Summary
Asymmetric cell division (ACD) gives rise to two daughter cells with distinct fates. ACD is widely used during development and by many types of adult stem cells during tissue homeostasis and regeneration. ACD can be regulated by extrinsic cues, such as signaling molecules, as well as by intrinsic factors, such as organelles and cortex proteins. The recent discovery of asymmetric histone inheritance during stem cell ACD has revealed another intrinsic mechanism by which ACD produces two distinct daughters. In this review, we will discuss these findings in the context of cell cycle regulation, as well as other studies of ACD, to begin understanding the underlying mechanisms and biological relevance of this phenomenon.
Keywords: stem cell, asymmetric cell division, histone, epigenetic inheritance
Introduction
Stem cells have a remarkable ability to both self-renew and generate daughter cells capable of differentiating to replace lost or damaged tissue. To achieve this balance, many different types of stem cells utilize asymmetric cell division (ACD) to generate two distinct daughter cells capable of self-renewal and differentiation, respectively. As a crucial mechanism for tissue homeostasis and repair, ACD must be tightly controlled to prevent excessive differentiation or proliferation, which can lead to diseases ranging from tissue dystrophy to cancer. In order to minimize risk of disease, stem cells utilize a variety of epigenetic mechanisms to regulate their proper activity.
Epigenetic phenomena refer to modifications made to DNA or DNA-associated proteins that do not alter DNA sequences but do lead to inheritable changes in gene expression. Many epigenetic phenomena affect changes in gene expression via altering DNA structure and/or packaging. In eukaryotic cells, genomic DNA primarily achieves higher order structure through interactions with the histone octamer. The fundamental building blocks of the octamer are the histone proteins H3, H4, H2A and H2B, which come together in the form of one (H3–H4)2 tetramer and two (H2A-H2B) dimers. The histone octamer is capable of wrapping 147 base pairs of DNA to create a structure known as the nucleosome, the fundamental unit used to form higher order chromatin structures inside the nucleus [1–8]. Post-translational modifications of histones can further modulate the compaction and accessibility of surrounding DNA. Together, histones and their post-translational modifications represent important epigenetic mechanisms for regulating gene expression in numerous developmental contexts [7, 9]. For example, epigenetic mechanisms have been recognized as important regulators for stem cell activities in multiple stem cell lineages [10]. Many studies have demonstrated that epigenetic modifications are inheritable, both through cell divisions and across generations [11, 12]. However, with the exception of covalent modifications made to DNA, little is known about the molecular mechanisms and cellular basis of epigenetic inheritance [13, 14] and how it may contribute to ACD.
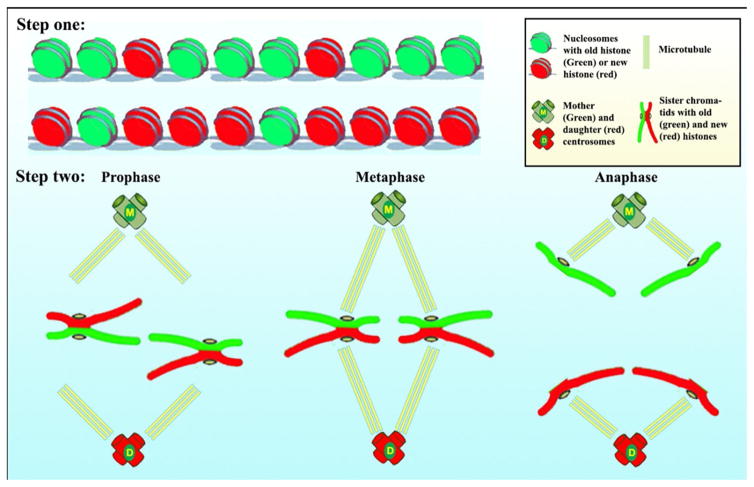
Recent investigations into ACD of the Drosophila male germline stem cell (GSC) have revealed that old histone 3 (H3) is selectively segregated to the self-renewed GSC, whereas new H3 is enriched in the differentiating daughter cell (gonialblast, GB) [15]. Notably, this asymmetry shows both cellular and molecular specificity. From a cellular perspective, asymmetric histone inheritance is specific to asymmetrically dividing GSCs, as symmetrically dividing progenitor germ cells show symmetric inheritance pattern. From a molecular perspective, asymmetric histone inheritance is specific to H3, as the histone variant H3.3 does not exhibit an asymmetric inheritance pattern during GSC ACD. Since H3 is incorporated during DNA replication (S phase), whereas H3.3 is incorporated independent of replication, we propose a two-step model (Figure 1) in which step one involves the establishment of asymmetry: during S phase, old H3 are retained in one set of sister chromatids, while new H3 are differentially incorporated into the other set of sister chromatids. Step two involves asymmetry recognition: during mitosis (M phase), the set of sister chromatids containing old H3 is segregated to GSCs, while the set of sister chromatids enriched with new H3 is segregated to the differentiating daughter cell. We hypothesize that the mitotic machinery is regulated in ACD to recognize the epigenetic asymmetry between sister chromatids to ensure proper attachment by spindle followed by segregation.
Figure 1. A two-step model to explain the asymmetric H3 inheritance in Drosophila male GSC ACD.
Step one: The old (green) and the new (red) histone H3 differentially deposited on the two chromatids at the gene locus. Step two: The sister chromatids with asymmetric H3 are asymmetrically segregated. The mother centrosome is in proximity with the stem cell niche.
The process of asymmetric histone inheritance likely involves coordination of a wide variety of cellular mechanisms throughout the cell cycle. To better understand how various cellular machineries may contribute to this phenomenon, we will discuss how the major cell cycle events may impact asymmetric H3 establishment and segregation. In the first part of this review, we will discuss current knowledge about the roles of DNA replication in histone incorporation. We will discuss intrinsic asymmetries during the process of DNA replication and their potential contributions to establishing asymmetric histone incorporation. In the second part of this review, we will discuss how mitotic machinery may communicate with the epigenetically distinct sister chromatids to allow for their differential recognition and segregation during M phase.
Step one: establishment of histone asymmetry during DNA replication (S phase)
Recycling of old histones during DNA replication
A critical question in the field of epigenetics concerns understanding how histones are efficiently recycled during DNA replication to ensure that the desired epigenetic state is passed on to each of the two daughter cells. In the case of a symmetric cell division, epigenetic information must be passed evenly to both daughter cells in order to preserve the same gene expression as that of the mother cell. Conversely, in the case of an ACD, epigenetic information could be asymmetrically partitioned in order to produce two daughter cells with distinct cell fates. In either instance (symmetric or asymmetric), the process of DNA replication presents a uniquely difficult challenge for cells to effectively maintain epigenetic information, because nucleosomes must be disassembled and stripped from DNA in order to allow for unhindered progression of the replication fork [16, 17]. In the wake of the replication fork, nucleosomes must be reassembled on newly synthesized DNA. The process of replication-coupled nucleosome assembly occurs via coordination of two key processes: recycling of old histones and incorporation of new histones [18–21] (Figure 2). Although significant progress has been made in unraveling the processes by which new histones are incorporated, much less is known about how old histones are recycled. As old histones contain post-translational modifications capable of defining cell type- and stage-specific gene expression, a better understanding of how they are recycled should provide invaluable insight into our understanding of epigenetic inheritance. Pioneering efforts have demonstrated that old histones are indeed recycled and that reconstitution of histone octamer structure occurs almost immediately after passage of the replication fork [19–21]. Several models have been proposed to explain the molecular mechanisms that underlie recycling old histones at the replication fork.
