Abstract
Background
Dietary protein restriction is recommended for patients with moderate to severe renal insufficiency. Long-term data on the relationship between dietary protein sources and risk for incident kidney disease in individuals with normal kidney function are largely missing.
Objective
To assess the association between dietary protein sources and incident chronic kidney disease (CKD)
Design
Prospective cohort
Setting
Atherosclerosis Risk in Communities (ARIC) study participants from 4 U.S. communities
Subjects
11,952 adults aged 44-66 years in 1987-1989 who were free of diabetes mellitus, cardiovascular disease and had an estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2.
Main outcome measure
A 66-item food frequency questionnaire was used to assess food intake. CKD stage 3 was defined as a decrease in eGFR of ≥25% from baseline resulting in an eGFR of less than 60 mL/min/1.73 m2; CKD-related hospitalization; CKD-related death; or end-stage renal disease. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using Cox proportional hazards regression.
Results
During a median follow-up of 23 years, there were 2,632 incident CKD cases. Red and processed meat consumption was associated with increased CKD risk (HRQ5 vs. Q1: 1.23, 95% CI: 1.06, 1.42, ptrend = 0.01). In contrast, higher dietary intake of nuts, legumes and low-fat dairy products was associated with lower CKD risk (nuts: HRQ5 vs. Q1: 0.81, 95% CI: 0.72, 0.92, ptrend <0.001; low-fat dairy products: HRQ5 vs. Q1: 0.75, 95% CI: 0.65, 0.85, ptrend <0.001; legumes: HRQ5 vs. Q1: 0.83, 95% CI: 0.72, 0.95, ptrend=0.03).
Conclusion
There were varied associations of specific dietary protein sources with risk of incident CKD, with red and processed meat being adversely associated with CKD risk and nuts, low-fat dairy products and legumes being protective against the development of CKD.
Keywords: dietary protein, protein sources, chronic kidney disease
Introduction
The role of dietary protein intake in kidney disease has been debated for decades.1-3 In 1923, Addis and Drury were among the first to describe a relationship between level of dietary protein and rates of urea excretion.4 This was followed by the observation that higher dietary intake of protein resulted in elevated rates of creatinine and urea excretion in a dog model as a consequence to meat feeding.5 Excretion rates were attributed to changes in glomerular filtration rate (GFR) based on renal blood flow alterations in response to increased protein intake.6-8 Therefore the concern arose that higher consumption of dietary protein may contribute to chronic renal disease through increased glomerular pressure (‘intrarenal hypertension’) and hyperfiltration that may predispose even healthy people to progressive glomerular sclerosis and deterioration of renal function.1
To this point, not much is known on the role of various dietary protein sources on renal functioning and incident kidney disease.9, 10 Most clinical evidence stems from trials in patients with moderate to severe kidney insufficiency.11 Pathophysiological mechanisms supporting different effects of various protein sources may involve lower concentrations of amino acids that cause renal vasodilation and lesser stimulation of vasodilator prostaglandins.12 Moreover, dietary protein sources vary in their non-protein constituents which may in part explain differential health effects. There is growing evidence showing the adverse health effects of red and processed meat intake specifically and its association with cardiovascular disease.13-16 On the other hand, a diet with a higher proportion of plant protein may be beneficial to persons with chronic kidney disease (CKD).17, 18 Unfortunately detailed analyses of specific food groups instead of single nutrients are largely missing.9, 10 Such investigations are warranted as these provide a more adequate assessment of the complexities of diet-disease associations.19
The primary aim of this study was to examine the association between individual protein-rich food groups on the risk of incident stage 3 CKD in a large, community-based cohort of middle-aged adults with normal renal function at baseline. We hypothesized that animal-derived food groups such as red or processed meat would be associated with a higher risk of incident stage 3 CKD whereas plant-based proteins such as nuts and legumes would be related to a lower risk.
Methods
Study Population
The Atherosclerosis Risk in Communities Study (ARIC) is a community-based prospective cohort study of 15,792 adults aged 45-64 years at baseline from four U.S. communities (Washington County, MD; suburban Minneapolis, MN; Jackson, MS; and Forsyth County, NC).20 For this analysis, only white and black adults were included. Individuals with diabetes (defined as self-reported diabetes, fasting blood glucose ≥126 mg/dL, non-fasting blood glucose ≥200 mg/dL, or use of diabetes medication) or cardiovascular disease (a history of myocardial infarction, heart failure, coronary bypass surgery, or angioplasty) at baseline were not included (Appendix Figure 1). Participants with an estimated GFR (eGFR) <60 mL/min/per 1.73 m2 at baseline or prior to baseline were also excluded (n=108). Last, we excluded individuals with incomplete dietary information or with extreme caloric intake (<600 kcal or >4,200 kcal per day for men, <500 kcal or >3,600 kcal per day for women) or with missing data on covariates of interest (n=841). Our final study population consisted of 11,952 participants at the baseline examination (visit 1, 1987-1989).
The ARIC study was approved by the Institutional Review Boards (IRB) of all participating institutions, including University of Minnesota, Johns Hopkins University, University of North Carolina, and University of Mississippi Medical Center. Written documentation of informed consent was obtained from all participants at each clinical site.
Assessment of Dietary Protein Intake
Dietary protein intake was assessed using an interviewer-administered, 66-item food frequency questionnaire (FFQ) adapted from the 61-item FFQ developed by Willett et al. and validated in ARIC.21, 22 The FFQ was administered to all subjects at visit 1 (baseline, 1987–1989) and visit 3 (1993–1995). As major dietary protein sources, the following food groups were identified: unprocessed red meat, processed red meat, red and processed meat intake (combined), poultry, fish and seafood, eggs, high-fat dairy products, low-fat dairy products, nuts, and legumes (Appendix Table 1). Vegetable protein intake was defined as the difference between total and animal protein intake.
Cumulative updating of the FFQ was used to reduce within-person variation and to represent long-term dietary intake.15, 23 Specifically, visit 1 FFQ data were used for those who developed kidney disease or were censored before visit 3. Otherwise, for those who did not develop kidney disease and those who were not censored before visit 3, the average of visits 1 and 3 FFQ data were used. In analysis, quintiles of dietary protein sources were created.
