Abstract
The diagnosis of Neuropsychiatric systemic lupus erythematosus disease (NPSLE) is challenging. The Automated Neuropsychological Assessment Metrics (ANAM) has been shown to be an accessible and promising tool for evaluating possible NPSLE in adult and childhood lupus. In this review, we present information about the development and use of Ped-ANAM; the benefit of using Ped-ANAM in children with and without NPSLE in the assessment and follow up of their disease condition; and the correlation of Ped-ANAM to imaging studies such as magnetic resonance imaging (MRI). PedANAM was validated in children with cSLE in different studies. Cognitive performance can be a challenging clinical feature to efficiently assess. However, research with the Ped-ANAM has produced a Cognitive Performance Score (CPS) that allows for a reliable and efficient estimation of cognitive ability and the presence of cognitive limitations that children with cSLE may show. Compared with traditional neurocognitive assessment tools, Ped-ANAM-CPS offers a promising alternative to overcome the difficulties that practitioners previously faced.
Introduction
Systemic lupus erythematosus (SLE) is a chronic disease which can affect multiple organ systems, often resulting in central nervous system (CNS), kidney, skin and hematological involvement [1]. Neuropsychiatric Systemic Lupus Erythematosus (NPSLE) [2] manifestations are more frequent among individuals with disease onset during childhood (cSLE) compared with SLE diagnosed during adulthood (aSLE). Irrespective of age of onset, NPSLE is notoriously difficult to diagnose. Indeed, there is a dearth of serological, imaging or immunological biomarkers to verify the presence and course of NPSLE.
Cognitive ability is thought to reflect overall brain health and has been the focus of many NPSLE studies. Typically, global cognition is categorized into a number of cognitive domains: simple or complex attention, memory, visual-spatial processing, language, reasoning/ problem-solving, psychomotor speed, and executive functions [3]. This review aims at providing an overview of the current medical literature relevant to the assessment of cognitive ability and the presence of NPSLE in children.
Neuropsychiatric SLE – Epidemiology, Pathology, Risk factors and Impact
NPSLE can affect the central, peripheral, and autonomic nervous systems, resulting in headaches, strokes, psychiatric disturbance, and cognitive dysfunction [4-7]. Given the lack of specific diagnostic tests, NPSLE remains largely a diagnosis of exclusion. Epidemiological studies suggest that NPSLE often occurs early in the course of SLE, and may even be the initial manifestation, but it may also develop at any time during the disease course, even in the setting of apparent quiescence of cSLE activity in other organ systems [9]. Estimates for NPSLE during the disease course range from 30-95% in cSLE and 21-70% in aSLE [10-12]. NPSLE early after the diagnosis and during the course of disease may both be more common in cSLE than aSLE [13, 14].
Although the pathological mechanisms that result in NPSLE have not been entirely elucidated, it has been shown that both gray and white matter structures are affected [15]. Blood-brain barrier impairment, autoantibodies and cytokines are all thought to play major roles in mediating NPSLE manifestations via thrombosis, vasculopathy, inflammation, neuronal cell compromise or death [16].
There are several studies implicating antiphospholipid antibodies (aPLs) in the development of NPSLE in some patients, specifically cognitive impairment [5, 17]. aPLs may promote micro-thrombus formation and non-inflammatory vasculopathy, resulting in ischemic damage in the brain [5]. These antibodies have also been associated with an increased incidence of white matter hyper-intensities as seen on magnetic resonance imaging (MRI) [4, 18, 19] and may be more frequent in SLE patients with seizures, stroke, acute transverse myelopathy, and cognitive impairments [20-22].
Other autoantibodies have also been found to be associated with cognitive abnormalities in SLE, such as methyl-D-aspartate receptor (also known as the NMDA receptor or NMDAR), which is a tetrametric glutamate receptor and ion channel protein found in nerve cells composed of NR2 subunits. Among others, NMDAR is involved in long-term potentiation, a process fundamental to learning and memory formation [23]. Anti-NR2 receptor antibodies, which cross-react with double stranded DNA, may mediate apoptotic cell death of neurons, regulate synaptic strength and neuronal plasticity, Supported by animal studies and some studies in children and adults, NR2 antibodies mayv contribute to cognitive compromise with SLE [24-27].
Anti-ribosomal P antibodies have been linked to psychosis and depression [28, 29] and might be involved in cognitive decline in cSLE [30-32]. There are likely other brain-reactive antibodies that are involved in the development of NPSLE, possibly contributing to the diverse clinical and imaging features of NPSLE. Notably, all brain-reactive antibodies must reach the brain tissues to exert their detrimental effects. Therefore, regional or global breaches of the blood-brain barrier need to occur [25, 33], either chronic or intermittent in nature.
Imaging in NPSLE
MRI is considered the imaging modality of choice for NPSLE. Although NPSLE can be present despite normal conventional MRI, imaging abnormalities are present in 25-75% of adult NPSLE patients; the number of imaging findings positively correlates with disease activity and patient age [34]. Neuroimaging studies in patients with cSLE describe gray and white matter alterations using different techniques of MRI [35-39], with cerebral and cerebellar volume loss reported in about 81% of the children [40]. White matter lesions may be most prominently located in prefrontal or frontal regions with cSLE [40], i.e. brain regions where myelination is ongoing during childhood and adolescence [41-43]. Functional MRI (fMRI) has shown differential brain activation patterns in children with cSLE compared to healthy controls, especially when cognitive ability is diminished [36, 38]. At the present time, results of advanced imaging research have limited value when managing cSLE in clinical practice. Future research is needed to more closely correlate imaging findings with the presence and clinical course of NPSLE and in turn develop targeted medical therapies.
Autopsy studies of patients with NPSLE
The relationship between the pre-mortem MRI findings and post-mortem histopathologic features in fatal NPSLE was examined in a study by (Sibbitt, W.L. et al) [44]. Pre-mortem MRI findings included small focal white matter lesions, moderate to severe cortical atrophy and ventricular dilation, with acute cerebral edema, acute leukoencephalopathy and parenchymal calcifications. Common post-mortem gross anatomical findings were cerebral infarction, focal or generalized cerebral edema, intracranial hemorrhage, intracranial calcifications, cyst formation, and focal atrophy. Histological features in fatal NPSLE included micro-thrombi, diffuse endothelial injury to small vessels, endothelial hyperplasia, frequent obvious focal or generalized brain edema; all suggesting breakdown of the blood-brain barrier. These histopathologic findings indicate that NPSLE is associated with cerebrovascular injury, disease activity, thromboembolism, focal and diffuse brain ischemia, edema, hemorrhage, brain infarcts, and parenchymal injury commonly related to small vessels vasculopathy despite absence of inflammatory changes in some cases [44-47]. Notably, autopsy findings failed to support a single pathogenic mechanism; rather a potpourri of pathology seems to be present with cognitive impairment [48].
