Abstract
A systematic literature review was conducted to identify predictors of poor adult retention in HIV medical care in developed and developing countries. An electronic search was conducted with MEDLINE (OVID), PubMED, EBSCO, SCOPUS, and Cochrane databases, as well as manual searches. Original, quantitative, adult studies in English, published between 1995 and 2015 were included. Only those with a focus on predictors of retention in care were reported on. Of the 345 articles identified, thirty were included following an independent assessment by two raters. In developed countries, the most frequently cited predictors of poor retention were active substance use and demographic factors. In developing countries, physical health factors were most frequently associated with poor retention in care. The results from this review suggests primary concerns for poor retention include substance use and physical health factors. Other psychosocial factors, such as psychiatric illness and social/welfare factors, were also found to be relevant.
Keywords: Retention, HIV, Predictors, Adult, Developing countries, Developed countries
Introduction
The Human Immunodeficiency Virus (HIV) Care and Treatment Cascade outlines the proportion of people living with HIV (PLWHIV) across a number of domains: living with HIV, linked to/engaged in care, retained in care, on treatment, and virologically suppressed [1]. The Centers for Disease Control and prevention [2] in the US estimates that only 50% of PLWHIV receive regular medical care, compared to an estimate of 73% in Australia [3]. The World Health Organization (WHO) estimates that most PLWHIV are lost to follow up (LTFU) within the first few years of starting treatment. Their synthesis of the available data suggests that the average retention rate 12 months after initiating medication ranges from 64 to 94%, and can reach 60% at 60 months in resource-limited countries [1]. The relevance of factors beyond the medical model has long been recognized in the context of optimal HIV management, including mental health, substance use, and social/welfare influences (‘psychosocial factors’). In the interest of providing holistic HIV care, understanding the role of these psychosocial factors in managing engagement with and retention in medical care is important.
Since their inception in the 1990s, significant improvements have been made to Antiretroviral Therapy (ART), and subsequently developed Highly Active Antiretroviral Therapy (HAART), with respect to efficacy, dosage and how well they are tolerated. In developed countries HAART is widely available, in contrast to resource-limited countries where there are significantly fewer medication options. Early findings from a recent clinical trial, the Strategic Timing of AntiRetroviral Treatment (START), across 35 countries indicate that starting medication early and maintaining adherence is beneficial from an individual health perspective [4]. Maintaining medication adherence affords optimal suppression of the virus and ensures a functioning immune system, leaving individuals less vulnerable to other infections or illnesses. Recent evidence also points to a strong link between virological suppression and a decrease in the risk of HIV transmission [5], which is the basis for the WHO global public health initiative ‘Treatment as Prevention’ [1]. Thus, retaining HIV+ individuals in medical treatment/care to increase HAART adherence is key in the maintenance of individual health and the eradication of new infections.
Multiple HIV treatment guidelines address adherence to treatment and retention in HIV care. The US Department of Health and Human Services recommends those who are newly diagnosed with HIV should immediately commence HAART, access medical care every three to four months, and continue this regime until they are virologically suppressed [6]. The US Health Resources and Services Administration (HRSA) defines successful retention in medical care as at least two visits [including blood collection to determine viral load (VL)] in a calendar year, spaced at least three months apart [6, 7]. This guideline is consistent with the European AIDS Clinical Society Guidelines (EACS; [8]). More broadly, the WHO [1] has globally recommended that those who are stable on medication be reviewed clinically every 3–6 months.
While the topic of retention in care has been extensively studied and reviewed, there lacks a synthesis of the literature to date to assist researchers and clinicians to identify and understand the key predictors of poor retention in HIV care. Only once retention in care has been improved can other factors, such as medication adherence, be tackled. By retaining people in care we can also address other factors such as physical health and psychological comorbidities, and other psychosocial factors which may impact both physical and mental health. In this way, developing our understanding of the key factors which are implicated in poor retention in care is required. The current review aims to address this gap in the literature.
Method
Systematic Literature Search
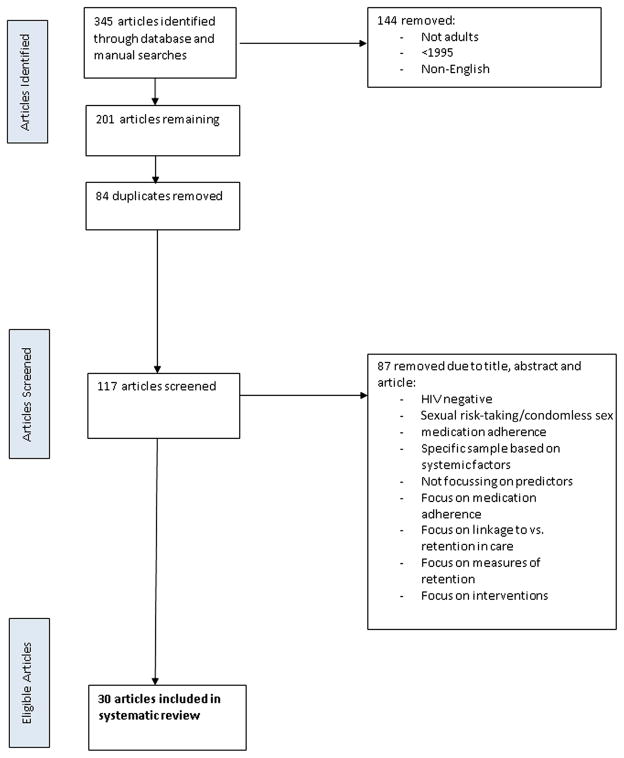
Search terms and associated synonyms reflecting predictors of retention in care for HIV+ populations were identified. Synonyms were identified for key themes of interest, and five databases (MEDLINE [OVID], PubMED, EBSCO, SCO-PUS, and Cochrane) were searched using the following terms: (predict* OR factor* OR caus* OR Component* OR Correlat* OR Determinant*) – Title, AND (retention* OR retain* OR engage* OR continuum OR treatment cascade) – Title, AND (HIV) – keyword. Manual searches through reference lists of relevant articles were also conducted. Duplicates were deleted, and articles were then screened by title, abstract and article content by two authors (SB and TNJ), with articles not meeting the agreed upon criteria removed. Figure 1 shows the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P; [9]) flowchart of the systematic literature search process.
Fig. 1.