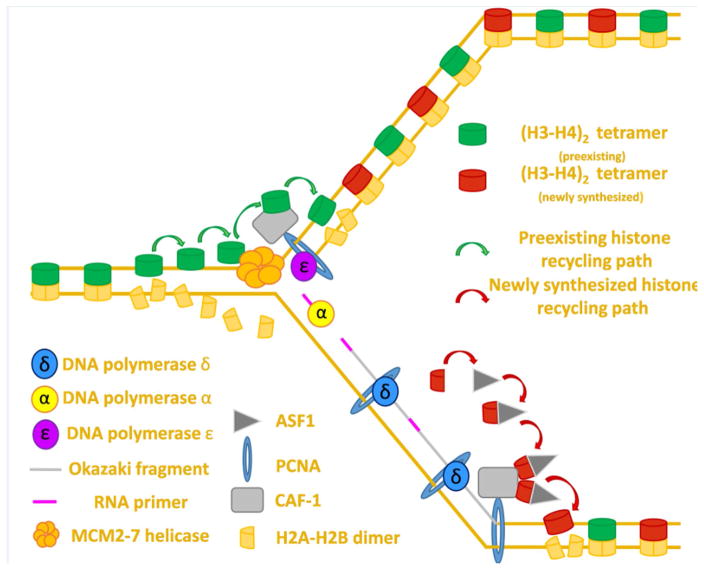
Figure 2. Proposed mechanism for directed histone recycling during DNA replication.
DNA replication overview: Polymerase ε synthesizes the leading strand continuously whereas polymerase δ synthesizes the lagging strand in discontinuous segments known as Okazaki fragments. Polymerase α synthesizes an RNA primer at the start of the leading strand (not shown) and at the start of each new Okazaki fragment.
Old histone recycling: The MCM2–7 helicase sits at the foremost edge of the replication fork where its primary function is to unwind non-replicated DNA. The MCM2 protein can bind (H3–H4)2 tetramers dissociated in the wake of the advancing fork to allow for their subsequent deposition on nascent DNA. Due to its ability to bind (H3–H4)2 tetramers, CAF-1 may help coordinate the deposition of preexisting histones after fork passage. CAF-1 is recruited to the edge of the advancing fork by PCNA, which also serves as a processivity factor for the replicative polymerases δ and ε.
New histone deposition: Histone chaperone ASF-1 binds (H3–H4) dimers in the cytoplasm and coordinates their import into the nucleus where ASF-1 transfers (H3–H4) dimers to CAF-1. CAF-1 then coordinates new H3–H4 deposition onto nascent DNA. The subsequent incorporation of two (H2A–H2B) dimers reforms the nucleosome structure and signifies the end of the process of replication coupled histone deposition.
Three models to explain old histone reincorporation at the replication fork
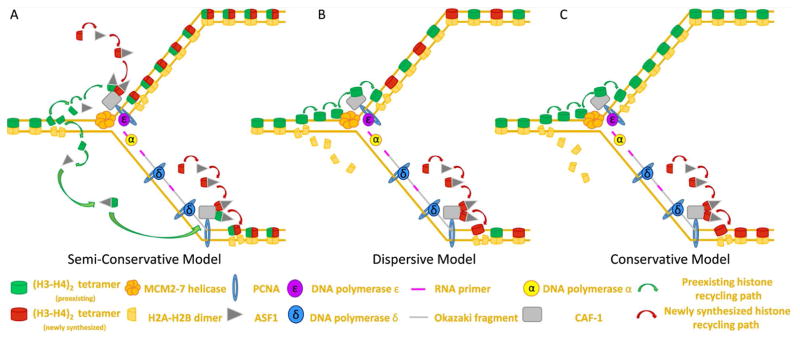
Early insights into histone composition of the nucleosome prompted researchers to propose that histones, like DNA, could be inherited via a semi-conservative mechanism. As stated previously, the octamer is composed of one (H3–H4)2 tetramer and two (H2A-H2B) dimers. Since most post-translational modifications known to impact gene expression are found at the N-termini of H3 and H4, many studies investigating histone inheritance have focused on understanding the behavior of the (H3–H4)2 tetramer at the replication fork [22]. Under the semi-conservative model, the (H3–H4)2 tetramer would be split into two dimers, each of which would contain the relevant epigenetic information necessary to propagate the parental chromatin state to each of the newly synthesized sister chromatids [9, 23] (Figure 3A). However, several findings have provided evidence suggesting that semi-conservative histone inheritance is not the primary means by which cells transmit epigenetic information globally during DNA replication. First, mass spectrometry analysis of nucleosome particles has demonstrated that (H3–H4) dimers are not symmetrically modified in (H3–H4)2 tetramers [24]. Because of this asymmetry in tetramer modifications, dimers resulting from tetramer splitting would not contain all original post-translational modifications. Second, (H3–H4)2 tetramers rarely split, meaning that mixed tetramers comprised of old and new (H3–H4) dimers rarely ever form [25]. Interestingly, previous studies have revealed that tetramers containing the histone variant H3.3 shows a higher frequency of splitting when compared to H3-containing tetramers (~23% for H3.3 vs. ~3% for H3). Furthermore, subsequent studies have demonstrated that splitting events appear to be associated with cell-type specific enhancer elements [26]. It is unlikely that semi-conservative histone inheritance regulates epigenetic inheritance in a genome-wide manner. However, these collective findings suggest the possibility that this inheritance pattern may contribute to the symmetric propagation of a desired epigenetic state onto the two sister chromatids at particular genomic loci.
Figure 3. Proposed models for directed histone recycling at the replication fork.
(A) Semi-conservative model: Old (H3–H4)2 tetramers are split at the replication fork, possibly by ASF1, with the resulting (H3–H4) dimers being inherited equally on both the leading and the lagging strand.
(B) Dispersive model: Old (H3–H4)2 tetramers remain together at the fork and are randomly partitioned between leading vs. lagging strand to ensure global symmetry of old vs. new on newly synthesized daughter strands.
(C) Conservation model: Old (H3–H4)2 tetramers remain un-split at the fork and are inherited as a tetramer. In this model, tetramers are biased in their inheritance such that either the leading strand (shown) or the lagging strand (not shown) inherits a majority of the old (H3–H4)2 tetramers.
