Abstract
Objectives
To quantify circulating fibroblast activation protein (cFAP) and dipeptidyl peptidase 4 (cDPP4) protease activities in patients with rheumatoid arthritis (RA), systemic sclerosis (SSc), and a control group with mechanical back pain and to correlate plasma levels with disease characteristics.
Methods
Plasma was collected from patients with RA (n=73), SSc (n=37) and control subjects (n=26). DPP4 and FAP were quantified using specific enzyme activity assays.
Results
Median cDPP4 was significantly lower in the RA group (p=0.02), and SSc group (p=0.002) compared with controls. There were no significant differences in median cFAP between the three groups. DPP4 and FAP demonstrated a negative correlation with inflammatory markers and duration of disease. There were no associations with disease subtypes in RA, including seropositive and erosive disease. Decreased cDPP4 was found in SSc patients with myositis. Plasma FAP was lower in RA patients receiving prednisone (p=0.001) or leflunomide (p=0.04), but higher with biologic agents (p=0.01). RA patients receiving leflunomide also had decreased cDPP4 (p=0.014). SSc patients receiving prednisone (p=0.02) had lower cDPP4 but there was no association with cFAP.
Conclusions
No association was found between cFAP and RA or SSc. Plasma DPP4 was decreased in RA and SSc when compared with controls. cDPP4 and cFAP correlated negatively with inflammatory markers and there were no significant correlations with disease characteristics in this RA cohort.
Keywords: proteases, biomarkers, inflammation, enzymes, fibroblasts, lymphocytes
Introduction
Dipeptidyl peptidase 4 (DPP4; DPP-IV) is a multifunctional cell surface glycoprotein, also known as CD26 or adenosine deaminase binding protein. It is expressed by a wide range of cell types, primarily lymphocytes, endothelial cells and epithelial cells. DPP4 has a rare peptidase activity that preferentially cleaves proline or alanine dipeptides from the N-terminal of polypeptides, often altering their bioactivity. Its substrates include chemokines and neuropeptides, such as stromal cell-derived factor-1 (SDF-1; CXCL12), CXCL10, RANTES (CCL5), neuropeptide Y and substance P. DPP4 binds adenosine deaminase, promotes T cell proliferation and provides a costimulatory signal for T cell activation 1–3.
Fibroblast activation protein (FAP) is closely related to DPP4 and has a similar protease function. In contrast to DPP4, it is expressed only by activated fibroblasts and some macrophages in sites of tissue remodelling and wound healing 4–8. In addition to DPP4 - like activity, it possesses a post-proline endopeptidase activity that exerts a gelatinase activity 9–11, inactivates fibroblast growth factor (FGF)-21 12, 13, processes alpha-2 antiplasmin into a more active form 11, and has a role in the invasion of cells in collagenous matrices 14.
DPP4 and FAP also circulate in soluble form in blood 1, 15–18. DPP4 and FAP are each measured by a specific, direct enzymatic assay 19–23, but FAP has also been measured in antibody-based assays such as ELISA 9, 24. Previous studies have shown decreased levels of circulating DPP4 (cDPP4) in patients with rheumatoid arthritis (RA), systemic lupus erythematosus, systemic sclerosis (SSc) and inflammatory bowel disease, and following acute ischaemic stroke, compared with controls23,25. Similarly, expression of DPP4 is lower in RA synovial fluid, compared with osteoarthritis synovial fluid 26. Conversely, T cell surface expression of DPP4 in blood is increased in RA compared with controls 27. Circulating FAP (cFAP) levels have been shown to vary in two other disease states. In patients with acute coronary syndromes, levels were lower than controls 28, 29. Conversely, both intrahepatic FAP and cFAP are elevated in patients with cirrhosis 19, 24 and in many patients with severe fibrosis 20, 30. Circulating levels of cFAP has not been investigated previously in RA or SSc.
Increased expression of FAP has been demonstrated on RA synovial fibroblasts (RASF) 31. It has been hypothesised that RASF has a key role in the pathogenesis of RA, having the potential to migrate between joints, leading to destruction of previously unaffected cartilage 32. It is possible that FAP has a role in this process.
The roles of DPP4 and FAP in autoimmune and inflammatory disease are unclear. The primary aim of this study was to use validated, specific, direct enzymatic assays 19–22 to compare plasma levels of cFAP and cDPP4 in patients with RA, SSc, and a control group with mechanical back pain. The secondary outcomes of interest were the correlations between cFAP and cDPP4 levels and disease characteristics.
Materials and Methods
The research protocol for this project was approved by the Western Sydney Local Health District Human Research Ethics Committee and conforms to the provisions of the World Medical Association’s Declaration of Helsinki. Informed consent was obtained from all participants. Patients were recruited from inpatients and outpatient clinics at Westmead Hospital and from private rheumatology consulting rooms. Plasma was collected in EDTA tubes from patients with RA, SSc and control subjects with mechanical back pain. Mechanical back pain was the chosen control because such patients were similar to the subjects regarding demographic parameters. Patients were excluded if they were under 18 years of age, had a history of malignancy, or were unable to provide written consent. Samples were separated by centrifugation at 1500 rpm for 10 minutes, and stored at −80°C. Routine clinical biochemistry and immunology blood tests were performed by commercial pathology laboratories, including liver and renal function tests, erythrocyte sedimentation rate (ESR), C-reactive protein (CPR), rheumatoid factor (RF), anti-cyclic citrullinated peptide (CCP) antibodies, antinuclear antibodies (ANA) and antibodies to extractable nuclear antigens (ENA).