PRISMA-P flowchart of article selection
Eligibility Criteria
Searches were limited to adult populations, published articles between 1995 and 2015 (to identify those written in the ART and HAART era), and those in English. Articles related to retention in medical care (as opposed to medication adherence) were included in the review. Furthermore, articles which specifically identified predictors of retention were included, whereas those focusing on measuring retention or interventions to improve retention were not. Articles which focused on specific samples were included in the review, provided systemic factors which may impact results were not present; for example, incarcerated populations where clients were mandated to attend appointments. Articles relating to a range of adult populations were included, on the basis that they could offer insight into retention issues from a broader context.
Original quantitative articles only (retrospective and prospective studies) were included while reviews were not, and studies which focused on psychosocial, demographic, health status, and welfare issues were included to provide a breadth of knowledge and understanding regarding all possible predictors of retention. One article, Tobias et al. [10], employed a mixture of quantitative and qualitative methods and was included because of the appropriateness of the quantitative methodology.
Study Appraisal and Selection
The inclusion and exclusion criteria were defined by SB and TNJ. The first 10 articles were jointly reviewed to establish consistency, with the following 107 reviewed separately and then compared. Cohen’s Kappa was used as a measure of interrater reliability, (K = 0.731, p <0.0001), and was considered to be substantial according to guidelines published by Landis and Koch [11]. Through consultation, agreement was reached regarding the articles the raters originally disagreed upon. In this context, the semantics associated with articles required clear definition, such that both raters were certain about the differences between articles focusing on access versus linkage versus engagement versus retention to/in care. A total of 30 articles were included in the review (see Fig. 1).
Data Extraction and Review
Data were extracted from the articles by the primary author (SB) using the PRISMA-P protocol [9]. This standardized method of extracting data obtained information regarding the quality of the study (appropriate use of measures and analyses, internal and external validity, any ethical concerns, minimization of any bias), and confirmed the research met the inclusion criteria agreed upon by SB and TNJ. Table 1 summarizes the relative strengths and weaknesses of the included articles, and includes information on the study design, the definition of retention (and whether this was an appropriate definition given overarching guidelines), and whether the estimation of retention was appropriate (that is, if the study was multi-site and could therefore account for transfers to other clinics so as not to underestimate retention). In addition, Table 1 outlines the generalizability of results, and whether appropriate data analyses were conducted to ascertain predictors as distinct from correlates. Studies employing hierarchical regression models to analyze data were considered preferable because of their capacity to identify the variables which account for the most variance. In contrast, bivariable correlational designs lack this rigor and therefore only provide superficial information about the relationships under investigation. The presence or absence of a broad range of predictor variables and adequate management of missing data, as well as accounting for additional contextual factors (e.g. recidivism in an incarcerated population) are also included.
Table 1.
Review of methodological strengths and weaknesses of included studies
| Study design (see key) | N | Appropriate definition of retention | Multi-site (appropriate estimation of retention) | Non-specific sample (generalizability of findings) | Appropriate data analyses | Broad range of variables measured/no missing data | All additional contextual factors accounted for | |
|---|---|---|---|---|---|---|---|---|
| Developed countries | ||||||||
| Adams [22] | R | 561 | + | − | − | + | − | − |
| Althoff [14] | P | 867 | + | + | − | + | + | − |
| Blank [19] | P | 921 | + | − | − | − | + | ? |
| Dombrowski [23] | P | 247 | − | − | + | + | + | ? |
| Giordano [15] | R | 2619 | + | + | − | + | + | ? |
| Horberg [20] | R | >23,000 | + | + | + | − | − | ? |
| Kelly [26] | P | 168 | + | − | + | + | + | ? |
| Lourenço [16] | R | 7621 | + | + | + | + | − | ? |
| Mcmahon [18] | R | 4966 | − | − | + | − | − | ? |
| Noysk [12] | R | 6152 | + | − | + | + | − | ? |
| Rebeiro [17] | P | 61,438 | + | + | + | + | + | ? |
| Richey [21] | C | 99 | + | − | + | − | − | ? |
| Scahfer [24] | P | 251 | − | − | + | + | + | ? |
| Tedaldi [7] | P | 1441 | + | + | + | + | − | ? |
| Tobias [10] | P | 1000 | + | + | + | + | + | ? |
| Waldrop-Valverde [25] | P | 210 | + | − | − | + | + | ? |
| Developing countries | ||||||||
| Allam [37] | P | 1690 | − | − | − | + | + | ? |
| Boyles [27] | P | 1803 | + | − | + | + | − | ? |
| Charurat [28] | P | 5760 | + | + | + | + | − | ? |
| Janssen [29] | R | 223 | + | − | + | + | − | ? |
| Koole [30] | R | 4147 | + | + | + | + | − | ? |
| Krumme [40] | P | 610 | + | − | + | + | − | − |
| Mekuria [31] | R | 836 | + | + | + | + | − | ? |
| Mutasa-Apollo [32] | R | 3919 | + | + | + | + | − | ? |
| Palombi [38] | R | 3749 | + | + | + | + | − | ? |
| Thida [33] | R | 5718 | + | + | + | + | − | ? |
| Ugoji [34] | R | 5176 | + | + | + | + | − | ? |
| Vella [35] | R | 2835 | + | + | + | + | − | ? |
| Vuylsteke [39] | R | 414 | + | + | − | + | − | + |
| Yang [36] | R | 822 | + | − | + | + | − | ? |
R retrospective study, P prospective study, C cross-sectional study, + identified in the article, – not identified in the article, ? not addressed in the article
Results
The database and manual searches yielded a total of 345 articles, with 30 retained for final review (see Fig. 1 for a full appraisal of the article selection process). Of the 30 remaining, all listed retention in care as a dependent/outcome variable. One study, Noysk et al. [12], operationalized retention in care as the duration of episodes on or off HARRT as defined by pharmacy dispensing records. While this is associated with medication, it does not relate to adherence per se but instead explores medication dispensing which is consistent with other measures of retention. In most other studies, attendance to medical appointments, including phlebotomy, was used to measure retention in care.
There were stark differences between the predictors identified for developing versus developed countries, which speaks to inherent access differences; for example, developed countries offer comparatively greater access to medical care than their developing counterparts. Furthermore, the priorities for HIV+ individuals in developed countries will likely be different to their developing counterparts; for example, those in developing nations may struggle more with access to appropriate medical care. Table 2 provides a broad overview of the studies retained for analysis for developed and developing countries, including the setting, design, participants and primary outcomes (predictors of retention identified). Tables 3 and 4 identify the predictors for developed and developing countries respectively. There were 16 articles which focused on developed countries and 14 which targeted developing countries, as defined by the United Nations World Economic Situation and Prospects [13]. The predictors of retention in care have been categorized under the broad banners of developed and developing countries, and are presented from higher to lower frequency in the articles included in the review. Results are also divided, where relevant, into predictors of poor retention followed by predictors of retention.