Therefore, two additional models have been proposed to explain how histone incorporation might occur during DNA replication: the dispersive model (Figure 3B) and the conservative model (Figure 3C). Both models propose that the (H3–H4)2 tetramer is inherited as an intact unit. However, the dispersive model proposes that old (H3–H4)2 tetramers are segregated randomly at the fork such that approximately half of the (H3–H4)2 displaced from the parental strand are recycled to the leading strand and the other half are incorporated onto the lagging strand (Figure 3B). On the other hand, the conservative model hypothesizes that (H3–H4)2 tetramers are biased in their incorporation at the fork such that either the leading strand (Figure 3C) or the lagging strand preferentially inherits more of the old (H3–H4)2 tetramers recycled from the parental strand. Early investigations suggest that preexisting histones are incorporated in a conservative manner, displaying a clear strand preference during recycling events [27–29]. Subsequent experiments indicate that old histones are preferentially reincorporated to the leading strand [30]. Different from these early findings, later explorations argue instead for a random dispersive model of histone inheritance. The debate between non-random (conservative) vs. random (dispersive) mode of histone incorporation eventually shift in favor of the dispersive model, as experiments using heavy isotope labeling reveal no clear bias in histone deposition [19, 31, 32]. However, these models are not mutually exclusive in nature, and evidence for the dispersive model does not wholly disprove the existence of conservative histone incorporation. Indeed, while ample evidence exists to demonstrate dispersive histone inheritance on a more global scale, certain observations, such as the presence of nucleosomal oligomers containing old histones on nascent chromatin in the absence of new protein synthesis and the tendency for adjacent histone octamers to co-segregate during replication, suggest that a smaller scale, old histones can coordinate their incorporation in a conservative manner to either the leading or the lagging strand [33]. Finally, because many of these questions are extremely challenging to study in an in vivo context, it remains possible that different cell types in a multicellular system may utilize different histone incorporation modes, possibly as a mechanism to achieve differential epigenetic outcomes during development.
Roles of histone chaperones and replication machinery components in old histone recycling and new histone deposition
To better understand how histone inheritance could be used to both establish and maintain cell fate choices during development, a significant amount of effort has been directed towards understanding the molecular players involved in histone recycling and deposition at the replication fork. Previous studies have demonstrated that (H3–H4) dimers are initially bound in the cytoplasm by the histone chaperone ASF1 and subsequently transported into the nucleus where ASF1 interacts with and transfers the (H3–H4) dimer to another histone chaperone, CAF-1 [34]. CAF-1 is capable of binding to multiple (H3–H4) dimers and is recruited to the advancing replication fork via interactions with Proliferating cell nuclear antigen (PCNA) [35, 36]. By binding multiple dimers, recent studies have demonstrated that CAF-1 facilitates the assembly of the new (H3–H4)2 tetramer prior to their deposition (Figure 2) [37].
In addition to roles in new histone deposition, recent evidence has suggested that the chaperones also contribute to efficient recycling of old histones. Recent co-immunoprecipitation studies suggest that ASF1 can form a complex with (H3–H4) dimers bearing post-transcriptional modifications characteristic of old histones [38, 39]. Such finding suggests that ASF1 may play a role in splitting the (H3–H4)2 tetramer. Furthermore, CAF-1 has been shown to bind to (H3–H4)2 tetramer, suggesting a potential route by which unsplit tetramers are recycled onto newly synthesized DNA (Figure 2) [40].
Another key component of the replication machinery which has essential roles in both DNA replication and epigenome maintenance is mini-chromosome maintenance 2 (MCM2) protein, a subunit of the MCM2–7 replicative helicase. MCM2 has been shown to bind old (H3–H4)2 tetramers following their dissociation during DNA replication. By binding to (H3–H4)2 tetramers along the DNA-binding surface, the MCM2 protein is able to retain preexisting histones at the replication fork to ensure their rapid and faithful deposition onto newly synthesized DNA [41]. Interestingly, in eukaryotes, the MCM2–7 helicase consistently associates with the leading strand. The proximity of the leading strand towards the MCM2 protein raises the intriguing possibility that this close association may bias reincorporation of old histones onto the leading strand. This bias may serve as one explanation for the leading strand preference to recycle old histones shown by previous studies (Figure 3C). In addition to the MCM2–7 helicase, many other components of the DNA replication machinery show preferential association with either the leading strand or the lagging strand, which could act synergistically to bias histone inheritance (Table 1, Figure 2) [42].
Table 1.
| Replication Component | Strand enrichment |
|---|---|
| DNA polymerase ε | Leading strand |
| CDC45 | Leading strand |
| MCM2–7 | Leading strand |
| MCM10 | Leading strand |
| GINS | Leading strand |
| DNA polymerase δ | Lagging strand |
| DNA polymerase α | Lagging strand |
| DNA ligase | Lagging strand |
| PCNA | Lagging strand |
| Rfc1 | Lagging strand |
| RPA | Lagging strand |
| FEN1 | Lagging strand |
| DNA2 | Lagging strand |
| RNase H | Lagging strand |
Recent research efforts have uncovered a developmental program in C. elegans which may utilize molecular asymmetries associated with replication-coupled nucleosome assembly to generate distinct cell fates during development. It has been demonstrated that a gain-of-function mutation in an H3-encoding gene (his-9) is sufficient to disrupt neuronal bilateral asymmetry in the developing worm. The his-9 (Q125ochre) mutation eliminates two residues, leucine 126 and isoleucine 130, which are thought to engage in H3-H3 interaction. The nature of this mutation would disrupt (H3–H4)2 tetramer formation and inhibit CAF-1-mediated nucleosome assembly at the replication fork. Consistently, a similar loss of neuronal asymmetry phenotype could be recapitulated upon knocking down either components of worm CAF-1 complex or pcn-1, which encodes the PCNA ortholog, the key players in replication-coupled nucleosome assembly. Together these results suggest that replication-coupled nucleosome assembly has an essential role in regulating asymmetric cell fate decision (neuron vs. muscle) during development [43].
Even though the precise mechanisms behind the loss of asymmetric cell fate described above remain elusive, authors hypothesize that this may result from nucleosome density differences on leading vs. lagging strands. Based on elevated density of PCNA molecules present on lagging strand, it has been proposed that the lagging stand could contain a higher nucleosomal density when compared to the leading strand [42, 44, 45]. If nucleosomal density is different at critical developmental genes, it could serve as a mechanism to drive differential gene expression and distinct cell fate decisions during development. In addition to nucleosomal density, it is conceivable that many variable features of nucleosomal structure, ranging from the presence of histone variants (H3 vs. H3.3) to the enrichment of different post-translational modifications, may help to bias cell fate decisions from ACD. Regardless of the nature of the asymmetry, any epigenetic difference established during DNA replication would be ineffectual in shaping cell fate decisions without proper recognition by the mitotic machinery.