Assessment of Incident Kidney Disease
The primary endpoint for this study was incident stage 3 CKD through December 31, 2012. A detailed description of creatinine measurements at all ARIC study visits has been previously published.24, 25 eGFR was calculated using the 2009 Chronic Kidney Disease Epidemiology (CKD-EPI) equation using creatinine.26 Incident stage 3 CKD was assessed from baseline (1987-1989) through December 31, 2012 and defined as a decrease in eGFR of at least 25% from baseline resulting in an eGFR of less than 60 mL/min/per 1.73 m2, International Classification of Disease-9/10 code for a CKD-related hospitalization identified by surveillance of hospitalizations, International Classification of Disease-9/10 code for a CKD-related death identified by linkage to the National Death Index, or end-stage renal disease (ESRD) defined as transplant or dialysis as indicated by entry in the U.S. Renal Data System (USRDS) registry.27
In sensitivity analyses, we evaluated associations with incident stage 3 CKD defined using visit-based measures only from visits 1-4, i.e. eGFR of less than 60 mL/min/per 1.73 m2 accompanied by a decrease in eGFR of at least 25% from baseline.27
Covariates
Information on height, weight, waist, and hip circumference, current smoking, alcohol intake, education level, and use of anti-hypertensive and lipid-lowering medication was obtained using standardized procedures at baseline.20 Baecke's physical activity questionnaire and scoring systems were used to assess sports-related physical activity and leisure-related physical activity.28 Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of anti-hypertensive medication. Participants provided blood samples for the assessment of blood glucose and the lipid profile.20 Diabetes status was defined as self-reported diabetes, physician diagnosis of fasting blood glucose ≥126 mg/dL, non-fasting blood glucose ≥200 mg/dL, or use of diabetes medication.
Statistical Analysis
Mean values and standard deviations (SD) for continuous variables and proportions for categorical variables were used to describe baseline characteristics (visit 1, 1987-1989) of the study participants. Cox proportional hazards regression was used to assess the association of dietary protein sources with stage 3 CKD incidence, accounting for time from the baseline examination to the date of the first outcome event, death, loss to follow-up, or December 31, 2012. Hazard ratios were calculated to estimate risk of CKD for participants in quintiles 2 to 5 of dietary food or protein intake relative to participants in the lowest quintile of dietary food or protein intake (reference group). Multivariable regression models were used to account for potential confounding. Model 1 was adjusted for age, race (in combination with ARIC study center), sex, education level, and total caloric intake. Model 2 additionally adjusted for high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol, lipid-lowering medication use, systolic blood pressure, anti-hypertensive medication use, alcohol intake, current smoker, physical activity index, leisure-related physical activity, total carbohydrate intake, body mass index, and waist-to-hip ratio. Tests for linear trend across quintiles were conducted by modeling the median value within each quartile. We tested for potential interactions by sex and race and conducted stratified analyses by sex and race. Sensitivity analyses were conducted to examine the association between dietary protein intake and incident CKD using visit-based measures only (9 years of follow-up; visits 1-4).
To characterize the distribution of dietary intake of total protein and sources of protein, we have plotted frequency histograms. In addition, to visualize the continuous association between total protein as well as specific sources of protein with risk of incident CKD, we plotted linear splines with four knots at the 20th, 40th, 60th, and 80th percentiles, corresponding to the quintiles. The 10th percentile of dietary intake of protein was used as the reference point in all spline models. We truncated the data at the 99th percentile to minimize the influence of extreme values.
Last, we investigated the effect of replacing red and processed meat with legumes, dairy, fish and seafood, or nuts. This substitution analysis was conducted by including the continuous forms of dietary protein variables in the same multivariable model (legumes, red and processed meat, fish and seafood, eggs, nuts, low-fat dairy, high-fat dairy, and poultry) in addition to other macronutrients and all the covariates in Model 2. The substitution effect estimates were calculated as the difference between the coefficient for the substituted food source of dietary protein and the coefficient for red meat, and the confidence intervals were calculated using the respective variances and covariance between these two factors.15, 29, 30
Statistical analyses were performed using Stata statistical software version 13.0 (StataCorp, College Station, Texas, USA). A two-sided p-value of 0.05 was considered statistically significant.
Results
There were no substantial differences in average age, blood pressure, total, or LDL-cholesterol across quintiles of total protein intake (Table 1). Participants in the highest quintile of total protein intake had higher caloric intake, body mass index, consumed more alcohol, and were more likely to have a college education. Mean total protein intake was 41.1 grams (15.9% of total caloric intake) in the lowest quintile compared to 109.5 grams (19.5% of total caloric intake) in the highest quintile. Participants who developed incident CKD were slightly older, more likely to be male, had higher blood pressure, cholesterol, and body mass index (Appendix Table 2). Total protein and animal protein intake at baseline was similar in persons who did and did not develop incident CKD.
Table 1. Baseline characteristics according to quintile of total protein consumption, ARIC, 1987-1989 (N=11,952).
| Quintiles of Total Protein Consumption (g/day)a | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-value for linear trend | |
| (n=2,391) | (n=2,390) | (n=2,391) | (n=2,390) | (n=2,390) | ||
| Age, years | 53.8 (5.8) | 53.8 (5.7) | 53.8 (5.7) | 53.8 (5.7) | 53.7 (5.6) | 0.43 |
| Female, % | 64.2 | 56.9 | 57.8 | 55.4 | 47.0 | <0.001 |