Cognitive Function in cSLE
As part of the expected brain maturation process, cognitive skills advance steadily during childhood, with the most pronounced gains made during infancy and early childhood [49, 50]. cSLE is generally diagnosed in children older than five years of age, most commonly around mid-adolescence, thus after the time of the most acute developmental increases in cognition. Unfortunately, the burden of chronic cSLE may compromise cognitive abilities in the developing child or adolescent. Indeed, mildly impaired cognitive ability commonly leads to deficits in executive functioning, visual organization and visual-motor functioning, and psychomotor speed. These findings can be seen even in children with SLE who do not have previous severe NPSLE manifestations, organ damage or apparent disease activity [40, 50-53]. There remain considerable gaps in our understanding of the impact of cSLE on cognitive ability over time. Arguably, surveillance should commence at the time of diagnosis as this would allow for evaluation of the effects of cSLE on cognitive development over time [49] .
However, quantifying cognitive ability and delineating abnormal cognitive function are not trivial tasks. Formal neurocognitive testing is considered the current gold standard for assessing cognitive functioning. Other currently proposed tools, and potentially more feasible approaches for clinical use, include questionnaire-based methods and computerized neuropsychological batteries (CNBs).
Formal Neurocognitive Testing
A variety of different tests have been used in the past to diagnose NPSLE, likely contributing to the highly differing estimates of NPSLE prevalence in the medical literature. Thus, the American College of Rheumatology [4] proposed a 1-hour battery of standardized tests to be used for formal neurocognitive testing in aSLE. This battery has been found reliable and valid to identify cognitive impairment in NPSLE as defined by the ACR case definitions [4, 54].
Given that several tests of the ACR battery are only suited for administration to adults, an alternative battery for cSLE has been proposed [55]. The cSLE battery particularly assesses working memory, psychomotor speed, attention, and visuoconstructional ability as these cognitive domains are thought to be particularly affected by cSLE in prior research (Table 1). All standardized tests included in this battery are validated and provide age-normed scores derived from large, demographically diverse normative samples [55].
Table 1.
Battery of Standardized Tests Recommended for cSLE
| Standardized Test | Functional cognitive domain assessed |
|---|---|
| WAIS-III Working Memory Index | Working memory |
| WAIS-III Processing Speed Index | Processing speed |
| WAIS-III Block Design Design | analysis and synthesis |
| WAIS-III Picture Completion | Visual scanning and discrimination |
| WAIS-III similarities | Abstract verbal reasoning |
| California Verbal Learning Test-2nd Edition Long Delay Free | Recall Verbal recall |
| California Verbal Learning Test-2nd Edition Discrimination | Verbal recognition |
| Rey Complex Figure Delayed Recall | Non-verbal recall |
| Animal Naming | Categorical fluency |
| Boston Naming Test | Confrontation naming |
| Wisconsin Card Sorting Test Conceptual Response | Problems solving |
| Wisconsin Card Sorting Test Perseverative Response | Executive function |
| Stroop Word Color Test Interference | Executive function |
| Trails A total time | Psychomotor processing speed |
| Trails B total time | Executive function |
Definitions of neurocognitive impairment
Standardized test performance is commonly reported as standardized means (Z scores) and standard deviations (SD), using the performance of normative populations as reference standards.
Based on performance on formal neurocognitive testing, cognitive ability may be categorized across a spectrum from global impairment to within the normal range. There are at least four suggested approaches to define neurocognitive impairment: (a) performance more than 2 SD below the standardized mean in at least one of the cognitive domains under consideration; (b) performance more than 1 SD below the standardized mean in at least two cognitive domains [56]; (c) performance more than 2 SD below the standardized mean in at least one of the cognitive domains plus low performance at least 1.5 SD below published norms in one or more other cognitive domains [57]; and (d) performance more than 1.5 SD below the mean in at least two cognitive domains [40].
Limitations of Formal Neurocognitive Testing
Limitations of the cSLE Battery for use in clinical settings include a 3-hour time requirement for completion and the need for specially trained administration personnel. In addition, formal neurocognitive testing performance may be influenced by fatigue and language proficiency and ideally, intervals between testing sessions should be at least 6 month and better 12 months in order to avoid overt practice effects [55, 58, 59].
Other Approaches to Screen for Impairments of Cognitive Ability in Children
The Mini Mental Status Exam (MMSE) is a cognitive screening test that, in a modified version, can be used in children four years and older [60, 61]. Although it is a brief and practical tool to screen for the severity of neurocognitive deficits over times, the MMSE is not sensitive enough to serve as a screening tool for reduced cognitive ability by itself; rather the MMSE needs to be combined with other tests to yield sufficient sensitivity desirable for a screening test [60].
School records and academic performance can provide insights into cognitive compromise in cSLE. Multi-domain neurocognitive compromise and severe academic deficits during the first year of the disease have been reported in some cSLE patients, in addition to concomitant increases in depressive symptoms on child self-report scales and internalizing behaviors [62]. Children with a history of NPSLE may experience school failure due to attention and learning difficulties [63], and long-term consequences that interfere with their subsequent cognitive development [64]. Therefore, a school history can provide important cues about a child’s cognitive abilities but certainly does not substitute for formalized testing.
Detecting cognitive impairments in pediatric rheumatology clinical practice based on physician perception or parent unstructured report is inaccurate and insensitive [65]. However, obtaining the caregiver’s perception of the child’s cognitive function by completing the Pediatric Perceived Cognitive Function-43 questionnaire (PedsPCF-43) may provide additional cues to a child’s cognitive ability [66]. This questionnaire has been validated for use in cSLE [67] and probes select cognitive domains such as attention, memory retrieval, and working memory [68, 69].
Computerized Battery to Measure Cognitive Ability
There has been considerable work in recent years to develop and test computerized neuropsychological batteries to efficiently assess and screen cognitive function [70]. Advantages of computerized neuropsychological batteries include automatized, highly standardized, and increased precision of reaction time measurement; real-time administration and automatized scoring; and the ability to generate multiple alternate versions to minimize practice effects [70]. Examples of CNBs used for adults include CNS Vital Signs, Cogstate, Cambridge Neuropsychological Testing Automated Battery (CANTAB), and the Automated Neuropsychological Assessment Metrics (ANAM) [71].
The CANTAB is a computerized neuropsychological battery originally developed to support a diagnosis of dementia in the elderly [72] but it has also been used in pediatric populations as young as 4 years of age [73, 74]. The CANTAB emphasizes the assessment of executive functioning; includes measures of planning, set-shifting, spatial working memory and non-verbal memory span. The 90-minute administration time of the CANTAB may be challenging for young children [49], making it less practical.