DPP4 Enzyme Assay
The DPP4 assay using the protease substrate H-Gly-Pro-p-nitroaniline (pNA) (Sigma-Aldrich, St Louis, MO, USA) and chromogenic standards in a linear range of 0–60 nmol of pNA was as recently described 19, 21. Briefly, triplicate plasma samples of 10 μl were incubated at 37°C and read at 405 nm in a FluoStar plate reader (BMG Labtech, Germany). Three in-house controls were used to normalise the final data. One unit of DPP4 activity produces 1.0 micromole of pNA per minute. The intra-assay CV was 3.5 ± 2.5 % and the inter-assay CV was 4.2 ± 3.5 %.
DPP4 contributes 95–98% of the hydrolysis of H-Gly-Pro-pNA by plasma or serum 15, 19, 22, 33. The source of the residual 2–5% hydrolysis of H-Gly-Pro based substrates is unknown, but is unlikely to be DPP8, DPP9, FAP, DPP7 or prolyl endopeptidase 15, 33.
FAP Enzyme Assay
Plasma levels of FAP were measured using an in-house enzyme activity assay that has been described and validated recently 19, 20. The validation included establishing specificity by showing that the substrate is not hydrolyzed by plasma or tissue sourced from the FAP-negative mouse or by plasma from a FAP-negative human 19. The FAP-specific substrate 3144-AMC, duplicate fluorescent standards in a linear range of 0–600 pmol of amino methylcourmarin (AMC) and triplicate plasma samples of 5 μl of a 1/5 dilution in PBS were incubated at 37°C then read in a Fluostar plate reader at excitation 355 nm and emission 450 nm. Three in-house controls in each assay and were used to normalise the final data. The intra-assay coefficient of variation (CV) is 6.2 ± 3.5% and the inter-assay CV is 19.7 ± 8.35%.
Statistical Analyses
Data were analysed using parametric and nonparametric statistical methods using IBM SPSS version 21. Mann-Whitney tests were used for group comparisons and Spearman rank correlations to assess relationships between parameters. P values below 0.05 were considered statistically significant.
Results
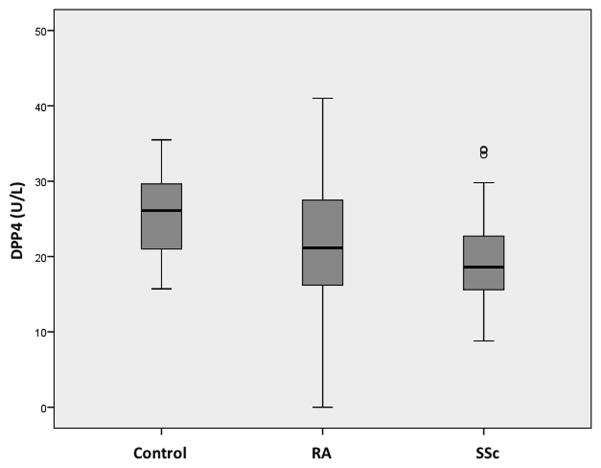
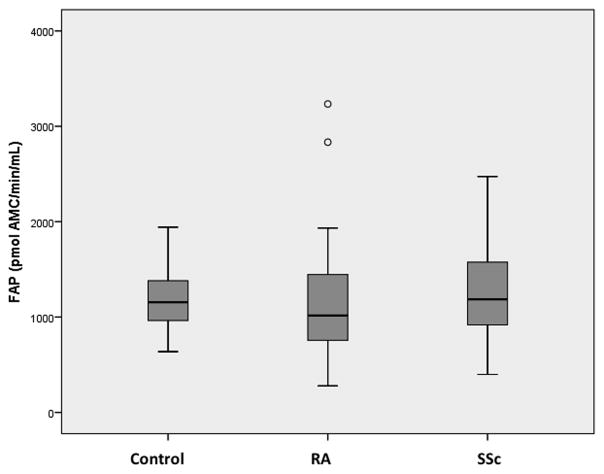
73 patients with RA, 37 with SSc and 26 control subjects were recruited for the study (Table 1). The groups were similar in terms of age and comorbidities, but there was a female predominance in the RA and SSc groups compared with the control group. Median (interquartile range; IQR) plasma DPP4 activity was significantly less in RA (21.2 U/L (16.2–27.5 U/L), p=0.02) and SSc (18.6 U/L (15.6–22.7 U/L), p=0.002) than control (26.14 U/L (21.0–29.7 U/L)) (Fig. 1). The difference in DPP4 activity between RA and SSc was not statistically significant (p=0.22). There was no significant difference in median (IQR) plasma FAP activity between the control group (1077.3 pmol AMC/min/mL (955.5–1363.5 pmol AMC/min/mL)) and RA (1009.5 pmol AMC/min/mL (755.8–1443.2 pmol AMC/min/mL), p=0.10) or SSc (1186.4 pmol AMC/min/mL (917.2–1575.3 pmol AMC/min/mL), p=0.86) (Fig. 2). The cDPP4 and cFAP levels were comparable to previously published data 19, 34.
