Abstract
Higher levels of physical activity (PA) have been linked to better neurocognitive functioning in many populations. The current study examines the longitudinal association between PA and neurocognitive functioning among HIV-infected and HIV-uninfected persons. Community-dwelling adults (N=291) self-reported level of PA and completed a comprehensive neuropsychological battery at two to four study visits (Mean follow-up time=2.6 years). Participants were divided into three PA groups: "No PA" (no PA at any visit), "Consistent PA" (PA at >=50% of visits), and "Inconsistent PA" (PA <50% of visits). A mixed effect model, adjusting for significant covariates showed that all PA groups had statistically significant, yet modest, neurocognitive decline over time; and, the Consistent PA group began with, and maintained, significantly better neurocognitive function compared to the other two PA groups. This effect was evident among both HIV-uninfected and HIV-infected persons, despite the fact that HIV-infected persons showed lower baseline neurocognitive function. PA is a modifiable lifestyle behavior that may help to protect against neurocognitive impairment regardless of HIV status, however, given the proportion of HIV-infected individuals who evidence neurocognitive difficulties, a focus on increasing PA seems warranted.
Keywords: HIV/AIDS, cognition, physical activity, exercise, neurocognitive impairment, HIV-associated neurocognitive disorders
Resumen
La actividad física (AF) ha sido asociada con un mejor funcionamiento neurocognitivo en varios grupos. Este estudio examinó la asociación longitudinal entre la AF y el funcionamiento neurocognitivo en personas con y sin infección del VIH. Adultos viviendo en la comunidad (N=291) proporcionaron información acerca de sus niveles de AF y completaron una batería neuropsicológica exhaustiva. Los participantes completaron entre dos y cuatro visitas relacionadas con el estudio (tiempo de seguimiento promedio = 2,6 años) y fueron divididos en tres grupos de AF: “Ninguna AF” (Ninguna AF durante todas las visitas del estudio), “AF Consistente” (AF durante 50% o más de las visitas del estudio), y “AF Inconsistente” (AF durante menos del 50% de las visitas del estudio). Un modelo estadístico mixto, ajustando por el efecto de variables externas, indicó que hubo una reducción estadísticamente significativa, pero poco pronunciada, en el funcionamiento neurocognitivo en todos los grupos. Además, el grupo con AF Consistente demostró un mejor funcionamiento neurocognitivo en comparación con los otros dos grupos de AF al comienzo del estudio, el cual se mantuvo durante el seguimiento. A pesar de que las personas con VIH demostraron un funcionamiento neurocognitivo más bajo al comienzo del estudio que las personas sin VIH, el efecto de AF fue demostrado en los dos grupos. Es importante recalcar que la AF es un factor de vida modificable que podría proteger contra los daños neurocognitivos independientemente de si las personas tienen o no VIH. Dada la proporción de personas con VIH que demuestran problemas neurocognitivos relacionados con esta enfermedad, será importante enfocar los esfuerzos investigativos en desarrollar formas de incrementar la AF en este grupo de personas.
Introduction
Antiretroviral therapy (ART) has improved a number of health outcomes and extended the lifespan of individuals infected with human immunodeficiency virus (1, 2). Yet, HIV-associated neurocognitive impairment (NCI) continues to be prevalent, with approximately half of community-dwelling HIV-infected adults experiencing NCI (3). Although the most severe form of HIV-associated neurocognitive disorders (HAND), HIV-associated dementia (HAD), is on the decline, there is evidence that the milder forms of HAND have increased in prevalence (3). Even mild NCI can lead to problems with everyday functioning, including medication adherence, driving, and financial management (4–7). Moreover, HIV infection and aging may have additive or synergistic negative effects on the central nervous system (CNS), leading to increased rates of NCI among older HIV-infected cohorts (8, 9). Thus, as the population of HIV-infected adults lives longer, the need to address the longer-term impact of NCI is increasing. Identifying modifiable lifestyle factors that may protect neurocognition across the lifespan may inform development of prevention and intervention methods for this population.
One promising lifestyle factor that may prevent NCI and improve the integrity of the CNS is physical activity (PA). The long-term benefits of PA on neurocognitive outcomes among HIV-uninfected older adults are well established (10–12). Epidemiological studies have indicated that midlife PA is associated with a lower risk of neurocognitive decline and dementia later in life (13, 14). Moreover, PA intervention trials have consistently shown improved cognitive function in older HIV-uninfected adults who participate in aerobic activity as compared to control groups (10, 15–20). Although the mechanisms by which PA affects neurocognition are not yet completely elucidated, research has suggested direct effects on the CNS, such as increased gray and white matter volume (10, 16, 18, 21), changes in functional brain activity and strengthening of brain connections (22–27), and increases in cerebral perfusion (15, 28–31) and neurotrophic factors (32, 33) that support angiogenesis and neuroplasticity. Indirect effects via the reduction of comorbidities (e.g., diabetes, hypertension) may also contribute to the decreased risk of cognitive decline (34, 35).
The relatively limited research on PA and cognition among those living with HIV is consistent with what has been shown among HIV-uninfected adults; higher levels of PA are associated with decreased concurrent risk for NCI (36–38). Moreover, work by our group among older adults living with HIV has shown that active lifestyles (including PA) are associated with better cognition and that PA is associated with better cognition and everyday functioning among older adults living with HIV (39, 40). Neuroimaging work has recently revealed that those who are physically active, as opposed to sedentary, have better executive functioning scores, and executive functioning was significantly associated with larger putamen volumes in adjusted models (40, 41). Despite this growing research, we are unaware of any longitudinal studies that explore the relationship between cognition and PA among those living with HIV and such studies are necessary to ascertain directionality and to determine if PA modifies neurocognitive trajectories over time in this vulnerable population. Additionally, no studies to date have compared the neurocognitive benefits of PA among those with HIV as compared to those without HIV.