Automated Neuropsychological Assessment Metrics (ANAM)
ANAM is a computerized neuropsychological battery, comprised of a large library of cognitive tests that can be used with normative databases for individuals ranging from 9 years to 85 years of age. The ANAM is the product of 30 years of research and development on serial cognitive function testing by U.S. military [75]. The ANAM was originally developed for the use with adults but early versions have been used in single studies in patients as young as age 13 [76, 77]. The success of the ANAM in assessing cognitive change and screening for cognitive impairment across a wide range of military and civilian applications has led to its increasing clinical use [70]. The ANAM has also been widely validated for use in adults with SLE [78, 79]. The ANAM measures a wide range of cognitive functions, including simple and complex reaction time, attention, processing speed, working memory, visual-spatial processing, and short-term recognition memory. It includes a large battery of tests but specific batteries of tests have been validated for specific groups and purposes in comparison with many diseases including SLE [80, 81]. Some of the most commonly used test scales in the ANAM include; Simple reaction time, Code substitution, Matching grids, Matching to sample, Mathematical processing, Continuous performance test, Code substitution delayed, and Sternberg memory test. Depending on the intent of ANAM use in a specific setting and the ANAM subtest included in the testing battery, the administration of the ANAM can take up to 30-40 minutes [82].
Pediatric Automated Neuropsychological Assessment Metrics (Ped-ANAM)
A pediatric version of ANAM (Ped-ANAM) has been developed for use with younger individuals. To date, most of the studies evaluating the Ped-ANAM have been conducted in cSLE and children with concussion [83, 84]. The PedANAM battery is comprised of original ANAM tests modified to include age-appropriate stimuli and testing procedures (e.g., reading level lowered, test stimuli and instructions simplified, increased time allowed to respond, etc.), and in addition to the ANAM subtests described above it includes Procedural reaction time, Logical reasoning, and Spatial processing (Table 2) [56]. The software can be obtained from Vista Life Sciences (Parker CO, USA) [85, 86].
Table 2.
Automated Neuropsychological Assessment Metrics pediatric battery (Ped-ANAM) subtests
| Ped-ANAM subset | Subtest description |
|---|---|
|
1. Simple reaction time
(SRT) |
20-item measure of reaction time. The test presents a simple stimulus on the screen (*) and the participant is required to press the left mouse key as quickly as possible following the presentation of the stimulus. This test is repeated at the end of the battery to assess both within-session reliability and the effect of fatigue on SRT performance. |
|
2. Procedural reaction
time (PRO) |
20-item measure of choice reaction time. The test presents a stimulus on the screen, a 2, 3, 4, or 5. The participant is required to press the blue (left) mouse key if a 2 or 3 is presented or the red [88] mouse key if a 4 or 5 is displayed. |
|
3. Code substitution
(CDS) & Code substitution delayed (CDD) |
The learning portion of this test assesses attention, concentration, and learning. In this test, a key containing a string of 9 symbols and 9 digits is displayed across the upper portion of the screen. Symbols and numbers are paired with a unique number located below a specific symbol. During the task, a “test” pair (i.e., a symbol and digit) is presented at the bottom of the screen, below the key containing the correct symbol number pairs. The objective is to indicate if the “test” pair matches the associated pair in the key at the top of the screen. The subject is instructed to try to remember the symbol-number pairs as they will be asked to recall them later. The delayed recall portion of this test is an explicit recognition memory task administered later in the test battery. For this subtest, the subject is presented only with a “test” pair and asked to remember whether the this symbol/number pairing is correct based on the earlier presented key. |
|
4. Logical reasoning
(LRS) |
This test consists of a practice and a real test in which the subject must decide if sentences presented on the screen make sense or not. Responses consist of pressing the blue (left) button if the sentence makes sense and the red [88] button if the sentence does not. |
|
5. Spatial processing
(SPD) |
Spatial processing is a test of spatial analysis and requires subjects to examine 2 bar graphs, one presented upright and one rotated 90°. They are then asked to decide if the 2 bar graphs are the same or different. |
|
6. Continuous
performance test (CPT) |
This is a test of sustained attention and working memory. Participants are asked to monitor a randomized sequence of numbers, 1–9. The numbers are presented one at a time in the center of the screen. Participants are asked to press a response button indicating whether or not the number presented on the screen matches the number that immediately preceded it. The subject is instructed to press the blue (left) mouse button if the number matches the previous stimuli or the red [88] button if the number does not match the previous stimuli |
|
7. Mathematical
processing (MTH) |
Mathematical processing is a test of arithmetic, attention, and processing speed. The subject is required to decide if a math problem presented on the screen is correct or incorrect. Each problem includes one mathematical operation (addition or subtraction) on single-digit numbers. The subject is instructed to indicate if the problem is correct by pressing the blue (left) button or if the problem is incorrect by pressing the red [88] button. |
| 8. Matching grids (MTG) | Matching grids is a test of visuospatial discrimination. Two 4 _ 4 grids are displayed side by side on the screen. The subject must then decide if the grids are exactly the same or different. The subject presses the blue (left) button if the grids are the same and the red [88] button if the grids are different. |
|
9. Matching to sample
(MSP) |
Matching to sample is a test of short-term memory, attention, and visuospatial discrimination. The subject is presented with a single design to study and remember. The design then disappears and the screen goes blank. Following a brief delay, 2 more designs appear on the screen. The subject must then decide which of the 2 designs matches the original. The subject presses the blue (left) button if the left comparison grid matches the original and the red [88] button if the right comparison grid matches the sample grid. |
|
10. Sternberg memory
search (STN) |
This is a test of sustained attention and working memory. Participants are presented with a set of 6 letters called the memory set (“secret code” in the pediatric version). They are allowed as long as they need to memorize the letters. Upon beginning the test, the code is removed from the screen and single letters begin appearing one at a time on the screen. The subject must then decide if the letter on the screen was contained in the secret code. The subject is instructed to press the blue (left) mouse button if the letter matches any of the letters in the secret code or the red [88] button if the letter does not match the code. |
Performance parameters
Ped-ANAM testing generates a series of metrics about a child’s test performance at each administration. Performance on each of the Ped-ANAM subtests can be gauged by four key metrics summarized in Table 3 [56, 87, 88]. Note that mean reaction time (MNc) is the primary variable of interest for the Simple Reaction Time [56, 88]. Resulting ANAM scores can be compared to age-matched norms derived from healthy individuals and children with juvenile idiopathic arthritis. This comparison allows for age-corrected standardized scores.
Table 3.
Ped-ANAM Performance Parameters and Cognitive Summary Scores
| Performance parameter per subtest | Abbreviation |
|---|---|
| 1. Accuracy, i.e. percentage of correct responses per subtest responses requested ( in %) | AC |
| 2. Speed; i.e. mean reaction time to produce a for correct responses (in seconds) | MNc |
| 3. Cognitive efficiency or throughput, i.e. percent correct answers divided by mean reaction time |
|
| 4. Consistency; i.e. coefficient of variation of reaction time for correct responses as SD of MNc divided by MNc | CVc |
Age and Language
Cognitive assessment using the Ped-ANAM has been conducted in children as young as 9 years of age. There is no upper age limit; thus Ped-ANAM can also be administered to adults, allowing for longitudinal assessment of cognition with the same measure into the adult years. Ped-ANAM was originally developed in English and current normative data is for the English version. Ideally cognitive assessment, including formal neuropsychological testing is conducted in the language with which an individual is most familiar and resulting test performance should be compared to reference groups matched on key demographic and culturally relevant factors. The Ped-ANAM interfaces used for cSLE have been cross-culturally adapted and translated to several languages. At present the following language versions are available: English (US, UK); French (Canada, France), German (Germany, Austria), Spanish (Spain), Portuguese (Brazil), Chinese, Hindi, and Arabic. Validation studies and collection of corresponding reference groups is underway.