Table 1.
Baseline Demographics
| Rheumatoid Arthritis | Systemic Sclerosis | Back Pain | |
|---|---|---|---|
| N | 73 | 37 | 26 |
| Median age - years (interquartile range) | 63.0 (53.0–68.0) | 54.0 (49.0–64.0) | 57.5 (41.0–66.0) |
| Median time since diagnosis – years (interquartile range) | 13.0 (5.5–22.5) | 5.0 (3.0–10.0) | - |
| Gender - female | 52 (71%) | 30 (81%) | 11 (42%) |
| Abnormal liver function tests | 11 (15%) | 7 (19%) | 8 (31%) |
| Impaired renal function (eGFR<60mL/min/1.73m2) | 7 (10%) | 3 (8%) | 5 (19%) |
| Diabetes | 8 (11%) | 1 (3%) | 4 (15%) |
| Osteoporosis | 26 (36%) | 6 (16%) | 4 (15%) |
| Hypertension | 30 (41%) | 9 (24%) | 5 (19%) |
|
| |||
| Rheumatoid factor positive | 54 (74%) | - | - |
| Anti-CCP antibody positive | 50 (72%)† | - | - |
| Erosions on X-ray | 46 (67%)† | - | - |
| Rheumatoid nodules | 20 (27%) | - | - |
| Anti-Scl70 antibody positive | - | 15 (11%) | - |
|
| |||
| Anti-centromere antibody positive | - | 7 (5%) | - |
| Anti-RNA polymerase III antibody positive | - | 4 (3%) | - |
| Limited cutaneous | - | 19 (53%)‡ | - |
| Joint involvement | - | 14 (62%) | - |
| Raynaud’s phenomenon | - | 37 (100%) | - |
| Digital ulcers | - | 18 (49%) | - |
| Pulmonary fibrosis | - | 20 (54%) | - |
| Pulmonary hypertension | - | 11 (30%) | - |
| Renal involvement | - | 4 (11%) | - |
| Muscle disease | - | 9 (24%) | - |
|
| |||
| Prednisone | 35 (48%) | 10 (27%) | - |
| Methotrexate | 53 (73%) | 5 (14%) | - |
| Leflunomide | 13 (18%) | 0 | - |
| Sulfasalazine | 14 (19%) | 0 | - |
| Hydroxychloroquine | 12 (16%) | 0 | - |
| Gold | 3 (4%) | 0 | - |
| Biologic disease modifying anti-rheumatic drug (DMARD) | 36 (49%) | 0 | - |
| Cyclosporin | 1 (1%) | 3 (10%) | - |
| Mycophenolate | 0 | 5 (14%) | - |
| Intravenous immunoglobulin | 0 | 2 (6%)‡ | - |
| Cyclophosphamide | 0 | 6 (17%)‡ | - |
N=69
N=36
Figure 1. Circulating DPP4 in Control, RA and SSc Patients.
Box plot displaying plasma DPP4 levels in RA and SSc patients, compared with controls. There are significantly decreased levels in RA (n=73; p=0.02) and SSc patients (n=37; p=0.002).
Figure 2. Circulating FAP in Control, RA and SSc Patients.
Box plot displaying plasma FAP levels in RA (n=73) and SSc (n=37) patients, compared with controls (n=26). There are no significant differences between the groups.
Secondary analyses are exploratory and as such are presented to inform future studies and explore potential associations. Plasma cDPP4 and cFAP were independent of gender across all groups (p=0.58 and p=0.78 respectively). Osteoporosis was more common in the RA group. The presence of diabetes or liver disease may affect DPP4 and FAP levels 19, 24, 35, 36. However, there was no significant difference in the frequency of abnormal liver function tests or diabetes between the groups. Furthermore, the presence of abnormal liver function tests was not associated with any significant differences in DPP4 (p=0.61) or FAP (p=0.08) across all patients. The presence of renal impairment (eGFR<60 mL/min/1.73m2) was not associated with any difference in DPP4 (p=0.77) or FAP (p=0.10) activity.
Rheumatoid Arthritis
In secondary outcome analyses for patients with RA, cDPP4 and cFAP enzyme activity levels were compared with disease characteristics, including RF and CCP antibody status, joint erosions and the presence of nodules. There were no statistically significant differences between the groups (Table 2). When cDPP4 and cFAP were correlated with current treatment, patients receiving prednisone or leflunomide had lower levels of cFAP (p= 0.01) and cDPP4 (p=0.04). There were too few patients receiving gold therapy to perform a meaningful comparison. cFAP was reduced in patients receiving biologic agents (p= 0.01). These included anti-TNF agents such as etanercept, infliximab and adalimumab, as well as anti-IL6 therapy (tocilizumab), anti-CD20 antibody (rituximab) and T cell costimulation modulator (abatacept) (Table 2). There were also some significant correlations with markers of disease activity. There was a negative correlation between the inflammatory markers, ESR and CRP, and cDPP4 and cFAP levels. There was no association with joint count, but there was a weak negative correlation with duration of disease, defined as the number of years since diagnosis (Table 3).