| Black, % | 23.3 | 22.6 | 22.7 | 22.1 | 23.5 | 0.96 |
| Current cigarette smoker, % | 28.3 | 26.7 | 24.3 | 24.6 | 26.4 | 0.04 |
| Education, % | <0.001 | |||||
| Less than high school | 24.8 | 20.3 | 18.9 | 18.9 | 19.1 | |
| High school or equivalent | 45.3 | 42.9 | 40.2 | 40.3 | 40.1 | |
| College or above | 29.9 | 36.8 | 41.0 | 40.8 | 40.8 | |
| Systolic blood pressure, mmHg | 119.6 (18.5) | 119.8 (17.9) | 119.6 (17.8) | 119.4 (18.0) | 120.0 (17.7) | 0.94 |
| Diastolic blood pressure, mmHg | 72.9 (11.4) | 73.0 (10.7) | 73.6 (11.0) | 73.3 (10.9) | 73.5 (11.0) | 0.02 |
| Hypertension, % | 30.7 | 30.8 | 32.6 | 31.0 | 28.7 | 0.21 |
| Anti-hypertensive medication, % | 22.6 | 22.2 | 24.5 | 23.1 | 20.3 | 0.16 |
| Total cholesterol, mg/dL | 214.0 (42.1) | 214.6 (40.5) | 214.1 (40.1) | 212.9 (40.9) | 212.6 (39.5) | 0.20 |
| LDL cholesterol, mg/dL | 136.3 (39.7) | 137.5 (38.5) | 136.8 (38.5) | 136.0 (39.3) | 136.4 (37.8) | 0.76 |
| Triglycerides, mg/dL | 115.5 (58.1) | 118.7 (58.3) | 119.0 (60.5) | 119.9 (61.2) | 122.3 (64.3) | 0.004 |
| HDL cholesterol, mg/dL | 54.5 (17.2) | 53.3 (17.2) | 53. 5 (17.0) | 52.8 (16.9) | 51.7 (17.0) | <0.001 |
| Lipid-lowering medication, % | 2.1 | 2.3 | 2.4 | 2.0 | 2.1 | 0.63 |
| eGFR, mL/min/per 1.73 m2 | 103.1 (13.8) | 103.3 (14.1) | 102.9 (13.9) | 103.3 (13.6) | 103.1 (13.3) | 0.93 |
| Body mass index, kg/m2 | 26.6 (5.1) | 26.8 (4.9) | 27.1 (5.0) | 27.2 (5.0) | 27.6 (5.1) | <0.001 |
| Waist-to-hip ratio | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | <0.001 |
| Leisure index | 2.3 (0.6) | 2.4 (0.6) | 2.4 (0.6) | 2.4 (0.6) | 2.4 (0.6) | <0.001 |
| Physical activity index | 2.4 (0.8) | 2.5 (0.8) | 2.5 (0.8) | 2.5 (0.8) | 2.5 (0.8) | <0.001 |
| Total caloric intake, kcal/day | 1,076 (332) | 1,337 (354) | 1,559 (388) | 1,792 (436) | 2,270 (606) | <0.001 |
| Alcohol intake, g/wk | 35.1 (85.5) | 40.7 (82.1) | 45.0 (95.2) | 47.0 (96.3) | 52.3 (103.3) | <0.001 |
| Total protein intake, g/day | 41.1 (7.3) | 57.2 (3.6) | 69.0 (3.3) | 82.3 (4.5) | 109.5 (18.3) | <0.001 |
| Total protein intake, %kcal | 15.9 (3.6) | 17.7 (3.5) | 18.2 (3.4) | 18.8 (3.4) | 19.5 (3.5) | <0.001 |
| Animal protein intake, g/day | 28.9 (7.0) | 42.3 (5.2) | 51.6 (5.7) | 62.5 (6.5) | 84.9 (17.6) | <0.001 |
| Vegetable protein intake, g/day | 12.2 (4.2) | 14.9 (4.5) | 17.4 (5.1) | 19.8 (5.4) | 24.5 (7.4) | <0.001 |
| Dietary fiber, g/d | 11.9 (5.6) | 14.4 (6.2) | 16.7 (6.4) | 19.2 (7.3) | 23.4 (9.3) | <0.001 |
| Total carbohydrate intake, g/day | 135.2 (54.3) | 155.7 (55.1) | 178.4 (59.6) | 199.9 (62.2) | 244.6 (76.0) | <0.001 |
| Carbohydrate intake, %kcal | 53.5 (9.5) | 49.9 (8.4) | 49.0 (7.9) | 48.1 (7.4) | 46.4 (7.1) | <0.001 |
| Total fat intake, g/day | 35.8 (11.3) | 47.7 (13.0) | 56.6 (14.7) | 66.2 (16.6) | 87.7 (25.2) | <0.001 |
| Total fat intake, %kcal | 30.1 (6.6) | 31.9 (6.1) | 32.4 (5.8) | 32.9 (5.6) | 34.0 (5.6) | <0.001 |
ARIC, Atherosclerosis Risk in Communities Study; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Protein consumption was estimated using cumulative average intake. For those who developed kidney disease or were censored from the analysis before visit 3, food frequency questionnaire data from visit 1 was used. Otherwise, for those who developed kidney disease or were censored from the analysis after study visit 3, the average of food frequency questionnaire data from visits 1 and 3 was used
A total of 2,632 cases of incident CKD cases were observed during a median follow-up of 23 years. Neither total protein nor animal protein consumption was significantly related to risk of incident CKD (Table 2). Participants consuming the highest amounts of vegetable protein sources were at lower risk for incident CKD compared to inidividuals with lowest intake (HRQ5 vs. Q1: 0.76, 95% CI, 0.64, 0.91; ptrend = 0.002).
Table 2. Association of total protein, animal protein, and vegetable protein consumption with incident chronic kidney disease, ARIC, 1987-2012.
| Quintiles of Protein Consumption a | P-value for linear trend | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Total protein | ||||||
| Person-years | 48,376 | 49,282 | 49,722 | 49,484 | 48,663 | |
| Events | 520 | 553 | 522 | 515 | 522 | |
| Range, g/d | <50.80 | 50.80-63.11 | >63.11-74.87 | >74.87-90.58 | >90.59 | |
| HR (95% CI)* | 1 (ref) | 0.99 (0.88, 1.12) | 0.92 (0.80, 1.04) | 0.90 (0.78, 1.03) | 0.91 (0.78, 1.07) | 0.10 |
| HR (95% CI)† | 1 (ref) | 0.98 (0.87, 1.11) | 0.89 (0.78, 1.02) | 0.89 (0.77, 1.02) | 0.89 (0.76, 1.05) | 0.08 |
| Animal protein | ||||||
| Person-years | 48,482 | 49,234 | 49,602 | 49,652 | 48,556 | |
| Events | 528 | 538 | 525 | 521 | 520 | |
| Range, g/d | <36.37 | 36.37-46.83 | >46.83-56.61 | 56.62-69.60 | >69.60 | |
| HR (95% CI)* | 1 (ref) | 0.96 (0.85, 1.09) | 0.94 (0.83, 1.07) | 0.94 (0.82, 1.07) | 0.95 (0.82, 1.10) | 0.41 |
| HR (95% CI)† | 1 (ref) | 0.95 (0.84, 1.08) | 0.92 (0.81, 1.04) | 0.92 (0.80, 1.05) | 0.91 (0.78, 1.06) | 0.19 |
| Vegetable protein | ||||||
| Person-years | 48,400 | 49,277 | 49,657 | 49,461 | 48,731 | |
| Events | 511 | 549 | 532 | 536 | 504 | |
| Range, g/d | <12.11 | 12.11-15.18 | >15.18-18.43 | >18.43-22.80 | >22.81 | |
| HR (95% CI)* | 1 (ref) | 0.95 (0.84, 1.08) | 0.87 (0.77, 0.99) | 0.85 (0.75, 0.98) | 0.75 (0.64, 0.88) | <0.001 |
| HR (95% CI)† | 1 (ref) | 0.94 (0.83, 1.06) | 0.89 (0.78, 1.01) | 0.85 (0.74, 0.98) | 0.76 (0.64, 0.91) | 0.002 |
ARIC indicates Atherosclerosis Risk in Communities Study; CI, confidence interval; HR, hazard ratio.
Protein consumption was estimated using cumulative average intake. For those who developed kidney disease or were censored from the analysis before visit 3, food frequency questionnaire data from visit 1 was used. Otherwise, for those who developed kidney disease or were censored from the analysis after study visit 3, the average of food frequency questionnaire data from visits 1 and 3 was used.
Model 1: Adjusted for age, race-center, sex, education level, and total caloric intake.
Model 2: Adjusted for variables in model 1 + high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol, lipid-lowering medication use, systolic blood pressure, anti-hypertensive medication use, alcohol intake, current smoker, physical activity index, leisure-related physical activity, total carbohydrate intake, body mass index, and waist-to-hip ratio.
