Abstract
Objective
To identify whether abnormal neural activity, in the form of epileptiform discharges and rhythmic or periodic activity, which we term here ictal-interictal continuum abnormalities (IICAs), are associated with delayed cerebral ischemia (DCI).
Methods
Retrospective analysis of continuous electroencephalography (cEEG) reports and medical records from 124 patients with moderate to severe grade subarachnoid hemorrhage (SAH). We identified daily occurrence of seizures and IICAs. Using survival analysis methods, we estimated the cumulative probability of IICA onset time for patients with and without delayed cerebral ischemia (DCI).
Results
Our data suggest the presence of IICAs indeed increases the risk of developing DCI, especially when they begin several days after the onset of SAH. We found that all IICA types except generalized rhythmic delta activity occur more commonly in patients who develop DCI. In particular, IICAs that begin later in hospitalization correlate with increased risk of DCI.
Conclusions
Thus, IICAs represent a new marker for identifying early patients at increased risk for DCI. Moreover, IICAs might contribute mechanistically to DCI and therefore represent a new potential target for intervention to prevent secondary cerebral injury following SAH.
Significance
These findings imply that IICAs may be a novel marker for predicting those at higher risk for DCI development.
Keywords: subarachnoid hemorrhage, delayed cerebral ischemia, ictal-interictal continuum, epileptiform discharges, cortical spreading depolarization, continuous EEG monitoring
1. Introduction
Subarachnoid hemorrhage (SAH) patients are at risk for early and late post-hemorrhage complications, including seizures, rebleeding and delayed cerebral ischemia. Among these, delayed cerebral ischemia (DCI) is the primary source of long-term neurologic disability (Hijdra et al. 1986; Roos et al. 2000; Dupont et al. 2010; Vergouwen et al. 2011; Rowland et al. 2012; Sánchez-Porras et al. 2013). Until recently most believed that DCI is caused by vasospasm of large cerebral arteries (Rowland et al. 2012). Consequently, transcranial doppler (TCD) monitoring to detect signs of vasospasm has been the chief modality for predicting DCI (Suarez et al. 2002; Naqvi et al. 2013).
However, increasing evidence shows that vasospasm alone predicts DCI poorly, and that other factors contribute to its development (Dreier et al. 2009, 2013a; Rowland et al. 2012; Woitzik et al. 2012). The post-SAH injured brain is often in a state of metabolic crises, in which a tenuous balance of low metabolic reserve coexists with increased metabolic demand (Macdonald 2014; Chung et al. 2016). If this increased demand can be met, then further injury may be avoided; otherwise, secondary brain injury ensues. We term this framework for understanding secondary brain injury the “metabolic supply-demand mismatch hypothesis for DCI”.
Seizures and to an even greater extent cortical spreading depolarizations (CSDs) increase metabolic demand (Dreier et al. 2013b). To meet this increased energy demand, cerebral blood flow in the healthy brain typically increases. However, this neurovascular coupling can be disturbed in the injured brain. In a rat model of SAH, CSDs are capable of causing a severe ischemic (inverse neurovascular) response that spreads together with the depolarization wave in brain tissue, a phenomenon known as spreading ischemia (Hartings et al. 2011) (Dreier et al., 1998). Spreading ischemia can aggravate the degree of ischemia already present in the injured brain (Shin et al. 2006; Strong et al. 2007; Bere et al. 2014), or can start from a relatively normal level of cerebral blood flow (Dreier et al. 1998). There are a number of different patterns and hybrid phenomena between CSDs and electrographic seizures (see for example, Fig. 1 in Fabricius et al or Fig. 2 in (Dreier et al. 2012), and in animals epileptiform activity is capable of triggering CSDs (Koroleva and Bures, 1983). Recent studies have shown that the reverse phenomenon can also be observed such that CSDs can trigger epileptiform discharges, possibly via facilitating synchrony (Eickhoff et al. 2014). The foregoing discussion highlights a series of complex links from CSD and (although less well studied) epileptiform abnormalities to secondary brain injury following SAH (Dreier et al. 2009; Claassen et al. 2013).
Continuous electroencephalography (cEEG) is increasingly used to monitor for early signs of DCI. Several groups have found that pathological low frequency activity increases and normal higher frequency activity decreases hours to days before DCI (Claassen et al. 2004a, 2005; Stuart et al. 2010; Foreman and Claassen 2012; Gollwitzer et al. 2015). Seizures occur in approximately 10–15% of SAH cases, and recent studies suggest that longer or more frequent seizures lead to worse outcomes (Claassen et al. 2013; De Marchis et al. 2016).