The primary objective of the current study was to examine the association between self-reported PA and objectively measured neurocognitive change in a large and diverse cohort of HIV-infected and HIV-uninfected persons. We hypothesized that both HIV-infected and HIV-uninfected participants who reported PA would show decreased risk for neurocognitive decline. We were also interested in examining whether the association between PA and neurocognitive decline was comparable between HIV-infected and HIV-uninfected persons given the increased risk for NCI in HIV.
Methods
Participants
The present study examined 235 HIV-infected and 56 HIV-uninfected community-dwelling adults recruited by the HIV Neurobehavioral Research Program (HNRP) for various cohort studies described elsewhere (42–44). Inclusion criteria for present analyses included having at least two visits with data for both self-reported PA and global neurocognition. Forty-nine participants had two visits, 42 had three visits, and 200 had four visits. All visits were between 5 months and 53 months from the prior visit with median follow-up time between visits of 10.9 (5.1, 36.3) months in the HIV-uninfected group and 12.0 (5, 52.8) months in the HIV-infected group. This is consistent with the annual visit structure for most of the studies from which these data were gathered. Median follow-up time over the full study period for the overall sample was 35 (24, 36) months. A participant’s earliest visit with both primary data points of interest was considered the baseline assessment for this study and was used to compare demographic variables between groups. Data from the total 1061 visits were used for analysis.
After providing informed consent, participants underwent comprehensive neurocognitive, neuromedical and psychiatric evaluations at baseline and follow-up visits.
Neurocognitive Function
Neurocognition was assessed via a well-validated standardized neurocognitive test battery that covers seven cognitive domains commonly affected by HIV (3). The domains and tests included Verbal Fluency, Working Memory, Speed of Information Processing, Learning, Recall, Executive Function, and Motor Function (45). Due to the repeated neuropsychological testing of participants, we used published norms for change to compute practice-adjusted scaled scores for each neuropsychological test (45). Practice-adjusted scaled scores for individual tests were averaged to compute domain and global practice-adjusted scaled scores.
Physical Activity
Physical activity (PA) was assessed via a staff-administered questionnaire developed by the HNRP to give an estimate of the recent metabolic rate of participants. Participants were asked to estimate time spent in PA in the last 72 hours. More specifically, this was defined as any PA in which the heart beats rapidly. A list of examples was given (i.e., running, jogging, lifting heavy weights, aerobics, hockey, football, soccer, squash, basketball, cross country, judo, roller blading/skating, vigorous swimming, vigorous long distance bicycling). We have chosen the term PA here to be more consistent with the general literature on this topic. All participants completed this measure at baseline and at least one follow-up visit. Due to the skewed distribution of the time spent in PA variable, for each study visit we dichotomized this variable into whether a participant reported any PA or not. This is consistent with the methodology previously used by our group using this measure (36). We then divided participants into three groups based on their responses across study visits. The “No PA” group, consisted of those who reported no PA across all study visits. The “Consistent PA” group, consisted of those who reported PA at more than half of their study visits. The “Inconsistent PA” group consisted of those who reported PA at some visits but less than half of the visits.
HIV Disease Characteristics
Current CD4+ count and plasma HIV viral load was determined from blood specimens taken at each visit. Plasma HIV viral loads were deemed “undetectable” below 50 copies/mL. Nadir CD4+ count was self-reported unless a study lab value was found to be lower. Hepatitis C co-infection was determined by antibody testing, Viral RNA, or prior diagnosis (n=22). AIDS diagnosis was made using CDC classification of 3 or C (46). Duration of HIV infection, ART use, and months exposed to ART were self-reported by participants to a member of the neuromedical team.
Psychiatric Characteristics and Physical Health Status
Current mood state was evaluated using the Beck Depression Inventory-II (BDI-II; (47). Substance use disorders (i.e., alcohol, amphetamine, cannabis, cocaine, hallucinogen, inhalant, sedative, opioid, PCP) and lifetime and current major depressive disorders were assessed through the computer-assisted Composite International Diagnostic Interview (CIDI), version 2.1 (48). Current and lifetime substance use disorder included any diagnosis of substance abuse and/or substance dependence to the substances listed above by DSM-IV criteria. Self-reported physical functioning and mental functioning were evaluated using the physical health summary (PHS) and mental health summary (MHS) scores, (49) derived from the Medical Outcomes Study HIV health Survey (50). Body Mass Index (BMI) was calculated using height and weight.
Statistical Analysis
To evaluate the association between PA and neurocognitive change by HIV status we conducted a series of mixed effect linear models on global and domain neurocognitive scale scores, with subject specific random intercepts and slopes in our overall sample. Mixed-effects models were used for these data to accommodate for within subject correlations that naturally occur in longitudinal studies. The mixed effect linear models on global and domain neurocognitive scores in the overall sample included terms for (1) HIV status; (2) PA group (i.e. “No PA”, “Consistent PA”, and “Inconsistent PA”); (3) time; (4) the two-way interactions between PA group and time, HIV status and time, and HIV status and PA group; (5) the three-way interaction among HIV status, PA and time; and (6) adjustments for demographic characteristics (age at baseline, education, gender, ethnicity) and significant covariates. To create a more parsimonious model, non-significant interaction terms were subsequently removed.
In order to identify significant covariates to be included in these mixed effect models we first considered psychiatric, substance use, and medical comorbidities that were associated with global scaled scores at p < 0.10 level and differed at the p < 0.10 level between PA groups and HIV status groups. We ran independent samples chi-square (or Fisher’s exact) tests for baseline covariates (lifetime substance use and major depression diagnoses, and Hepatitis C infection), and linear and logistic mixed effects models for time-dependent covariates (BDI-II total score, current major substance use and major depression diagnoses, MHS, PHS, and BMI). Covariates that differed by PA groups or HIV status in these univariable analyses and that also showed univariable association with changes in scaled scores (p < 0.10) were included in a mixed effect linear model on global neurocognitive scaled scores along with terms for HIV status, PA group, time, and demographic characteristics (age at baseline, education, gender, ethnicity). We then removed non-significant covariates in a systematic backward selection with significance level α = 0.05.