Table 2.
Subgroup Analysis in RA
| Plasma Level Median (IQR) | ||||
|---|---|---|---|---|
|
| ||||
| Disease Characteristic and number of subjects | DPP4 (U/L) | P | FAP (pmol AMC/min/mL) | P |
| Rheumatoid factor | 0.48 | 0.11 | ||
| 54 Positive | 21.3 (9.4) | 1037.9 (668.9) | ||
| 19 Negative | 20.8 (12.3) | 788.7 (705.7) | ||
| Anti-CCP antibody | 0.27 | 0.18 | ||
| 50 Positive | 20.9 (10.4) | 1037.4 (688.9) | ||
| 19 Negative | 21.5 (10.7) | 842.5 (518.4) | ||
| Erosions on X-ray | 0.82 | 0.21 | ||
| 46 Present | 21.2 (9.8) | 1051.5 (685.5) | ||
| 23 Absent | 21.1 (13.6) | 913.4 (506.3) | ||
| Rheumatoid nodules | 0.33 | 0.50 | ||
| 20 Present | 20.9 (8.8) | 1013.2 (666.7) | ||
| 53 Absent | 21.4 (12.6) | 1037.4 (747.4) | ||
|
| ||||
| Treatment and number | ||||
|
| ||||
| Prednisone | 0.08 | 0.001 | ||
| 35 Yes | 20.0 (10.6) | 826.7 (455.4) | ||
| 38 No | 21.5 (12.0) | 1167.7 (627.1) | ||
| Methotrexate | 0.51 | 0.39 | ||
| 53 Yes | 20.9 (7.2) | 1018.8 (689.6) | ||
| 20 No | 23.8 (17.9) | 849.7 (796.0) | ||
| Leflunomide | 0.01 | 0.04 | ||
| 13 Yes | 16.2 (8.2) | 838.2 (310.5) | ||
| 60 No | 21.5 (10.6) | 1051.2 (701.9) | ||
| Sulfasalazine | 0.61 | 0.27 | ||
| 14 Yes | 20.9 (6.6) | 1085.8 (580.3) | ||
| 59 No | 21.2 (13.6) | 984.9 (741.1) | ||
| Hydroxychloroquine | 0.21 | 0.72 | ||
| 12 Yes | 20.4 (11.3) | 1101.9 (745.6) | ||
| 61 No | 21.2 (12.2) | 989.8 (700.7) | ||
| Biologic DMARD | 0.08 | 0.01 | ||
| 36 Yes | 22.6 (12.9) | 1081.3 (678.1) | ||
| 37 No | 20.7 (10.3) | 895.3 (734.7) | ||
Table 3.
Markers of Disease Activity
| Rheumatoid Arthritis | |||||
|---|---|---|---|---|---|
|
| |||||
| ESR | Duration of CRP | Disease | Tender/swollen Joint Count | ||
| DPP4 | Correlation coefficient | −0.322 | −0.429 | −0.247 | 0.016 |
| P | 0.03 | <0.001 | 0.04 | 0.90 | |
|
| |||||
| FAP | Correlation coefficient | −0.426 | −0.352 | −0.058 | 0.123 |
| P | 0.003 | 0.002 | 0.63 | 0.31 | |
|
| |||||
| Systemic Sclerosis | |||||
|
| |||||
| DPP4 | Correlation coefficient | −0.454 | −0.288 | −0.233 | - |
| P | 0.039 | 0.110 | 0.116 | ||
|
| |||||
| FAP | Correlation coefficient | −0.412 | −0.323 | −0.137 | - |
| P | <0.001 | 0.001 | 0.15 | ||
Systemic Sclerosis
In the secondary analyses, plasma DPP4 and FAP levels were compared with disease characteristics in SSc. There was a lower level of cDPP4 in patients with myositis (p=0.03). There were no statistically significant correlations found with other disease characteristics (Table 4).
Table 4.