Step two: histone asymmetry recognition during mitosis (M phase)
Asymmetrically dividing cells could utilize a cohort of trans-factors (chromatin-independent) and cis-modifications (chromatin-bound) to recognize and segregate epigenetically distinct sister chromatids into the two daughter cells. In light of the discovered asymmetric centrosome inheritance as a trans-factor [46] and differential phosphorylation at Threonine 3 of H3 (H3T3P) at sister chromatids as a cis-modification [47], we herein consider potential cellular mechanisms which could act together to read out the epigenetic difference between the two sets of sister chromatids.
Trans asymmetry I: asymmetric inheritance of mother vs. daughter centrosomes
Centrosomes, each of which consists of two centrioles, serve as the main microtubule-organizing center (MTOC) and play a pivotal role in regulating cell cycle progression. Centriole duplication occurs during DNA replication to produce one mother centrosome and one daughter centrosome, both of which have MTOC activity to nucleate microtubules in order to form the mitotic spindle. Centriole matures with the accumulation of γ-tubulin ring complexes and other pericentriolar matrix proteins [48]. The increase of γ-tubulin allows greater MTOC activity for the more mature centriole. Microtubule nucleation sites are preferentially placed on, or near, the more mature centriole [49–51]. Mother centrosome and daughter centrosome can be distinguished structurally and functionally [46, 52–54]. For example, the mother centrosome contains the oldest centriole and has more appendages at the distal/sub-distal end than the daughter centrosome, as shown by electron microscopy.
Intriguingly, in several ACD model systems, centrosomes exhibit a variety of asymmetric behaviors, including orientation, segregation and maturation [55, 56]. In Drosophila male GSCs, the mother centrosome containing the more mature centriole is consistently located towards the GSC-niche interface, while the daughter centrosome migrates to the opposite side of the cell, leading to spindle orientated in perpendicular to the GSC-niche interface [46, 57]. This arrangement of mother and daughter centrosome locations is maintained because the mother centrosome nucleates significantly more astral microtubules compared to the daughter centrosome, which is thought to anchor the mother centrosome at the GSC-hub interface [46]. Together, the consistent positioning of centrosomes ensures spindle polarity for the asymmetric outcome of GSC ACD, which serves as an important trans-factor in this system.
Potential trans asymmetry II: asymmetric kinetochore components
The kinetochore is a massive and highly organized multiprotein structure assembled at the outer surface of centromeres, and it serves as an important trans-factor for proper attachment and segregation of sister chromatids. Because the kinetochore orchestrates the attachment from spindle microtubule to chromatids, it could coordinate centrosome polarity with sister chromatid asymmetry during ACD. A large number of proteins have been identified to participate in kinetochore assembly, and these can be categorized into several intimately interacting complexes. In general, three major kinetochore complexes are known: the centromere-associated inner kinetochore, the microtubule-interacting outer kinetochore, and the bridging complex connecting the inner and outer complexes. Among them, the inner kinetochore stays with centromeres, while the outer kinetochore assembles upon mitotic entry to interact with the mitotic spindle [58].
Using budding yeast S. cerevisiae, it has been reported that several kinetochore components show asymmetric partitioning pattern in a lineage-specific manner. In this experimental design, diploid yeast cells carrying differentially tagged kinetochore genes (one allele tagged with YFP and another allele tagged with CFP) were induced to undergo meiosis to give rise to haploid spores. Each spore contains only one allele (either YFP allele or CFP allele), but both YFP- and CFP-tagged kinetochore proteins. Remarkably, upon mating and entry into mitosis, either YFP-tagged or CFP-tagged kinetochore protein which is no longer synthesized by the lack of coding allele, shows ~ 2-fold asymmetric inheritance between the two daughter cells. Intriguingly, this asymmetry is detected in all tested kinetochore components spanning from inner to outer kinetochore complex, suggesting that the entire kinetochore is possibly assembled asymmetrically. Furthermore, this asymmetry appears to be specific to the kinetochore components, as non-kinetochore components tested using the same experimental strategy all show symmetric pattern. Notably, starting from the first ACD after germination, a spore gives rise to a mother and a bud, and hereafter. After multiple rounds of ACD, among the many mothers only one lineage maintains the mother identity descended directly from the very first mother. Another unique characteristic for this kinetochore asymmetry is that it is specifically detected in the ACD of this mother cell lineage, but not those descended from the bud cells, [59]. This inheritance pattern very much resembles the adult stem cell system in which only one daughter cell from stem cell ACD retains stemness, whereas all derivative cells from the other daughter cell are either progenitor cells or differentiated cells [60]. This phenomenon suggests that asymmetric kinetochore inheritance may also exist in asymmetrically dividing stem cells in multicellular organisms and is, therefore, an interesting question to pursue in future studies.
Although the function of this kinetochore asymmetry is unclear, it is speculated to be related to the asymmetrically inherited spindle pole body (centrosome equivalent) in S. cerevisiae [61, 62]. However, the lineage that shows asymmetric kinetochore inheritance is only a subset of those asymmetrically segregating spindle pole bodies. Another hypothetical function of this kinetochore asymmetry is to partition a particular centromere or centromere-associated proteins into a specific lineage. During meiosis in female metazoan species, a phenomenon called ‘meiotic drive’ has been reported to select the allele with the strongest kinetochore to become oocyte, while the remaining alleles become degenerative polar bodies [63, 64]. During this selection process, the stronger kinetochore often associates with longer centromere [65]. However, such function in mitosis, especially in ACD, has not been reported. It is conceivable that kinetochore asymmetry may provide a mechanism for selective sister chromatid segregation. Further investigations are necessary to address how kinetochore asymmetry might be linked to centrosome asymmetry and how they cooperate during ACD.
Potential cis asymmetry I: centromere-specific chromatin structure
The centromere represents a specialized region of the chromosome [66], which serves not only as an assembly platform for kinetochore, but also as an active participant in spindle checkpoint important for sister chromatid segregation [67]. A hallmark of all eukaryotic centromeric nucleosomes is the centromere-specific H3 variant CENP-A/CenH3, which is structurally different from the canonical H3 [68, 69]. Studies using atomic force microscopy have revealed that some native CENP-A nucleosomes have only half the height of H3-containing octamers, leading to the hypothesis of ‘tetrasome’ [70] or ‘hemisome’ [71, 72] structure. In Cid (Drosophila CENP-A ortholog) overexpressing cells, ectopic kinetochores could be detected at non-centromeric region [73], suggesting that CENP-A is the key determinant of centromere identity and kinetochore assembly [74].
At normal centromeric region, CENP-A/Cid-containing nucleosomes are normally interspersed with H3-containing nucleosomes [75, 76]. The differences between CENP-A- and H3-containing nucleosomes likely change the chromosomal structure at the centromeric region. Both CENP-A and H3 nucleosomes are enriched with specific post-translational modifications which have been shown to play important roles in regulating the structure and function of the centromere. For example, phosphorylation at Ser68 of CENP-A regulates its binding to chaperons HJURP [77] necessary for new CENP-A incorporation. A similar function has also been found for the centromere-enriched H3K4me2 [78]. In addition, H3K9me2 is present at the pericentromeric domain and has been shown to recruit heterochromatin protein 1 alpha (HP1α) for heterochromatin formation and gene expression silencing [79].