Less is known about ictal-interictal continuum (IICAs), which include sporadic epileptiform discharges (spikes and sharp waves), periodic epileptiform discharges, and rhythmic patterns (Sivaraju and Gilmore 2016). IIICAs share some features with seizures and are common in all types of acute brain injury, including ischemic stroke, but their significance is less clear (de Curtis and Avanzini 2001; Claassen et al. 2004a; Chong and Hirsch 2005; Staley et al. 2011; Maciel and Gilmore 2016). Critically ill patients with periodic discharges tend to have poorer outcomes in some studies, though not consistently (Pohlmann-Eden et al. 1996; Claassen et al. 2006; Ong et al. 2012; Crepeau et al. 2013; Punia et al. 2015). The presence of early epileptiform abnormalities makes subsequent electrographic seizures more likely (Shafi et al. 2012; Westover et al. 2015). However, the time course of epileptiform abnormalities and its impact on secondary neuronal injury has yet to be explored.
Evidence suggests that lateralized periodic discharges (LPDs) originate from the peri-lesional zone (Schwartz et al. 1973). Indirect observations indicate that LPDs increase local cerebral blood flow and cause focal hypermetabolism on PET imaging and microdialysis recordings of lactate/pyruvate ratios (Theodore et al. 1983; Pohlmann-Eden et al. 1996; Struck et al. 2016; Vespa et al. 2016). These studies suggest that, like CSDs, abnormal neural activity is metabolically taxing.
In light of these observations, we hypothesize that IICAs are associated with increased risk of DCI in SAH patients, perhaps via increased metabolic stress (directly or indirectly, e.g. via triggering CSDs) on the injured brain. Two testable predictions of this hypothesis are that IICAs will be more common in SAH patients with DCI, and that IICAs will generally precede DCI. To test these predictions, we compared the prevalence and time-course of IICAs in patients with and without DCI.
2. Methods
2.1 Study population
We evaluated EEG reports and medical records from 124 consecutive ICU patients at a tertiary care center (Massachusetts General Hospital Neurosciences ICU) who met study inclusion criteria between September 2011 and January 2015. Inclusion criteria were: age ≥18 years; Hunt Hess 4–5 or Fisher group 3 non-traumatic SAH; continuous EEG data (cEEG) was available lasting at least 24 hours; and cEEG monitoring had not been discontinued more than 24 hours before any clinically diagnosed DCI events. We excluded patients who developed status epilepticus (convulsive or non-convulsive). EEG monitoring for ischemia detection was performed as part of a clinical protocol in all Hunt Hess 4–5 and Fisher group 3 patients as part of routine medical care. The recommended protocol was to begin recording within 48 hours of admission and continue for 10 days, although clinicians were allowed to override the recommended duration if they felt it would interfere with clinical care. In practice, the median duration (±standard deviation) of recordings was 7 (±3.1) days with a median start date of 2 (±1.8) days post-SAH. Retrospective collection and analysis of clinical data was performed under a protocol approved by the local institutional review board.
2.2 EEG recordings
cEEG data was recorded using conventional 10–20 scalp electrode placement.
2.3 EEG Report review
Interictal continuum abnormalities were classified according to standardized, validated nomenclature (Mani et al. 2012; Hirsch et al. 2013) as: seizures, sporadic epileptiform discharges (EDs), lateralized or generalized periodic discharges (LPDs and GPDs), lateralized or generalized rhythmic delta activity (LRDA and GRDA). The presence or absence of these abnormalities on each day as documented in the daily clinical EEG reports was tallied for each patient with “day of bleed” marked as day 0.
2.4 DCI classification
DCI events were diagnosed according to an international consensus definition (Vergouwen et al. 2010) as either (1) new focal neurologic deficits and/or decrease in the Glasgow Coma Scale of at least 2 points, persisting for a minimum of one hour, not explained by other causes (e.g. complications of a procedure, spike in intracranial pressure, re-rupture, hydrocephalus, seizures, systemic or metabolic abnormalities) by means of clinical assessment, imaging or laboratory data, or (2) the presence of cerebral infarction on CT or MRI imaging of the brain that was not present on any neuroimaging done within the first 48 hours following early aneurysm occlusion, and not attributable to other causes such as surgical clipping or endovascular treatment.