In detailed analyses, higher intake of red and processed meat was significantly associated with an increased risk for incident CKD (HRQ5 vs. Q1: 1.23; 95% CI, 1.06, 1.42, ptrend = 0.01; Table 3). Individuals with the highest levels of dietary intake of low-fat dairy products and legumes had a lower risk of incident CKD (HRQ5 vs. Q1: 0.75; 95% CI, 0.65, 0.85, ptrend <0.001 and HRQ5 vs. Q1: 0.83; 95% CI, 0.72, 0.95, ptrend=0.03). In addition, higher nut consumption was associated with reduced CKD risk (HRQ5 vs. Q1: 0.81; 95% CI, 0.72, 0.92, ptrend <0.001). Higher levels of fish and seafood consumption was not significantly associated with lower risk of incident CKD, although the test for trend was statistically significant (ptrend = 0.01). Dietary intake of poultry, eggs and high-fat dairy products were not significantly associated with incident CKD. When we investigated the association of major dietary protein sources with CKD risk by sex and race, our results did not change significantly (pinteraction by sex = 0.60; pinteraction by race = 0.91).
Table 3. Association of food sources of protein with incident chronic kidney disease, ARIC, 1987-2012.
| Quintiles of Protein Consumption a | P-value for linear trend | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Red meat | ||||||
| Person-years | 51,458 | 50,381 | 46,369 | 50,169 | 47,151 | |
| Events | 524 | 520 | 515 | 528 | 545 | |
| Median, svg/d | 0.17 | 0.35 | 0.57 | 0.78 | 1.15 | |
| HR (95% CI)* | 1 (ref) | 1.05 (0.93-1.19) | 1.12 (0.99-1.27) | 1.12 (0.98-1.27) | 1.27 (1.11-1.46) | <0.001 |
| HR (95% CI)† | 1 (ref) | 1.01 (0.89-1.14) | 1.07 (0.94-1.21) | 1.06 (0.93-1.21) | 1.19 (1.03-1.36) | 0.02 |
| Processed meat | ||||||
| Person-years | 56,652 | 48,075 | 47,322 | 47,079 | 46,399 | |
| Events | 554 | 482 | 505 | 514 | 577 | |
| Median, svg/d | 0.03 | 0.14 | 0.29 | 0.53 | 1.00 | |
| HR (95% CI)* | 1 (ref) | 1.03 (0.91-1.16) | 1.07 (0.95-1.21) | 1.09 (0.96-1.24) | 1.25 (1.09-1.43) | 0.002 |
| HR (95% CI)† | 1 (ref) | 0.98 (0.87-1.11) | 1.00 (0.88-1.13) | 0.99 (0.87-1.13) | 1.12 (0.98-1.29) | 0.16 |
| Red and processed meat | ||||||
| Person-years | 50,795 | 50,096 | 49,163 | 48,670 | 46,803 | |
| Events | 495 | 510 | 521 | 540 | 566 | |
| Median, svg/d | 0.30 | 0.62 | 0.92 | 1.28 | 1.93 | |
| HR (95% CI)* | 1 (ref) | 1.09 (0.96-1.24) | 1.13 (0.99-1.28) | 1.22 (1.07-1.39) | 1.36 (1.18-1.56) | <0.001 |
| HR (95% CI)† | 1 (ref) | 1.03 (0.91-1.17) | 1.05 (0.93-1.20) | 1.11 (0.97-1.27) | 1.23 (1.06-1.42) | 0.01 |
| Poultry | ||||||
| Person-years | 60,072 | 57,204 | 59,868 | 18,964 | 49,418 | |
| Events | 699 | 606 | 624 | 179 | 524 | |
| Median, svg/d | 0.13 | 0.28 | 0.43 | 0.50 | 0.71 | |
| HR (95% CI)* | 1 (ref) | 0.88 (0.79, 0.99) | 0.88 (0.79, 0.98) | 0.83 (0.71, 0.98) | 0.93 (0.83, 1.05) | 0.12 |
| HR (95% CI)† | 1 (ref) | 0.92 (0.82, 1.02) | 0.90 (0.80, 1.00) | 0.85 (0.72, 1.01) | 0.94 (0.84, 1.06) | 0.26 |
| Fish and seafood | ||||||
| Person-years | 51,557 | 48,650 | 46,426 | 49,625 | 49,267 | |
| Events | 570 | 546 | 501 | 502 | 513 | |
| Median, svg/d | 0.07 | 0.14 | 0.23 | 0.35 | 0.64 | |
| HR (95% CI)* | 1 (ref) | 1.01 (0.89, 1.13) | 0.96 (0.85, 1.08) | 0.86 (0.76, 0.97) | 0.89 (0.78, 1.01) | 0.003 |
| HR (95% CI)† | 1 (ref) | 0.98 (0.87, 1.10) | 0.97 (0.86, 1.09) | 0.85 (0.75, 0.97) | 0.89 (0.78, 1.01) | 0.01 |
| Eggs | ||||||
| Person-years | 65,463 | 37,876 | 49,151 | 58,751 | 34,261 | |
| Events | 692 | 400 | 520 | 635 | 384 | |
| Median, svg/d | 0.03 | 0.10 | 0.14 | 0.43 | 0.72 | |
| HR (95% CI)* | 1 (ref) | 0.93 (0.82, 1.05) | 0.97 (0.86, 1.08) | 0.98 (0.88, 1.09) | 0.94 (0.83, 1.08) | 0.76 |
| HR (95% CI)† | 1 (ref) | 0.93 (0.82, 1.05) | 0.94 (0.84, 1.06) | 0.96 (0.86, 1.07) | 0.92 (0.81, 1.06) | 0.33 |
| High-fat dairy | ||||||
| Person-years | 48,866 | 52,059 | 47,937 | 50,524 | 46,064 | |
| Events | 513 | 536 | 501 | 561 | 521 | |
| Median, svg/d | 0.13 | 0.35 | 0.57 | 0.90 | 1.61 | |
| HR (95% CI)* | 1 (ref) | 0.95 (0.84, 1.08) | 0.91 (0.80, 1.03) | 0.95 (0.84, 1.07) | 0.90 (0.79, 1.03) | 0.24 |
| HR (95% CI)† | 1 (ref) | 0.96 (0.85, 1.08) | 0.93 (0.82, 1.06) | 0.96 (0.84, 1.08) | 0.93 (0.81, 1.06) | 0.32 |
| Low-fat dairy | ||||||
| Person-years | 48,236 | 47,894 | 42,299 | 50,024 | 50,048 | |
| Events | 603 | 532 | 531 | 493 | 473 | |
| Median, svg/d | 0.00 | 0.22 | 0.60 | 1.07 | 2.04 | |
| HR (95% CI)* | 1 (ref) | 0.87 (0.77, 0.98) | 0.81 (0.71, 0.91) | 0.72 (0.64, 0.82) | 0.72 (0.63, 0.82) | <0.001 |
| HR (95% CI)† | 1 (ref) | 0.88 (0.78, 0.99) | 0.81 (0.72, 0.91) | 0.74 (0.65, 0.84) | 0.75 (0.65, 0.85) | <0.001 |
| Nuts | ||||||
| Person-years | 59,643 | 39,092 | 48,463 | 48,763 | 49,541 | |
| Events | 708 | 415 | 523 | 484 | 502 | |
| Median, svg/d | 0.03 | 0.10 | 0.21 | 0.43 | 0.86 | |
| HR (95% CI)* | 1 (ref) | 0.88 (0.78, 0.99) | 0.87 (0.78, 0.98) | 0.78 (0.70, 0.88) | 0.77 (0.68, 0.87) | <0.001 |
| HR (95% CI)† | 1 (ref) | 0.89 (0.78, 1.00) | 0.88 (0.79, 0.99) | 0.82 (0.73, 0.92) | 0.81 (0.72, 0.92) | <0.001 |
| Legumes | ||||||
| Person-years | 56,812 | 42,547 | 49,648 | 53,035 | 43,485 | |
| Events | 582 | 442 | 556 | 605 | 447 | |
| Median, svg/d | 0.07 | 0.14 | 0.21 | 0.36 | 0.68 | |
| HR (95% CI)* | 1 (ref) | 0.94 (0.83-1.06) | 0.99 (0.88-1.12) | 0.96 (0.85-1.08) | 0.82 (0.72-0.94) | 0.02 |
| HR (95% CI)† | 1 (ref) | 0.95 (0.83-1.07) | 0.97 (0.86-1.10) | 0.96 (0.85-1.08) | 0.83 (0.72-0.95) | 0.03 |
ARIC indicates Atherosclerosis Risk in Communities Study; CI, confidence interval; HR, hazard ratio; and svg/d, servings/day.