We also examined the association of PA to global and domain neurocognitive change within our HIV-infected group adjusting for the effect of significant HIV-disease characteristics following a similar methodology as presented above. The main multivariable model included terms for (1) PA group; (2) time; (3) the interaction between PA group and time; and (4) adjustments for demographic characteristics (age at baseline, education, gender, ethnicity) and significant covariates. In subsequent analyses we removed any non-significant interaction terms.
Covariates examined within the HIV-infected group included similar psychiatric, substance use and medical comorbidities as in the overall sample, along with HIV-disease characteristics that were significantly associated (p< 0.10) with either global neurocognitive change or PA group among HIV-infected participants. HIV-disease characteristics were considered for inclusion based on their baseline values (i.e., duration of HIV infection, AIDS, nadir CD4, ART status, duration ART) or as time-dependent covariates (CD4 cell count, plasma and CSF RNA).
All multivariable models are based on the subset of participants that had available data for all the covariates that were considered for inclusion in multivariable analyses.
Results
Baseline Sample Characteristics by HIV status
Table 1 shows demographic, substance use, psychiatric and physical health characteristics of participants with and without HIV infection at the baseline visit, as well as HIV disease characteristics of the HIV-infected group. The HIV-infected group was comprised of a higher percentage of ethnic/racial minorities; had higher scores on a measure of current depressed mood; a higher proportion of persons with a history of major depression, self-reported physical and mental functioning; a lower proportion of persons with Hepatitis-C infection; and lower BMI.
Table 1.
Cohort characteristics by HIV status (N=291)
| Variable | HIV Serostatus | |||
|---|---|---|---|---|
| HIV-infected (n=235) |
HIV-uninfected (n=56) |
Test Statistics | p | |
| Demographics | ||||
| Age (years) | 49.2 (9.8) | 51.6 (9.4) | t=−1.69, df=289 | 0.09 |
| Education (years) | 13.1 (3.1) | 13.8 (2.9) | t=−1.55, df=289 | 0.12 |
| Male | 73% | 63% | χ2=2.02, df=1 | 0.15 |
| Ethnicity | χ2=7.24, df=2 | 0.03 | ||
| White | 59% | 77% | ||
| Black | 20% | 7% | ||
| Other | 21% | 16% | ||
| Psychiatric Characteristics | ||||
| Beck Depression Inventory-II | 8.5 (3.0–15.0) | 4.0 (1.0–9.3) | t=2.41, df=288 | 0.02 |
| Lifetime Substance Use Disorder |
66% | 66% | χ2=0, df=1 | >0.99 |
| Current Substance Use Disorder |
4% | 0% | FET | 0.22 |
| Lifetime Major Depressive Disorder |
58% | 38% | χ2=6.97, df=1 | <0.01 |
| Current Major Depressive Disorder |
8% | 2% | FET | 0.14 |
| Mental Health Summary Score | 51.9 (43.1–58.4) | 60.8 (54.6–63.4) |
t=−5.63, df=32.2 |
<0.01 |
| Physical Health | ||||
| Characteristics | ||||
| Physical Health Summary Score |
50.7 (39.0–57.6) | 58.5 (53.7–61.9) |
t=−5.77, df=43.1 |
<0.01 |
| Body Mass Index | 25.7 (23.2–28.8) | 28.0 (24.9–31.2) | t=−2.30, df=278 | 0.02 |
| Hepatitis-C Infection | 23% | 52% | χ2 =16.4, df=1 | <0.01 |
| HIV Disease Characteristics | ||||
| Duration of Infection (years) | 14.9 (9.9–19.1) | --- | --- | --- |
| Nadir CD4 Count | 158 (50–259) | --- | --- | --- |
| Current CD4 Count | 542 (368–794) | --- | --- | --- |
| Detectable Plasma Viral Load | 27% | --- | --- | --- |
| AIDS Diagnosis | 63% | --- | --- | --- |
| ART Prescribed | 85% | --- | --- | --- |
| Months Exposed to ART | 87.8 (40–141) | --- | χ2=0.32, df=2 | 0.85 |
| Physical activity | ||||
| Consistent | 20% | 19% | ||
| Inconsistent | 34% | 38% | ||
| None | 46% | 43% | ||
| Neurocognition | ||||
| Global Scaled Score | 9.4 (7.8–10.8) | 10.2 (8.8–11.6) | t=−2.66, df=289 | <0.01 |
Notes. Mean (SD), Median (IQR), or % reported. ART = antiretroviral therapy. FET = Fisher’s exact test.
Missing Demographic data. Beck Depression Inventory-II: 0.3%, Lifetime and Current Substance Use Disorder: 1%, Lifetime and Current Major Depressive Disorder: 1%, Mental and Physical Health Summary Score: 26.1%, Body Mass Index: 3.8%, Duration of Infection: 0.9%, Nadir CD4: 0.4%, Current CD4: 2.6%, Detectable Plasma Viral Load: 5.5%, AIDS: 0.9%, ART Prescribed: 0.9%, Months Exposed to ART: 2.1%.
Time in Physical Activity
For visits with reported PA in the last 72 hours, the Consistent PA group reported spending an average of 178 (190) minutes in PA and the Inconsistent PA group reported spending 185 (207) minutes (F=0.10, df=1, 269, p=0.75).
