networks. In agreement with this idea, we have observed microtubule sliding in mammalian cells [8]; furthermore, human kinesin-1 heavy chain (KIF5B) fully rescues microtubule sliding in Drosophila S2 cells [6]. As neurons are the most polarized cell type in animals, microtubule sliding is essential for neurons providing the mechanical force for cell shape changes during neuronal differentiation and regeneration.
The interplay between kinesin-1 and dynein in microtubule organization
Besides the plus-end motor kinesin-1, minus-end motor cytoplasmic dynein has also been implicated as a motor for microtubule movement in neurons and tissue culture cells.
Using cultured rat sympathetic neurons, the Baas group has shown that dynein is important for the transport of microtubule fragments released from centrosomes, and for the anterograde transport of microtubules in axons [23–27]. Dehmelt and colleagues demonstrated that dynein pushes bundled microtubules outwards in Neuro2A neuroblastoma cell and primary hippocampal neurons and this movement is important for neurite initiation [28]. Directional movement and bending of microtubules is associated with and dependent on cortical dynein in COS7 cells, and Neuro2A cells [29]. The Vallee group showed that dynein is required for microtubule advance into the growth cone during axonal outgrowth in both chick DRG neurons and rat hippocampal neurons [30]. This is further supported by a study from the Miller group showing that en masse microtubule anterograde translocation was abolished upon dynein inhibition in chick DRG neurons [31].
The popular model of dynein-driven microtubule movement is that the dynein complex binds to an immobilized stable scaffold, such as the cortical F-actin, and pushes microtubules in the cytoplasm [28, 31, 32] (Figure 4). Alternatively, dynein can potentially bind to two microtubules simultaneously using its two motor domains and slide them apart. Such possibility was demonstrated in vitro [33], although it is unclear whether it happens in cells.
Figure 4. A model of microtubule motors driving microtubule sliding at different stages.
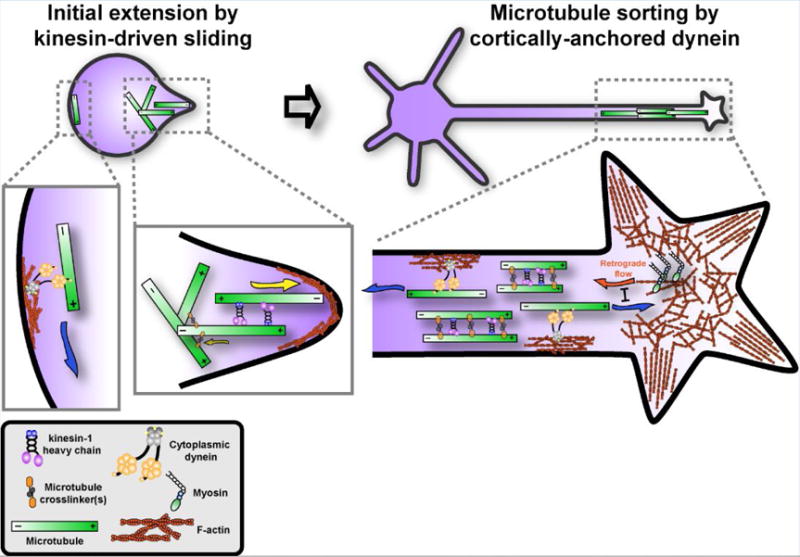
Kinesin-1 drives anti-parallel microtubules sliding apart in early-stage neurons, inducing the formation of initial neurites in spherical-shaped young neurons (left panel). At later stages, cytoplasmic dynein anchors at cell cortex by interacting with F-actin and sorts out the microtubules of wrong orientation (minus-ends out), and slides microtubules of right orientation (plus-ends out) towards the growth cone to counteract actin retrograde flow driven by myosins (right panel). Both kinesin-driven and dynein-driven microtubule sliding can be regulated by microtubule crosslinker(s).