Protein consumption was estimated using cumulative average intake. For those who developed kidney disease or were censored from the analysis before visit 3, food frequency questionnaire data from visit 1 was used. Otherwise, for those who developed kidney disease or were censored from the analysis after study visit 3, the average of food frequency questionnaire data from visits 1 and 3 was used.
Model 1: Adjusted for age, race-center, sex, education level, and total caloric intake.
Model 2: Adjusted for variables in Model 1 + high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol, lipid-lowering medication use, systolic blood pressure, anti-hypertensive medication use, alcohol intake, current smoker, physical activity index, leisure-related physical activity, total carbohydrate intake, body mass index, and waist-to-hip ratio.
When we characterized the shape and asssociations of total protein intake and of selected food groups with CKD risk and frequency in the overall population (Figure 1A-F), total protein consumption was not related to CKD risk. However, there was a higher risk of CKD among persons with elevated red and processed meat intake and a lower risk among persons with higher consumption of low-fat dairy products. Dietary intake of nuts, fish and seafood and legumes were not significantly associated with lower CKD risk. Replacing red and processed meat in the diet with legumes, low-fat dairy products or nuts was significantly associated with a lower risk of CKD (Table 4).
Figure 1. Frequency histograms and adjusted hazard ratiosa for the association between dietary intake of (A) total protein, (B) legumes, (C) red and processed meat, (D) fish and seafood, (E) nuts, and (F) low-fat dairy products and incident chronic kidney disease.
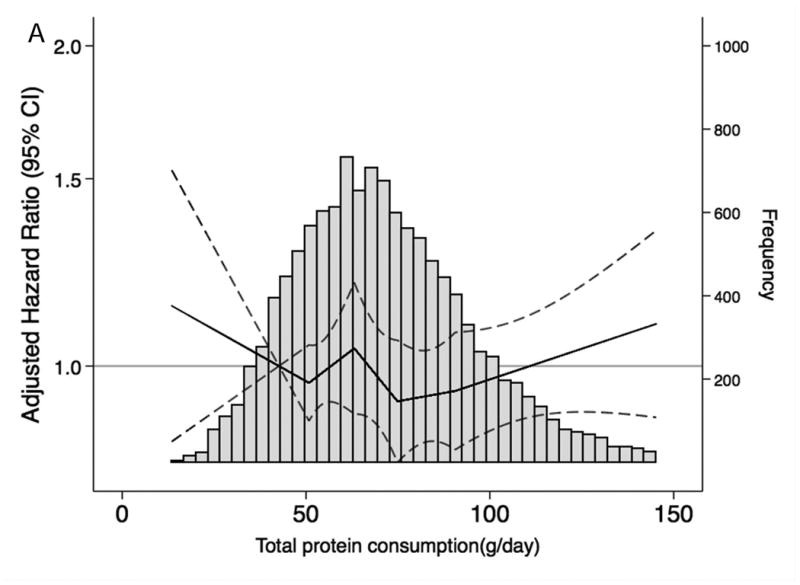
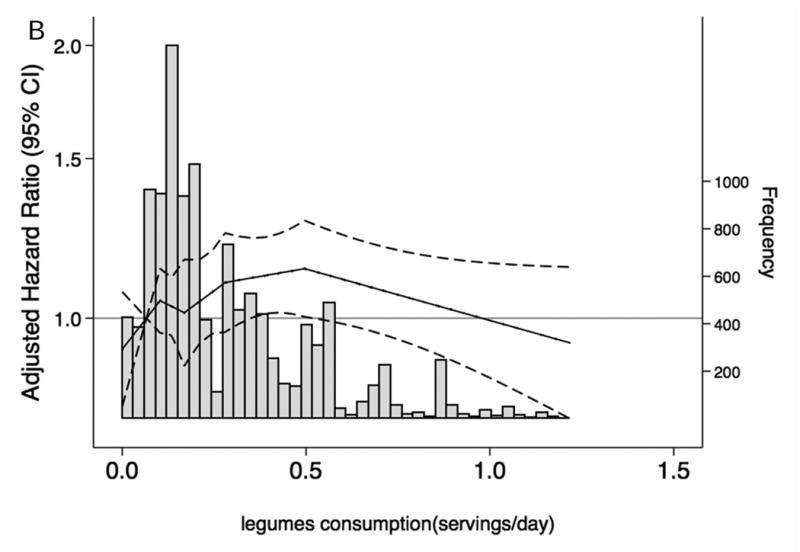
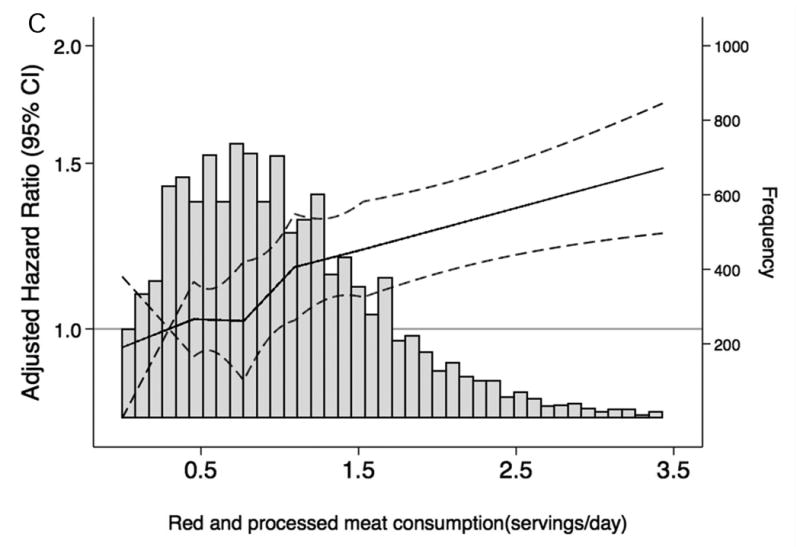
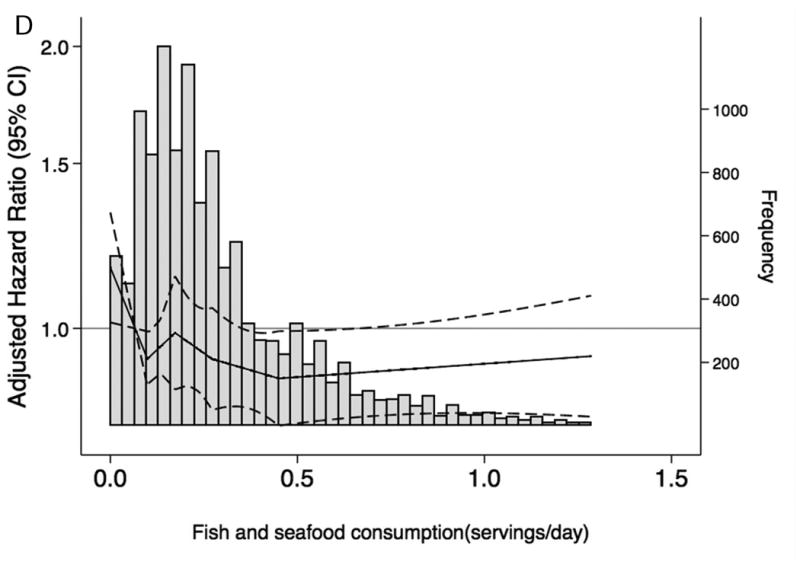
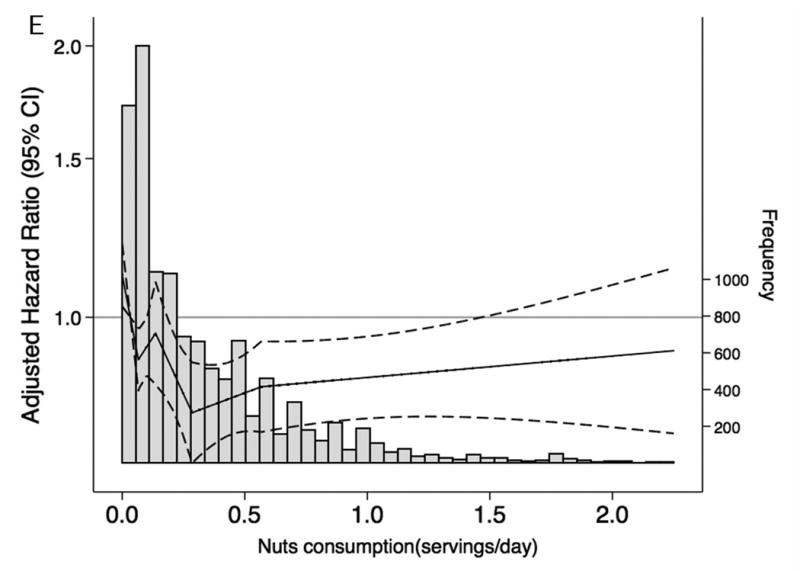
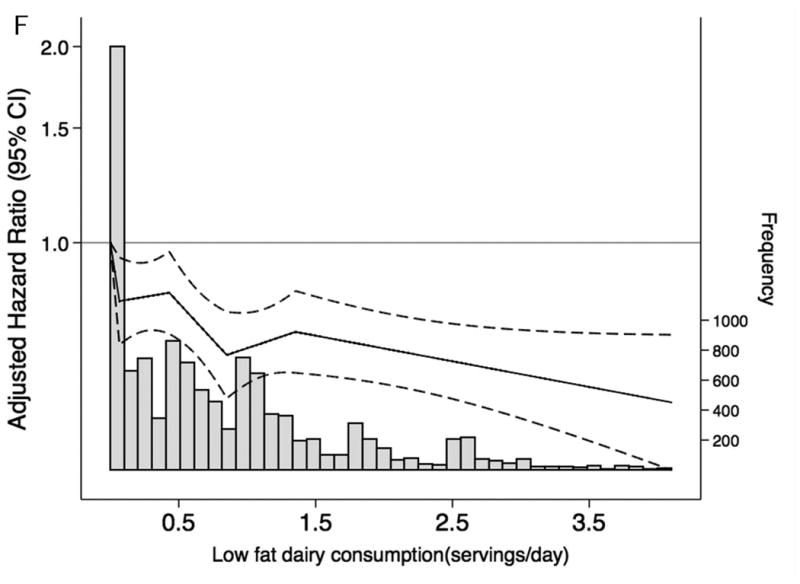