Physical Activity and Neurocognitive Change by HIV Status
Analyses aimed at identifying significant baseline and time-dependent covariates that might impact the association between PA and neurocognitive change by HIV status showed that Hepatitis C (t = −3.59, df = 731, p<0.001), physical health score (t = 1.66, df = 498, p=0.10), and current substance diagnosis (t = 1.68, df = 708, p=0.09) were associated with global neurocognition. Of these covariates, Hepatitis C (χ2 = 16.4, df = 1, p<0.001) and physical health score (t = −3.40, df = 498, p<0.001) were significantly associated with HIV status and Hepatitis C with PA group (χ2 = 6.7, df = 2, p=0.04). None of these variables, however, remained significant when entered in a model of global neurocognition along with demographic characteristics, HIV status, PA and time, and thus were not included in subsequent multivariable models.
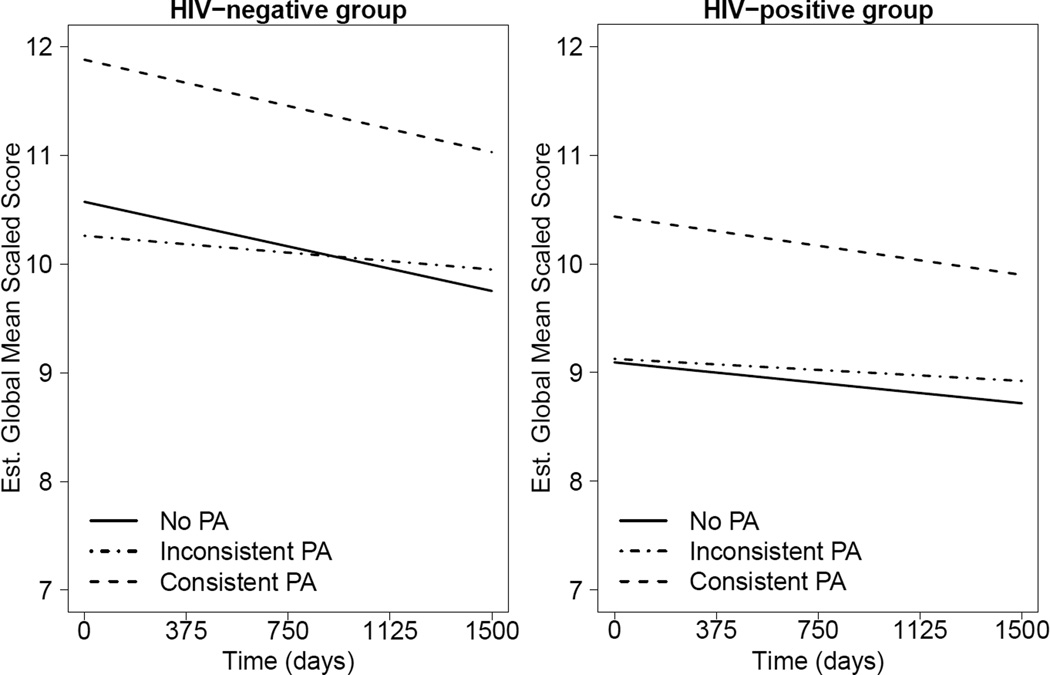
Results from the multivariable model examining the association between PA and global neurocognitive change by HIV status including terms for HIV status, PA group, time and their interaction, along with adjustments for demographics showed no significant interactions (ps>0.45; see Figures 1a and 1b). Thus, interaction terms were removed from the model. These results revealed a main effect of time, PA group, and HIV, indicating that neurocognitive function declined significantly over time in all groups. The Consistent PA group had significantly better neurocognitive function than both the No PA (Estimate = 1.28, SE = 0.33, t = 3.83, df = 492, p<0.001) and the Inconsistent PA groups (Estimate = 1.19, SE = 0.34, t = 3.50, df = 492, p<0.001), with no significant differences between the latter two (Estimate = −0.08, SE = 0.27, t = −0.31, df = 492, p=0.76). The HIV-infected group had worse neurocognitive function across time than the HIV-uninfected group (Estimate = −1.24, SE = 0.40, t = −3.05, df = 492, p=0.002).
Figure 1.
a and b. Mixed-effect linear model examining the longitudinal relationship between neurocognitive change (i.e., practice adjusted global scaled scores) and PA group (No PA, Consistent PA, Inconsistent PA) by HIV status. The HIV-uninfected group (Figure 1a) demonstrated better overall neurocognitive function than the HIV-infected group (Figure 1b), and there were no significant differences in neurocognitive change over time between the PA groups as evidenced by similar slopes across PA groups. PA=Physical Activity.