Table 2.
Predictors of retention in HIV care—developed and developing countries
| First author (article #) | Study dates | Country/Setting | Study design | Participants (N) | Predictors of retention |
|---|---|---|---|---|---|
| Developed countries | |||||
| Adams [22] | 2005–2011 | US (Philadelphia) | Retrospective cohort study | PLWHIV postpartum women (561) | Linkage to care; physical health |
| Althoff [14] | January 2008–March 2011 | US/prisoners recently released | Prospective cohort study | PLWHIV prisoners recently released (867) | Demographics; substance use; support |
| Blank [19] | Unspecified | US/eight clinical services | Prospective cohort study | PLWHIV women of colour (921) | Demographics; health beliefs; mental health; physical health; social/welfare |
| Dombrowski [23] | April 2013–April 2014 | US/public health facility | Prospective cohort study | Diagnosed with HIV for ≥6 months with either (a) no CD4 count or VL results for ≥12 months, or (b) VL > 500 copies/mL (247) | Mental health; substance use |
| Giordano [15] | 1997–1998 | US/Database of veterans with HIV | Retrospective cohort study | PLWHIV veterans (2619) | Demographics; physical health; substance use |
| Horberg [20] | 2010–2012 | US/HIV multi-site clinic | Retrospective cohort study | PLWHIV ( > 23,000) | Demographics |
| Kelly [26] | January 2006–September 2007 | US (Texas)/outpatient HIV clinic | Prospective observational | Diagnosed with HIV in previous 90 days and not yet have completed an outpatient visit with a care provider (168) | Social support (not significant) |
| Lourenço [16] | 2011 | Canada (BC)/database including residents diagnosed with HIV | Retrospective cohort study | PLWHIV (7621) | Substance use |
| McMahon [18] | February 2011–June 2013 | Australia (VIC)/HIV clinics | Retrospective cohort study | PLWHIV (4966) | Health beliefs; mental health; time |
| Noysk [12] | July 1996–June 2012 | Canada (BC)/database including residents living with HIV | Retrospective cohort study | PLWHIV (6152) | Demographics; physical health; substance use |
| Rebeiro [17] | 2000–2008 | US and Canada/epidemiological database | Retrospective | PLWHIV ≥18 yo, receiving care in US or Canada with ≥1 CD4 or VL result (61,438) | Demographics; social/welfare; substance use |
| Richey [21] | Unspecified | US/public health facility | Prospective study | PLWHIV newly diagnosed by a public health ED (99) | Demographics; linkage to care; physical health |
| Schafer [24] | April 2010–April 2011 | US/HIV clinic | Cross-sectional cohort study | PLWHIV (251) | Domestic violence |
| Tedaldi [7] | 2000–2011 | US/HIV clinics | Prospective observational cohort study | PLWHIV (1441) | Physical health; social/welfare |
| Tobias [10] | October 2003–July 2005 | US/multi-site HIV clinics | Prospective, semi-structured interviews and review of blood results | PLWHIV (1000) | Health beliefs; mental health; substance use; support |
| Waldrop-Valverde [25] | August 2009–May 2011 | US/HIV clinics in Florida | Prospective cohort study | Outpatient PLWHIV (210) | Cognitive impairment; support |
| Developing countries | |||||
| Allam [37] | January–December 2008 | India/high caseload HIV clinics | Prospective cohort study | PLWHIV (≥15 years of age) initiated on ART during the study period (1690) | Demographics; physical health |
| Boyles [27] | June 2005–May 2009 | South Africa/patients of a rural ART program | Prospective cohort study | PLWHIV initiated on ART during the study period (1803) | Demographics; physical health; process/clinic factors |
| Charurat [28] | March 2005–July 2006 | Nigeria/five health facilities | Prospective cohort study | PLWHIV initiated on ART during the study period (5760) | Demographics; physical health |
| Janssen [29] | Jan 2010–Jan 2012 | Gabon/HIV clinics | Retrospective cohort study | PLWHIV (233) | Physical health |
| Koole [30] | 2003–2010 | Tanzania, Uganda, Zambia/ART clinics | Retrospective cohort study | PLWHIV initiated on ART during the study period (4147) | Demographics; physical health |
| Krumme [40] | July 2006–August 2008 | Rwanda/ART clinics | Prospective cohort study | PLWHIV initiated on ART during the study period (610) | Mental health |
| Mekuria [31] | May 209–April 2012 | Ethiopia/Healthcare facilities | Retrospective cohort study | PLWHIV treatment naïve (836) | Physical health |
| Mutasa-Apollo [32] | 2007–2009 | Zimbabwe/multi-site clinics | Retrospective cohort study | PLWHIV initiated on ART during the study period (3919) | Demographics; physical health |
| Palombi [38] | Mozambique, Milawi, Guinea-Conakry/public sector HIV clinics | Retrospective cohort study | PLWHIV initiated on ART during the study period (3749) | Physical health; process/clinic factors | |
| Thida [33] | June 2005–October 2011 | Myanmar/Integrated HIV Care Program | Retrospective cohort study | PLWHIV (5718) | Demographics; physical health |
| Ugoji [34] | 2005–2009 | Nigeria/HIV treatment clinics | Retrospective cohort study | PLWHIV (5176) | Demographics; physical health |
| Vella [35] | March 2004–May 2006 | South Africa/public HIV clinics | Retrospective cohort study | PLWHIV initiated on ART during the study period (2835) | Demographics; physical health; process/clinic factors |
| Vuylsteke [39] | January–December 2010 | Ivory Coast/HIV clinics | Retrospective cohort study | PLWHIV sex workers (414) | Demographics; process/clinic factors |
| Yang [36] | Until June 2013 | China | Retrospective cohort study | PLWHIV (822) | Demographics; physical health |
Table 3.