DCI diagnoses using this definition were determined using a multi-step process of (1) prospective daily structured research coordinator interview with the clinical team, (2) independent medical record review by three of the authors (ESR, MBW, SFZ) blinded to cEEG findings, (3) consensus adjudication by the same three authors in cases of uncertainty or disagreement.
Patients were followed for the duration of their hospitalization. If a patient experienced a DCI event at any point during their hospitalization, this was documented, even after the cessation of EEG recording. Given our focus on the temporal relationship between IICAs and DCI, patients with DCI events occurring >24 hours after discontinuation of cEEG monitoring were excluded from analysis (see Study Population above).
2.4 Data analysis
For data analysis, we used the mstate package45 written in R (The R Foundation) and Matlab, including the Matlab Statistics Toolbox (MathWorks; Natick, MA). To estimate the proportion of the population with IICAs on a given day relative to the time of SAH onset, we used survival analysis. We estimated cumulative distribution functions for the onset of each IICA type using the Kaplan-Meier method. A key advantage of Kaplan-Meier and survival analysis methods is the ability to handle right-censored data, which in our data arose from the variable time of discontinuation of cEEG monitoring in cases where the final outcome of interest (e.g., an IICA or DCI event) had not occurred by the time monitoring was discontinued. We recorded the times from SAH to beginning of EEG monitoring and the duration monitoring for patients in DCI and non-DCI groups, and tested for significant differences using the two-sample Kolmogrov-Smirnov test.
To investigate the relationship between IICAs, seizures and DCI, we separated the data into those that did and did not develop DCI, and calculated the cumulative distribution function (CDF) for the two groups separately. We evaluated statistical differences in the CDFs using bootstrapping: We calculated 1000 CDF curves for DCI and non-DCI groups using bootstrap sampling, subtracted the non-DCI curve from the DCI curve to obtain 1000 difference curves, and then calculated the distribution of these difference curves, which we term the coverage interval. This procedure yields coverage intervals for each day after SAH. To interpret these coverage intervals, suppose that most bootstrap CDF difference curves are greater than zero a given number of days following SAH. This would provide evidence that the cumulative incidence of epileptiform discharges is greater in patients with DCI at that timepoint. For the difference between CDFs for the DCI and non-DCI groups to be declared statistically significant, we required 95% of the coverage interval for the difference curves to be greater than zero. Alternatively, when this condition is satisfied, we say that the curves are statistically “well separated.”
In cases where the CDFs for DCI and non-DCI groups do become statistically separated, we observed that the difference is maximal at the end of the observation period, day 20. We therefore compared curves at day 20 to determine whether CDFs for DCI and non-DCI groups ever became statistically separated. In addition, in instances where the CDFs were significantly separated at day 20, we defined the “time of separation” for the two CDFs as first day on which the CDFs became statistically separated.
When evaluating the time relationship between IICA onset and DCI occurrence, we used a one-tail, binomial test to assess whether a significant IICA event preceded the DCI event in a statistically significant number of cases.
3. Results
3.1 Prevalence of IICAs
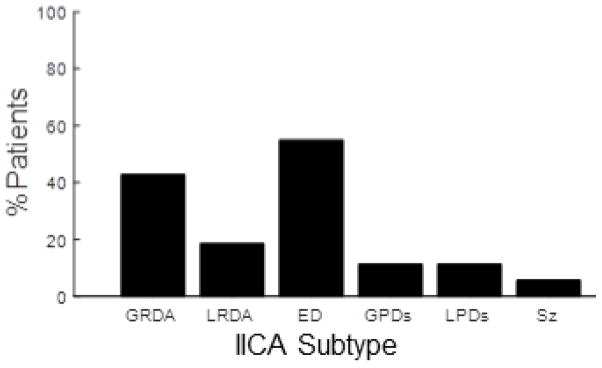
Figure 1 shows the number of patients with any occurrence of seizures and each IICA pattern during EEG monitoring. Over all recording days (mean ± standard deviation time 6.9±3.1 days), 5.6% (n=7) of patients have at least one electrographically documented seizure (one patient has two seizures). Sporadic epileptiform discharges appear in more than half of cases (54.8%). Lateralized and generalized periodic discharges both occur in 11% of cases. Generalized rhythmic delta activity, often with frontal predominance, is seen in 43.5%. Lateralized rhythmic delta activity is less common, occurring in 18.5% of cases. Polymorphic generalized slowing is seen in 92% of patients, and lateralized, polymorphic slowing is seen in 61%.