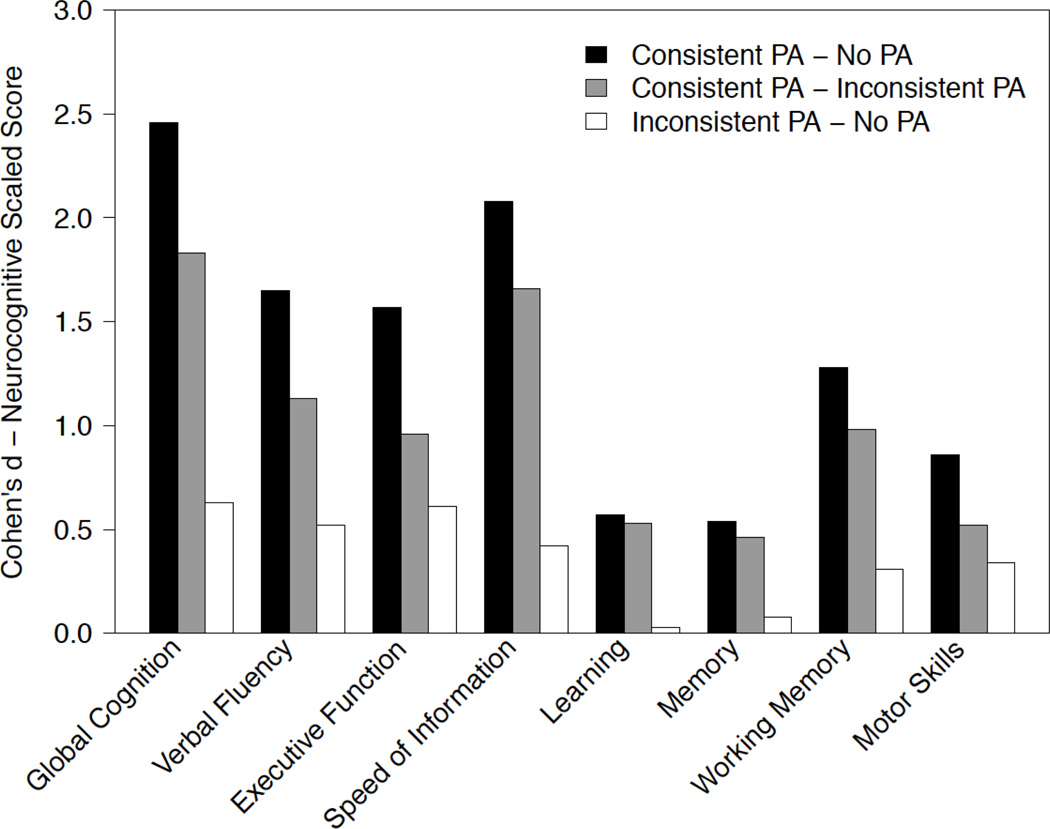
Comparable analyses by cognitive domain also showed no significant interactions and thus these terms were removed from the final models. In five of seven neurocognitive domains (Verbal Fluency, Working Memory, Speed of Information Processing, Executive Function, and Motor Function), the Consistent PA group showed significantly less neurocognitive decline than the No PA Group. Effect sizes between the three PA groups derived from these analyses are shown in Figure 2.
Figure 2.
Effect sizes, measured by Cohen’s d, for estimating group differences in mean practice-adjusted scaled scores for the entire sample. Values were obtained from multivariable mixed-effect linear models examining the longitudinal relationship between neurocognitive change and PA group (No PA, Consistent PA, Inconsistent PA). PA=Physical Activity.
Physical Activity and Neurocognitive Change among HIV-infected Participants
Univariable analyses of baseline and time-dependent covariates (i.e., psychiatric, physical and HIV disease characteristics) showed detectable HIV plasma viral load (χ2 = 6.1, df = 2, p=0.05) and Hepatitis C co-infection (χ2 = 5.8, df = 2, p=0.05) were associated with PA, and detectable HIV CSF viral load was associated with global neurocognition (t = −2.09, df = 298, p=0.04). Only detectable HIV CSF viral load remained significant in a multivariable model that also included terms for demographics, PA group and time, and thus HIV CSF viral load was included in subsequent models.
The multivariable model examining the association between PA group and global neurocognitive change within the HIV-infected group included terms for PA group, time and their interaction, along with adjustment for demographic characteristics and significant covariates. This model showed no significant PA X time interaction (χ2 = 2.03, df = 2, p=0.36), and thus the interaction term was removed from the model. The final model is presented in Table 2 and yielded a similar pattern of findings to those in the overall group. Namely, global neurocognitive function declined over time in all PA groups, and the Consistent PA group showed better global neurocognitive function across time than both the No PA (t=3.88, df=252, p<0.001) and the Inconsistent PA groups (t=2.85, df=252, p<0.01), with no significant differences between the latter two (t=1.19, df=252, p=0.24).
Table 2.
Mixed-effect linear model examining change in neurocognitive function by physical activity (PA) group and demographic and HIV disease-related covariates among the HIV sample (n=118)
| Term | Estimate (SE) |
T statistic (df = 254) |
p |
|---|---|---|---|
| Age | −0.07 (0.02) | −3.96 | <0.001 |
| Education | 0.23 (0.06) | 3.80 | <0.001 |
| Gender (female) | 1.33 (0.39) | 3.42 | <0.001 |
| Race/Ethnicity (white) | 0.57 (0.27) | −1.99 | 0.048 |
| Detectable CSF RNA | −0.40 (0.16) | −2.55 | 0.01 |
| PA groupa | |||
| Consistent | 1.78 (0.46) | 3.88 | <0.001 |
| Inconsistent | 0.42 (0.35) | 1.19 | 0.24 |
| Time (+ 30 days) | −0.01 (0.002) | −3.21 | <0.001 |
Note.
Reference group = No Physical Activity.
Comparable models by neurocognitive domain showed results consistent with those on global neurocognition in most domains except for Learning, Recall, and Motor function, which were not significant. Again, we saw performance declined over time with Consistent PA being significantly associated with higher scaled scores in comparison with the other two PA groups (all ps<0.05).
Discussion
The present study demonstrates that HIV-infected and HIV-uninfected persons who consistently engage in PA maintain better neurocognitive function over time than those who participate in PA inconsistently or do not engage in PA. This finding is consistent with previous research documenting that inconsistent or no PA is associated with concurrent risk for NCI in HIV-infected adults (36, 37). As would be expected, HIV-infected persons had worse neurocognitive function overall. Yet, the strength of the association between PA and neurocognitive decline was comparable among HIV-infected and HIV-uninfected persons, even after adjusting for a number of potential confounds including differences in demographic characteristics, and physical and mental health status. Moreover, the relationship between PA and cognition was not better explained by these factors or HIV disease characteristics when examining the HIV-infected individuals alone. The cognitive domains that demonstrated a similar relationship between PA and cognition included Verbal Fluency, Working Memory, Speed of Information Processing, Executive Function, and Motor function, whereas Recall and Learning were not significantly associated with PA. Additionally, we did not find a significant difference in rate of decline among the PA groups.