Summary of predictors of retention in care, and associated articles—developed countries
| Predictor(s) of retention in care | Number of articles in which predictor(s) is/are cited | Referenced in first author (article #) |
|---|---|---|
| Substance use | 7 | Althoff [14], Dombrowski [23], Giordano [15], Lourenço [16], Noysk [12], Rebeiro [17], Tobias [10] |
| Demographic | 7 | Althoff [14], Blank [19], Giordano [15], Horberg [20], Noysk [12], Rebeiro [17], Richey [21] |
| Physical health | 6 | Adams [22], Blank [19], Giordano [15], Noysk [12], Richey [21], Tedaldi [7] |
| Mental health | 4 | Blank [19], Dombrowski [23], McMahon [18], Tobias [10] |
| Support | 4 | Althoff [14], Kelly [26], Tobias [10], Waldrop-Valverde [25] |
| Health beliefs | 3 | Blank [19], McMahon [18], Tobias [10] |
| Social/welfare | 3 | Blank [19], Rebeiro [17], Tedaldi [7] |
| Cognitive impairment | 1 | Waldrop-Valverde [25] |
| Domestic violence | 1 | Schafer [24] |
| Linkage to care | 2 | Adams [22], Richey [21] |
| Time | 1 | McMahon [18] |
Table 4.
Summary of predictors of retention in care, and associated articles—developing countries
| Predictor(s) of retention in care | Number of articles in which predictor(s) is/are cited | Referenced in first author (article #) |
|---|---|---|
| Physical health | 11 | Allam [37], Boyles [27], Charurat [28], Janssen [29], Koole [30], Mekuria [31], Mutasa-Apollo [32], Palombi [38], Thida [33], Ugoji [34], Vella [35], Yang [36] |
| Demographic | 9 | Allam [37], Boyles [27], Charurat [28], Koole [30], Mutasa-Apollo [32], Thida [33], Ugoji [34], Vella [35], Vuylsteke [39], Yang [36] |
| Process/clinic factors | 6 | Boyles [27], Koole [30], Mutasa-Apollo [32], Palombi [38], Vella [35], Vuylsteke [39] |
| Mental health | 1 | Krumme [40] |
Developed Countries
Substance Use
Substance use was the most cited predictor of poor retention in the literature in developed nations, and was referred to in seven of the studies examined. Althoff et al. [14] theorized a model of predisposing and needs factors in a sample of detainees recently released from jail, but not mandated to attend HIV treatment. This study used VL testing as a proxy for retention in care, a practice used by other researchers in this field. They also utilized the Behavioral Model for Vulnerable Populations to suggest that predisposing (e.g. demographics, mental health, and substance use) and needs (e.g. medical comorbidity, addiction severity and psychiatric severity) factors interacted with others (e.g. jail and community services) to influence health behaviors; in this instance, medication adherence and retention in care. Using logistic regression, the authors concluded that substance use, among other factors, was significantly associated with poorer sustained retention in care.
Other studies which identified a relationship between active substance use and poor retention include Giordano et al. [15], who reviewed a cohort of US Veterans, and Lourenço et al. [16] who reported the greatest attrition rate from medical care among their substance using population above all others. Noysk et al. [12] noted that intravenous drug use (IVDU) in particular was associated with poor retention, while Rebeiro et al. [17] noted that those within the IVDU HIV transmission risk group were more likely, than their non-IVDU counterparts, to dropout of care. Tobias et al. [10] identified that those classified as receiving “no care” (i.e. those who did not see a doctor for their HIV after diagnosis, or had not seen an HIV specialist within the past 6 months) were more likely to report binge drinking or illicit drug use in the previous 30 days.
Demographics
Various demographic characteristics were also identified as strong predictors of retention in care. Those factors associated with poorer retention in care included male sex [20], female sex [12, 14, 16, 17], having young children [19], younger age [12, 15, 16, 20, 21], and being from an ethnic minority group [17, 19]. Noysk et al. [12] further identified aboriginality was a predictor of poor retention in their Canadian study. Conversely, Horberg et al. [20] found that certain ethnic minorities (e.g. Latinos) were more likely to be retained than others (e.g. African Americans, or Anglo Americans).
HIV-Disease Progression and Physical Co-morbidities
Some authors identified that physical health, rather than psychosocial, factors were pertinent to a person’s capacity to remain engaged in care. Giordano et al. [15] identified that those with Hepatitis C (HCV) coinfection and higher CD4 counts (>350 × 106/L) were less likely to be retained in care. Those with HCV coinfection were also more likely to present with active substance use, which would likely have further interfered in retention. Conversely, Tedaldi et al. [7] identified that those with lower baseline CD4 counts were less likely to be retained in care. Adams et al. [22] conducted a retrospective analysis of postpartum mothers and found those who were recently diagnosed and engaged in low prenatal care were less likely to remain retained in HIV care during the postpartum period.
Psychiatric Co-morbidities
Psychiatric illness, besides substance use, has been identified in a number of the studies reviewed as being a significant predictor of poor retention in care. Tobias et al. [10] noted that subjects with poorer mental health scores were less likely to be retained in care. McMahon et al. [18] further noted that active psychiatric illness at the last clinical visit was associated with not returning for care. Conversely, Blank et al. [19] noted in their study that those who reported at least 14 “mentally unhealthy” days per month were more likely to remain retained in care.
Social/Welfare
In their study of retention among a US cohort of women of color, Blank et al. [19] identified that those who had dependent children <18 years old and those living in institutional facilities were less likely to remain retained in care. Tedaldi et al. [7] noted that patients who were publicly insured were less likely to be retained in care. Dom-browski et al. [23] also identified that cost (e.g. lack of insurance) was a significant barrier to them continuously accessing care despite, as the authors noted, the study being conducted in a US state with universal access to care.
Miscellaneous (Health Beliefs, Support, Domestic Violence, Practical Factors, Linkage to Care)
Other factors have also been identified as predictors of poor retention in care. Health beliefs appear to play an important role, and include feeling hopeless about treatment for HIV [19], or feeling well and being too busy [18]. In addition, perceiving ‘wellness’ as a barrier to seeking treatment, not trusting the medical system or not ‘bothering’ because there is no cure were identified by Tobias et al. [10] as relevant beliefs which impacted retention. Schafer et al. [24] also identified that intimate partner violence could have a role, such that patients who feel threatened by their partner are less likely to remain retained in care.
Practical factors (e.g. access to transport to attend appointments, ease of obtaining an appointment with an HIV specialist) were also identified as barriers to patients remaining in care [23]. These authors found that this was more of an obstacle than other psychosocial issues such as depression and substance use, despite these being highly prevalent. In addition, Adams et al. [22] and Richey et al. [21] also noted in their respective studies that early linkage to care was associated with longer term retention in care.