Subgroup Analysis in SSc
| Plasma Level Median (IQR) | ||||
|---|---|---|---|---|
|
| ||||
| Disease Characteristic and number of subjects | DPP4 (U/L) | P | FAP (pmol AMC/min/mL) | P |
| Antibody status | 0.11 | 0.97 | ||
| 7 Anti-centromere positive | 24.5 (13.5) | 1218.8 (825.3) | ||
| 15 Anti-Scl70 positive | 18.6 (15.9) | 1192.4 (713.7) | ||
| Cutaneous involvement | 0.06 | 0.35 | ||
| 19 Limited | 20.0 (6.7) | 1186.4 (648.4) | ||
| 18 Diffuse | 14.9 (13.7) | 1192.4 (917.3) | ||
| Joint involvement | 0.06 | 0.53 | ||
| 14 Present | 16.1 (7.6) | 1047.4 (1074.6) | ||
| 23 Absent | 20.8 (9.7) | 1218.8 (600.5) | ||
| Digital ulcers | 0.26 | 0.41 | ||
| 18 Present | 18.6 (8.7) | 1079.1 (887.7) | ||
| 19 Absent | 20.6 (11.3) | 1269.9 (645.7) | ||
| Pulmonary fibrosis | 0.36 | 0.78 | ||
| 20 Present | 18.6 (9.8) | 1189.4 (800.9) | ||
| 17 Absent | 20.0 (9.6) | 1044.1 (741.9) | ||
| Pulmonary hypertension | 0.50 | 0.23 | ||
| 11 Present | 19.3 (12.8) | 1114.0 (602.7) | ||
| 26 Absent | 18.6 (10.0) | 1189.4 (780.9) | ||
| Renal involvement | 0.38 | 0.35 | ||
| 4 Present | 16.4 (16.0) | 1445.8 (1255.5) | ||
| 33 Absent | 19.3 (7.9) | 1150.3 (738.2) | ||
| Muscle disease | 0.03 | 0.24 | ||
| 9 Present | 13.3 (9.2) | 1728.9 (1021.5) | ||
| 28 Absent | 20.3 (10.2) | 1132.2 (591.4) | ||
|
| ||||
| Treatment | ||||
|
| ||||
| Prednisone | 0.02 | 0.09 | ||
| 10 Yes | 13.2 (7.9) | 819.9 (1093.0) | ||
| 27 No | 20.6 (10.5) | 1218.8 (594.5) | ||
| Methotrexate | 0.42 | 0.11 | ||
| 5 Yes | 17.0 (10.8) | 1761.9 (1473.3) | ||
| 32 No | 19.7 (9.0) | 1168.4 (746.8) | ||
| Mycophenolate | 0.35 | 0.48 | ||
| 5 Yes | 21.6 (16.3) | 917.2 (834.7) | ||
| 32 No | 18.5 (7.8) | 1192.4 (658.4) | ||
| Cyclophosphamide | 0.25 | 0.33 | ||
| 6 Yes | 14.8 (11.7) | 1097.2 (675.1) | ||
| 30 No | 19.7 (9.5) | 1244.4 (725.0) | ||
Similarly to the RA group, there was a negative correlation between inflammatory markers and cDPP4 and cFAP. There was no association with disease duration (Table 3). Decreased cDPP4 levels were found in patients receiving prednisone treatment (Table 4).
Discussion
This study is the first investigation of cFAP in RA and SSc. Our data did not demonstrate any significant difference in cFAP levels in patients with RA or SSc versus controls. Consistent with the published literature on a small cohort of 35 patients 27, our data demonstrates that cDPP4 levels are decreased in patients with RA and SSc, compared with controls. The enzyme activity levels of DPP4 and FAP were measured.
There were negative correlations of cDPP4 and cFAP enzyme activities with inflammatory markers. In previous reports, some studies have shown a similar negative correlation 37, whereas one has reported no correlation with CRP or ESR 25. There are some differences between that latter study cohort and the patients included in our study. Their patients had shorter median disease duration (5.5 years versus 13 years), and in our study we demonstrated a weak negative correlation between cDPP4 and disease duration. Furthermore, Ulusoy et al excluded patients receiving leflunomide or biologic agents 25. All patients in that study were receiving corticosteroids with a mean prednisolone dose of 7.5 mg/day 25, as compared with 47.9% in our cohort. Our data indicate that treatment may affect levels of cDPP4 and cFAP; this may have affected the overall association seen in our data as all of our RA patients were receiving treatment, often with combination therapy.
Tamaki et al have previously reported decreased levels of soluble DPP4 in a cohort of 56 patients with SSc compared with controls 38, which is in keeping with our results. However, they also found significantly decreased levels in patients with diffuse cutaneous disease compared with limited cutaneous disease and a negative association with the modified Rodnan’s total skin thickness scores 38. In our cohort of 37 SSc patients, median cDPP4 levels appeared to be greater in patients with limited cutaneous disease (20.0 U/L) vs diffuse cutaneous disease (14. 9U/L), but this difference was not statistically significant (p=0.06). Decreased expression of DPP4 has been demonstrated on skin biopsy and dermal fibroblasts from patients with SSc compared with controls 39, and it might therefore be expected that patients with more extensive skin disease could have lower cDPP4 levels.
Although there are several hypotheses concerning the decrease in circulating protease levels in inflammatory diseases, the source of cDPP4 is not known 40. It has been suggested that the process of shedding DPP4 from the cell surface is inhibited in inflammation 40. This could account for the observation that the number of lymphocytes expressing DPP4 is increased in RA, whereas plasma levels are decreased 41. DPP4 may play a dual role in RA and inflammatory disease. On one hand, its peptidase activity inactivates important cytokines and neuropeptides such as substance P and SDF-1, which are responsible for promoting recruitment and retention of activated T cells within inflamed joints. Experiments in murine models have shown that inhibition of DPP4 leads to increased invasion of RASF into cartilage. In vitro, inhibition of the peptidase activity of DPP4 and FAP can lead to increased levels of active forms of the chemokines CXCL10 and CXCL12 (SDF-1) 42,43. However, DPP4 expression on T cells is strongly upregulated in RA and it is thought to act as a T cell co-stimulatory molecule 3, and, in a very large human cohort, lower risk of RA has been associated with DPP4 inhibition 44. It may be that the bound and soluble forms of DPP4 have differing roles in inflammation.