The association of PA to global and domain neurocognitive functioning did not differ by HIV status. This is particularly encouraging given results from prior intervention studies in HIV-uninfected persons that show that PA can improve neurocognition. Participants who reported consistent PA over time had significantly better neurocognitive functioning as compared to those who did not, but we did not find a significant difference between our Inconsistent PA group and our No PA group. The nature of our PA variable only allowed for self-reported examination of time engaged in rather vigorous PA (mostly identified as traditional “exercise” or sporting activities) in the past 72 hours. The variable did not take into account duration of a given PA session or which specific type of PA the participant engaged in. Furthermore, this variable may also have missed relatively strenuous PA that may occur in the context of the individual’s life (e.g., walking to work, construction as a job). These limitations of our measure may be one reason why we were not able to observe differences between the Inconsistent and No PA groups.
Although participants who were consistently physical active began with and maintained better neurocognitive function, rates of decline were similar across PA groups. One possible explanation for this finding is that we did not intervene on PA behavior. Our available data do not allow ascertainment of when participants began their PA practices; thus, the major benefit of PA may have occurred prior to the study time period. Additionally, participants may have begun, altered or ended PA during the study, obscuring the relationship between time and PA. Further longitudinal observational studies and intervention studies are needed, along with longer follow-up periods and objective measurement of PA (e.g., using a pedometer or accelerometer), in order to better examine the effect of PA on neurocognitive trajectories over time. Specifically, if there were a dose response relationship that may thwart decline, this level of PA could be prescribed. This dose may be higher or even lower than current CDC guidelines for PA.
PA may benefit neurocognition through both indirect and direct impacts on the CNS. The indirect benefit of PA on the brain may be via reduced neurocognitive risk factors, such as diabetes, high blood pressure and hyperlipidemia (51). Studies have shown that aerobic PA can improve body composition, reduce waist circumference and weight, and improve lipid levels in those with HIV infection (52), which are risk factors for NCI in HIV-infected adults (53). The cognitive domains of Speed of Information Processing and Executive Function are linked to metabolic and cerebrovascular disease (20), both of which are also risk factors for neurodegeneration and cognitive decline (54). Many of the cognitive domains for which we found an association with consistent PA are mediated by frontal and subcortical brain systems that are commonly affected in HIV and by cerebrovascular disease (55, 56). Thus, the indirect effects that PA exerts on cerebrovascular and metabolic risk factors may help to explain our current findings (35).
PA also has direct impacts on the brain, as studies have shown that PA reduces oxidative stress and inflammatory markers, and increases neurogenesis, angiogenesis, and synaptogenesis in the brain (57, 58). The directionality between PA and neurocognition remains unclear as it might be the case that participants with better neurocognitive function are better able to adhere to a specific PA regimen. As suggested above, randomized control trials examining the effect of PA on neurocognitive function in HIV-infected adults are needed and warranted given the large effect sizes observed among HIV-uninfected populations (59).
Our study was limited by the use of self-report to measure PA. Self-reported data are subject to bias; however, self-report of PA captures some behaviors that may not be identified by accelerometry (60). Seventy-two hours is a short period to assess PA; yet, the validity of this period of recall is similar to that of self-report measure of PA over longer recall periods (61). Our questionnaire was short and did not specify the daily frequency or duration, and the quantity of PA in various categories (e.g., leisure vs. work-related PA). Although we were able to report recent PA behavior over several visits, we are unable to assess when PA habits were started and/or suspended. Our decision to divide our sample into three groups (Consistent PA, No PA, and Inconsistent PA) based on the report of PA across visits was done to simplify interpretation of our findings. We considered other groupings, but decided on the presented approach as it appeared to best encapsulate the groups of people who showed a consistent pattern of behavior across visits (Consistent PA and No PA), while also depicting data of a less clear third group. Interestingly, we found that the inconsistent PA group performed similarly to the No PA group, lending support to the notion that consistent PA may be to key in maintaining health benefits (62, 63). Finally, it is important to note neurocognitive functioning declined in both HIV+ and HIV− groups, but this decline was very small. On average, the change in cognition over the entire study period (up to 5 years) was predicted to be approximately 0.42 SS. Considering that SS have a mean of 10 and standard deviation of 3, a change in 0.42 SS may be of little clinical significance as it represents less than a quarter of a standard deviation. This degree of change is expected given that our analyses used scaled scores that are not corrected for age and it is well accepted that most neurocognitive abilities show some small decline with age. While our models adjusted for age at baseline, the impact of advancing age over the study period on cognition was not accounted for.
It is also important to note that the HIV-uninfected adults who participated in this study had a significantly higher rate of Hepatitis C infection than even our HIV-infected group; therefore, our results may not generalize to the larger HIV-uninfected population. The strength of this study includes the assessment of a large, well-characterized cohort and the extensive and well-validated neurocognitive battery over time.
In addition, we did not address the important issue of nutrition in this survey. Cross-behavior regulation studies show that individuals who exercise consistently report better compliance with healthy diet practices (64). Adults living with HIV are also reported to have poor diet quality, including higher fat consumption and lower fiber intake compared to HIV-uninfected adults (65, 66). Greater central obesity, a key factor modulated by diet, is associated with a higher rate of NCI in HIV-infected individuals (53) Substantial evidence indicates that diet interventions, such as the well-known Mediterranean diet, may slow cognitive decline over time or lead to cognitive improvement in domains such as working memory and executive function (67). Therefore, subsequent work in this field should optimally examine both PA and diet effects on neurocognition in HIV-infected cohorts.
