Abstract
BACKGROUND
A positive surgical margin (PSM) following radical prostatectomy (RP) for prostate cancer (PCa) is associated with increased risk of biochemical recurrence. We sought to examine whether the pathologist is an independent predictor of PSMs.
METHODS
We performed a retrospective review of 3,557 men who underwent RP for localized PCa at our institution from 2003–2015. We evaluated 29 separate pathologists. Univariate and multivariable logistic regression were used to test variables previously shown to influence PSM rates.
RESULTS
Overall rate of PSM was 18.9%. Compared to patients without PSM, patients with PSM had higher BMI (mean 28.8 vs. 28.3), Gleason ≥ 7 (84% vs. 66%), extracapsular extension (51% vs. 20%), and median PSA (5.9 vs. 5.1 ng/ml) (all p < 0.05). Univariate logistic regression showed surgeon experience, pathologist experience, and pathologist GU fellowship training were all predictors of PSMs (all p < 0.05). Multivariable regression analysis confirmed decreased surgeon experience, increased pathologist experience, higher pathologic Gleason score, higher pathologic stage, and higher PSA were significant predictors of PSMs. Increasing surgeon experience was associated with decreased odds of PSM (OR 0.79 per 1 SD increase, 95% CI [0.70 – 0.89]). In contrast, increasing pathologist experience was associated with increased odds of PSM (OR 1.11 per 1 SD increase, 95% CI [1.03 – 1.19]). The relationship between pathologist experience and PSM appeared to be non-linear (Figure 2).
CONCLUSIONS
Greater pathologist experience appears to be associated with greater odds of PSMs following radical prostatectomy, even after controlling for case mix, pathologist fellowship training, and surgeon experience. Based on these findings, pathologists with less experience reviewing RP specimens may consider requesting re-review by a dedicated GU pathologist.
Keywords: Prostate cancer, urology, pathology, interobserver variability
1. Introduction
Prostate cancer (PCa) is the most commonly diagnosed non-skin cancer in the United States and radical prostatectomy (RP) is the most common treatment option for men with localized disease [1]. Analysis of the surgical margin following RP, defined as tumor cells present at the inked margin of a resected specimen [2], is frequently used to assist in risk stratification and guide subsequent therapies. Over several decades, there has been continued downward stage migration due to prostate specific antigen (PSA) screening [3]. Consequently, the rates of positive surgical margins (PSM) after RP have been decreasing over the past 25 years and contemporary rates range from 10–30% [4,5].
PSMs following RP can be a significant source of anxiety for patients and increases the risk of biochemical recurrence (BCR) [6,7] and secondary therapies [8]. While some data suggest PSMs independently predict PCa specific mortality [9], most adjusted analyses do not show similar observations [10,11]. Some expert guidelines (ASCO/AUA/EAU) suggest men with PSMs should consider adjuvant radiation therapy [12,13]; however, this is not commonly done [14,15]. For these reasons, accurate interpretation of the surgical margin has a critical role for patient counseling, prognosis, and treatment decisions [16].
PSA, clinical stage, pathologic stage, and volume of tumor are consistently associated with a higher PSM rate [5,17–19]. The role of the pathologist on PSMs has also been examined. Interpretation of surgical margins is subject to inter-observer variability with multiple studies suggesting 8–26% rates of discordance among pathologists [20,21].
We hypothesize the individual pathologist and pathologist experience are associated with PSMs following RP.
2. Methods
2.1 Study Design and Data Collection
We performed a retrospective, single-center, observational cohort study on 3,557 men who were treated with robotic-assisted laparoscopic prostatectomy (RALP) for localized PCa at the University of Chicago Medical Center and Weiss Hospital between April 2003 and January 2015. Men were excluded if surgery was aborted (n=38; 1%), most often due to intraoperative positive lymph nodes, or if they received neoadjuvant therapy (n=57; 1.5%)
All patients provided informed consent. Data were collected and stored in an IRB-approved, HIPAA-compliant database [22]. Data include patient demographics, pre-operative variables, biopsy data, intra-operative information, pathological variables, patient-reported quality-of-life outcomes, and recurrence information.