In this first investigation of cFAP in RA and SSc, no significant differences in cFAP levels were detected. This may be related to the more restricted expression of FAP compared with DPP4, as FAP is found only on activated fibroblasts and circulating levels may therefore show little variation in RA or SSc, despite increased expression by RASF. By contrast, cFAP is increased in patients with liver cirrhosis 19, 24. This may be a reflection of the relatively large size of the liver compared with joint tissue, such that upregulation of FAP in the chronically fibrotic liver leads to an increase in circulating levels that is sufficiently large to be detected in plasma. Concordant with our data here, cFAP remains low in patients with arterial thrombosis 29 and in patients with steatosis and little or no liver fibrosis 20.
Our data indicated a weak positive correlation between plasma DPP4 and FAP levels (Spearman rank correlation coefficient = 0.390; p<0.001). As the two enzymes are expressed by different cell types, a reason for this correlation is unclear. The levels of both proteases drop with inflammation, but the mechanism for this is unknown.
Although larger than a concordant previous study 27, our sample size was a limitation. It is possible that some differences in disease subtypes were not detected as the sample was too small to reach statistical significance. Another limitation was that not all of the patients with RA had radiographs performed at the time of sample collection. In these cases, the presence or absence of erosions was determined by examining the medical records and previous imaging studies. It is therefore possible that in some patients cDPP4 and cFAP levels might have changed since the imaging occurred. In the context of intense efforts to construct disease biomarker panels, this study importantly adds to the understanding of which human diseases can alter cDPP4 and cFAP levels.
Conclusion
Plasma levels of DPP4 were decreased in RA and SSc when compared with controls., whereas cFAP was not associated with either RA or SSc. Both cDPP4 and cFAP demonstrated a negative correlation with inflammatory markers. There were no significant correlations between cDPP4 or cFAP and disease characteristics in RA, but some trends were seen with disease subtypes in SSc. The roles of DPP4 and FAP in inflammatory disease are not well understood, and further research is required to understand their mechanism of action and potential as biomarkers of disease.
Acknowledgments
Financial Support
MDG was supported by project grants 632822 and 1105238 from the Australian National Health and Medical Research Council and grants from Perpetual Trustees and the Rebecca L. Cooper Medical Research Foundation. WWB is supported by NIH R01 CA163930-01A1.
We thank Haesung Bak and Marina Ali of Rheumatology Department, Westmead Hospital, for assistance with patient recruitment and laboratory work, respectively, and Karen Byth of Westmead Hospital for statistics assistance.
Abbreviations
- cDPP4
Circulating dipeptidyl peptidase 4
- cFAP
circulating fibroblast activation protein
- RA
rheumatoid arthritis
- SSc
systemic sclerosis
- SDF-1
stromal cell-derived factor-1
- CXCL
chemokine ligand
- RASF
rheumatoid arthritis synovial fibroblasts
- EDTA
ethylenediaminetetraacetic acid
- ESR
erythrocyte sedimentation rate
- CPR
C-reactive protein
- RF
rheumatoid factor
- CCP
anti-cyclic citrullinated peptide
- ANA
antibodies; antinuclear antibodies
- ENA
extractable nuclear antigens
- pNA
p-nitroaniline
- AMC
amino methylcoumarin
- CV
coefficient of variation
- IQR
interquartile range
- eGFR
estimated glomerular filtration rate
- DMARD
disease modifying anti-rheumatic drug
Footnotes
Author CONTRIBUTIONS
Design experiment, collect data, analyse data, write paper: PS, WL, NM, MG
Generate data, analyse data: AR
Project design, provision of know-how: WB
Data provision, data interpretation: HE, GH, DS
Potential Conflicts of Interest
Professor William W. Bachovchin is a director of Arisaph Pharmaceuticals Inc., which provided the FAP-specific substrate 3144-AMC.