The following sections will summarize the measurement properties of the Ped-ANAM when used in cSLE.
Feasibility
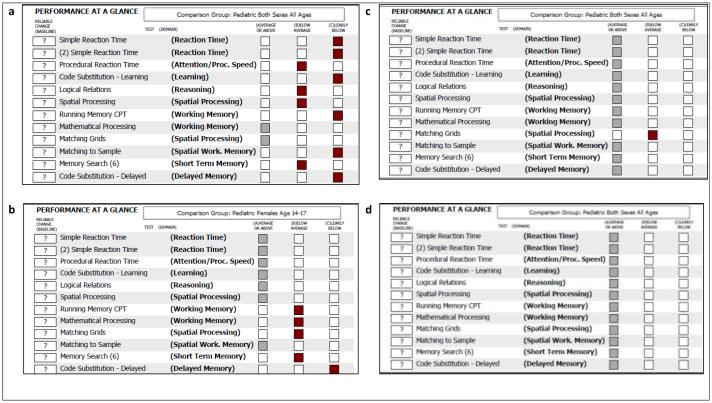
The cSLE battery of the Ped-ANAM requires, on average, 20-30 minutes for completion. Previous studies support that completion of the Ped-ANAM can be achieved by children with cSLE during regular clinics visits [56, 88]. However, the test environment should be quiet, free from distractions, and ensure uninterrupted completion of the Ped-ANAM battery. Although the Ped-ANAM allows for automated administration, it is our experience that valid and reliable test administration is best accomplished when a test administrator is present and readily available to reinforce the rationale for test administration, clarify any potential misunderstanding of test instructions, and ensure that responding to Ped-ANAM stimuli occurs without interruption (e.g. the patient should not communicate via text messaging, make phone calls, or talk with other individuals while taking the Ped-ANAM). Such procedures are supported by consensus statements on appropriate use of computerized neuropsychological testing [71]. Standardized test directions on the Ped-ANAM instruct the individual to try their best, but additional emphasis of this point by the test administrator is highly advisable and particularly important in pediatric groups, where individuals may be prone to distraction. Figure 2 summarizes the results of in-clinic testing of selected cSLE patients.
Figure 2.
Ped-ANAM Performance throughput Report - Case examples
Test-retest reliability & Learning Effect
Test stimuli are randomly generated session-to-session to provide an almost infinite number of repeated measures testing sessions. It is recommended to have children complete the battery at least two times to provide a stable baseline estimate of performance (without confound of practice effects) to track cognitive change over time [89]. Repeat administration of the Ped-ANAM in cSLE has minimal practice effect [56, 90, 91].
Criterion and Construct Validity
Our earlier studies suggest that the Ped-ANAM is a sensitive screening tool, yielding accuracy of 97% for identify children with cSLE and clinically relevant cognitive impairment [88].
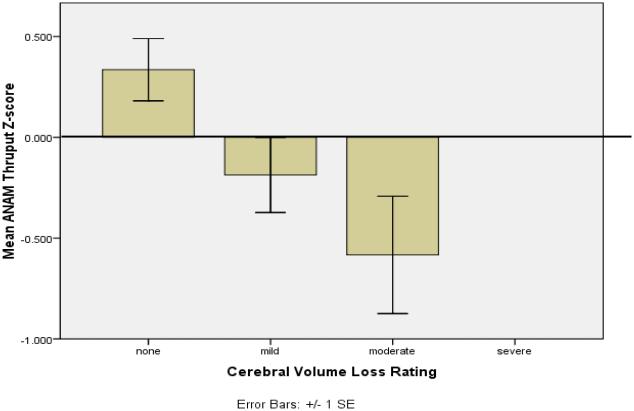
Ped-ANAM performance in cSLE is moderately correlated with the performance on formal neuropsychological testing [38, 56]. Interestingly, this association is complemented by the results of imaging studies. Muscal et al. [40] showed that low Ped-ANAM performance is associated with reduced brain volumes in cSLE patients (Figure 1). Further, Ped-ANAM performance is correlated with functional neuronal network activation using functional magnetic resonance imaging [38].
Figure 1.
Relationship of PedANAM performance and Brain Imaging in cSLE
Responsiveness to change
Serial Ped-ANAM administration in cSLE is especially sensitive to changes in visuoconstructional ability, followed by those in processing speed and attention, and to a lesser degree in working memory, using results of formal neuropsychological testing as standard criterion [88]. However, minimal clinical important differences in specific Ped-ANAM performance parameters have not been established.
Ped-ANAM Cognitive Performance Scores
ANAM is a data-rich tool that produces a large number of performance metrics per test. Current studies of pediatric SLE have focused on examination of traditional throughput scores readily available from the Ped-ANAM clinical report. Such scores provide an assessment of cognitive efficiency accounting for both speed and accuracy of performance and have been shown to be sensitive indicators of cognitive inefficiency in cSLE. Previous studies of ANAM in adults with Lupus and other autoimmune disorders [81] have found that statistically accounting for simple reaction time (e.g., motor speed) in throughput scores allowed for increased sensitivity when measuring higher cognitive processing and resulted in increased specificity when distinguishing between different autoimmune disorders. Future studies may consider exploring similar metrics in pediatric populations.
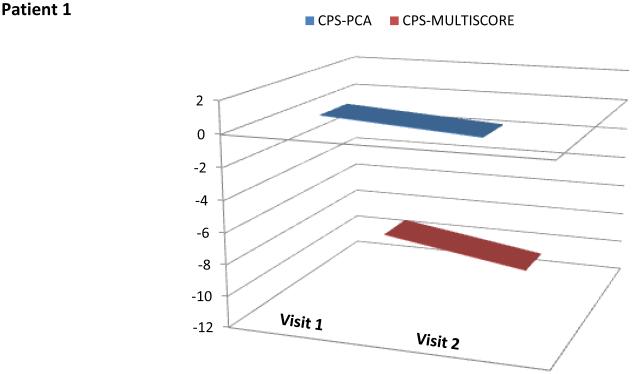
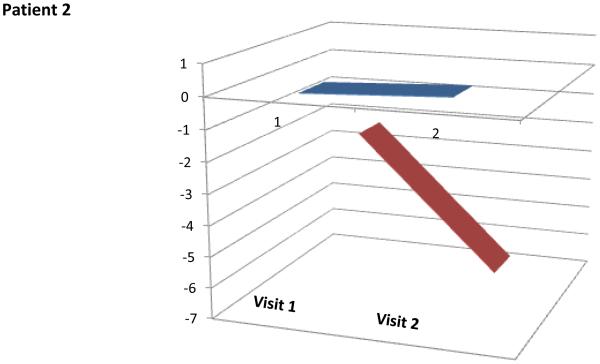
These performance metrics have been combined in various ways to produce meaningful summary statistics (e.g., using regression models or averaged ANAM performance scores) [79]. Recently, several cognitive performance scores (Ped-ANAM-CPS) have been developed to summarize various Ped-ANAM test scores into a single score [67, 92].. However, based on preliminary validation and considering its ease of use, two scores, the PedANAM-CPSmultiscore and the PedANAMCPSPCA, appear to be best suited as global measures of PedANAM performance [67, 92]. The higher the PedANAM-CPSPCA, the better the cognitive ability. In contrast, a higher PedANAM-CPSmultiscore, the worse correlates to a worse overall performance on the PedANAM. Real time examples of cSLE patients are summarized in (Figure 3). Whether the Ped-ANAM-CPS can be used in children with other diseases affecting cognition requires further study.