Interestingly, kinesin-driven and dynein-driven sliding models are not mutually exclusive. A recent study from our group showed that kinesin-1 and dynein activities are coordinated and function at different developmental stages to ensure normal neurite outgrowth and correct organization of microtubules in the axons of Drosophila larval brain neurons [34]. In this study, we showed that at early stages of initial neurite outgrowth, kinesin-1-driven microtubule sliding breaks the spherical symmetry of undifferentiated neurons, and drives initial neurite extensions with minus-ends of microtubules pushing out the plasma membrane at the tips of the processes. This sliding can be favored in the anterograde direction, as more microtubules tangled or crosslinked in the cell body restrict the retrograde sliding and provide the mechanical support for initial neurite extension (Figure 4, left panel). Later, cytoplasmic dynein becomes essential for microtubule sorting, establishing the uniform (plus-ends out) orientation of microtubules in the axons by sliding ‘minus-end-out’ microtubules toward the cell body (Figure 4, right panel. It can potentially also participate in outgrowth by moving plus-end out microtubules toward the tips, as demonstrated by other labs [23–26, 30, 31]. Dynein sorting function requires its recruitment to actin cortex, as depolymerization of F-actin phenocopied dynein inhibition, while artificial recruitment of dynein to the plasma membrane bypassed the requirement of F-actin [34]. This is consistent with a recent report from the Hoogenraad group showing that initially the neurites of rat hippocampal and cortical neurons contain microtubules of mixed polarity; in later stages the mixed orientation in axons changes to the uniform (plus-ends-out), while the mixed orientation in dendrites is preserved [35]. It suggests that during polarization the sorting machinery removes minus-end-out microtubules only from axons. Besides its function of sorting minus-ends-out microtubules, dynein may mediate the forward movement of plus-ends-out microtubules, antagonizing the myosin-mediated actin retrograde flow and neurite retraction [26, 30, 31] (Figure. 4, right panel).
What triggers this transition from kinesin-driven to dynein-driven sliding is not unknown. The easiest explanation is that the transition is simply triggered by the changing geometry of the developing neuron. Cortical dynein alone in a spherical cell cannot initiate process formation because by definition it would only move microtubules parallel to the cell surface, while kinesin-1 slides microtubules to form initial neurite extension by pushing against the plasma membrane (Figure. 4, left panel). After the initial process is formed, dynein tethered to the cortex of the process started to drive both the outgrowth by pushing the plus-ends-out microtubules forward, and sorting by removing the minus-ends-out microtubules, as the force produced by the cortical dynein in the process will be directed parallel to the axis of the axon. At the same time, the ability of kinesin-1 to slide microtubules would be constrained because kinesin-1 cannot slide parallel microtubules in the axons, but bundles them (Figure 2B, and Figure 4, right panel). Thus, simply geometry can explain the shift from kinesin-driven to dynein-driven neurite extension. Besides the cell geometry, biochemical regulation can also contribute to the switch between kinesin-1 and dynein sliding activity (See next section “Developmental regulation of microtubule sliding”).
Developmental regulation of microtubule sliding
As microtubule sliding is essential for axon elongation and regeneration, it is not surprising that sliding is developmentally regulated. Microtubule sliding is only active in young growing neurons, and becomes undetectable as they mature [15, 16]. Downregulation of microtubule sliding is not caused by inhibition of kinesin-1, since the kinesin-1-dependent organelle transport is still active in mature neurons [9, 15, 16, 36]. We demonstrated that a “mitotic” kinesin Pavarotti (a kinesin-6 family member known in other organisms as MKLP1, CHO1 or KIF20A) is a potent negative regulator of kinesin-1-driven sliding of cytoplasmic microtubules [17], most likely crosslinking anti-parallel microtubules in neurons. Because Pavarotti contains multiple nuclear localization signals (NLS) at its C-terminal tail and it is mainly localized in the nucleus in interphase cells [37, 38], it is likely that translocation of Pavarotti from the nucleus to the cytoplasm causes inhibition of microtubule sliding. This speculation is supported by the fact that ectopic expression of NLS-mutated Pavarotti resulted in dramatic inhibition of microtubule sliding and axon growth [17].