References
- 1.Gorrell MD, Gysbers V, McCaughan GW. CD26: A multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol. 2001;54:249–64. doi: 10.1046/j.1365-3083.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 2.Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci. 2005;108:277–92. doi: 10.1042/CS20040302. [DOI] [PubMed] [Google Scholar]
- 3.Waumans Y, Baerts L, Kehoe K, Lambeir A-M, De Meester I. The dipeptidyl peptidase family, prolyl oligopeptidase and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front Immunol. 2015;6:387–405. doi: 10.3389/fimmu.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990;87:7235–9. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchou J, Zhang PJ, Bi Y, et al. Fibroblast activation protein expression by stromal cells and tumor-associated macrophages in human breast cancer. Hum Pathol. 2013;44:2549–57. doi: 10.1016/j.humpath.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorrell MD, Park JE. Fibroblast activation protein alpha. In: Rawlings NL, Salvesen G, editors. Handbook of Proteolytic Enzymes 3rd Edition. Elsevier; San Diego: 2013. pp. 3395–401. [Google Scholar]
- 7.Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding Fibroblast Activation Protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin Appl. 2014;8:454–63. doi: 10.1002/prca.201300095. [DOI] [PubMed] [Google Scholar]
- 8.Kelly T, Huang Y, Simms AE, Mazur A. Fibroblast Activation Protein-alpha: A key modulator of the microenvironment in multiple pathologies. In: Kwang WJ, editor. International Review of Cell and Molecular Biology. Academic Press; 2012. pp. 83–116. [DOI] [PubMed] [Google Scholar]
- 9.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein: A dual-specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274:36505–12. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 10.Levy MT, McCaughan GW, Abbott CA, et al. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology. 1999;29:1768–78. doi: 10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- 11.Christiansen VJ, Jackson KW, Lee KN, McKee PA. Effect of fibroblast activation protein and alpha2-antiplasmin cleaving enzyme on collagen types I, III, and IV. Arch Biochem Biophys. 2007;457:177–86. doi: 10.1016/j.abb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunshee DR, Bainbridge TW, Kljavin NM, et al. Fibroblast Activation Protein cleaves and inactivates Fibroblast Growth Factor 21. J Biol Chem. 2016;291:5986–96. doi: 10.1074/jbc.M115.710582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coppage AL, Heard KR, DiMare MT, et al. Human FGF-21 Is a substrate of fibroblast activation protein. PLoS ONE. 2016;11:e0151269. doi: 10.1371/journal.pone.0151269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang XM, Yu DMT, McCaughan GW, Gorrell MD. Fibroblast activation protein increases apoptosis, cell adhesion and migration by the LX-2 human stellate cell line. Hepatology. 2005;42:935–45. doi: 10.1002/hep.20853. [DOI] [PubMed] [Google Scholar]
- 15.Durinx C, Lambeir AM, Bosmans E, et al. Molecular characterization of dipeptidyl peptidase activity in serum - Soluble CD26/dipeptidyl peptidase IV is responsible for the release of X-Pro dipeptides. Eur J Biochem. 2000;267:5608–13. doi: 10.1046/j.1432-1327.2000.01634.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee KN, Jackson KW, Christiansen VJ, Lee CS, Chun JG, McKee PA. Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood. 2006;107:1397–404. doi: 10.1182/blood-2005-08-3452. [DOI] [PubMed] [Google Scholar]
- 17.Lee KN, Jackson KW, Christiansen VJ, Dolence EK, McKee PA. Enhancement of fibrinolysis by inhibiting enzymatic cleavage of precursor alpha2-antiplasmin. J Thromb Haemost. 2011;9:987–96. doi: 10.1111/j.1538-7836.2011.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong PF, Gall MG, Bachovchin WW, McCaughan GW, Keane FM, Gorrell MD. Neuropeptide Y is a physiological substrate of fibroblast activation protein: Enzyme kinetics in blood plasma and expression of Y2R and Y5R in human liver cirrhosis and hepatocellular carcinoma. Peptides. 2016;75:80–95. doi: 10.1016/j.peptides.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Keane FM, Yao T-W, Seelk S, et al. Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs. FEBS Open Bio. 2014;4:43–54. doi: 10.1016/j.fob.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams KH, Vieira De Ribeiro AJ, Prakoso E, et al. Lower serum fibroblast activation protein shows promise in the exclusion of clinically significant liver fibrosis due to non-alcoholic fatty liver disease in diabetes and obesity. Diabetes Res Clin Pract. 2015;108:466–72. doi: 10.1016/j.diabres.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Williams KH, Vieira de Ribeiro AJ, Prakoso E, et al. Circulating dipeptidyl peptidase-4 activity correlates with measures of hepatocyte apoptosis and fibrosis in non-alcoholic fatty liver disease in type 2 diabetes mellitus and obesity: A dual cohort cross-sectional study. J Diabetes. 2015;7:809–19. doi: 10.1111/1753-0407.12237. [DOI] [PubMed] [Google Scholar]
- 22.Matheeussen V, Lambeir A-M, Jungraithmayr W, et al. Method comparison of dipeptidyl peptidase IV activity assays and their application in biological samples containing reversible inhibitors. Clin Chim Acta. 2012;413:456–62. doi: 10.1016/j.cca.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Baerts L, Brouns R, Kehoe K, et al. Acute Ischemic Stroke Severity, Progression, and Outcome Relate to Changes in Dipeptidyl Peptidase IV and Fibroblast Activation Protein Activity. Translational Stroke Research. 2016:1–8. doi: 10.1007/s12975-016-0493-3. [DOI] [PubMed] [Google Scholar]