Figure 3.
PedANAM-CPSPCA and CPSmulitscore values over time in patients with NPSLE
Screening tool for clinical relevant low cognitive ability
Our earlier studies suggest that the Ped-ANAM is a sensitive screening tool, yielding accuracy of 97% for identify children with cSLE and clinically relevant cognitive impairment [88]. Indeed, a PedANAM-CPSmultiscore of > 4.63 or a PedANAMCPSPCA,of < 0.25 are over 80% sensitive to capture children with lower than expected cognitive ability [67, 92]. Future studies with larger numbers of normal controls will be needed to confirm the above cut-off values.
Summary
Children with cSLE are at a considerable risk of experiencing NPSLE symptoms. In this setting, monitoring cognitive ability as a reflection of overall brain health appears crucial. While formal neuropsychological testing remains the standard criterion for quantifying cognitive ability, its routine use is limited by administration time, availability of trained personnel, and practice effects. Recent validation studies of Ped-ANAM in cSLE indicate that serial administration of the Ped-ANAM constitutes a useful tool for screening and monitoring cognitive functioning.
Legend Figure 1 Brain volumes and PedANAM performance
Traditional neuropsychological battery (TNPB) and Ped-ANAM were administered on the same day to 22 adolescents with SLE. The summary scores were correlated with demographic/clinical variables potentially related to cognitive performance to examine differential sensitivity of Ped-ANAM and TNPB. Ped-ANAM Summary throughput Scores are Lower in Adolescents with Cerebral Volume Loss [93].
Legend Figure 2 PedANAM Performance Reports for select cSLE patients
Panel a: a 17 year old female with normal cognition but lack of motivation to complete the PedANAM as observed by the administrator. Panel b: an 18 year old female with neurocognitive impairment, altered mental status and severe working memory deficits. Panel c: 22 years old female with cognitive impairment, decreased processing speed and markedly reduced working memory after treatment with IV cyclophosphamide. Panel d: 18 years old female without neurocognitive impairments.
Legend Figure 3 PedANAM Cognitive Performance Scores in cSLE patients with acute NPSLE before and on treatment
Patient 1: 18 year of female with neurocognitive impairment, altered mental status and severe working memory deficits (also see Panel b of Figure 2). At the time of visit 1 the PedANAM-CPSPCA and PedANAM-CPSMultiscore are both very low. Visit 2 occurred 11 weeks after Visit 1. Between visits the patient received pulse methylprednisolone and cyclophosphamide IV. The patient appeared modestly improved. PedANAM-CPSPCA and PedANAM-CPSMultiscore are both improved almost in parallel. X axes indicate the PedANAM-CPS Score and the Y axes indicate the number of patient visit.
Patient 2: 22 year old female on treatment for NPSLE (see Panel c of Figure 2) at the time of Visit 1 the patient was just discharged after 3 weeks of hospitalization. Visit 2 occurred 12 weeks after Visit1 and the patient received treatment with cyclophosphamide IV and rituximab and pulse methylprednisolone. PedANAM-CPSPCA remained constant and PedANAM-CPSmultiscore markedly improved, in line with clinical perception of patient improvement.
Significance and Innovation.
The Screening for Cognitive function in Children with NPSLE
Review about the use of Pediatric Automated Neuropsychological Assessment Metrics (Ped-ANAM).
Correlation between Ped-ANAM and other modalities for diagnosis of Neurocognitive impairments.
Comparison between different Neurocognitive Testing tools.
Acknowledgement
We would like to thank, Mrs. Kasha Wiley for her support as research coordinator on this study; Drs. Ji Li , Hai-Mei Liu, Felice Mizan, Macy Zou, Bader-Meunier , Simone Thiemi Kishimoto ,Nahlah AlGhasham, Salah AlKharraz and Najla AlJabri for participating in the translation of the PedANAM. Special thanks for Lori white the Director, Customer Relations in Vista Life Sciences, Inc. for her great support in ANAM software related inquires. Drs. Frank Zelko, Jun Ying, Natasha M. Ruth, for their contributions in Ped-ANAM network.
Funding acknowledgement: This study is supported by grants from the NIH U01 AR059509to HB
Contributor Information
Ashwaq AlE’ed, Division of Rheumatology, Department of Pediatrics, Qassim University College of Medicine; Qassim, Saudi Arabia; ashwaqaleed@qumed.edu.sa.
Patricia Vega-Fernandez, Division of Rheumatology, Department of Pediatrics, Emory University; pvegafe@emory.edu.
Eyal Muscal, Baylor College of Medicine and Pediatric Rheumatology Center, Texas Children’s Hospital, Houston, Texas; emuscal@bcm.tmc.edu.
Claas Hinze, Department of Pediatric Rheumatology and Immunology University Hospital Münster: Münster, Germany; claas.hinze@gmail.com.
Lori B. Tucker, Division of Rheumatology, BC Children’s Hospital, Vancouver BC; ltucker@cw.bc.ca.
Simone Appenzeller, Division of Rheumatology, Department of Medicine, Faculty of Medical Science, State University of Campinas, Brazil; appenzellersimone@yahoo.com.
Brigitte Bader-Meunier, Département d’Immunologie et Rhumatologie pédiatrique, Hôpital Necker-Enfants Malades, Assistance Publique-Hôpitaux de Paris (AP-HP), Paris, France; Institut Imagine, Paris, France; INSERM 1163, Paris, France; brigitte.bader-meunier@nck.aphp.fr.
Johannes Roth, Division of Pediatric Rheumatology, Children’s Hospital of Eastern Ontario, Department of Pediatrics, University of Ottawa, jroth@cheo.on.ca.
Vicenç Torrente-Segarra, Physician Attending,Pediatric Rheumatology Unit, Hospital Sant Joan de Déu, Esplugues Llobregat (Spain) ; vtorrente@hsjdbcn.org.
Marisa S Klein-Gitelman, Ann & Robert H. Lurie Children's Hospital of Chicago, Northwestern Feinberg School of Medicine; klein-gitelman@northwestern.edu.
Deborah M Levy, Division of Rheumatology, Department of Pediatrics, Hospital for Sick Children and University of Toronto, Toronto, ON; Deborah.levy@sickkids.ca.