Based on the unique features and essential roles of the centromeric region during mitosis, it has been proposed that epigenetic difference between the sister chromatid centromeres could ensure that stem cells will retain their epigenetic information through many divisions [80]. Although the contribution of particular post-translational modifications of CENP-A/Cid to ACD remain elusive, a recent finding shows that differential phosphorylation on H3 enriched at peri-centromeric region regulates asymmetric histone inheritance in Drosophila male GSCs [47]. This shows how temporal changes at the peri-centromeric chromatin structure could help establish a platform distinguished by the mitotic machinery.
Cis asymmetry II: sequential phosphorylation of H3T3 orchestrates asymmetric sister chromatid segregation
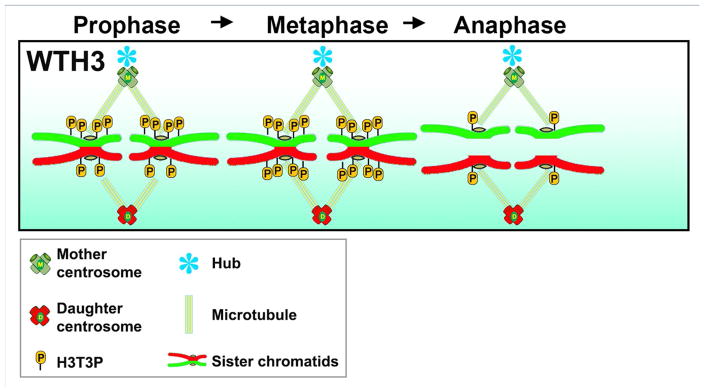
A transient phosphorylation at Thr3 of H3 (H3T3P) [81–83] was found to distinguish old vs. new H3-enriched sister chromatids in prophase GSCs [47]. Across multiple species, H3T3P has been found to be tightly regulated in both spatio- and temporal-specific manner. First, immunostaining shows that H3T3P is highly enriched at the peri-centromeric region. Second, H3T3P can only be detected during mitosis from the onset of prophase to anaphase [47, 82, 84]. In Drosophila GSCs, an additional level of temporal regulation appears to differentiate sister chromatids enriched with different levels of old vs. new H3, i.e., sister chromatids enriched with old H3 acquire more H3T3P in prophase compared to sister chromatids enriched with new H3 (Figure 4).
Figure 4.
A sequential phosphorylation at T3 of H3 (old) prior to H3 (new) ensures asymmetric sister chromatid segregation.
The sequential phosphorylation of old vs. new H3-enriched sister chromatids appears to play a role in the proper recognition and segregation of sister chromatids, as perturbation of H3T3P leads to disruption of the H3 segregation pattern. Specifically, mutations of T3 to the un-phosphorylatable residue alanine (H3T3A) or to the phosphomimetic aspartic acid (H3T3D) both disrupt the temporally asymmetric phosphorylation, resulting in a randomization of H3 inheritance patterns (Figure 5). Intriguingly, randomization of H3 inheritance patterns is accompanied by a series of germline defects, including GSC maintenance and progenitor germ cell differentiation defects. Consistently, progressively decreased male fertility was found in both H3T3A- or H3T3D-expressing testes. Compromising the activity of the Haspin kinase, which phosphorylates T3 of H3, enhances the H3T3A- induced phenotypes, but suppresses the H3T3D-induced phenotypes. As a consequence of enrichment of H3T3P at the peri-centromeric region during mitosis, this particular phosphorylation could assist asymmetric histone segregation by facilitating differential communication between sister chromatids and the polarized spindle.
Figure 5.
Both dominant negative H3T3A and phosphomimetic H3T3D randomize the segregation pattern of sister chromatids.
Indeed, the Haspin kinase has been shown to regulate the mitotic spindle across species. For example, the yeast Haspin homolog ALK1/2 has been demonstrated to potentially regulate mitotic spindle polarity [85]. It has also been reported that knockdown of Haspin in human cells ([81, 82]; reviewed by [86]) or in Xenopus [83] results in mitotic spindle defects. These reported defects upon inactivation of Haspin could be caused by failure to phosphorylate its target H3T3 or other substrates from the mitotic machinery. Interestingly, the ‘reader’ of the H3T3P called Survivin belongs to the chromosomal passenger complex, whose function has been implicated in microtubule-kinetochore interaction to align metaphase chromosomes in mammalian cells [81–83]. Further investigation is needed to understand how this temporally and spatially controlled phosphorylation is regulated in such a precise manner and how H3T3P communicates with the mitotic machinery to ensure asymmetric segregation of epigenetically distinct sister chromatids [87]. Previous studies have demonstrated that H3T3P is part of a combinatorial histone modification pattern at the mitotic chromosome, in cis with H3K4me3 and H3R8me2 [88], suggesting that particular modifications on old histone-enriched chromatid could facilitate an earlier recruitment of the Haspin kinase.
Interaction between trans-factors and cis-modifications
In order to achieve the asymmetric outcome, the polarized trans-regulators from the mitotic machinery must interact with the critical cis-modifications at sister chromatids in a tightly regulated manner. The different MTOC activities between mother and daughter centrosomes could allow for asymmetric microtubule dynamics. Meanwhile, sister chromatids with different epigenetic signatures could be poised for differential attachment by spindle microtubules during ACD. This asymmetric attachment is likely driven by asymmetries in sister centromeres and possibly through differential nucleation and strength of the outer kinetochore. Therefore, if the temporal asymmetry of the mitotic machinery coincides with the sequential modifications at the centromere region of sister chromatids, the outcome of mitosis would be greatly biased toward the asymmetric outcome. This centrosome →kinetochore →centromere→ sister chromatids axis could serve as a fundamental cellular basis for ACD.
Interestingly, recent work in mouse hematopoietic stem cell (HSC) lineage reveal that the coordination between mitotic spindle and histone modifications maintains proper stem cell activity during aging. Aged HSCs lose asymmetric distribution of the H4K16ac and the spindle polarity, which is associated with elevated RhoGTPase Cdc42 activity. Intriguingly, treatment with a Cdc42 inhibitor restores both polarities and rejuvenates aged HSCs. While the mechanisms how asymmetric H4K16ac distribution contributes to higher stem cell activity is currently unknown, this finding suggests that asymmetric histone inheritance along with their specific modifications during ACD could be conserved in mammals [89].
Concluding Remarks
Two fundamental biological processes are necessary for any live cell: DNA replication and cell division. Our current understanding of these two processes focuses primarily on how they can be reliably propagated. The prevailing view of DNA replication is that it duplicates the genome in preparation for being symmetrically partitioned during cell division, in order for one mother cell to give birth to two identical daughter cells. However, these principles cannot explain the development of any multi-cellular organism for which different cell types must be generated in a temporal- and spatio-specific manner and organized with a high degree of coordination. In other words, if DNA replication and cell division are consistently carried out in a symmetric manner, it would lead to a lump of identical tumor-like cells instead of a living organism. Despite the recognition that generating distinct cell types is crucial for animal development, tissue homeostasis and regeneration, it is extremely difficult to study this question at single-cell resolution.