a Dietary intake of total protein and sources of protein are modeled using linear spline terms with knots at the 20th, 40th, 60th, and 80th percentiles. The 10th percentile of dietary intake of protein was used as the reference point, and data were truncated at the 99th percentile. The solid lines represent hazard ratios adjusted for age, race-center, sex, education level, total caloric intake, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol, lipid-lowering medication, systolic blood pressure, anti-hypertensive medication use, alcohol intake, current smoker, physical activity index, leisure-related physical activity, total carbohydrate, body mass index, and waist-to-hip ratio.
Table 4. Hazard ratiosa for incident chronic kidney disease associated with substituting one serving red and processed meat with one serving of another food source of dietary protein.
| Substituted Food | HR (95% CI) | P-value |
|---|---|---|
| Legumes | 0.69 (0.57, 0.83) | <0.001 |
| Low-fat dairy | 0 .80 (0.73, 0.87) | <0.001 |
| Fish and seafood | 0.86 (0.73, 1.02) | 0.08 |
| Nuts | 0.82 (0.73, 0.92) | 0.001 |
HR, hazard ratio; CI, confidence interval
The substitution analysis was conducted by including the continuous forms of dietary protein variables in the same multivariable model, and calculating the difference in their coefficients plus their covariance to estimate the hazard ratios and 95% confidence intervals. Regression model included legumes, red and processed meat, fish and seafood, eggs, nuts, low-fat dairy, high-fat dairy, and poultry intake. Model was adjusted for age, race-center, sex, education level, total caloric intake, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol, lipid-lowering medication use, systolic blood pressure, anti-hypertensive medication use, alcohol intake, current smoker, physical activity index, leisure-related physical activity, total carbohydrate intake(%kcal), total fat intake(%kcal), body mass index, and waist-to-hip ratio.
When we defined incident stage 3 CKD using visit-based measures only (eGFR <60 mL/min/1.73 m2 and ≥25% eGFR decline), vegetable protein remained significantly associated with incident CKD (HRQ5 vs. Q1: 0.69; 95% CI, 0.55, 0.86, ptrend = 0.005) (Appendix Table 3). Consumption of eggs, dairy products, nuts and legumes was related to a lower CKD risk, whereas dietary intake of red and processed meat tended to be associated with a higher risk but the test for trend was not significant (Appendix Table 4).