Microtubule sliding is downregulated in fully grown mature neurons; however, sliding can be reactivated by axonal injury. Reactivation depends on the formation of antiparallel microtubules near the injury site, and is essential for driving initial axonal regeneration [16]. It will be interesting to see whether suppression of kinesin-6 could stimulate axonal regeneration and if so, it can be potentially used for treatment of neurodegenerative diseases as well as spinal cord and brain injuries.
Another example of developmental regulation of microtubule sliding is observed during the mouse myogenesis. Microtubule movement has been demonstrated in mouse myoblast C2C12 cells during myogenesis and differentiation [39]. Straube and colleagues demonstrated that a novel isoform of microtubule-associated protein MAP4, oMAP4, functions as a microtubule cross-linker inhibiting microtubule sliding during myogenic differentiation [39]. oMAP4’s ability to inhibit sliding is mostly regulated by its expression level: expression is upregulated during myogenic differentiation, when microtubule sliding is greatly reduced [39]. Thus, although microtubule crosslinking could serve as a general braking mechanism, the regulators themselves could be cell type specific (oMAP4 during myogenic differentiation versus kinesin-6 in neurons).
Moreover, developmental downregulation of microtubule sliding observed in neurons [15–17] may help to resolve the long-standing debate whether tubulin is transported as a polymer or as subunits in the axons [40, 41]. Microtubule movement along the axons has been reported in Xenopus embryonic neurons [42, 43] and rat sympathetic neurons [23, 44]; but no microtubule movement could be detected in rat neuron-like PC-12 cells [45], chick DRG neurons [46], and mouse sensory neuron [47]. It is interesting to note that neurons in which microtubule movement was reported are characterized by fast neurite outgrowth (>25 μm/hour) [15, 42, 48–50]. As we reported, the microtubule movement rate is highly correlated with the neurite growth rate [15, 17]. Therefore, the discrepancies in the previous studies are likely explained by different neurite outgrowth rate at different development stages: the high level of microtubule sliding is only detected during fast outgrowth; whereas during slow neurite outgrowth, microtubule sliding becomes undetectable and/or easily obscured by microtubule dynamics.
The role of F-actin in the growth cone
Despite the fact that F-actin is at the extreme periphery of the leading edge of the growth cone, numerous studies have shown that it is dispensable for neurite outgrowth; instead, disruption of F-actin leads to faster neurite outgrowth, probably by allowing microtubules penetrate into the growth cone to drive the extension [18, 51–57]. Consistent with these findings, we showed that both fragmentation of F-actin by Cytochalasin D and its total depolymerization by Latrunculin B stimulate neurite outgrowth in Drosophila embryonic neurons. In the absence of F-actin at the neurite tip, sliding microtubules push directly against the cell membrane to make membrane protrusion [15, 17]. In addition, it has been shown that in rat hippocampal neurons local disruption of F-actin (Cytochalasin D and Latrunculin B) favors the axonal specification and global disruption of F-actin leads to formations of multiple long axons [18]. Disruption of F-actin also caused defects of axon targeting and branching [54, 55]. Thus, F-actin in the growth cone plays an important role in outgrowth control and growth cone steering by allowing microtubule penetration in part of the growth cone towards local guidance cues [58, 59]. Uncoupling of microtubules and F-actin in the growth cone, such as in mutants of the microtubule- and actin-cross-linker, spectraplakin/short stop, led to mistargeting of axons in vivo [60, 61].