The radical prostatectomy specimens were processed in accordance to the College of American Pathologists (CAP) and International Society of Urological Pathology (ISUP) recommendations [23,24]. Briefly, the entire outer surface of the prostate was inked using 2 different colors to identify right and left outer margins. The prostatic apex was amputated and sectioned perpendicular to the inked surface and the prostatic base was submitted serially in a perpendicular fashion. The remainder of the prostate was serially sectioned transversely at 3–5 mm intervals and submitted for processing either partially (at least 50%) or entirely (100%) in quadrants. Partial sampling is by submitting alternate slices. Sections of bilateral seminal vesicles, including its proximal portions, were also submitted. A margin was considered positive if a cancer gland extends into the inked outer surface.
2.2 Statistical Analysis
A PSM was defined as tumor at the inked margin of the resected specimen. We included variables shown to be associated with PSM [5,19,25–27], including age, race, body mass index (BMI), pathologic Gleason score, pathologic tumor stage, pre-operative PSA level, surgeon and pathologist experience (defined as the number of cases the surgeon/pathologist had performed prior to the date of each case), fellowship training in genitourinary pathology, and year of surgery. Pathologist and surgeon experience and BMI were standardized to their respective means, such that the values of each variable corresponded to their standard deviation from the mean. PSA values were log transformed for use in regression analyses.
There were 19 pathologists who evaluated at least 25 cases during the study period with 9 pathologists having < 25 cases and therefore grouped together in the “low volume” group. The number of cases seen by each pathologist who evaluated > 25 cases varied from 39 to 606 cases. Pathologist #10 represents a consolidation of multiple low-volume pathologists from an affiliated hospital.
Mean and standard deviation were used to report continuous normally distributed variables; median and IQR were used for continuous non-normally distributed variables. We used univariate logistic regression to analyze the relationship between each variable of interest and PSMs, followed by multivariable logistic regression using predictor variables with p < 0.1 from univariate models to control for confounders and assess the relationship between the individual pathologist and PSMs. To account for the clustering due to multiple cases seen by each pathologist, the standard errors were adjusted with the use of the sandwich estimator of variance. The models produced were assessed for interactions between individual pathologists and surgeons, and between individual pathologists and pathologic parameters. To further explore its effects, pathologist experience was also modeled as a restricted cubic spline with 5 knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles [28], and plots of the probability of PSM versus pathologist experience were generated with all other variables in the model set to their means (for continuous variables) and to the most common category (for categorical variables). All statistical analyses were performed using Stata 13.1 (StataCorp, College Station, TX, USA) with a two-sided significance level set at p < 0.05.
3. Results
3.1 Cohort Description
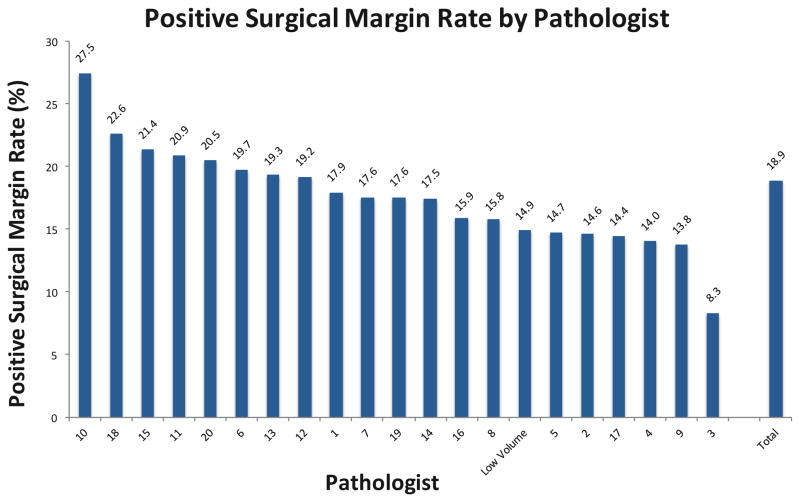
The mean age was 60 years old (SD 7.1) and median PSA was 5.2 ng/ml (IQR 4.1–7.2; Table 1). Following surgery, Gleason score 6 was identified in 1,088 (31%), Gleason 7 in 2,213 (62%), Gleason 8 in 131 (4%), and Gleason 9 in 124 (3%). Pathologic stage was pT2a in 320 (9%), pT2b in 304 (9%), pT2c in 2,099 (57%), pT3a in 728 (20%), and pT3b in 193 (5%). PSM was identified in 672 (18.9%). PSM rates by pathologist varied from 8.3% to 27.5% (Figure 1). Among the 29 pathologists examined, the mean number of cases evaluated was 153 (SD 145). Among the 5 surgeons examined, the mean number of cases performed was 799 (SD 584).