Predictors of Retention
Even though most studies have focused on barriers to retention, some have focused on predictors to retention in care. A number of authors have also looked at the role of certain factors in improving retention rates; for example, social and/or professional support. Tobias et al. [10] noted that professional support (such as case management from HIV and/or mental health services) was associated with greater retention in care. Althoff et al. [14] also noted that access to case management was an important factor to improve retention in their population of recently released inmates. Waldrop-Valverde et al. [25] also identified that social support mediates the relationship between cognitive impairment and retention in care, such that those who are cognitively impaired and have some support are more likely to be retained in care. Conversely, Kelly et al. [26] noted in their study of newly diagnosed patients that social support did not significantly predict retention in care.
Developing Countries
HIV-Disease Progression and Physical Co-morbidities
HIV-disease progression [27–36], and lower body mass index (BMI) [30, 32, 33, 37, 38] were cited in eleven of the 14 articles in developing countries. Lower baseline CD4 counts or higher WHO stage of the disease was usually associated with higher rates of attrition [34], however in a number of articles, the authors noted that higher CD4 counts were related to poorer retention in care [27, 36]. Koole et al. [30] and Mekuria et al. [31] found that those with a poorer level of functionality (e.g. those who were bedridden) were also less likely to remain retained in medical care. Charurat et al. [28] also noted that those with either low or high baseline CD4 counts were more likely to be lost to follow-up (LTFU). Time of commencing ART was also a factor of interest; Janssen et al. [29] found that those who initiated ART early were more likely to remain retained in care, and Ugoji et al. [34] further noted that individuals who recently commenced ART, compared to those who initiated more than six months prior, were more likely to be retained in care.
Demographic
Demographic factors were identified in nine of the 14 articles which focused on resource-limited countries, suggesting it is a key predictor of poor retention in developing populations. Factors such as sex, age, and education level were frequently cited in these articles. For example, a number of articles noted that male sex was associated with an increased risk of attrition from care [28, 32–35, 37], as was younger age [27, 30, 34], and lower levels of education [28, 36, 39].
Miscellaneous (Process/Clinic Factors, Mental Health)
Other factors associated with poor retention in care were cited far less frequently than those already mentioned in the literature reviewed in resource-rich countries. Boyles et al. [27] notes that those patients who commenced medication as an inpatient or when pregnant were at greater risk of LTFU. The authors suggest this may be because patients were required to commence ART at a faster rate than usual, and perhaps before they were psychologically ready, thereby limiting their capacity to effectively prepare for it. Vuylsteke et al. [39] also noted that patients who had not received ART adherence counseling at baseline were less likely to remain engaged in regular medical care, and that those who commenced medication later in the study period were more likely to become LTFU. The authors suggested the latter might be explained by increased workload within the clinic in the absence of any increase in resources [39]. This finding was replicated by Palombi et al. [38], and likewise Koole et al. [30] who found that retention rates decreased as the number of years the clinic had been in operation increased. Likewise, Vella et al. [35] noted that clinics with part-time (compared to full-time) nursing and medical staff demonstrated poorer retention rates. Mutasa-Apollo et al. [32] also note that those initiating medication at primary healthcare facilities (compared to district hospitals) were more likely to remain retained, and the authors suggest this is likely because rural patients would travel to urban hospitals to initiate treatment under medical specialists and then likely transfer back to rural facilities.
Only one study in developing countries included in the review investigated the potential role of mental health, and found that mental health concerns were a factor in retention rates in developing countries. Krumme et al. [40] found that higher rates of depression were associated with higher rates of dropout from care, and that this effect was not mediated by suboptimal medication adherence as hypothesized.
Discussion
The 30 articles included in this systematic review highlight the complexity of the issues surrounding retention in care. The factors related to retention clearly differ between developed and developing countries. This issue is not unique to HIV and is well documented throughout the chronic disease literature; however there are factors specific to the HIV+ population which require particular attention.
The primary predictor of poor retention identified in developed countries in this review is active substance use [10, 12, 14–17]. We already know that active substance use impacts the frontal cortex, among other structures, which is responsible for a person’s capacity to plan and make decisions [41]. The limbic system (the ‘reward center’) is also implicated in substance use, meaning intoxicated individuals not only experience a heightened sense of reward and pleasure, but also potentially lack the capacity to effectively care for themselves in the short, medium and long-term, depending on the severity of their use [41]. Disorganization, impulsivity, lack of stable housing, and other physical health and social problems associated with substance use are also likely to contribute to poor retention in care. In the context of retention, it is clear that effectively treating substance use in order to alter the impact of substances on the brain could better equip a person to make decisions and manage their healthcare, potentially improving their capacity to remain engaged in medical treatment. Active substance use can also impact certain populations more than others. In many developed countries (for example, Australia) the prevalence of HIV in MSM populations is higher than the general population, and substance use is common within the community, in part due to its (often positive) impact on the frequency and intensity sexual experiences [42], as well as the management of psychological distress [43]. In this way, developing our knowledge and treatment approaches to substance use and its antecedents, we may be able to improve individuals’ psychological wellbeing in many facets, and potentially positively impact retention rates in HIV medical care. A goal for future research might be to ascertain the amount of variance accounted for by substance use in a regression model of multiple predictors on retention in care, so as to explore this association further.
Demographic factors were also highly predictive of retention rates in the literature surveyed, both in developed and resource-constrained countries. In particular, sex, age, education level, and race and ethnicity all appear to play a large role in retention rates, across the board. Health perceptions were also identified as important; Blank et al. [19] noted that “feeling hopeless” about one’s health status impacted retention. It is possible this is associated with the construct of self-efficacy or locus of control, and may moderate relationships with other variables. For example, younger people may not fully appreciate the necessity for ongoing medical care or feel they lack the capacity to ‘commit’; conversely, older people may feel as though there is “no point” in managing their care. Further research is required to explore these relationships.
Health status factors, including how unwell a person was, were also highly correlated with retention in care. Studies identifying competing results have been reviewed here, such that some authors [7, 13] noted that those with lower baseline CD4 counts were less likely to be retained, while others [21, 27] found the opposite to be true. Adams et al. [22], in their study of postpartum women, also found that those who were recently diagnosed and those who had not engaged well with prenatal care were less likely to remain retained in the postpartum period. It is possible that high or low CD4 counts might influence a person’s perception of “feeling well” or “feeling unwell”, and that this might be a risk factor for not attending appointments. It is evident that these mixed findings are apparent in both developed and developing countries. Clearly further research is warranted to explore these discrepancies, with a number of potentially influential factors including health beliefs, level of social support and other demographic variables requiring examination.