Future studies would benefit from the use of objective measurements of PA, such as pedometers, accelerometers, or supervised PA, to assess which types, intensity, and frequency of PA yield the most neurocognitive benefit over time. With the longitudinal design of our study we are able to start making stronger inferences about the potential directionality of findings; yet, the observational nature of the study pre-empts more definite conclusions. PA intervention studies would be best suited to make a more direct assessment of the relationship between time and the neurocognitive benefits of PA. Additional research is also needed to understand the complex underlying neural mechanisms whereby PA may protect or improve neurocognitive function in the HIV-infected population. We owe it to persons living with HIV infection to more definitively be able to say what dose may be needed in order to thwart neurocognitive decline and the specific ways that PA may ameliorate neurocognitive impairment and decline.
Individuals, both with and without HIV infection, who consistently reported PA maintained higher neurocognitive functioning over time. These results suggest that PA may represent a modifiable lifestyle factor that promotes positive neurocognitive outcomes in the HIV-infected population, which represents a group at increased risk for neurocognitive problems. In our study the relationship between PA and neurocognition was similar between HIV-uninfected and HIV-infected participants, which is promising because of extensive research showing that PA enhances cognition in HIV-uninfected adults. PA inventions trials in HIV-infected persons are needed to identify and promote the optimal neuroprotective PA regimen for this population.
Acknowledgments
Funding/Support: The following NIH grants provided funding for the current work: P30MH062512 and P01DA012065. Dr. Marquine is funded by K23MH105297.
Appendix
The San Diego HIV Neurobehavioral Research Program group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Terry Alexander, R.N., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Terry Jernigan, Ph.D., Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S., Christi Kao, M.S.
Footnotes
Compliance with Ethical Standards:
Financial Disclosure: Catherine A. Dufour declares that she has no conflict of interest. María J. Marquine declares that she has no conflict of interest. Pariya L. Fazeli declares that she has no conflict of interest. Anya Umlauf declares that she has no conflict of interest. Brook L. Henry declares that he has no conflict of interest. Zvinka Zlatar declares that she has no conflict of interest. Jessica L. Montoya declares that she has no conflict of interest. Ronald J. Ellis declares that he has no conflict of interest. Igor Grant declares that he has no conflict of interest. David J. Moore declares that he has no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of California, San Diego, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Disclaimer: The views expressed herein are those of the authors and do not reflect the official policy or position of the US government.
References
- 1.Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect Dis. 2015;15(7):810–818. doi: 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Senate Special Committee on Aging. Older Americans: The Changing Face of HIV/AIDS in America. Washington, D. C.: 2013. [Google Scholar]
- 3.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 6.Marcotte TD, Heaton RK, Wolfson T, et al. The impact of HIV-related neuropsychological dysfunction on driving behavior. The HNRC Group. J Int Neuropsychol Soc. 1999;5(7):579–592. doi: 10.1017/s1355617799577011. [DOI] [PubMed] [Google Scholar]
- 7.Thames AD, Arentoft A, Rivera-Mindt M, Hinkin CH. Functional disability in medication management and driving among individuals with HIV: A 1-year follow-up study. J Clin Exp Neuropsychol. 2013;35(1):49–58. doi: 10.1080/13803395.2012.747596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: Incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43(11):2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 9.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63(5):822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 11.Gajewski PD, Falkenstein M. Physical activity and neurocognitive functioning in aging - a condensed updated review. Eur Rev Aging Phys Act. 2016;13:1. doi: 10.1186/s11556-016-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakash RS, Voss MW, Erickson KI, Kramer AF. Physical activity and cognitive vitality. Annu Rev Psychol. 2015;66:769–797. doi: 10.1146/annurev-psych-010814-015249. [DOI] [PubMed] [Google Scholar]
- 13.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol Med. 2009;39(1):3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 14.Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J Intern Med. 2011;269(1):107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 15.Chapman SB, Aslan S, Spence JS, et al. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci. 2013;5(75):1–9. doi: 10.3389/fnagi.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colcombe S, Erickson K, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):M176–M180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 17.Colcombe S, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemoun G, Thibaud M, Roumagne N, et al. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement Geriatr Cogn Disord. 2010;29(2):109–114. doi: 10.1159/000272435. [DOI] [PubMed] [Google Scholar]
- 20.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colcombe S, Erickson K, Scalf P, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 22.Burdette JH, Laurienti PJ, Espeland MA, et al. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2(23):1–10. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGregor KM, Zlatar Z, Kleim E, et al. Physical activity and neural correlates of aging: A combined TMS/fMRI study. Behav Brain Res. 2011;222(1):158–168. doi: 10.1016/j.bbr.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voelcker-Rehage C, Godde B, Staudinger UM. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front Hum Neurosci. 2011;5(26):1–12. doi: 10.3389/fnhum.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voss MW, Erickson KI, Prakash RS, et al. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48(5):1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voss MW, Prakash RS, Erickson KI, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2(32):1–17. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zlatar ZZ, Towler S, McGregor KM, et al. Functional language networks in sedentary and physically active older adults. J Int Neuropsychol Soc. 2013;19(6):625–634. doi: 10.1017/S1355617713000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murrell CJ, Cotter JD, Thomas KN, Lucas SJ, Williams MJ, Ainslie PN. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: Effect of age and 12-week exercise training. Age. 2013;35(3):905–920. doi: 10.1007/s11357-012-9414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JC, Paulson ES, Cook DB, Verber MD, Tian Q. Detecting changes in human cerebral blood flow after acute exercise using arterial spin labeling: implications for fMRI. J Neurosci Methods. 2010;191(2):258–262. doi: 10.1016/j.jneumeth.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Swain RA, Harris AB, Wiener EC, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 31.Thomas BP, Yezhuvath US, Tseng BY, et al. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO. J Magn Reson Imaging. 2013;38(5):1–16. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 33.Voss MW, Erickson KI, Prakash RS, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer's disease? Mol Psychiatry. 2013;18(8):864–874. doi: 10.1038/mp.2012.162. [DOI] [PubMed] [Google Scholar]