Tresa Roebuck-Spencer, Cognitive Science Research Center, Department of Psychology, University of Oklahoma, Norman, OK, USA; tresa@ou.edu.
Hermine Brunner, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine.
References
- 1.Brunner HI, et al. Sensitivity of the Systemic Lupus Erythematosus Disease Activity Index, British Isles Lupus Assessment Group Index, and Systemic Lupus Activity Measure in the evaluation of clinical change in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1999;42(7):1354–60. doi: 10.1002/1529-0131(199907)42:7<1354::AID-ANR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Muscal E, et al. Cognitive impairment in children with systemic lupus erythematosus: Assessing diagnostic practices and research needs in the CARRA network. Arthritis and Rheumatism. 2008;58(9):S250–S251. [Google Scholar]
- 3.Ainiala H, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001;45(5):419–23. doi: 10.1002/1529-0131(200110)45:5<419::aid-art360>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.ACR NPSLE Criteria The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Hanly JG, et al. A prospective analysis of cognitive function and anticardiolipin antibodies in systemic lupus erythematosus. Arthritis Rheum. 1999;42(4):728–34. doi: 10.1002/1529-0131(199904)42:4<728::AID-ANR16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 6.Tan EM, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 7.Brey RL, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58(8):1214–20. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 8.Ward MM, Studenski S. The time course of acute psychiatric episodes in systemic lupus erythematosus. J Rheumatol. 1991;18(4):535–9. [PubMed] [Google Scholar]
- 9.Vandam AP, et al. Psychiatric-Symptoms before Systemic Lupus-Erythematosus Is Diagnosed. Rheumatology International. 1994;14(2):57–62. doi: 10.1007/BF00300248. [DOI] [PubMed] [Google Scholar]
- 10.Sibbitt WL, Jr., et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29(7):1536–42. [PubMed] [Google Scholar]
- 11.Denburg SD, Denburg JA. Cognitive dysfunction and antiphospholipid antibodies in systemic lupus erythematosus. Lupus. 2003;12(12):883–90. doi: 10.1191/0961203303lu497oa. [DOI] [PubMed] [Google Scholar]
- 12.Tucker LB, et al. Adult- and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Br J Rheumatol. 1995;34(9):866–72. doi: 10.1093/rheumatology/34.9.866. [DOI] [PubMed] [Google Scholar]
- 13.Brunner HI, et al. Risk factors for damage in childhood-onset systemic lupus erythematosus: cumulative disease activity and medication use predict disease damage. Arthritis Rheum. 2002;46(2):436–44. doi: 10.1002/art.10072. [DOI] [PubMed] [Google Scholar]
- 14.Brunner HI, et al. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis and Rheumatism. 2008;58(2):556–562. doi: 10.1002/art.23204. [DOI] [PubMed] [Google Scholar]
- 15.Sakic B, et al. Immunosuppression prevents neuronal atrophy in lupus-prone mice: evidence for brain damage induced by autoimmune disease? J Neuroimmunol. 2000;111(1-2):93–101. doi: 10.1016/s0165-5728(00)00364-7. [DOI] [PubMed] [Google Scholar]
- 16.Devinsky O, Petito CK, Alonso DR. Clinical and neuropathological findings in systemic lupus erythematosus: the role of vasculitis, heart emboli, and thrombotic thrombocytopenic purpura. Ann Neurol. 1988;23(4):380–4. doi: 10.1002/ana.410230411. [DOI] [PubMed] [Google Scholar]
- 17.Harel L, et al. Neuropsychiatric manifestations in pediatric systemic lupus erythematosus and association with antiphospholipid antibodies. J Rheumatol. 2006;33(9):1873–7. [PubMed] [Google Scholar]
- 18.Valdes-Ferrer SI, et al. Cerebral changes in SLE with or without antiphospholipid syndrome. a case-control MRI study. J Neuroimaging. 2008;18(1):62–5. doi: 10.1111/j.1552-6569.2007.00183.x. [DOI] [PubMed] [Google Scholar]
- 19.Tomietto P, et al. General and specific factors associated with severity of cognitive impairment in systemic lupus erythematosus. Arthritis Rheum. 2007;57(8):1461–72. doi: 10.1002/art.23098. [DOI] [PubMed] [Google Scholar]
- 20.Sabet A, et al. Neurometabolite markers of cerebral injury in the antiphospholipid antibody syndrome of systemic lupus erythematosus. Stroke. 1998;29(11):2254–60. doi: 10.1161/01.str.29.11.2254. [DOI] [PubMed] [Google Scholar]
- 21.Afeltra A, et al. Neuropsychiatric lupus syndromes: relationship with antiphospholipid antibodies. Neurology. 2003;61(1):108–10. doi: 10.1212/01.wnl.0000058904.94330.a7. [DOI] [PubMed] [Google Scholar]
- 22.Avcin T, et al. A followup study of antiphospholipid antibodies and associated neuropsychiatric manifestations in 137 children with systemic lupus erythematosus. Arthritis Rheum. 2008;59(2):206–13. doi: 10.1002/art.23334. [DOI] [PubMed] [Google Scholar]
- 23.Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–73. doi: 10.1093/brain/awl082. Pt 7. [DOI] [PubMed] [Google Scholar]
- 24.Martel MA, Wyllie DJ, Hardingham GE. In developing hippocampal neurons, NR2B-containing N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience. 2009;158(1):334–43. doi: 10.1016/j.neuroscience.2008.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeGiorgio LA, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7(11):1189–93. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 26.Kozora E, et al. Antibodies against N-methyl-D-aspartate receptors in patients with systemic lupus erythematosus without major neuropsychiatric syndromes. J Neurol Sci. 2010;295(1-2):87–91. doi: 10.1016/j.jns.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emmer BJ, et al. Perfusion MRI in neuro-psychiatric systemic lupus erthemathosus. J Magn Reson Imaging. 2010;32(2):283–8. doi: 10.1002/jmri.22251. [DOI] [PubMed] [Google Scholar]
- 28.Ghirardello A, et al. Anti-ribosomal P protein antibodies and neuropsychiatric systemic lupus erythematosus: cross-sectional vs. prospective studies. Lupus. 2010;19(6):771–3. doi: 10.1177/0961203309353914. [DOI] [PubMed] [Google Scholar]
- 29.Mostafa GA, et al. The role of measurement of serum autoantibodies in prediction of pediatric neuropsychiatric systemic lupus erythematosus. J Neuroimmunol. 2010;227(1-2):195–201. doi: 10.1016/j.jneuroim.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Brunner HI, et al. Blood-based candidate biomarkers of the presence of neuropsychiatric systemic lupus erythematosus in children. Lupus Sci Med. 2014;1(1):e000038. doi: 10.1136/lupus-2014-000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lapa AT, et al. Reduction of cerebral and corpus callosum volumes in childhood-onset systemic lupus erythematosus. A volumetric magnetic resonance imaging analysis. Arthritis Rheumatol. 2016 doi: 10.1002/art.39680. [DOI] [PubMed] [Google Scholar]
- 32.Press J, et al. Antiribosomal P antibodies in pediatric patients with systemic lupus erythematosus and psychosis. Arthritis Rheum. 1996;39(4):671–6. doi: 10.1002/art.1780390420. [DOI] [PubMed] [Google Scholar]