Stem cells provide a promising opportunity to study this important question given the high-throughput feature of ACD during post-embryonic stage. The finding of asymmetric histone inheritance during Drosophila male GSC ACD has shed light on this fundamental question in biology, i.e., how to break the symmetry of cell division to give rise to different cells? This finding also provokes many intriguing questions, such as coordinating the differential deposition of old vs. new histones onto sister chromatids among different replication forks in S-phase; identifying key modifications of old histones recruiting Haspin kinase for earlier phosphorylation; defining any differences at sister centromeres and corresponding responsibility for differential kinetochore assembly; and determining how microtubules interact with epigenetically distinct sister chromatids. We might then investigate the biological significance of all these molecular and cellular mechanisms, as well as how they are generally used in other cell types and in other organisms. Probing and understanding these questions will undoubtedly revolutionize our current understanding of the important roles of ACD in determining cell fate of stem cells and other cell types during development.
Trend Box.
Stem cells segregate cell fate determinants unequally during the asymmetric cell division (ACD), including histones. To achieve this, it requires a whole set of coordinated machineries for multiple processes, especially for DNA replication and mitosis.
In Drosophila male germline stem cells (GSC), pre-existing histone H3 are predominantly retained by the daughter cell that remain stem cell identity.
The incorporation of new and old histones during DNA replication plays an important role in transmitting epigenetic information and may be utilized to influence cell fate decisions during development.
During mitosis, a transit H3 modification, threonine 3 phosphorylation (H3T3P), distinguishes pre-existing from newly synthesized H3. Disruption of the H3T3P signal randomizes H3 inheritance patterns and lead to germline defects.
Key mitotic machineries, including centrosome and kinetochore, display a morphological and functional asymmetry in multiple model systems during the ACD.
Outstanding Questions box.
Is asymmetric histone inheritance a general mechanism during the ACD in different organisms?
How to coordinate the differential deposition of old vs. new histone onto the sister chromatids among different replication forks in S-phase?
What are other modifications on the old histones to mark them as old and recruit Haspin for earlier phosphorylation? Does haspin has other substrates except for H3 Thr3?
Do the sister kinetochores assemble at the same time, or different for the sister chromatids with old versus new histones? How will microtubule-kinetochore attachment error be fixed during the process?
What cellular pathways are linked to normal cell division (symmetric division) regulation versus those in response to in ACD? (e.g. there could be some specific checkpoint mechanisms, like the spindle orientation check point found in Drosophila male GSCs)
DNA Replication Summary.
The earliest steps of DNA replication begin with the binding of the origin recognition complex (ORC1–6 protein) to a sequence of DNA known as an origin of replication (ORI) and the subsequent recruitment of the Cdc6 protein [91]. After ORC proteins have successfully bound, Cdt1 and the MCM2–7 helicase complex are recruited to the complex. Cdt1, together with ORC1–6 and Cdc6, load the MCM2–7 helicase complex onto un-replicated DNA. A second MCM2–7-Cdt1 complex is recruited soon after but is loaded in a mechanistically distinct manner, resulting in two helicase complexes positioned next to each other but are oriented in opposite directions. The loading of the second MCM2–7 helicase signifies the formation of the pre-replication complex (Pre-RC). Following Pre-RC formation, a rise in S-phase cyclins stimulates DDK (Dbf4-dependent kinase) and CDK (Cyclin-dependent kinase) activity which help to recruit additional proteins to form the entire preinitiation complex. These factors include MCM10, CDC45, RECQL4, treslin, GINS, DNA topoisomerase-2 binding protein (TOPBP1) and DNA polymerase ε. Activity of the CDK and DDK kinases eventually converts the inactive MCM2–7 helicase into the active CMG (CDC45-GINS-MCM2–7) complex. Helicase activation triggers the recruitment of additional proteins such as replication factor C (RFC), proliferating cell nuclear antigen (PCNA), replication protein A (RPA) as well as additional polymerases α and δ. and Once active, the now intact replisome complex moves outward from the ORI, unwinding double-stranded DNA as it goes. The firing of origins signifies the first of many symmetry breaking events which serve to differentiate one newly synthesized strand of DNA from the other [92].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arents G, et al. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc Natl Acad Sci USA. 1991;88(22):10148–52. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arents G, Moudrianakis EN. Topography of the histone octamer surface: repeating structural motifs utilized in the docking of nucleosomal DNA. Proc Natl Acad Sci USA. 1993;90(22):10489–93. doi: 10.1073/pnas.90.22.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxevanis AD, Godfrey JE, Moudrianakis EN. Associative behavior of the histone (H3–H4)2 tetramer: dependence on ionic environment. Biochemistry. 1991;30(36):8817–23. doi: 10.1021/bi00100a013. [DOI] [PubMed] [Google Scholar]
- 4.Moudrianakis EN, Arents G. Structure of the histone octamer core of the nucleosome and its potential interactions with DNA. Cold Spring Harb Symp Quant Biol. 1993;58:273–9. doi: 10.1101/sqb.1993.058.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Andrews AJ, Luger K. Nucleosome structure(s) and stability: variations on a theme. Annu Rev Biophys. 2011;40:99–117. doi: 10.1146/annurev-biophys-042910-155329. [DOI] [PubMed] [Google Scholar]
- 6.Luger K, et al. Crystal structure of the nucleosome core particle at 2.8[thinsp]A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 7.Kornberg RD, Thonmas JO. Chromatin Structure: Oligomers of the Histones. Science. 1974;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- 8.McGinty RK. Nucleosome structure and function. 2015;115(6):2255–73. doi: 10.1021/cr500373h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–99. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- 10.Avgustinova A, Benitah SA. Epigenetic control of adult stem cell function. Nat Rev Mol Cell Biol. 2016;17(10):643–658. doi: 10.1038/nrm.2016.76. [DOI] [PubMed] [Google Scholar]
- 11.Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol. 2009;10(3):192–206. doi: 10.1038/nrm2640. [DOI] [PubMed] [Google Scholar]
- 12.Heard E, Martienssen RA. Transgenerational Epigenetic Inheritance: myths and mechanisms. Cell. 2014;157(1):95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin C, Zhang Y. Mechanisms of epigenetic inheritance. Curr Opin Cell Biol. 2007;19(3):266–72. doi: 10.1016/j.ceb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science. 2010;330(6004):612–6. doi: 10.1126/science.1191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran V, et al. Asymmetric division of Drosophila male germline stem cell shows asymmetric histone distribution. Science. 2012;338(6107):679–82. doi: 10.1126/science.1226028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKnight SL, Miller OL., Jr Electron microscopic analysis of chromatin replication in the cellular blastoderm Drosophila melanogaster embryo. Cell. 1977;12(3):795–804. doi: 10.1016/0092-8674(77)90278-1. [DOI] [PubMed] [Google Scholar]