Table 1.
Clinical and pathological features
| Parameter | Overall, no. (%) |
|---|---|
| Number of Patients | 3,557 |
| Age (years), mean (SD) | 59.6 (7.1) |
| BMI, mean (SD) | 28.4 (4.7) |
| Race (%) | |
| Caucasian | 2,688 (75) |
| African American | 531 (15) |
| Asian | 130 (4) |
| Hispanic and Other | 49 (1) |
| Year of surgery (%) | |
| 2003–2005 | 379 (11) |
| 2006–2008 | 1,299 (37) |
| 2009–2011 | 1,155 (32) |
| 2012–2015 | 724 (20) |
| Biopsy Gleason Sum (%) | |
| 6 | 1,670 (47) |
| 7 | 1,489 (42) |
| 8 | 214 (6) |
| 9 | 105 (3) |
| 10 | 2 (0.06) |
| Pathologic Gleason Sum (%) | |
| 6 | 1,088 (31) |
| 7 | 2,213 (62) |
| 8 | 131 (4) |
| 9 | 123 (3) |
| Clinical Stage (%) | |
| cT1a / cT1b | 12 (0.3) |
| cT1c | 2,460 (69) |
| cT2a | 633 (18) |
| cT2b | 228 (6) |
| cT2c | 76 (2) |
| cT3a | 24 (0.7) |
| cT3b | 4 (0.1) |
| Pathologic Stage (%) | |
| pT2a | 320 (9) |
| pT2b | 304 (9) |
| pT2c | 2,009 (56) |
| pT3a | 728 (20) |
| pT3b | 193 (5) |
| PSA, ng/ml, median (IQR) | 5.2 (4.1–7.2) |
Figure 1.
Positive surgical margin rate by pathologist. Pathologists are numbered by volume of cases with pathologist 1 having the smallest volume and pathologist 20 having the largest.
3.2 Predictors of Positive Surgical Margins
On univariate logistic regression, higher BMI, less surgeon experience, greater pathologist experience, pathologist completion of a GU fellowship, higher pathologic Gleason score, higher pathologic stage, and higher PSA were all associated with a higher rate of PSM (all p-values < 0.05; Supplementary Table 3). There were no significant associations between surgical margin status and age, race, or year of surgery (all p-values > 0.05). Compared to pT2a disease, higher pathologic stage was associated with increased odds of PSM: pT2b OR 3.3, 95% CI [1.9–5.7]; pT2c OR 2.9, 95% CI [1.7–5.1]; pT3a OR 10.9, 95% CI [6.2–19.4]. pT3b disease was associated with the highest odds of PSM (OR 12.3; 95% CI [6.0–25.2]).