- 33.Yoshio T, et al. IgG anti-NR2 glutamate receptor autoantibodies from patients with systemic lupus erythematosus activate endothelial cells. Arthritis Rheum. 2013;65(2):457–63. doi: 10.1002/art.37745. [DOI] [PubMed] [Google Scholar]
- 34.Brooks WM, et al. Neurochemistry of brain lesions determined by spectroscopic imaging in systemic lupus erythematosus. J Rheumatol. 1997;24(12):2323–9. [PubMed] [Google Scholar]
- 35.Mortilla M, et al. Brain study using magnetic resonance imaging and proton MR spectroscopy in pediatric onset systemic lupus erythematosus. Clinical and Experimental Rheumatology. 2003;21(1):129–135. [PubMed] [Google Scholar]
- 36.DiFrancesco MW, et al. Functional magnetic resonance imaging assessment of cognitive function in childhood-onset systemic lupus erythematosus: a pilot study. Arthritis Rheum. 2007;56(12):4151–63. doi: 10.1002/art.23132. [DOI] [PubMed] [Google Scholar]
- 37.Jones JT, et al. Childhood-onset lupus with clinical neurocognitive dysfunction shows lower streamline density and pairwise connectivity on diffusion tensor imaging. Lupus. 2015;24(10):1081–6. doi: 10.1177/0961203315572718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiFrancesco MW, et al. Functional neuronal network activity differs with cognitive dysfunction in childhood-onset systemic lupus erythematosus. Arthritis Res Ther. 2013;15(2):R40. doi: 10.1186/ar4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gitelman DR, et al. Brain morphometric changes associated with childhood-onset systemic lupus erythematosus and neurocognitive deficit. Arthritis Rheum. 2013;65(8):2190–200. doi: 10.1002/art.38009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muscal E, et al. Neurocognitive deficits and neuroimaging abnormalities are prevalent in children with lupus: clinical and research experiences at a US pediatric institution. Lupus. 2010;19(3):268–279. doi: 10.1177/0961203309352092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klingberg T, et al. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10(13):2817–21. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 42.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 43.Filley CM. The behavioral neurology of cerebral white matter. Neurology. 1998;50(6):1535–40. doi: 10.1212/wnl.50.6.1535. [DOI] [PubMed] [Google Scholar]
- 44.Sibbitt WL, Jr., et al. Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Semin Arthritis Rheum. 2010;40(1):32–52. doi: 10.1016/j.semarthrit.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson RT, Richardson EP. The neurological manifestations of systemic lupus erythematosus. Medicine (Baltimore) 1968;47(4):337–69. doi: 10.1097/00005792-196807000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Hanly JG, Walsh NM, Sangalang V. Brain pathology in systemic lupus erythematosus. J Rheumatol. 1992;19(5):732–41. [PubMed] [Google Scholar]
- 47.Ellis SG, Verity MA. Central nervous system involvement in systemic lupus erythematosus: a review of neuropathologic findings in 57 cases, 1955--1977. Semin Arthritis Rheum. 1979;8(3):212–21. doi: 10.1016/s0049-0172(79)80009-8. [DOI] [PubMed] [Google Scholar]
- 48.Hanly JG, et al. Short-term outcome of neuropsychiatric events in systemic lupus erythematosus upon enrollment into an international inception cohort study. Arthritis & Rheumatism-Arthritis Care & Research. 2008;59(5):721–729. doi: 10.1002/art.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy DM, Ardoin SP, Schanberg LE. Neurocognitive impairment in children and adolescents with systemic lupus erythematosus. Nature Clinical Practice Rheumatology. 2009;5(2):106–114. doi: 10.1038/ncprheum0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luna B, et al. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–72. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 51.Monastero R, et al. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. Journal of the Neurological Sciences. 2001;184(1):33–39. doi: 10.1016/s0022-510x(00)00492-5. [DOI] [PubMed] [Google Scholar]
- 52.Fisk JD, et al. Patterns of cognitive impairment in patients with systemic lupus erythematosus. Br J Rheumatol. 1993;32(6):458–62. doi: 10.1093/rheumatology/32.6.458. [DOI] [PubMed] [Google Scholar]
- 53.Williams TS, et al. Neurocognitive impairment in childhood-onset systemic lupus erythematosus: measurement issues in diagnosis. Arthritis Care Res (Hoboken) 2011;63(8):1178–87. doi: 10.1002/acr.20489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozora E, Ellison MC, West S. Reliability and validity of the proposed American College of Rheumatology neuropsychological battery for systemic lupus erythematosus. Arthritis Rheum. 2004;51(5):810–8. doi: 10.1002/art.20692. [DOI] [PubMed] [Google Scholar]
- 55.Ross GS, et al. A proposed framework to standardize the neurocognitive assessment of patients with pediatric systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62(7):1029–33. doi: 10.1002/acr.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunner HI, et al. Initial validation of the pediatric automated neuropsychological assessment metrics for childhood-onset systemic lupus erythematosus. Arthritis & Rheumatism-Arthritis Care & Research. 2007;57(7):1174–1182. doi: 10.1002/art.23005. [DOI] [PubMed] [Google Scholar]
- 57.Ad Hoc Committee on Lupus Response Criteria: Cognition, S.-c. et al. Proposed response criteria for neurocognitive impairment in systemic lupus erythematosus clinical trials. Lupus. 2007;16(6):418–25. doi: 10.1177/0961203307079044. [DOI] [PubMed] [Google Scholar]
- 58.Tiersky LA, et al. Neuropsychology of chronic fatigue syndrome: a critical review. J Clin Exp Neuropsychol. 1997;19(4):560–86. doi: 10.1080/01688639708403744. [DOI] [PubMed] [Google Scholar]
- 59.Heilbronner RL, et al. Official position of the American Academy of Clinical Neuropsychology on serial neuropsychological assessments: the utility and challenges of repeat test administrations in clinical and forensic contexts. Clin Neuropsychol. 2010;24(8):1267–78. doi: 10.1080/13854046.2010.526785. [DOI] [PubMed] [Google Scholar]
- 60.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 61.Ouvrier RA, et al. The value of the Mini-Mental State Examination in childhood: a preliminary study. J Child Neurol. 1993;8(2):145–8. doi: 10.1177/088307389300800206. [DOI] [PubMed] [Google Scholar]
- 62.Wyckoff PM, et al. Neuropsychological assessment of children and adolescents with systemic lupus erythematosus. Lupus. 1995;4(3):217–20. doi: 10.1177/096120339500400310. [DOI] [PubMed] [Google Scholar]
- 63.Zelko F, et al. Academic outcomes in childhood-onset systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012;64(8):1167–74. doi: 10.1002/acr.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki M, et al. Initial validation of a novel protein biomarker panel for active pediatric lupus nephritis. Pediatr Res. 2009;65(5):530–6. doi: 10.1203/PDR.0b013e31819e4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vega-Fernandez P, et al. Value of questionnaire-based screening as a proxy for neurocognitive testing in childhood-onset systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2014;66(6):943–8. doi: 10.1002/acr.22247. [DOI] [PubMed] [Google Scholar]