- 35.Nation DA, Hong S, Jak AJ, et al. Stress, exercise, and Alzheimer’s disease: A neurovascular pathway. Med Hypotheses. 2011;76(6):847–854. doi: 10.1016/j.mehy.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dufour CA, Marquine MJ, Fazeli PL, et al. Physical exercise is associated with less neurocognitive impairment among HIV-infected adults. J Neurovirol. 2013;19(5):410–417. doi: 10.1007/s13365-013-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fillipas S, Oldmeadow LB, Bailey MJ, Cherry CL. A six-month, supervised, aerobic and resistance exercise program improves self-efficacy in people with human immunodeficiency virus: a randomised controlled trial. Aust J Physiother. 2006;52(3):185–190. doi: 10.1016/s0004-9514(06)70027-7. [DOI] [PubMed] [Google Scholar]
- 38.Honn VJ, Para MF, Whitacre CC, Bornstein RA. Effect of exercise on neuropsychological performance in asymptomatic HIV infection. AIDS Behav. 1999;3(1):67–74. [Google Scholar]
- 39.Fazeli PL, Marquine MJ, Dufour C, et al. Physical activity is associated with better neurocognitive and everyday functioning among older adults with HIV disease. AIDS Behav. 2015;19(8):1470–1477. doi: 10.1007/s10461-015-1024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fazeli PL, Woods SP, Heaton RK, et al. An active lifestyle is associated with better neurocognitive functioning in adults living with HIV infection. J Neurovirol. 2014;20(3):233–242. doi: 10.1007/s13365-014-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortega M, Baker LM, Vaida F, Paul R, Basco B, Ances BM. Physical activity affects brain integrity in HIV+ individuals. J Int Neuropsychol Soc. 2015;21(10):880–889. doi: 10.1017/S1355617715000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heaton RK, Grant I, Butters N, et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1(3):231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 43.Rippeth JD, Heaton RK, Carey CL, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- 44.Woods SP, Conover E, Rippeth JD, et al. Qualitative aspects of verbal fluency in HIV-associated dementia: a deficit in rule-guided lexical-semantic search processes? Neuropsychologia. 2004;42(6):801–809. doi: 10.1016/j.neuropsychologia.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Cysique LA, Franklin D, Jr, Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol. 2011;33(5):505–522. doi: 10.1080/13803395.2010.535504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro KG, Ward JW, Slutsker L, Buehler JW, Jaffe HW, Berkelman RL. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. US Department of Health and Human Services; 1992. [PubMed] [Google Scholar]
- 47.Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. Vol. 1. San Antonio, TX: Psychological Corporation; 1996. p. 82. [Google Scholar]
- 48.Wittchen HU. Reliability and validity studies of the WHO--Composite International Diagnostic Interview (CIDI): A critical review. J Psychiatr Res. 1994;28(1):57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 49.Revicki DA, Sorensen S, Wu AW. Reliability and validity of physical and mental health summary scores from the Medical Outcomes Study HIV Health Survey. Med Care. 1998;36(2):126–137. doi: 10.1097/00005650-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV) Qual Life Res. 1997;6(6):481–493. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]
- 51.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Brien K, Nixon S, Tynan AM, Glazier R. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2010;(8):CD001796. doi: 10.1002/14651858.CD001796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78(7):485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popa-Wagner A, Buga A-M, Popescu B, Muresanu D. Vascular cognitive impairment, dementia, aging and energy demand. A vicious cycle. J Neural Transm (Vienna) 2015;122(S1):47–54. doi: 10.1007/s00702-013-1129-3. [DOI] [PubMed] [Google Scholar]
- 55.DeCarli C, Murphy DGM, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in healthy adults. Neurology. 1995;45(11):2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 56.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117(6):1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 57.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol. 2010;30(4):493–503. doi: 10.1007/s10571-009-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 60.Senso MM, Anderson CP, Crain AL, Sherwood NE, Martinson BC. Self-reported activity and accelerometry in 2 behavior maintenance trials. Am J Health Behav. 2014;38(2):254–264. doi: 10.5993/AJHB.38.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han JL, Dinger MK. Validity of a self-administered 3-day physical activity recall in young adults. Am J Health Educ. 2009;40(1):5–13. [Google Scholar]
- 62.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psych Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 63.Shahandeh M, Roshan VD, Hosseinzadeh S, Mahjoub S, Sarkisian V. Chronic exercise training versus acute endurance exercise in reducing neurotoxicity in rats exposed to lead acetate. Neural Regen Res. 2013;8(8):714–722. doi: 10.3969/j.issn.1673-5374.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fleig L, Kerschreiter R, Schwarzer R, Pomp S, Lippke S. 'Sticking to a healthy diet is easier for me when I exercise regularly': Cognitive transfer between physical exercise and healthy nutrition. Psychol Health. 2014;29(12):1361–1372. doi: 10.1080/08870446.2014.930146. [DOI] [PubMed] [Google Scholar]
- 65.Duran AC, Almeida LB, Segurado AA, Jaime PC. Diet quality of persons living with HIV/AIDS on highly active antiretroviral therapy. J Hum Nutr Diet. 2008;21(4):346–350. doi: 10.1111/j.1365-277X.2008.00886.x. [DOI] [PubMed] [Google Scholar]
- 66.Ziegler TR, McComsey GA, Frediani JK, Millson EC, Tangpricha V, Eckard AR. Habitual nutrient intake in HIV-infected youth and associations with HIV-related factors. AIDS Res Hum Retroviruses. 2014;30(9):888–895. doi: 10.1089/aid.2013.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. Adherence to a mediterranean-style diet and effects on cognition in adults: A qualitative evaluation and systematic review of longitudinal and prospective trials. Front Nutr. 2016;3:22. doi: 10.3389/fnut.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]