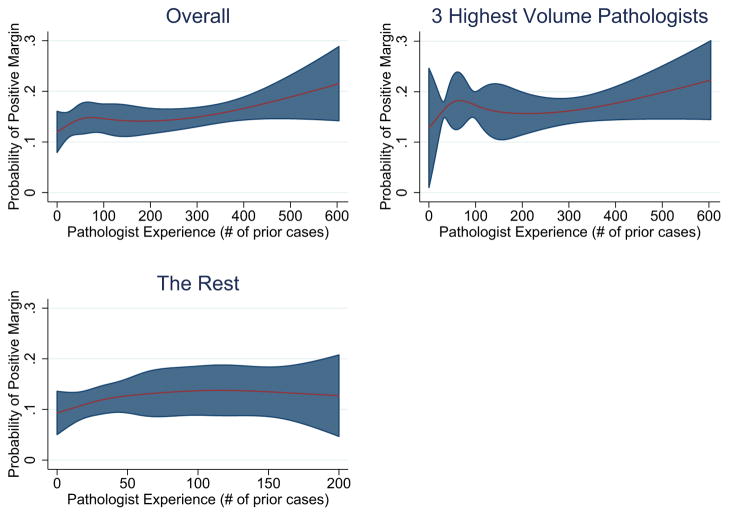
Multivariable regression showed less surgeon experience, greater pathologist experience, higher pathologic Gleason score, higher pathologic stage, and higher PSA all remained significantly associated with PSMs (all p-values < 0.05; Table 2). Individual surgeon and pathologist effects were not statistically significant after controlling for other important covariates. The individual surgeon and pathologist also did not have an effect on the relationship between pathologist experience and PSMs, so these covariates were dropped from the model for parsimony. There were no significant interactions between individual pathologists and individual surgeons, or between pathologist and any pathologic parameter (all p > 0.05). Greater surgeon experience was associated with decreased PSM rates (OR 0.79 per 1 SD increase, 95% CI [0.70–0.89], p < 0.001), while greater pathologist experience exhibited the opposite relationship (OR 1.11 per 1 SD increase, 95% CI [1.03–1.19], p = 0.008). However, further analysis of the relationship between pathologist experience and PSMs indicates early case experience up to approximately 50 cases is associated with an increase in PSMs, followed by a plateau as case experience increases from 75 to 250, followed by a rise as experience increases beyond 300 cases (Figure 2).
Table 2.
Multivariate logistic regression analysis of the association between clinical and pathological parameters and PSM rates
| Parameter | Overall | OR (95% CI) | P value |
|---|---|---|---|
| BMI, mean (SD) | 28.4 (4.7) | ||
| Standardized** | 1.07 (0.98–1.16) | 0.12 | |
| Surgeon Experience | |||
| Standardized** | 0.79 (0.70 – 0.89) | <0.001 | |
| Pathologist Experience | |||
| Standardized** | 1.11 (1.03 – 1.19) | 0.008 | |
| GU Fellowship Completed | |||
| No | 2,357 | 1 (Reference) | |
| Yes | 1,200 | 1.15 (0.88 – 1.49) | 0.3 |
| PSA, median (IQR) | 5.2 (4.1–7.2) | 1.28 (1.03 – 1.59) ‡ | 0.025 |
| Gleason Sum | |||
| 6 | 1,088 | 1 (Reference) | <0.001 |
| 7 | 2,213 | 1.65 (1.28 – 2.13) | |
| 8 | 131 | 1.49 (0.97 – 2.29) | |
| 9 | 124 | 2.26 (1.47 – 3.49) | |
| Pathologic Stage | |||
| 2a | 320 | 1 (Reference) | <0.001 |
| 2b | 304 | 2.92 (1.77 – 4.84) | |
| 2c | 2,009 | 2.50 (1.48 – 4.24) | |
| 3a | 728 | 7.64 (4.43 – 13.19) | |
| 3b | 193 | 6.79 (3.52 – 13.11) | |
BMI, Surgeon experience, and pathologist experience were standardized to their respective means for regression analysis
PSA was log transformed for regression analysis
Figure 2.
Plot of pathologist case experience and PSMs with pathologist experience modelled as a restricted cubic spline. Highlighted areas indicate 95% confidence bounds. Early experience up to approximately 50 cases reveals a moderate increase in PSM rates. This is followed by a plateau in PSMs between approximately 75 cases and 250 cases. After this plateau, there is a significant rise in PSMs with increasing pathologist experience. This relationship is most pronounced for the pathologists with the greatest number of cases in the dataset.