- 17.Sogo JM, et al. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J Mol Biol. 1986;189(1):189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- 18.Worcel A, Han S, Wong ML. Assembly of newly replicated chromatin. Cell. 1978;15(3):969–77. doi: 10.1016/0092-8674(78)90280-5. [DOI] [PubMed] [Google Scholar]
- 19.Jackson V, Chalkley R. A new method for the isolation of replicative chromatin: selective deposition of histone on both new and old DNA. Cell. 1981;23(1):121–34. doi: 10.1016/0092-8674(81)90277-4. [DOI] [PubMed] [Google Scholar]
- 20.Alabert C, Groth A. Chromatin replication and epigenome maintenance. Nat Rev Mol Cell Biol. 2012;13(3):153–67. doi: 10.1038/nrm3288. [DOI] [PubMed] [Google Scholar]
- 21.Jackson V, Chalkley R. Histone synthesis and deposition in the G1 and S phases of hepatoma tissue culture cells. Biochemistry. 1985;24(24):6921–30. doi: 10.1021/bi00345a026. [DOI] [PubMed] [Google Scholar]
- 22.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–95. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu B, Reinberg D. Epigenetic inheritance: Uncontested? Cell Research. 2011;21(3):435–441. doi: 10.1038/cr.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voigt P, et al. Asymmetrically modified nucleosomes. Cell. 2012;151(1):181–93. doi: 10.1016/j.cell.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu M, et al. Partitioning of histone H3–H4 tetramers during DNA replication-dependent chromatin assembly. Science. 2010;328(5974):94–8. doi: 10.1126/science.1178994. [DOI] [PubMed] [Google Scholar]
- 26.Huang C, et al. H3.3–H4 tetramer splitting events feature cell-type specific enhancers. PLoS Genet. 2013;9(6):e1003558. doi: 10.1371/journal.pgen.1003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riley D, Weintraub H. Conservative segregation of parental histones during replication in the presence of cycloheximide. Proc Natl Acad Sci USA. 1979;76(1):328–32. doi: 10.1073/pnas.76.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weintraub H. Cooperative alignment of nu bodies during chromosome replication in the presence of cycloheximide. Cell. 1976;9(3):419–22. doi: 10.1016/0092-8674(76)90086-6. [DOI] [PubMed] [Google Scholar]
- 29.Seale RL. Studies on the mode of segregation of histone nu bodies during replication in HeLa cells. Cell. 1976;9(3):423–9. doi: 10.1016/0092-8674(76)90087-8. [DOI] [PubMed] [Google Scholar]
- 30.Seidman MM, Levine AJ, Weintraub H. The asymmetric segregation of parental nucleosomes during chrosome replication. Cell. 1979;18(2):439–49. doi: 10.1016/0092-8674(79)90063-1. [DOI] [PubMed] [Google Scholar]
- 31.Russev G, Hancock R. Assembly of new histones into nucleosomes and their distribution in replicating chromatin. Proc Natl Acad Sci USA. 1982;79(10):3143–7. doi: 10.1073/pnas.79.10.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson V, Chalkley R. Histone segregation on replicating chromatin. Biochemistry. 1985;24(24):6930–8. doi: 10.1021/bi00345a027. [DOI] [PubMed] [Google Scholar]
- 33.Annunziato AT. Assembling chromatin: the long and winding road. Biochim Biophys Acta. 2013;1819(3–4):196–210. doi: 10.1016/j.bbagrm.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Burgess RJ, Zhang Z. Histone chaperones in nucleosome assembly and human disease. Nat Struct Mol Biol. 2013;20(1):14–22. doi: 10.1038/nsmb.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith S, Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989;58(1):15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- 36.Tagami H, et al. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116(1):51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- 37.Liu WH, et al. CAF-1-induced oligomerization of histones H3/H4 and mutually exclusive interactions with ASF1 guide H3/H4 transitions among histone chaperones and DNA. Nucleic Acids Research. 2012;40(22):11229–11239. doi: 10.1093/nar/gks906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groth A, et al. Regulation of replication fork progression through histone supply and demand. Science. 2007;318(5858):1928–31. doi: 10.1126/science.1148992. [DOI] [PubMed] [Google Scholar]
- 39.Jasencakova Z, et al. Replication stress interferes with histone recycling and predeposition marking of new histones. Mol Cell. 2010;37(5):736–43. doi: 10.1016/j.molcel.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 40.Winkler DD, et al. Yeast CAF-1 assembles histone (H3–H4)(2) tetramers prior to DNA deposition. Nucleic Acids Res. 2012;40(20):10139–49. doi: 10.1093/nar/gks812. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Huang H, et al. A unique binding mode enables MCM2 to chaperone histones H3–H4 at replication forks. Nat Struct Mol Biol. 2015;22(8):618–26. doi: 10.1038/nsmb.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu C, et al. Strand-Specific Analysis Shows Protein Binding at Replication Forks and PCNA Unloading from Lagging Strands when Forks Stall. Mol Cell. 2014;56(4):551–63. doi: 10.1016/j.molcel.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakano S, Stillman B, Horvitz HR. Replication-coupled chromatin assembly generates a neuronal bilateral asymmetry in C. elegans. Cell. 2011;147(7):1525–36. doi: 10.1016/j.cell.2011.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibahara K, Stillman B. Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell. 1999;96(4):575–85. doi: 10.1016/s0092-8674(00)80661-3. [DOI] [PubMed] [Google Scholar]
- 45.SW, Stillman B. THE DNA REPLICATION FORK IN EUKARYOTIC CELLS. Annual Review of Biochemistry. 1998;67(1):721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 46.Yamashita YM, et al. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315(5811):518–21. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie J, et al. Histone H3 Threonine Phosphorylation Regulates Asymmetric Histone Inheritance in the Drosophila Male Germline. Cell. 2015;163(4):920–33. doi: 10.1016/j.cell.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meraldi P, Nigg EA. The centrosome cycle. FEBS Lett. 2002;521(1–3):9–13. doi: 10.1016/s0014-5793(02)02865-x. [DOI] [PubMed] [Google Scholar]
- 49.Reina J, Gonzalez C. When fate follows age: unequal centrosomes in asymmetric cell division. Philos Trans R Soc Lond B Biol Sci. 2014;369(1650) doi: 10.1098/rstb.2013.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piel M, et al. Centrosome-dependent exit of cytokinesis in animal cells. Science. 2001;291(5508):1550–3. doi: 10.1126/science.1057330. [DOI] [PubMed] [Google Scholar]