Other key predictors of retention identified in this review were health beliefs, psychiatric illness, support, social/welfare issues, clinic factors, and others. The prevalence of psychiatric illness, especially mood disorders, in an HIV+ population is well documented [44], as is the impact this can have on self-care including attendance to medical appointments and medication adherence [40]. Health beliefs in particular appear to have strong correlations (e.g. feeling too well or too busy; [18]). In addition, professional support in the form of case management appeared to positively assist vulnerable populations (e.g. those who were incarcerated; [14]) to remain engaged in care. Other practical factors, including the ease with which patients can attend appointments [23], and clinic factors (e.g. the number of patients enrolled in a clinic and associated staff workloads; [35]); and the availability of medication adherence counselling prior to initiation [39] were also noted. These point to the subtle interplay between psychological and social factors impacting retention, and acknowledge the role of both in researching this area.
In resource-limited countries, compared to developed countries, it appears that fundamental factors such as weight, disease progression, and education level are the key factors which impact upon retention in care, whereas in developed countries other factors, such as active substance use, is the primary factor which interferes in optimal retention in care. This points to inherent differences between developed and developing countries with respect to access to adequate care and medication, and the overall differences between patient needs in both contexts.
Of interest is the absence of quantification of the role of perceived stigma and/or discrimination in impacting an individual’s capacity or willingness to remain retained in medical HIV care. This is despite numerous findings regarding the importance of stigma and/or discrimination in HIV [45], including its impact on self-efficacy and perceived capacity to manage one’s own health [46]. While attitudes and understanding about HIV and its transmission in developed countries have greatly improved over the past 30 years, stigma and/or discrimination regarding HIV remains an issue for some, and it was therefore expected to have been identified as a predictor. Despite this, none of the studies included in this review explicitly investigated the potential role of stigma in retention in care. Likewise, it was not identified as a retention-interfering factor in the developing countries literature, which is perhaps even more surprising. In their study, Mutasa-Apollo et al. [32] noted that those HIV-infected patients in rural settings were more likely to travel to urban areas for their medical treatment, and it is possible this is related (at least in part) to concerns around stigma and/or discrimination.
A broad limitation with the articles reviewed is that factors are only able to be identified as determinants if they are investigated. It is probable that some influences, such as substance use and mental health, are more frequently studied than others, and indeed it may be impossible to identify and include all possible determinants in one study. Furthermore, other aspects such as clinic factors or stigma/discrimination may in fact be highly influential in retention in care, and have been previously identified in the literature, but are not a key variable in the studies included in this review.
There are several limitations with the specific studies reviewed, which have been summarized in Table 1. A large proportion of the studies included in this review employed a retrospective cohort design; this was especially true in developing countries, and is likely due to limitations in conducting prospective studies. An issue with retrospective studies is the potential risk that key variables are missed, as the analysis can depend on data routinely collected (for example, it is unlikely that perceived stigma and/or discrimination would have been routinely collected). A further limitation is that some of the studies reviewed employed bivariable correlational analyses, while others provided more detailed predictive regression models. Study designs involved in prediction (hierarchical regression models) provide the opportunity to test for the influence of multiple variables on outcomes. This exploration allows researchers to identify the variables which account for the most variance in outcomes. In practical terms, this means finances and interventions can be targeted accordingly. Future research should attempt to specifically measure predictors of retention, for the purposes of identifying those ‘at-risk’ of dropping out and intervening as early as possible.
A key issue with the data reviewed involves definitional terms; there appears to be a lack of consistency regarding the terminology in this area. For example, the terms ‘engagement in care’ and ‘retention in care’ are often ill-de-fined and used interchangeably, and this impacted the search strategy and the analysis of the articles to ensure authors’ definition of retention in care matched our own. Likewise, definitions of retention in care varied slightly between studies, despite there being clear guidelines stipulated by the HRSA and EACS [7, 8] in developed countries. It seems there are no/limited guidelines specifically related to developing countries, which may in itself be an issue, in addition to the varied definitions of LTFU which were employed by studies.
There are other limitations with some of the specific studies reviewed, the most common of which relates to the definition and means of measuring retention in care. Inconsistencies were noted in the ways in which retention was measured; some used VL tests as a proxy for retention [14, 17], while others [19] measured attendance to HIV medical appointments. Tobias et al. [10] used self-reported use of medical services as the outcome variable in their analysis, perhaps not the most rigorous test of this variable and open to bias (for example, social desirability or memory/recall bias). The definition of LTFU also needs to be carefully considered; for example, Yang et al. [36] noted that patients who had transferred their care to another clinic were counted as LTFU, suggesting their retention rates may be underestimated. This points to the risk of bias in any research study, which may be difficult to eliminate entirely, but could be managed by identifying a homogenous and reliable way of measuring retention in order to standardize conclusions.
An issue with some of the studies presented in this review relates to a lack of data sharing between clinical sites, such that retention rates may be underestimated. It is possible that those who are LTFU at one site may well be retained at another. In addition, some studies [14] acknowledge that additional contextual factors specific to their sample potentially impacted rates (e.g. recidivism in an incarcerated population). A further limitation of the studies reviewed is that generalizability of findings was often low because of specific sample characteristics, e.g. veterans [15] or postpartum women [22]. The majority of the studies reviewed also did not explicitly address the issue of power in the context of their statistical analyses; it is possible to infer adequate power in those studies with large sample sizes, however this is not ideal.
Inherent in this investigation into predictors of retention is the necessity to focus/direct interventions appropriately so as to maximize retention rates. This review therefore serves as a foundation, to facilitate a thorough understanding of these factors in order to fully achieve this goal. Furthermore, given the bulk of the literature in developed countries is from the US/Canada, it would be important to conduct studies in other developed countries to ascertain whether there are any differences, given the different nature of the epidemic across the globe, and fundamental differences in access to medical care and medication. There were no articles included from Europe, for example, which satisfied all eligibility criteria. This may point to a gap in our knowledge, or that HIV retention research in developed countries is concentrated on the United States and Canada.
The current review also has some limitations. Firstly, the review is limited by the variables which have been studied, and therefore cannot provide an exhaustive evaluation of all potential influences on retention in care. The results from this review should therefore only be interpreted within specific parameters; that is, as a synthesis of available data, not necessarily of all influences on retention in care. Secondly, the different terminology used in literature in this area potentially complicated the inclusion/exclusion criteria. Thirdly, while investigating efficacious interventions to improve retention is beyond the scope of the present review, this is a possibility for future consideration. However, this review does synthesize the relevant data from both developing and developed countries, whereas previous reviews have focused on one region only, and it therefore makes a valuable contribution to the evidence-base on this topic. The results presented here should guide future research, however do not offer firm conclusions given the heterogeneity of samples within the studies undertaken in developed and developing countries.