4. Discussion
We demonstrate the individual pathologist’s experience is independently associated with rate of PSMs following RP. There appears to be a learning curve among pathologists for identifying PSMs with increasing odds of finding PSMs as experience is accrued. The relationship was most pronounced for the pathologists with the greatest caseload.
These findings illustrate the influence an individual pathologist can have on surgical margin status and suggest that strategies would be helpful to standardize evaluation of PSMs. Further research is needed to better understand what association the individual pathologist’s evaluation has on long-term outcomes after RP.
Various authors have reported interobserver variability rates for surgical margin status between 8% and 26% [20,21,29]. Factors influencing the determination of surgical margins include tumor distortion along the actual margin due to crushing or thermal artifacts, irregular tracking or disruption of the inking of the specimen, tears of the extraprostatic soft tissue associated with processing of the specimen, and narrow margins in which malignant cells were close to, but did not touch, the inked margin [16]. Concordance for margin status is higher between expert genitourinary pathologists and at high volume institutions [30]. However, differences of opinion may exist among experts, even in cases that are free from the complicating factors listed above and there is no true gold standard for PSMs [16]. Some institutions have implemented re-review of difficult cases with multi-disciplinary teams as a method of addressing discordance between pathologists [31]. Though we did not prospectively assess interobserver concordance in this study, the relationship between increasing pathologist experience and increasing PSM rates in our study may be resultant of improving accuracy with experience. Therefore, it we highlight the importance of pathologists regularly reviewing RP cases.
There are multiple limitations to our study. All analyses were performed retrospectively, subject to the limitations of this approach. Other studies have utilized prospective review of prostate samples by expert pathologists [16] and inclusion of similar data in our study may have bolstered our understanding of pathologist accuracy. Although we utilize the AJCC TNM staging for prostate cancer at our institution as has been reported in several other recent series [32,33], we recognize that other institutions may use different staging systems. Not all prostate specimens were submitted in their entirety, which may impact positive margin rates. Most prostatectomy specimens were reviewed by one pathologist. While our center has a daily surgical pathology quality assurance (QA) consensus conference, equivocal margins are not routinely or regularly reviewed unless specifically presented by a junior or senior faculty member. Our study does not assess long-term clinical outcomes. Experience of both the surgeon and the pathologist in our sample only includes cases in our series and does not include any experience from prior centers or cases prior to our dataset. However, the 3 highest volume surgeons in our series performed all of their RPs at our institution and comprise >95% of all surgeries in our cohort.
5. Conclusion
We demonstrate pathologist’s experience is an independent factor for PSM, even when controlling for fellowship training, surgeon experience, pathologic stage, Gleason score, PSA, and BMI. Based on these findings, pathologists with less experience reviewing RP specimens may consider requesting re-review by a dedicated GU pathologist.
Supplementary Material
Highlights.
Greater pathologist experience was associated with greater odds of PSMs after RP
Increasing surgeon experience was associated with decreased odds of PSM
The relationship between pathologist experience and PSMs was non-linear
Acknowledgments
This study was supported in part by the National Institute of Diabetes, Digestive, and Kidney Diseases grant #5T35DK062719-28 and in part by National Institute of Health CTSA grant UL1 TR000430.