- 51.Uzbekov R, Prigent C. Clockwise or anticlockwise? Turning the centriole triplets in the right direction! FEBS Lett. 2007;581(7):1251–4. doi: 10.1016/j.febslet.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 52.Rebollo E, et al. Functionally unequal centrosomes drive spindle orientation in asymmetrically dividing Drosophila neural stem cells. Dev Cell. 2007;12(3):467–74. doi: 10.1016/j.devcel.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 53.Rusan NM, Peifer M. A role for a novel centrosome cycle in asymmetric cell division. J Cell Biol. 2007;177(1):13–20. doi: 10.1083/jcb.200612140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamashita YM. The centrosome and asymmetric cell division. Prion. 2009;3(2):84–8. doi: 10.4161/pri.3.2.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamashita YM, Fuller MT. Asymmetric centrosome behavior and the mechanisms of stem cell division. J Cell Biol. 2008;180(2):261–6. doi: 10.1083/jcb.200707083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamashita YM. Regulation of asymmetric stem cell division: spindle orientation and the centrosome. Front Biosci. 2009;14:3003–11. doi: 10.2741/3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301(5639):1547–50. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 58.Cheeseman IM. The kinetochore. Cold Spring Harb Perspect Biol. 2014;6(7):a015826. doi: 10.1101/cshperspect.a015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorpe PH, Bruno J, Rothstein R. Kinetochore asymmetry defines a single yeast lineage. Proc Natl Acad Sci USA. 2009;106(16):6673–8. doi: 10.1073/pnas.0811248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thorpe PH, Bruno J, Rothstein R. Modeling stem cell asymmetry in yeast. Cold Spring Harb Symp Quant Biol. 2008;73:81–8. doi: 10.1101/sqb.2008.73.010. [DOI] [PubMed] [Google Scholar]
- 61.Liakopoulos D, et al. Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell. 2003;112(4):561–74. doi: 10.1016/s0092-8674(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 62.Pereira G, et al. Modes of spindle pole body inheritance and segregation of the Bfa1p-Bub2p checkpoint protein complex. EMBO J. 2001;20(22):6359–70. doi: 10.1093/emboj/20.22.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pardo-Manuel de Villena F, Sapienza C. Female meiosis drives karyotypic evolution in mammals. Genetics. 2001;159(3):1179–89. doi: 10.1093/genetics/159.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pardo-Manuel de Villena F, Sapienza C. Nonrandom segregation during meiosis: the unfairness of females. Mamm Genome. 2001;12(5):331–9. doi: 10.1007/s003350040003. [DOI] [PubMed] [Google Scholar]
- 65.Chmatal L, et al. Centromere strength provides the cell biological basis for meiotic drive and karyotype evolution in mice. Curr Biol. 2014;24(19):2295–300. doi: 10.1016/j.cub.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dunleavy E, Pidoux A, Allshire R. Centromeric chromatin makes its mark. Trends Biochem Sci. 2005;30(4):172–5. doi: 10.1016/j.tibs.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Westhorpe FG, Straight AF. The Centromere: Epigenetic Control of Chromosome Segregation during Mitosis. Cold Spring Harb Perspect Biol. 2014;7(1) doi: 10.1101/cshperspect.a015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miell MD, et al. CENP-A confers a reduction in height on octameric nucleosomes. Nat Struct Mol Biol. 2013;20(6):763–5. doi: 10.1038/nsmb.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McKinley KL, I, Cheeseman M. The molecular basis for centromere identity and function. Nat Rev Mol Cell Biol. 2016;17(1):16–29. doi: 10.1038/nrm.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams JS, et al. Fission yeast Scm3 mediates stable assembly of Cnp1/CENP-A into centromeric chromatin. Mol Cell. 2009;33(3):287–98. doi: 10.1016/j.molcel.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dalal Y, et al. Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS Biol. 2007;5(8):e218. doi: 10.1371/journal.pbio.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furuyama T, Henikoff S. Centromeric nucleosomes induce positive DNA supercoils. Cell. 2009;138(1):104–13. doi: 10.1016/j.cell.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heun P, et al. Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores. Dev Cell. 2006;10(3):303–15. doi: 10.1016/j.devcel.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amor DJ, et al. Building the centromere: from foundation proteins to 3D organization. Trends Cell Biol. 2004;14(7):359–68. doi: 10.1016/j.tcb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Zinkowski RP, Meyne J, Brinkley BR. The centromere-kinetochore complex: a repeat subunit model. J Cell Biol. 1991;113(5):1091–110. doi: 10.1083/jcb.113.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blower MD, Sullivan BA, Karpen GH. Conserved organization of centromeric chromatin in flies and humans. Dev Cell. 2002;2(3):319–30. doi: 10.1016/s1534-5807(02)00135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu Z, et al. Dynamic Phosphorylation of CENP-A at Ser68 Orchestrates Its Cell-Cycle-Dependent Deposition at Centromeres. Dev Cell. 2015;32(1):68–81. doi: 10.1016/j.devcel.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 78.Bergmann JH, et al. Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. EMBO J. 2011;30(2):328–40. doi: 10.1038/emboj.2010.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sullivan BA, Karpen GH. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat Struct Mol Biol. 2004;11(11):1076–83. doi: 10.1038/nsmb845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lansdorp PM. Immortal strands? Give me a break. Cell. 2007;129(7):1244–7. doi: 10.1016/j.cell.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 81.Yamagishi Y, et al. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330(6001):239–43. doi: 10.1126/science.1194498. [DOI] [PubMed] [Google Scholar]
- 82.Wang F, et al. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science. 2010;330(6001):231–5. doi: 10.1126/science.1189435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelly AE, et al. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330(6001):235–9. doi: 10.1126/science.1189505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dai J, et al. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes & Development. 2005;19(4):472–488. doi: 10.1101/gad.1267105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Panigada D, et al. Yeast haspin kinase regulates polarity cues necessary for mitotic spindle positioning and is required to tolerate mitotic arrest. Dev Cell. 2013;26(5):483–95. doi: 10.1016/j.devcel.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 86.Higgins JM. Haspin: a newly discovered regulator of mitotic chromosome behavior. Chromosoma. 2010;119(2):137–47. doi: 10.1007/s00412-009-0250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pirrotta V. Histone Marks Direct Chromosome Segregation. Cell. 2015;163(4):792–3. doi: 10.1016/j.cell.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 88.Markaki Y, et al. Phosphorylation of histone H3 at Thr3 is part of a combinatorial pattern that marks and configures mitotic chromatin. J Cell Sci. 2009;122(Pt 16):2809–19. doi: 10.1242/jcs.043810. [DOI] [PubMed] [Google Scholar]
- 89.Florian MC, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10(5):520–30. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Langston LD, et al. CMG helicase and DNA polymerase ε form a functional 15-subunit holoenzyme for eukaryotic leading-strand DNA replication. Proceedings of the National Academy of Sciences. 2014;111(43):15390–15395. doi: 10.1073/pnas.1418334111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mechali M. Eukaryotic DNA replication origins: many choices for appropriate answers. Nat Rev Mol Cell Biol. 2010;11(10):728–38. doi: 10.1038/nrm2976. [DOI] [PubMed] [Google Scholar]
- 92.Fragkos M, et al. DNA replication origin activation in space and time. Nat Rev Mol Cell Biol. 2015;16(6):360–74. doi: 10.1038/nrm4002. [DOI] [PubMed] [Google Scholar]