Figure 1.
Percent of patients with IICAs. This bar graph shows the total percentage of patients that showed an IICA at any point during EEG recording. Abbreviations are as follows: GRDA (generalized rhythmic delta activity), LRDA (lateralized rhythmic delta activity), ED (sporadic epileptiform discharges), GPDs (generalized periodic discharges), LPDs (lateralized periodic discharges).
3.2 Proportion of IICAs per EEG Day
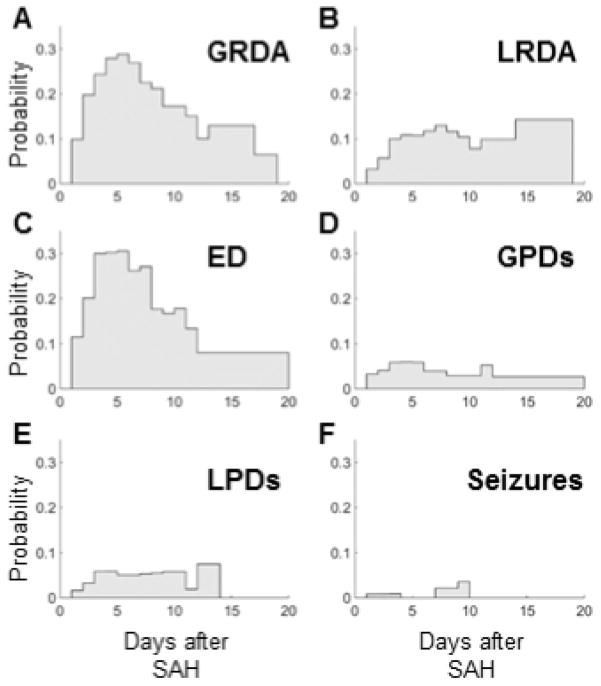
We next analyze the time course of the appearance and resolution of each type of IICA event within the population as a whole (Figure 2A–F). GRDA has a peak incidence on day 5 with a reduction of 50% by day 11 (Figure 2A). LRDA has bimodal peaks at day 7 and later at 14–19 (Figure 2B). EDs peak at day 7 and mostly dissipate by day 12 (Figure 2C). GPDs peak on day 5, but also exhibit a small uptick at day 11 (Figure 2D). LPDs are more broadly temporally distributed with slightly higher incidences on days 4, 9 and 12 (Figure 2E). Seizures occur both early (day 1–3) and late (day 7–9), similar to previous observations (Figure 2F) (Lin et al. 2003).
Figure 2.
Percent of IICAs per EEG day. The total number of recorded days with IICAs was tallied and the percentage of each IICA that occurred on a given EEG recording day was calculated. Abbreviations used in legend are the same as those described in Figure 1.
3.3 Time-course of the appearance IICAs
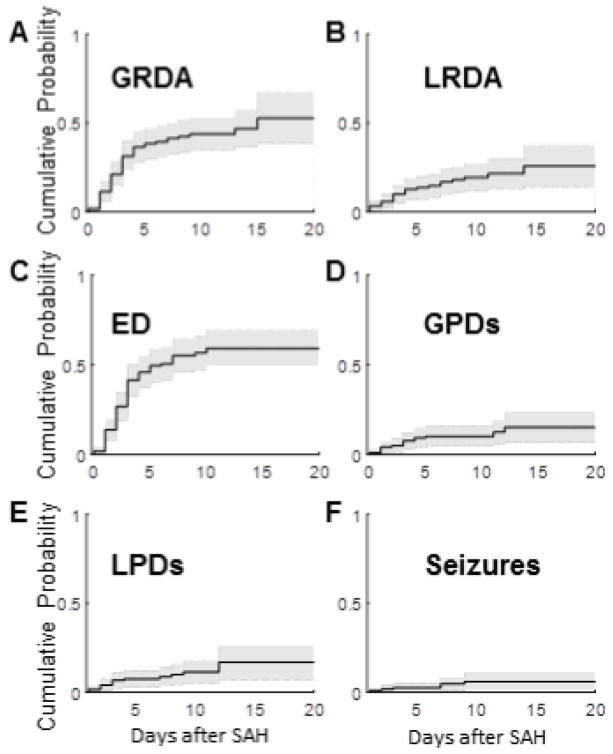
Next, we look at when the initial appearance of each IICA type occurs for each patient. The cumulative distribution curves exhibit these findings (Figure 3). Eighty-three percent of GRDA patterns occur by post-SAH day 4 (Figure 3A). LRDA accumulates at a relatively constant rate, slows around day 8, and stops accumulating at day 14 (Figure 3B). Most epileptiform discharges appear within the first 5 days after their SAH, and the emergence of new epileptiform discharges halts by day 10 (Figure 3C). The majority of patients who develop GPDs exhibit them early on in the course (≤day 5), but a small percentage of patients exhibit late GPDs at post-SAH day 11 and 12 (Figure 3D). Lateralized periodic discharges appear mostly prior to day 4, though a small proportion begin at day 7–9 and again at day 12 (Figure 3E). Seizures occur in a bimodal pattern, in days 1–3 and again in days 7–9 (Figure 3F).