Discussion
In this large, community-based cohort of middle-aged adults with normal kidney function at baseline, participants with the highest red and processed meat consumption had an increased risk for incident CKD, while individuals with high consumption of nuts, low-fat dairy products or legumes were at a lower risk for CKD. Replacing red and processed meat in the diet with other sources of dietary protein, including nuts, low-fat dairy products and legumes was associated with lower CKD risk.
The majority of scientific evidence on dietary protein intake stems from studies involving patients with kidney disease, most prominently the Modification of Diet in Renal Disease (MDRD) study.11 There, among patients with moderate renal insufficiency, over 3 years, no significant correlation between achieved protein intake and GFR decline was observed.11, 31 However, extension of this relationship to individuals with normal renal function is inappropriate as the damaged glomerulus responds differently to various stressors than the healthy glomerulus. To this point, there are only a few longitudinal studies on the effect of long-term, high consumption of protein on kidney function decline in persons with normal kidney function available.9, 10, 32, 33 Analyses of the Nurses' Health Study, the Women's Health Initiative, and the Cardiovascular Health Study suggest that total protein intake was not significantly associated with changes in eGFR in individuals with normal kidney function.9, 32, 33 Non-dairy animal protein, dairy protein, and vegetable protein were not found to be related to changes in eGFR in nurses but unfortunately detailed food group analyes were not undertaken.9 Additionally, almost all previous studies consisted of relatively small sample sizes of predominantly white women, and large, diverse prospective studies of adequate duration are needed for further clarification.9, 32 The most recent results of the Singapore Chinese Health Study indicate that red meat intake may increase the risk of ESRD.10
Our data derived from a US community-based cohort support no association between total or animal protein intake and stage 3 CKD, whereas vegetable protein was found to be protective. Given the hypothesized effect of specific types of protein on renal vasodilation, renal blood flow, and GFR, analyses of food groups instead of total total protein intake may be a more appropriate method.19 This approach follows early observations that showed renal blood flow increases in dogs as a consequence to meat feeding.5 Controlled short-term feeding studies in humans support that an increase in the GFR can be induced by animal protein, specifically protein from meat.34 In contrast, consumption of soy protein produced little or no effect.12, 35 Our observational results over 20 years add to the evidence that among animal protein sources, red and processed meat is positively related while low-fat dairy products are inversely related to risk of incident CKD. Among vegetable protein sources, nuts and legumes were associated with a lower risk for CKD.
Many longitudinal studies define CKD solely on biomarker results which may introduce a selection bias as persons developing the disease may be less likely to attend study visits. The outcome definition for this analysis incorporated surveillance measures, including the USRDS registry and ICD-9/10 codes for CKD-related hospitalizations and deaths. In sensitivity analyses, we also evaluated associations with incident stage 3 CKD defined using visit-based measures only with eGFR of less than 60 mL/min/per 1.73 m2 accompanied by a decrease in eGFR of at least 25% from baseline. Using this alternative definition of CKD, the results confirm that vegetable protein sources (overall as well as nuts and legumes specifically) and low-fat dairy products are inversely related to incident stage 3 CKD.
There are numerous potential mechanisms that could explain the varying associations of dietary protein sources with kidney function decline. Importantly, red and processed meat intake is associated with an increased risk of hypertension and diabetes-related phenotypes 36-38 whereas dairy products and nuts are related to a lower risk.39, 40 Other explanations for our results may involve differences in the metabolism among dietary protein sources, such as the lower nonvolatile acid load that can be found in proteins from plant sources.41-43
The strengths of our study include a large diverse, community-based, prospective cohort with long follow-up, and structured assessment of dietary intake and covariates. Nonetheless, there are several limitations. In this US population assessed in the mid 1990's, there was little variation in reported intake for some types of protein of interest for CKD prevention. The relationships examined in this study should be confirmed, ideally, in populations with greater vegetable and seafood protein intake (e.g., Mediterranean populations). Second, intake of any particular food, including red meat, seafood, and others, occurs within a complex pattern of food consumption and of lifestyle. Therefore, residual and unmeasured confounding may explain part of the results, although our analyses adjusted for many potentially confounding factors. Moreover, changing dietary habits and food supply (e.g. processed meat) over time may not have been adequately captured by our FFQs. Translation of these results into a ‘modern’ population is thus limited. Finally, as we excluded individuals with diabetes and existing cardiovascular disease from our study population, generalizability of our findings is limited.
In conclusion, high red and processed meat consumption was associated with an increased CKD risk, whereas consumption of nuts, legumes and low-fat dairy products was associated with a lower risk of CKD. These results emphasize the potential role of dietary protein sources rather than total protein intake for developing kidney disease.
Practical Application
A diet high in protein from certain protein sources, rather than total protein restriction, appears to be advantageous in the long-term. The risk and frequency of CKD increases with high red and processed meat consumption, whereas intake of nuts, legumes, and low-fat dairy products are beneficial. Consequently, analyses replacing red and processed meat in the diet with legumes, low-fat dairy products or nuts indicate potential gains for the prevention of CKD.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. Some of the data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Financial Support: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). There are no relationships with industry to declare. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dr. Selvin was supported by NIH/NIDDK grant K24DK106414. Dr. Rebholz was supported by NIH/NIDDK grant K01 DK107782.
Footnotes
Financial disclosures: none
Author contributions: Study conception and design: Bernhard Haring; Casey Rebholz
Generation, collection, assembly of data: Casey Rebholz; Josef Coresh; Elizabeth Selvin; Bernhard Haring
Statistical analysis: Casey Rebholz; Menglu Liang; Bernhard Haring
Data interpretation: all authors
Drafting of manuscript: Bernhard Haring
Critical revision of manuscript for important intellectual content and approval of the final version of the manuscript: all authors
Data Access, Responsibility and Analysis: Casey Rebholz and Bernhard Haring had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bernhard Haring, Department of Internal Medicine I, University of Würzburg, Würzburg 97080, Bavaria, Germany.
Elizabeth Selvin, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, Maryland, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21287.
Menglu Liang, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, Maryland, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21287.
Josef Coresh, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, Maryland, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21287.
Morgan E. Grams, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, Maryland, USA; Division of Nephrology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD 21287.
Natalia Petruski-Ivleva, Department of Epidemiology, University of North Carolina Gillings School of Global Public Health, Chapel Hill, NC 27599.
Lyn M. Steffen, Division of Epidemiology & Community Health, University of Minnesota School of Public Health, Minneapolis, MN 55454.
Casey M. Rebholz, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, Maryland, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21287.