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Authors’ Contributions:
JE Tallman: Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing
VT Packiam: Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing
KE Wroblewski: Data collection or management, Data analysis, Manuscript writing/editing
GP Paner: Data collection or management, Data analysis, Manuscript writing/editing
SE Eggener: Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing
Conflict of Interest: Dr. Paner receives textbook publication royalties from Amirsys, Inc. Dr. Eggener’s work is funded by the NIH. The remaining authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohori M, Wheeler TM, Kattan MW, Goto Y, Scardino PT. Prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 1995;154:1818–24. [PubMed] [Google Scholar]
- 3.Weiner AB, Etzioni R, Eggener SE. Ongoing Gleason grade migration in localized prostate cancer and implications for use of active surveillance. Eur Urol. 2014;66:611–2. doi: 10.1016/j.eururo.2014.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han M, Partin AW, Chan DY, Walsh PC. An evaluation of the decreasing incidence of positive surgical margins in a large retropubic prostatectomy series. J Urol. 2004;171:23–6. doi: 10.1097/01.ju.0000133181.94746.b5. [DOI] [PubMed] [Google Scholar]
- 5.Retèl VP, Bouchardy C, Usel M, Neyroud-Caspar I, Schmidlin F, Wirth G, et al. Determinants and effects of positive surgical margins after prostatectomy on prostate cancer mortality: a population-based study. BMC Urol. 2014;14:86. doi: 10.1186/1471-2490-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silberstein JL, Eastham JA. Significance and management of positive surgical margins at the time of radical prostatectomy. Indian J Urol. 2014;30:423–8. doi: 10.4103/0970-1591.134240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalfin HJ, Dinizo M, Trock BJ, Feng Z, Partin AW, Walsh PC, et al. Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int. 2012;110:1684–9. doi: 10.1111/j.1464-410X.2012.11371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong YM, Hu JC, Paciorek AT, Knight SJ, Carroll PR. Impact of radical prostatectomy positive surgical margins on fear of cancer recurrence: Results from CaPSURE??? Urol Oncol Semin Orig Investig. 2010;28:268–73. doi: 10.1016/j.urolonc.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Wright JL, Dalkin BL, True LD, Ellis WJ, Stanford JL, Lange PH, et al. Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality. J Urol. 2010;183:2213–8. doi: 10.1016/j.juro.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephenson AJ, Eggener SE, Hernandez AV, Klein Ea, Kattan MW, Wood DP, et al. Do margins matter? the influence of positive surgical margins on prostate cancer-specific mortality. Eur Urol. 2014;65:675–80. doi: 10.1016/j.eururo.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Mauermann J, Fradet V, Lacombe L, Dujardin T, Tiguert R, Tetu B, et al. The impact of solitary and multiple positive surgical margins on hard clinical end points in 1712 adjuvant treatment-naive pT2-4 N0 radical prostatectomy patients. Eur Urol. 2013;64:19–25. doi: 10.1016/j.eururo.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Störkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al. EAU guidelines on prostate cancer. Eur Assoc Urol. 2015 doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Sineshaw HM, Gray PJ, Efstathiou Ja, Jemal A. Declining Use of Radiotherapy for Adverse Features After Radical Prostatectomy: Results From the National Cancer Data Base. Eur Urol. 2015;68:1–7. doi: 10.1016/j.eururo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Maurice MJ, Zhu H, Abouassaly R. Low Utilization of Immediate and Delayed Postoperative Radiation for Prostate Cancer with Adverse Pathologic Features. J Urol. 2015;194:972–6. doi: 10.1016/j.juro.2015.03.122. [DOI] [PubMed] [Google Scholar]
- 16.Evans AJ, Henry PC, Van der Kwast TH, Tkachuk DC, Watson K, Lockwood Ga, et al. Interobserver variability between expert urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens. Am J Surg Pathol. 2008;32:1503–12. doi: 10.1097/PAS.0b013e31817fb3a0. [DOI] [PubMed] [Google Scholar]