Intriguingly, a recent paper by from the Svitkina lab [62] questioned these studies, and claimed that F-actin polymerization drives neurite extension. We cannot exclude the possibility that at certain stages of slow axonal outgrowth actin filaments do provide some driving force for axonal and/or dendritic outgrowth or steering. However, Chia et al. [62] did not directly show that actin polymerization indeed drives neurite extension. Instead, they showed that after treatment with Cytochalasin D or Latrunculin B, some F-actin remains as a cluster (“actin bulb”) at the collapsed growth cone of some growing neurites. They argued that actin polymerization is required for neurite extension. In fact Cytochalasin D binds to growing ends of F-actin and prevent addition of actin monomers (G-actin) [63], while Latrunculin B binds to G-actin and prevents F-actin assembly [64], which means both drugs effectively inhibit actin dynamics. The remnants of actin staining at the neurite tip after the drug treatment suggested that these actin filaments are stable, which, together with the bulk of other published data [15, 17, 18, 51–57], argues against their conclusion that actin polymerization drives the neurite extension. Furthermore, their interpretation failed to explain why actin-specific inhibitors increase rather than decrease the rate of axon outgrowth.
Cargo riding on moving microtubules: another form of hitchhiking
Cargo transport and microtubule sliding are two main and conserved functions of kinesin-1. It is possible that some cargoes can simply ride on sliding microtubules and get transported within the cells along microtubules. This hypothesis was first proposed based on the observations of organelles moving with sliding microtubules in amoeba Reticulomyxa [65–67]. More recently, an observation of concerted movement of peroxisomes in Drosophila S2 cells led to the speculation that they are attached to a moving microtubule [12]. Our recent study of Drosophila ooplasmic streaming revisited this hitchhiking hypothesis. We showed that kinesin-driven microtubule sliding is required for cytoplasmic streaming in Drosophila oocytes [22]. Unlike sliding in neurons, microtubule sliding in oocytes occurs between the cortically-anchored and free microtubules. This sliding generates unidirectional movement of cytoplasm. Inhibition of sliding by a kinesin mutation (KhcmutA, see section “Kinesin-1-driven microtubule sliding in neurons and non-neuronal cells”) resulted in a more diffuse pattern of posterior determinants in the oocyte. We propose that the cytoplasmic streaming refines the posterior determination by bringing determinants that are attached to moving microtubules to the posterior pole for proper anchorage [22]. This is consistent with previous studies that suggest streaming transports polarity determinants in the oocytes [68–70]. This “ride-on-microtubules” mode of transport can serve as a quick and efficient way of redistributing organelles and mRNAs/proteins in parallel with microtubule reorganization, especially in big cells, such as oocytes. More validation and future studies in other cell types and higher organisms are needed to further test this hypothesis.
Concluding remarks and future perspectives
With the recent studies from multiple groups, interphase microtubule sliding emerges as a novel driving force for neurite outgrowth and microtubule reorganization. Kinesin-1 and cytoplasmic dynein both play important roles in driving microtubule movements, and defining cell shape and microtubule organization/orientation. Furthermore, new studies reveal that motor-driven microtubule sliding is tightly controlled by developmental mechanisms. The new studies provide insights on the mechanism and regulation of this microtubule movement in neurons and other cells, and generate many more interesting questions: How do kinesin-1 and dynein switch their activities between microtubule sliding and cargo transport? How is dynein anchored to the cortex to slide microtubules in the axons? Is the activity of dynein-sorting mechanism differently regulated in axons versus dendrites? What regulates activity of cross-linkers inhibiting microtubule sliding in neurons and other cell types? More studies are needed to address these important questions.
Table 1.
Summary of microtubule sliding and related motors.