- 24.Uitte de Willige S, Malfliet JJMC, Janssen HLA, Leebeek FWG, Rijken DC. Increased N-terminal cleavage of alpha-2-antiplasmin in patients with liver cirrhosis. J Thromb Haemost. 2013;11:2029–36. doi: 10.1111/jth.12396. [DOI] [PubMed] [Google Scholar]
- 25.Ulusoy H, Kamanli A, Ilhan N, et al. Serum levels of soluble CD26 and CD30 and their clinical significance in patients with rheumatoid arthritis. Rheumatol Int. 2012;32:3857–62. doi: 10.1007/s00296-011-2302-3. [DOI] [PubMed] [Google Scholar]
- 26.Gotoh H, Hagihara M, Nagatsu T, Iwata H, Miura T. Activities of dipeptidyl peptidase II and dipeptidyl peptidase IV in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin Chem. 1989;35:1016–8. [PubMed] [Google Scholar]
- 27.Cordero OJ, Salgado FJ, Mera-Varela A, Nogueira M. Serum interleukin-12, interieukin-15, soluble CD26, and adenosine deaminase in patients with rheumatoid arthritis. Rheumatol Int. 2001;21:69–74. doi: 10.1007/s002960100134. [DOI] [PubMed] [Google Scholar]
- 28.Tillmanns J, Widera C, Habbaba Y, et al. Circulating concentrations of fibroblast activation protein α in apparently healthy individuals and patients with acute coronary syndrome as assessed by sandwich ELISA. Int J Cardiol. 2013;168:3926–31. doi: 10.1016/j.ijcard.2013.06.061. [DOI] [PubMed] [Google Scholar]
- 29.Uitte de Willige S, Malfliet JJMC, Deckers JW, Dippel DWJ, Leebeek FWG, Rijken DC. Plasma levels of soluble fibroblast activation protein in arterial thrombosis; determinants and cleavage of its substrate alpha-2-antiplasmin. Int J Cardiol. 2015;178:105–10. doi: 10.1016/j.ijcard.2014.10.091. [DOI] [PubMed] [Google Scholar]
- 30.Levy MT, McCaughan GW, Marinos G, Gorrell MD. Intrahepatic expression of the hepatic stellate cell marker fibroblast activation protein correlates with the degree of fibrosis in hepatitis C virus infection. Liver Internat. 2002;22:93–101. doi: 10.1034/j.1600-0676.2002.01503.x. [DOI] [PubMed] [Google Scholar]
- 31.Bauer S, Jendro MC, Wadle A, et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther. 2006;8:R171. doi: 10.1186/ar2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefevre S, Knedla A, Tennie C, et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med. 2009;15:1414–20. doi: 10.1038/nm.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu DMT, Ajami K, Gall MG, et al. The in vivo expression of dipeptidyl peptidases 8 and 9. J Histochem Cytochem. 2009;57:1025–40. doi: 10.1369/jhc.2009.953760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuchacovich M, Gatica H, Pizzo SV, Gonzalez-Gronow M. Characterization of human serum dipeptidyl peptidase IV (CD26) and analysis of its autoantibodies in patients with rheumatoid arthritis and other autoimmune diseases. Clin Exp Rheumatol. 2001;19:673–80. [PubMed] [Google Scholar]
- 35.Lee SA, Kim YR, Yang EJ, et al. CD26/DPP4 levels in peripheral blood and T cells in patients with Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2013;98:2553–61. doi: 10.1210/jc.2012-4288. [DOI] [PubMed] [Google Scholar]
- 36.Firneisz G, Varga T, Lengyel G, et al. Serum Dipeptidyl Peptidase-4 Activity in Insulin Resistant Patients with Non-Alcoholic Fatty Liver Disease: A Novel Liver Disease Biomarker. PLoS ONE. 2010;5:e12226. doi: 10.1371/journal.pone.0012226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busso N, Wagtmann N, Herling C, et al. Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol. 2005;166:433–42. doi: 10.1016/S0002-9440(10)62266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamaki Z, Kubo M, Yazawa N, et al. Serum levels of soluble CD26 in patients with scleroderma. J Dermatol Sci. 2008;52:66–9. doi: 10.1016/j.jdermsci.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Bou-Gharios G, Osman J, Atherton A, et al. Expression of ectopeptidases in scleroderma. Ann Rheum Dis. 1995;54:111–6. doi: 10.1136/ard.54.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordero OJ, Salgado FJ, Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother. 2009;58:1723–47. doi: 10.1007/s00262-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedo A, Duke-Cohan JS, Balaziova E, Sedova LR. Dipeptidyl peptidase IV activity and/or structure homologs: Contributing factors in the pathogenesis of rheumatoid arthritis? Arthritis Res Ther. 2005;7:253–69. doi: 10.1186/ar1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ospelt C, Mertens JC, Juengel A, et al. Inhibition of fibroblast activation protein and dipeptidyl peptidase IV increases cartilage invasion by rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2010;62:1224–35. doi: 10.1002/art.27395. [DOI] [PubMed] [Google Scholar]
- 43.Decalf J, Tarbell KV, Casrouge A, et al. Inhibition of DPP4 activity in humans establishes its in vivo role in CXCL10 post-translational modification: prospective placebo-controlled clinical studies. EMBO Mol Med. 2016;8:679–83. doi: 10.15252/emmm.201506145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population-based cohort study. Ann Rheum Dis. 2014;74:1968–75. doi: 10.1136/annrheumdis-2014-205216. [DOI] [PMC free article] [PubMed] [Google Scholar]