- 66.Lai JS, et al. Development of a Parent-Report Cognitive Function Item Bank Using Item Response Theory and Exploration of its Clinical Utility in Computerized Adaptive Testing. Journal of Pediatric Psychology. 2011;36(7):766–779. doi: 10.1093/jpepsy/jsr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vega-Fernandez P, et al. Cognitive performance scores for the pediatric automated neuropsychological assessment metrics in childhood-onset systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2015 doi: 10.1002/acr.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahone EM, et al. Parent and self-report ratings of executive function in adolescents with myelomeningocele and hydrocephalus. Child Neuropsychol. 2002;8(4):258–70. doi: 10.1076/chin.8.4.258.13510. [DOI] [PubMed] [Google Scholar]
- 69.Lai JS, et al. Parent-reported cognition of children with cancer and its potential clinical usefulness. Qual Life Res. 2014;23(4):1049–58. doi: 10.1007/s11136-013-0548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kane RL, Kay GG. Computerized assessment in neuropsychology: a review of tests and test batteries. Neuropsychol Rev. 1992;3(1):1–117. doi: 10.1007/BF01108787. [DOI] [PubMed] [Google Scholar]
- 71.Bauer RM, et al. Computerized neuropsychological assessment devices: joint position paper of the American Academy of Clinical Neuropsychology and the National Academy of Neuropsychology. Clin Neuropsychol. 2012;26(2):177–96. doi: 10.1080/13854046.2012.663001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luciana M. Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB) J Child Psychol Psychiatry. 2003;44(5):649–63. doi: 10.1111/1469-7610.00152. [DOI] [PubMed] [Google Scholar]
- 73.Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36(3):273–93. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- 74.Luciana M, Nelson CA. Assessment of neuropsychological function through use of the Cambridge Neuropsychological Testing Automated Battery: Performance in 4-to 12-year-old children. Developmental Neuropsychology. 2002;22(3):595–624. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- 75.Reeves DL, et al. ANAM genogram: historical perspectives, description, and current endeavors. Arch Clin Neuropsychol. 2007;22(Suppl 1):S15–37. doi: 10.1016/j.acn.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Johnson DR, et al. Reliability and construct validity of the Automated Neuropsychological Assessment Metrics (ANAM) mood scale. Arch Clin Neuropsychol. 2008;23(1):73–85. doi: 10.1016/j.acn.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Daniel JC, et al. Repeated measures of cognitive processing efficiency in adolescent athletes: implications for monitoring recovery from concussion. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12(3):167–9. [PubMed] [Google Scholar]
- 78.Holliday SL, et al. Validating a computerized neuropsychological test battery for mixed ethnic lupus patients. Lupus. 2003;12(9):697–703. doi: 10.1191/0961203303lu442oa. [DOI] [PubMed] [Google Scholar]
- 79.Roebuck-Spencer TM, et al. Use of computerized assessment to predict neuropsychological functioning and emotional distress in patients with systemic lupus erythematosus. Arthritis & Rheumatism-Arthritis Care & Research. 2006;55(3):434–441. doi: 10.1002/art.21992. [DOI] [PubMed] [Google Scholar]
- 80.Woodhouse J, et al. Efficacy of the ANAM General Neuropsychological Screening Battery (ANAM GNS) for detecting neurocognitive impairment in a mixed clinical sample. Clin Neuropsychol. 2013;27(3):376–85. doi: 10.1080/13854046.2012.762427. [DOI] [PubMed] [Google Scholar]
- 81.Hanly JG, et al. Assessment of cognitive function in systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis by computerized neuropsychological tests. Arthritis Rheum. 2010;62(5):1478–86. doi: 10.1002/art.27404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Proctor SP, Heaton KJ. Automated Neuropsychological Assessment Metrics Version 4 (ANAM4): Select Psychometric Properties and Administration Procedures. 2012 DTIC Document. [Google Scholar]
- 83.Segalowitz SJ, et al. Retest reliability in adolescents of a computerized neuropsychological battery used to assess recovery from concussion. Neurorehabilitation. 2007;22(3):243–251. [PubMed] [Google Scholar]
- 84.Kirkwood MW, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics. 2006;117(4):1359–71. doi: 10.1542/peds.2005-0994. [DOI] [PubMed] [Google Scholar]
- 85.Partners, V.L.a.V. 2016 [cited 2/10/2016; Available from: http://www.vistalifesciences.com/
- 86.Ped-ANAM: Administration Manual . Cognitive Science Research Center. University of Oklahoma, Norman, OK. CSRC; 2014. [Google Scholar]
- 87.Singer J, Denburg JA. Diagnostic criteria for neuropsychiatric systemic lupus erythematosus: the results of a consensus meeting. The Ad Hoc Neuropsychiatric Lupus Workshop Group. J Rheumatol. 1990;17(10):1397–402. [PubMed] [Google Scholar]
- 88.Brunner HI, et al. Validation of the Pediatric Automated Neuropsychological Assessment Metrics in childhood-onset systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2013;65(3):372–81. doi: 10.1002/acr.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reeves D, et al. ANAM-Pediatric Version (Ped-ANAM) User's Manual.; 2004. National Rehabilitation Hospital; Washington, DC: 2004. [Google Scholar]
- 90.Levy DM, et al. Longitudinal performance on the pediatric automated neuropsychological assessment metrics (Ped-ANAM) in childhood-onset systemic lupus erythematosus (cSLE) ARTHRITIS AND RHEUMATISM. 2008 WILEY-LISS DIV JOHN WILEY & SONS INC, 111 RIVER ST, HOBOKEN, NJ 07030 USA. [Google Scholar]
- 91.Muscal E, et al. Small practice effects on neurocognitive testing–markers of early cognitive impairment among adolescents with SLE? Pediatric Rheumatology. 2012;10(Suppl 1):A26. [Google Scholar]
- 92.Nguyen J, et al. Cross-validation of the Pediatric Automated Neuropsychological Assessment Metrics-Cognitive Performance Scores in the Screening of Neurocognitive Impairment in Childhood-Onset Systemic Lupus Erythematosus. In: Sons W, editor. American College of Rheumatology Annual Meeting. Wiley & Sons; San Francisco: 2015. p. 2045. [Google Scholar]
- 93.Muscal E, et al. Further Validation of the Pediatric Automated Neuropsychological Assessment Metrics for Adolescents with Lupus. 2009 [Google Scholar]
- 94.Mina R, et al. Validation of the lupus nephritis clinical indices in childhood-onset systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2015 doi: 10.1002/acr.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]