| Species | Cell type | Visualization method | Motors involved | Reference |
|---|---|---|---|---|
| Ustilago maydis | Single cell | GFP-α-tubulin | kinesin-1 and dynein | Straube et al., 2006 [7] |
| Drosophila melanogaster | S2 cells (tissue culture) | mCherry-tubulin and Dendra2-α-tubulin (photoconversion); | kinesin-1 | Jolly et al., 2010 [8] |
| Xenopus | Fibroblast (tissue culture) | Dendra2-Xenopus α-tubulin (photoconversion) | kinesin-1 | Jolly et al., 2010 [8] |
| Rat | kangaroo epithelial Ptk2 cells (tissue culture) | GFP-tubulin | Kinesin-1 | Jolly et al., 2010 [8] |
| Drosophila melanogaster | Embryonic neurons (primary culture) | tdEOS2-α-tubulin (photoconversion) | Kinesin-1 | Lu et al., 2013 [15] |
| Drosophila melanogaster | Larval motor neurons (primary culture) | tdEOS2-α-tubulin (photoconversion) | Kinesin-1 | Lu et al., 2015 [16] |
| Drosophila melanogaster | Sensory neurons underneath the pupal bristles in vivo | tdMaple3-α-tubulin (photoconversion) | Kinesin-1 | Winding et al., 2016 [6] |
| Caenorhabditis elegans | Motor neurons in vivo | EBP-2-GFP (plus-end microtubule marker) | Kinesin-1 | Yan et al., 2013 [21] |
| Mouse | Myoblast C2C12 (tissue culture) | mEos2-Tubulin (photoconversion) | Kinesin-1 and dynein | Mogessie et al., 2015 [39] |
| Amoeba Reticulomyxa | Single cell | DIC movie | Dynein | Koonce et al. 1987 [65]; Euteneuer et al., 1989 [66] |
| Rat | Sympathetic neurons (primary culture) | EGFP-tubulin and photobleaching | Dynein | He et al., 2005 [25] |
| Mouse/Monkey | Neuro-2a and Cos7 (tissue culture) | GFP-tagged and mCherry-tagged microtubule-associated protein MAP2c (Speckle and TIRF microscopy) | Dynein | Dehmelt et al., 2006 [28]; Mazel et al., 2014 [29] |
| Chick | Dorsal root ganglion neurons (primary culture) | EGFP-α-tubulin | Dynein | Grabham et al., 2007 [30] |
| Chick | Dorsal root ganglion neurons (primary culture) | Docked mitochondria as fiducial markers for bulk cytoskeleton movements | Dynein | Roossien et al., 2014 [31] |
| Drosophila melanogaster | Larval brain neurons (primary culture) and S2 cells (tissue culture) | GFP-CAMSAP (minus-end microtubule marker) and EB1-GFP (plus-end microtubule marker) | Dynein | Del Castillo et al., 2015 [14] |
Outstanding Questions.
Is there a specific mechanism that switches motors from the cargo-transporting to a microtubule-transporting mode?
How is actin dynamics at the growth cone coupled to microtubule sliding?
How is dynein anchored to the cortex of neurites?
Do differential regulation and/or anchoring of dynein contribute to differentiation of axons and dendrites?
Is microtubule hitchhiking a major mode of cargo transport in any cell type? What types of cargoes are translocated by this mechanism?
Trends.
Contrary to the textbook view, cytoplasmic microtubules in interphase cells are not static; they robustly slide in the cytoplasm moved by microtubule motors.
Two motors contribute to microtubule sliding: kinesin-1 that slides microtubules against each other, and cytoplasmic dynein that drives microtubule movement against cell cortex
Sliding microtubules can drive cell shape change by pushing against the plasma membrane.
Plus-end motor kinesin-1 and minus-end motor dynein cooperate to achieve the correct microtubule organization in the axons.
Microtubule sliding activity is developmentally regulated, independently of the global regulation of the motor activity.
F-actin in the growth cone of growing neurites antagonizes the neurite outgrowth by preventing sliding microtubules from penetration into the growth cone.