References
- 1.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307:652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- 2.King AJ, Levey AS. Dietary protein and renal function. J Am Soc Nephrol. 1993;3:1723–1737. doi: 10.1681/ASN.V3111723. [DOI] [PubMed] [Google Scholar]
- 3.Meyer TW, Lawrence WE, Brenner BM. Dietary protein and the progression of renal disease. Kidney Int Suppl. 1983;16:S243–247. [PubMed] [Google Scholar]
- 4.Addis T, Drury DR. THE RATE OF UREA EXCRETION: VII. THE EFFECT OF VARIOUS OTHER FACTORS THAN BLOOD UREA CONCENTRATION ON THE RATE OF UREA EXCRETION. J Biol Chem. 1923;55:629–638. [Google Scholar]
- 5.Jolliffe N, Smith HW. THE EXCRETION OF URINE IN THE DOG II. The Urea and Creatinine Clearances on a Mixed Diet. Am J Physiol. 1931;98:572–577. [Google Scholar]
- 6.Shannon JA, Jolliffe N, Smith HW. The Excretion of Urine in the Dog. VI. The Filtration and Secretion of Exogenous Creatinine. Am J Physiol. 1932;102:534–550. [Google Scholar]
- 7.Herrin RC, Rabin A, Feinstein RN. The Influence of Diet Upon Urea Clearance in Dogs. Am J Physiol. 1937;119:87–92. [Google Scholar]
- 8.Van Slyke DD, Rhoads CP, Hiller A, Alving A. The Relationship of the Urea Clearance to the Renal Blood Flow. Am J Physiol. 1934;110:387–391. [Google Scholar]
- 9.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460–467. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- 10.Lew QJ, Jafar TH, Koh HW, et al. Red Meat Intake and Risk of ESRD. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura H, Ito S, Ebe N, Shibata A. Renal effects of different types of protein in healthy volunteer subjects and diabetic patients. Diabetes Care. 1993;16:1071–1075. doi: 10.2337/diacare.16.8.1071. [DOI] [PubMed] [Google Scholar]
- 13.Larsson SC, Orsini N. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol. 2014;179:282–289. doi: 10.1093/aje/kwt261. [DOI] [PubMed] [Google Scholar]
- 14.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–883. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendinelli B, Palli D, Masala G, et al. Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct study. Diabetologia. 2013;56:47–59. doi: 10.1007/s00125-012-2718-7. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Wei G, Jalili T, et al. The Associations of Plant Protein Intake With All-Cause Mortality in CKD. Am J Kidney Dis. 2016;67:423–430. doi: 10.1053/j.ajkd.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scialla JJ, Appel LJ, Wolf M, et al. Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate levels in patients with chronic kidney disease: the Chronic Renal Insufficiency Cohort study. J Ren Nutr. 2012;22:379–388. doi: 10.1053/j.jrn.2012.01.026. e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goraya N, Wesson DE. Dietary Protein as Kidney Protection: Quality or Quantity? J Am Soc Nephrol. 2016;27:1877–1879. doi: 10.1681/ASN.2015111286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 22.Stevens J, Metcalf PA, Dennis BH, Tell GS, Shimakawa T, Folsom AR. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutrition Research. 1996;16:735–745. [Google Scholar]
- 23.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 24.Parrinello CM, Grams ME, Couper D, et al. Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem. 2015;61:938–947. doi: 10.1373/clinchem.2015.238873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grams ME, Rebholz CM, McMahon B, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64:214–221. doi: 10.1053/j.ajkd.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–693. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 29.Faerch K, Lau C, Tetens I, et al. A statistical approach based on substitution of macronutrients provides additional information to models analyzing single dietary factors in relation to type 2 diabetes in danish adults: the Inter99 study. J Nutr. 2005;135:1177–1182. doi: 10.1093/jn/135.5.1177. [DOI] [PubMed] [Google Scholar]
- 30.Song M, Fung TT, Hu FB, et al. Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Adler S, Caggiula AW, et al. Effects of dietary protein restriction on the progression of moderate renal disease in the Modification of Diet in Renal Disease Study. J Am Soc Nephrol. 1996;7:2616–2626. doi: 10.1681/ASN.V7122616. [DOI] [PubMed] [Google Scholar]
- 32.Beasley JM, Aragaki AK, LaCroix AZ, et al. Higher biomarker-calibrated protein intake is not associated with impaired renal function in postmenopausal women. J Nutr. 2011;141:1502–1507. doi: 10.3945/jn.110.135814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beasley JM, Katz R, Shlipak M, Rifkin DE, Siscovick D, Kaplan R. Dietary protein intake and change in estimated GFR in the Cardiovascular Health Study. Nutrition. 2014;30:794–799. doi: 10.1016/j.nut.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wetzels JF, Wiltink PG, van Duijnhoven EM, Hoitsma AJ, Koene RA. Short-term protein restriction in healthy volunteers: effects on renal hemodynamics and renal response to a meat meal. Clin Nephrol. 1989;31:311–315. [PubMed] [Google Scholar]
- 35.Kontessis P, Jones S, Dodds R, et al. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990;38:136–144. doi: 10.1038/ki.1990.178. [DOI] [PubMed] [Google Scholar]
- 36.Pan A, Sun Q, Bernstein AM, Manson JE, Willett WC, Hu FB. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: three cohorts of US men and women. JAMA Intern Med. 2013;173:1328–1335. doi: 10.1001/jamainternmed.2013.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borgi L, Curhan GC, Willett WC, Hu FB, Satija A, Forman JP. Long-term intake of animal flesh and risk of developing hypertension in three prospective cohort studies. J Hypertens. 2015;33:2231–2238. doi: 10.1097/HJH.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Manson JE, Buring JE, Sesso HD. Meat intake and the risk of hypertension in middle-aged and older women. J Hypertens. 2008;26:215–222. doi: 10.1097/HJH.0b013e3282f283dc. [DOI] [PubMed] [Google Scholar]
- 39.Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr. 2013;98:1066–1083. doi: 10.3945/ajcn.113.059030. [DOI] [PubMed] [Google Scholar]
- 40.Weng LC, Steffen LM, Szklo M, Nettleton J, Chambless L, Folsom AR. A diet pattern with more dairy and nuts, but less meat is related to lower risk of developing hypertension in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Nutrients. 2013;5:1719–1733. doi: 10.3390/nu5051719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engberink MF, Bakker SJ, Brink EJ, et al. Dietary acid load and risk of hypertension: the Rotterdam Study. Am J Clin Nutr. 2012;95:1438–1444. doi: 10.3945/ajcn.111.022343. [DOI] [PubMed] [Google Scholar]
- 42.Fagherazzi G, Vilier A, Bonnet F, et al. Dietary acid load and risk of type 2 diabetes: the E3N-EPIC cohort study. Diabetologia. 2014;57:313–320. doi: 10.1007/s00125-013-3100-0. [DOI] [PubMed] [Google Scholar]
- 43.Rebholz CM, Coresh J, Grams ME, et al. Dietary Acid Load and Incident Chronic Kidney Disease: Results from the ARIC Study. Am J Nephrol. 2015;42:427–435. doi: 10.1159/000443746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