Conclusions
As treatments improve we have an ageing HIV+ population, which is accompanied by an increase in comorbidities and amplifies the need for often complex monitoring and retention in care. Concentrating on physical health aspects alone can fail to account for the broader range of factors which impact an individual’s life. In this way, expanding this focus to retention in HIV care (vs. medication adherence alone) offers a more holistic view of HIV management and moves away from the pure medical model to account for psychological and physical comorbidities, and other psychosocial issues in conjunction with medication adherence.
The literature reviewed here points to a collection of factors which appear to predict retention in medical HIV care, both across developed and developing countries. Given the results from a broad range of settings, populations and countries, the capacity for one cohesive outcome from this review is unlikely. Individual countries battling the issue of retaining patients in medical HIV care may focus on the results relevant to them. It is also possible that organizations such as the WHO, whose role it is to oversee the broader implementation of HIV treatment on a global scale, may find the present findings particularly valuable. It is also clear that further research is required to quantify some of the constructs identified (e.g. stigma and/or discrimination), and to do so with other samples not previously reported in the literature. While we have a sound knowledge regarding the factors that contribute to poor retention, little remains understood regarding the complexity of interrelationships between these, and whether intervening in one part of the system will ultimately impact the outcome.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.World Health Organization. [Accessed 4 Aug 2016];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2016 http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1. [PubMed]
- 2.Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment—United States. [Accessed 12 Mar 2016];CDC. 2011 60(47):1618–1623. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6047a4.htm. [PubMed] [Google Scholar]
- 3.The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report. Sydney: The Kirby Institute, UNSW Australia; 2015. [Google Scholar]
- 4.National Institutes of Health. [Accessed 30 May 2016];Starting antiretroviral treatment early improves outcomes for HIV-infected individuals. https://www.nih.gov/news-events/news-releases/starting-antiretroviral-treatment-early-improves-outcomes-hiv-infected-individuals.
- 5.Charania MR, Marshall KJ, Lyles CM, Crepaz N, Kay LS, Koenig LJ, et al. Identification of evidence-based interventions for promoting HIV medication adherence: findings from a systematic review of US-based studies, 1996–2011. AIDS Behav. 2014;18(4):646–60. doi: 10.1007/s10461-013-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young B. The importance of retention in HIV care. [Accessed 28 Apr 2015];Medscape. 2014 http://www.medscape.com/viewarticle/831324.
- 7.Tedaldi EM, Richardson JT, Debes R, Young B, Chmiel JS, Durham MD, et al. Retention in care within 1 year of initial HIV care visit in a multisite US cohort: who’s in and who’s out? J Int Assoc Provid AIDS Care. 2014;13:232–41. doi: 10.1177/2325957413514631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European AIDS Clinical Society. [Accessed 28 Apr 2015];EACS European Guidelines for treatment of HIV infected adults in Europe. http://www.eacsociety.org/Guidelines.aspx.
- 9.Moher D, Shamseer L, Clarke D, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 10.Tobias CR, Cunningham W, Cabral HD, Cunningham CO, Eldred L, Naar-King S, et al. Living with HIV but without medical care: barriers to engagement. AIDS Patient Care STDs. 2007;21:426–34. doi: 10.1089/apc.2006.0138. [DOI] [PubMed] [Google Scholar]
- 11.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 12.Nosyk B, Lourenço L, Min JE, Shopin D, Lima VD, Montaner JS, et al. Characterizing retention in HAART as a recurrent event process: insights into ‘cascade churn’. AIDS. 2015;29(13):1681–9. doi: 10.1097/QAD.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The United Nations. [Accessed 7 Dec 2015];World economic situation and prospects. http://www.un.org/en/development/desa/policy/wesp/wesp_current/2014wesp_country_classification.pdf.
- 14.Althoff AL, Zelenev A, Meyer JP, Fu J, Brown SE, Vagenas P, et al. Correlates of retention in HIV care after release from jail: results from a multi-site study. AIDS Behav. 2013;17(Suppl 2):S156–70. doi: 10.1007/s10461-012-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials. 2009;10(5):299–305. doi: 10.1310/hct1005-299. [DOI] [PubMed] [Google Scholar]
- 16.Lourenço L, Colley G, Nosyk B, Shopin D, Montaner JSG, Lima VD. High levels of heterogeneity in the HIV cascade of care across different population subgroups in British Columbia, Canada. PLoS ONE. 2014;9:e115277. doi: 10.1371/journal.pone.0115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebeiro P, Althoff KN, Buchacz K, Gill J, Horberg M, Krentz H, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. JAIDS. 2013;62:356–62. doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon JH, Moore R, Eu B, Tee B-K, Chen M, El-Hayek C, et al. Clinic network collaboration and patient tracing to maximize retention in HIV care. PLoS ONE. 2015;10:e0127726. doi: 10.1371/journal.pone.0127726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blank AE, Fletcher J, Verdecias N, Garcia I, Blackstock O, Cunningham C. Factors associated with retention and viral suppression among a cohort of HIV+ women of color. AIDS Patient Care STDS. 2015;29(Suppl 1):S27–35. doi: 10.1089/apc.2014.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horberg MA, Hurley LB, Klein DB, Towner WJ, Kadlecik P, Antoniskis D, et al. The HIV care cascade measured over time and by age, sex, and race in a large national integrated care system. AIDS Patient Care STDs. 2015;29(11):582–90. doi: 10.1089/apc.2015.0139. [DOI] [PubMed] [Google Scholar]
- 21.Richey LE, Halperin J, Pathmanathan I, Van Sickels N, Seal PS. From diagnosis to engagement in HIV care: assessment and predictors of linkage and retention in care among patients diagnosed by emergency department based testing in an urban public hospital. AIDS Patient Care STDs. 2014;28(6):277–9. doi: 10.1089/apc.2014.0052. [DOI] [PubMed] [Google Scholar]