Cargoes can be transported by hitchhiking on moving microtubules.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirokawa N, et al. Kinesin superfamily motor proteins and intracellular transport. Nature reviews. Molecular cell biology. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 2.Rice S, et al. A structural change in the kinesin motor protein that drives motility. Nature. 1999;402:778–784. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]
- 3.Hackney DD, Stock MF. Kinesin’s IAK tail domain inhibits initial microtubule-stimulated ADP release. Nature cell biology. 2000;2:257–260. doi: 10.1038/35010525. [DOI] [PubMed] [Google Scholar]
- 4.Seeger MA, Rice SE. Microtubule-associated protein-like binding of the kinesin-1 tail to microtubules. The Journal of biological chemistry. 2010;285:8155–8162. doi: 10.1074/jbc.M109.068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navone F, et al. Cloning and expression of a human kinesin heavy chain gene: interaction of the COOH-terminal domain with cytoplasmic microtubules in transfected CV-1 cells. The Journal of cell biology. 1992;117:1263–1275. doi: 10.1083/jcb.117.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winding M, et al. Role of kinesin-1-based microtubule sliding in Drosophila nervous system development. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E4985–4994. doi: 10.1073/pnas.1522416113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straube A, et al. Conventional kinesin mediates microtubule-microtubule interactions in vivo. Molecular biology of the cell. 2006;17:907–916. doi: 10.1091/mbc.E05-06-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jolly AL, et al. Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12151–12156. doi: 10.1073/pnas.1004736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlan K, et al. The microtubule-binding protein ensconsin is an essential cofactor of kinesin-1. Current biology : CB. 2013;23:317–322. doi: 10.1016/j.cub.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling SC, et al. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H, et al. Microtubule binding by dynactin is required for microtubule organization but not cargo transport. The Journal of cell biology. 2007;176:641–651. doi: 10.1083/jcb.200608128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulic IM, et al. The role of microtubule movement in bidirectional organelle transport. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10011–10016. doi: 10.1073/pnas.0800031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ally S, et al. Opposite-polarity motors activate one another to trigger cargo transport in live cells. The Journal of cell biology. 2009;187:1071–1082. doi: 10.1083/jcb.200908075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Castillo U, et al. Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in Drosophila neurons. eLife. 2015;4 doi: 10.7554/eLife.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu W, et al. Initial Neurite Outgrowth in Drosophila Neurons Is Driven by Kinesin-Powered Microtubule Sliding. Current biology : CB. 2013;23:1018–1023. doi: 10.1016/j.cub.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu W, et al. Kinesin-1-powered microtubule sliding initiates axonal regeneration in Drosophila cultured neurons. Molecular biology of the cell. 2015;26:1296–1307. doi: 10.1091/mbc.E14-10-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.del Castillo U, et al. Pavarotti/MKLP1 regulates microtubule sliding and neurite outgrowth in Drosophila neurons. Current biology : CB. 2015;25:200–205. doi: 10.1016/j.cub.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira A, et al. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. The Journal of cell biology. 1992;117:595–606. doi: 10.1083/jcb.117.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaven R, et al. Drosophila CLIP-190 and mammalian CLIP-170 display reduced microtubule plus end association in the nervous system. Molecular biology of the cell. 2015;26:1491–1508. doi: 10.1091/mbc.E14-06-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan J, et al. Kinesin-1 regulates dendrite microtubule polarity in Caenorhabditis elegans. eLife. 2013;2:e00133. doi: 10.7554/eLife.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu W, et al. Microtubule-microtubule sliding by kinesin-1 is essential for normal cytoplasmic streaming in Drosophila oocytes. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E4995–5004. doi: 10.1073/pnas.1522424113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. Journal of cell science. 1995;108(Pt 8):2761–2769. doi: 10.1242/jcs.108.8.2761. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad FJ, et al. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. The Journal of cell biology. 1998;140:391–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Y, et al. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. The Journal of cell biology. 2005;168:697–703. doi: 10.1083/jcb.200407191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad FJ, et al. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nature cell biology. 2000;2:276–280. doi: 10.1038/35010544. [DOI] [PubMed] [Google Scholar]