- 17.Vesey SG, McCabe JE, Hounsome L, Fowler S. UK radical prostatectomy outcomes and surgeon case volume: based on an analysis of the British Association of Urological Surgeons Complex Operations Database. BJU Int. 2012;109:346–54. doi: 10.1111/j.1464-410X.2011.10334.x. [DOI] [PubMed] [Google Scholar]
- 18.Coelho RF, Chauhan S, Orvieto Ma, Palmer KJ, Rocco B, Patel VR. Predictive Factors for Positive Surgical Margins and Their Locations After Robot-Assisted Laparoscopic Radical Prostatectomy. Eur Urol. 2010;57:1022–9. doi: 10.1016/j.eururo.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Yossepowitch O, Briganti A, Eastham JA, Epstein J, Graefen M, Montironi R, et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol. 2014;65:303–13. doi: 10.1016/j.eururo.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 20.Ekici S, Ayhan A, Erkan I, Bakkalo!lu M, Ozen H. The role of the pathologist in the evaluation of radical prostatectomy specimens. Scand J Urol Nephrol. 2003;37:387–91. doi: 10.1080/00365590310014535. [DOI] [PubMed] [Google Scholar]
- 21.van der Kwast TH, Collette L, Van Poppel H, Van Cangh P, Vekemans K, DaPozzo L, et al. Impact of pathology review of stage and margin status of radical prostatectomy specimens (EORTC trial 22911) Virchows Arch. 2006;449:428–34. doi: 10.1007/s00428-006-0254-x. [DOI] [PubMed] [Google Scholar]
- 22.Harris Pa, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srigley JR. Key issues in handling and reporting radical prostatectomy specimens. Arch Pathol Lab Med. 2006;130:303–17. doi: 10.1043/1543-2165(2006)130. [303:KIIHAR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Magi-Galluzzi C, Evans AJ, Delahunt B, Epstein JI, Griffiths DF, van der Kwast TH, et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 3: extraprostatic extension, lymphovascular invasion and locally advanced disease. Mod Pathol. 2011;24:26–38. doi: 10.1038/modpathol.2010.158. [DOI] [PubMed] [Google Scholar]
- 25.Bott SRJ, Kirby RS. Avoidance and management of positive surgical margins before, during and after radical prostatectomy. Prostate Cancer Prostatic Dis. 2002;5:252–63. doi: 10.1038/sj.pcan.4500612. [DOI] [PubMed] [Google Scholar]
- 26.Vickers AJ, Bianco FJ, Serio AM, Eastham Ja, Schrag D, Klein Ea, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–7. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 27.Secin FP, Serio A, Bianco FJ, Karanikolas NT, Kuroiwa K, Vickers A, et al. Preoperative and Intraoperative Risk Factors for Side-Specific Positive Surgical Margins in Laparoscopic Radical Prostatectomy for Prostate Cancer. Eur Urol. 2007;51:764–71. doi: 10.1016/j.eururo.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 28.Harrell FE. Regression modeling strategies. With applications to linear models, logistic regression, and survival analysis. 2001 doi: 10.1007/978-1-4757-3462-1. [DOI] [Google Scholar]
- 29.Netto GJ, Eisenberger M, Epstein JI. Interobserver variability in histologic evaluation of radical prostatectomy between central and local pathologists: Findings of TAX 3501 multinational clinical trial. Urology. 2011;77:1155–60. doi: 10.1016/j.urology.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuroiwa K, Shiraishi T, Ogawa O, Usami M, Hirao Y, Naito S. Discrepancy Between Local and Central Pathological Review of Radical Prostatectomy Specimens. J Urol. 2010;183:952–7. doi: 10.1016/j.juro.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 31.Kuijpers CCHJ, Burger G, Al-Janabi S, Willems SM, van Diest PJ, Jiwa M. Improved quality of patient care through routine second review of histopathology specimens prior to multidisciplinary meetings. J Clin Pathol. 2016 doi: 10.1136/jclinpath-2015-203488. jclinpath-2015-203488. [DOI] [PubMed] [Google Scholar]
- 32.Bokhorst LP, Valdagni R, Rannikko A, Kakehi Y, Pickles T, Bangma CH, et al. A Decade of Active Surveillance in the PRIAS Study”: An Update and Evaluation of the Criteria Used to Recommend a Switch to Active Treatment. 2016:1–7. doi: 10.1016/j.eururo.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Anderson CB, Sternberg IA, Karen-Paz G, Kim PH, Sjoberg D, Vargas HA, et al. Age is associated with upgrading at confirmatory biopsy among men with prostate cancer treated with active surveillance. J Urol. 2015;194:1607–11. doi: 10.1016/j.juro.2015.06.084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.