- 22.Adams JW, Brady KA, Michael YL, Yehia BR, Momplaisir FM. Postpartum engagement in HIV care: an important predictor of long term retention in care and viral suppression. Clin Infect Dis. 2015;61(12):1880–7. doi: 10.1093/cid/civ678. [DOI] [PubMed] [Google Scholar]
- 23.Dombrowski JC, Simoni JM, Katz DA, Golden MR. Barriers to HIV care and treatment among participants in a public health HIV care relinkage program. AIDS Patient Care STDs. 2015;29:279–87. doi: 10.1089/apc.2014.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer KR, Brant J, Gupta S, Thorpe J, Winstead-Derlega C, Pinkerton R, et al. Intimate partner violence: a predictor of worse HIV outcomes and engagement in care. AIDS Patient Care STDs. 2012;26(6):356–65. doi: 10.1089/apc.2011.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldrop-Valverde D, Guo Y, Ownby RL, Rodriguez A, Jones DL. Risk and protective factors for retention in HIV care. AIDS Behav. 2014;18(8):1483–91. doi: 10.1007/s10461-013-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly JD, Hartman C, Graham J, Kallen MA, Giordano TP. Social support as a predictor of early diagnosis, linkage, retention, and adherence to HIV care: results from the steps study. J Assoc Nurses AIDS Care. 2014;25(5):405–13. doi: 10.1016/j.jana.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyles TH, Wilkinson LS, Leisegang R, Maartens G. Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PLoS ONE. 2011;6(5):e19201. doi: 10.1371/journal.pone.0019201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charurat M, Oyegunle M, Benjamin R, Habib A, Eze E, Ele P, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS ONE. 2010;5(5):e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janssen S, Wieten RW, Stolp S, Cremers AL, Rossatanga EG, Klipstein-Grobusch K, Belard S, Grobusch MP. Factors associated with retention to care in an HIV clinic in Gabon, Central Africa. PLoS ONE. 2015;10(10):e0140746. doi: 10.1371/journal.pone.0140746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koole O, Tsui S, Wabwire-Mangen F, Kwesigabo G, Menten J, Mulenga M, et al. Retention and risk factors for attrition among adults in antiretroviral treatment programmes in Tanzania, Uganda and Zambia. Trop Med Int Health. 2014;19(12):1397–410. doi: 10.1111/tmi.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mekuria LA, Prins JM, Yalew AW, Sprangers MAG, Nieuwkerk PT. Retention in HIV care and predictors of attrition from care among HIV-infected adults receiving combination anti-retroviral therapy in Addis Ababa. PLoS ONE. 2015;10(6):e0130649. doi: 10.1371/journal.pone.0130649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutasa-Apollo T, Shiraishi RW, Takarinda KC, Dzangare J, Mugurungi O, Murungu J, et al. Patient retention, clinical outcomes and attrition-associated factors of HIV-infected patients enrolled in Zimbabwe’s National Antiretroviral Therapy Programme, 2007–2010. PLoS ONE. 2014;9(1):e86305. doi: 10.1371/journal.pone.0086305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thida A, Tun STT, Zaw SKK, Lover AA, Cavailler P, Chunn J, et al. Retention and risk factors for attrition in a large public health ART program in Myanmar: a retrospective cohort analysis. PLoS ONE. 2014;9(9):e108615. doi: 10.1371/journal.pone.0108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ugoji C, Okere N, Dakum P, Ake-Uzoigwe R, Igboelina D, Ndembi N, Ekong E, Charurat M, Blattner WA. Correlates of patient retention in HIV care and treatment programs in Nigeria. Curr HIV Res. 2015;13(4):300–7. doi: 10.2174/1570162x13999150317155348. [DOI] [PubMed] [Google Scholar]
- 35.Vella V, Govender T, Dlamini S, Taylor M, Moodley I, David V, et al. Retrospective study on the critical factors for retaining patients on antiretroviral therapy in KwaZulu-Natal, South Africa. JAIDS. 2010;55(1):109–16. doi: 10.1097/QAI.0b013e3181e7744e. [DOI] [PubMed] [Google Scholar]
- 36.Yang GL, Yan J, Liu Y, Huang ZL, Long S. Retention in care and factors affecting it among people living with HIV/AIDS in Changsha city, China. Asia-Pacific J Public Health. 2015;27(2 suppl):86S–92S. doi: 10.1177/1010539514548758. [DOI] [PubMed] [Google Scholar]
- 37.Allam RR, Murhekar MV, Bhatnagar T, Uthappa CK, Chava N, Rewari BB, et al. Survival probability and predictors of mortality and retention in care among patients enrolled for first-line antiretroviral therapy, Andhra Pradesh, India, 2008–2011. Trans R Soc Trop Med Hyg. 2014;108:198–205. doi: 10.1093/trstmh/tru025. [DOI] [PubMed] [Google Scholar]
- 38.Palombi L, Marazzi MC, Guidotti G, Germano P, Buonomo E, Scarcella P, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral-treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48(1):115–22. doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 39.Vuylsteke B, Semdé G, Auld AF, Sabatier J, Kouakou J, Ettiégne-Traoré V, et al. Retention and risk factors for loss to follow-up of female and male sex workers on antiretroviral treatment in Ivory Coast: a retrospective cohort analysis. JAIDS. 2015;68:S99–106. doi: 10.1097/QAI.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krumme AA, Kaigamba F, Binagwaho A, Murray MB, Rich ML, Franke MF. Depression, adherence and attrition from care in HIV-infected adults receiving antiretroviral therapy. J Epidemiol Community Health. 2014;69:284–9. doi: 10.1136/jech-2014-204494. [DOI] [PubMed] [Google Scholar]
- 41.National Institute on Drug Abuse. [Accessed 20 Mar 2016];Drugs, brain and behavior: the science of addiction. https://www.drugabuse.gov/publications/drugs-brains-behavior-science-addiction/drugs-brain.
- 42.Hull P, Mao L, Kao S-C, Edwards B, Prestage G, Zablotska I, et al. Gay Community Periodic Survey: Sydney 2013. Sydney: National Centre in HIV Social Research, University of New South Wales; [Google Scholar]
- 43.Hatzenbuehler ML. How does sexual minority stigma “get under the skin”? A psychological mediation framework. Psychol Bull. 2009;135(5):707. doi: 10.1037/a0016441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav. 2012;16(8):2119–43. doi: 10.1007/s10461-012-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayles JN, Wong MD, Kinsler JJ, Martins D, Cunningham WE. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24(10):1101–8. doi: 10.1007/s11606-009-1068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson MO, Neilands TB, Dilworth SE, Morin SF, Remien RH, Chesney MA. The role of self-efficacy in HIV treatment adherence: validation of the HIV Treatment Adherence Self-Efficacy Scale (HIV-ASES) J Behav Med. 2007;30(5):359–70. doi: 10.1007/s10865-007-9118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]