- 27.Rao AN, et al. Sliding of centrosome-unattached microtubules defines key features of neuronal phenotype. The Journal of cell biology. 2016;213:329–341. doi: 10.1083/jcb.201506140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehmelt L, et al. A microtubule-based, dynein-dependent force induces local cell protrusions: Implications for neurite initiation. Brain cell biology. 2006;35:39–56. doi: 10.1007/s11068-006-9001-0. [DOI] [PubMed] [Google Scholar]
- 29.Mazel T, et al. Direct observation of microtubule pushing by cortical dynein in living cells. Molecular biology of the cell. 2014;25:95–106. doi: 10.1091/mbc.E13-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grabham PW, et al. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:5823–5834. doi: 10.1523/JNEUROSCI.1135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roossien DH, et al. Cytoplasmic dynein pushes the cytoskeletal meshwork forward during axonal elongation. Journal of cell science. 2014;127:3593–3602. doi: 10.1242/jcs.152611. [DOI] [PubMed] [Google Scholar]
- 32.Muresan V, Muresan Z. Unconventional functions of microtubule motors. Archives of biochemistry and biophysics. 2012;520:17–29. doi: 10.1016/j.abb.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanenbaum ME, et al. Cytoplasmic dynein crosslinks and slides anti-parallel microtubules using its two motor domains. eLife. 2013;2:e00943. doi: 10.7554/eLife.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Castillo U, et al. Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in neurons. eLife. 2015;4 doi: 10.7554/eLife.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yau KW, et al. Dendrites In Vitro and In Vivo Contain Microtubules of Opposite Polarity and Axon Formation Correlates with Uniform Plus-End-Out Microtubule Orientation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:1071–1085. doi: 10.1523/JNEUROSCI.2430-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu W, et al. Organelle transport in cultured Drosophila cells: s2 cell line and primary neurons. Journal of visualized experiments : JoVE. 2013 doi: 10.3791/50838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minestrini G, et al. Domains of the Pavarotti kinesin-like protein that direct its subcellular distribution: effects of mislocalisation on the tubulin and actin cytoskeleton during Drosophila oogenesis. Journal of cell science. 2002;115:725–736. doi: 10.1242/jcs.115.4.725. [DOI] [PubMed] [Google Scholar]
- 38.Minestrini G, et al. Localization of Pavarotti-KLP in living Drosophila embryos suggests roles in reorganizing the cortical cytoskeleton during the mitotic cycle. Molecular biology of the cell. 2003;14:4028–4038. doi: 10.1091/mbc.E03-04-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mogessie B, et al. A novel isoform of MAP4 organises the paraxial microtubule array required for muscle cell differentiation. eLife. 2015;4:e05697. doi: 10.7554/eLife.05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baas PW, Brown A. Slow axonal transport: the polymer transport model. Trends in cell biology. 1997;7:380–384. doi: 10.1016/S0962-8924(97)01148-3. [DOI] [PubMed] [Google Scholar]
- 41.Hirokawa N, et al. Slow axonal transport: the subunit transport model. Trends in cell biology. 1997;7:384–388. doi: 10.1016/S0962-8924(97)01133-1. [DOI] [PubMed] [Google Scholar]
- 42.Reinsch SS, et al. Microtubule polymer assembly and transport during axonal elongation. The Journal of cell biology. 1991;115:365–379. doi: 10.1083/jcb.115.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka EM, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axon elongation. The Journal of cell biology. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Brown A. Rapid movement of microtubules in axons. Current biology : CB. 2002;12:1496–1501. doi: 10.1016/s0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- 45.Lim SS, et al. Progressive and spatially differentiated stability of microtubules in developing neuronal cells. The Journal of cell biology. 1989;109:253–263. doi: 10.1083/jcb.109.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim SS, et al. A test of microtubule translocation during neurite elongation. The Journal of cell biology. 1990;111:123–130. doi: 10.1083/jcb.111.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okabe S, Hirokawa N. Differential behavior of photoactivated microtubules in growing axons of mouse and frog neurons. The Journal of cell biology. 1992;117:105–120. doi: 10.1083/jcb.117.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slaughter T, et al. Microtubule transport from the cell body into the axons of growing neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:5807–5819. doi: 10.1523/JNEUROSCI.17-15-05807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu WQ, Baas PW. The Growth of the Axon Is Not Dependent Upon Net Microtubule Assembly at Its Distal Tip. Journal of Neuroscience. 1995;15:6827–6833. doi: 10.1523/JNEUROSCI.15-10-06827.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu W, et al. Microtubule transport and assembly during axon growth. The Journal of cell biology. 1996;133:151–157. doi: 10.1083/jcb.133.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marsh L, Letourneau PC. Growth of Neurites without Filopodial or Lamellipodial Activity in the Presence of Cytochalasin-B. Journal of Cell Biology. 1984;99:2041–2047. doi: 10.1083/jcb.99.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forscher P, Smith SJ. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. The Journal of cell biology. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joshi HC, et al. Tension and compression in the cytoskeleton of PC 12 neurites. The Journal of cell biology. 1985;101:697–705. doi: 10.1083/jcb.101.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- 55.Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruthel G, Hollenbeck PJ. Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:2266–2274. doi: 10.1523/JNEUROSCI.20-06-02266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flynn KC, et al. ADF/cofilin-mediated actin retrograde flow directs neurite formation in the developing brain. Neuron. 2012;76:1091–1107. doi: 10.1016/j.neuron.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 58.Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. Journal of cell science. 2009;122:3595–3604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahn OI, Baas PW. Microtubules and Growth Cones: Motors Drive the Turn. Trends Neurosci. 2016;39:433–440. doi: 10.1016/j.tins.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee S, et al. short stop is allelic to kakapo, and encodes rod-like cytoskeletal-associated proteins required for axon extension. Journal of Neuroscience. 2000;20:1096–1108. doi: 10.1523/JNEUROSCI.20-03-01096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee S, Kolodziej PA. Short Stop provides an essential link between F-actin and microtubules during axon extension. Development. 2002;129:1195–1204. doi: 10.1242/dev.129.5.1195. [DOI] [PubMed] [Google Scholar]
- 62.Chia JX, et al. Neurite outgrowth is driven by actin polymerization even in the presence of actin polymerization inhibitors. Molecular biology of the cell. 2016;27:3695–3704. doi: 10.1091/mbc.E16-04-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casella JF, et al. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981;293:302–305. doi: 10.1038/293302a0. [DOI] [PubMed] [Google Scholar]
- 64.Spector I, et al. Latrunculins - Novel Marine Macrolides That Disrupt Microfilament Organization and Affect Cell-Growth. 1. Comparison with Cytochalasin-D. Cell motility and the cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- 65.Koonce MP, et al. Active Sliding between Cytoplasmic Microtubules. Nature. 1987;328:737–739. doi: 10.1038/328737a0. [DOI] [PubMed] [Google Scholar]
- 66.Euteneuer U, et al. Microtubule Bundles of Reticulomyxa Networks Are of Uniform Polarity. Eur J Cell Biol. 1989;49:373–376. [PubMed] [Google Scholar]
- 67.Orokos DD, et al. Organelles are transported on sliding microtubules in Reticulomyxa. Cell motility and the cytoskeleton. 2000;47:296–306. doi: 10.1002/1097-0169(200012)47:4<296::AID-CM4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 68.Glotzer JB, et al. Cytoplasmic flows localize injected oskar RNA in Drosophila oocytes. Current biology : CB. 1997;7:326–337. doi: 10.1016/s0960-9822(06)00156-4. [DOI] [PubMed] [Google Scholar]
- 69.Forrest KM, Gavis ER. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Current biology : CB. 2003;13:1159–1168. doi: 10.1016/s0960-9822(03)00451-2. [DOI] [PubMed] [Google Scholar]
- 70.Mische S, et al. Direct observation of regulated ribonucleoprotein transport across the nurse cell/oocyte boundary. Molecular biology of the cell. 2007;18:2254–2263. doi: 10.1091/mbc.E06-10-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]