Figure 3.
Cumulative distributions for first EEG day of each IICA type. These plots show the cumulative distributions for the first day on which an IICA appeared for each patient. A. Generalized rhythmic delta activity (GRDA), B. Lateralized rhythmic delta activity (LRDA), C. Epileptiform Discharges (ED), D. Generalized periodic discharges (GPDs), E. Lateralized periodic discharges (LPDs), F. Seizures (Sz).
3.4 Relationship of IICA appearance and development of DCI event
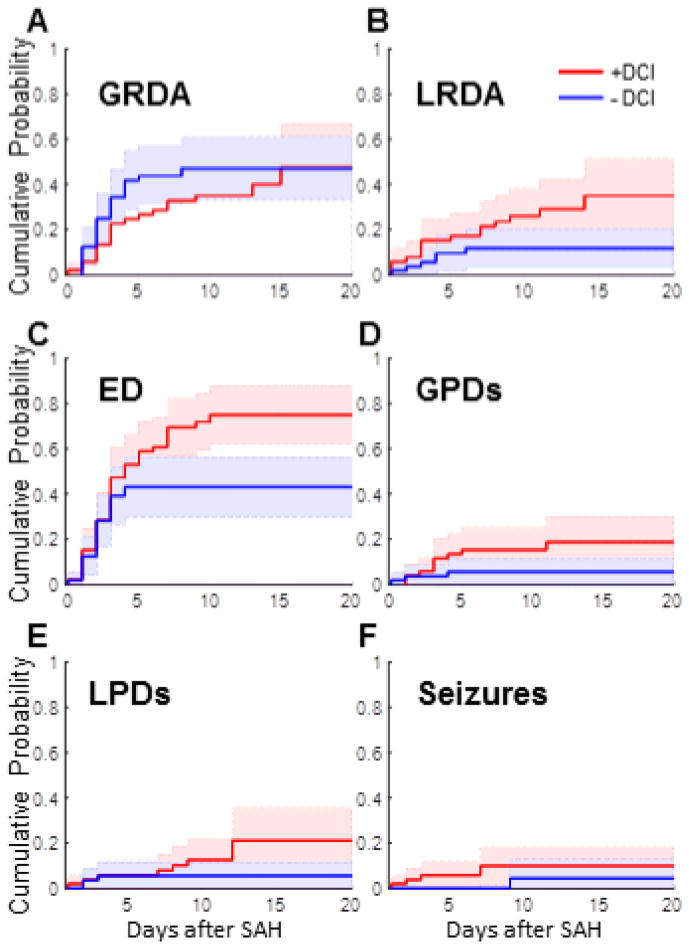
We next investigate whether patients with DCI have higher rates of IICAs. In our study population, 42.7% patients had DCI, similar to previously reported rates (Karamchandani et al. 2014). First, there is no significant difference between DCI and non-DCI groups in the time after SAH before EEG monitoring began (1.7±1.1 vs 1.7±1.2 days), or in the total duration of EEG monitoring (6.9±2.9 vs. 7.0±3.0) as measured by the two-sample KS test (p =0.001). We determine separate cumulative distributions for each IICA type for patients with and without DCI events. LRDA is statistically more common in patients with DCI (Figure 4B). Separation between the curves for those with and without DCI begins early, and the difference passes our threshold of statistical significance on day 9.
Figure 4.
Presence of IICAs on patients with/without ischemic events The cumulative distributions here are also for the first day on which an IICA appeared for each patient, but separated into those who developed DCI (red) and those who did not (blue). Abbreviations are the same as those described in Figure 3. As discussed in the text, all IICAs except seizures and GRDA were significantly higher in the subset who later developed DCI events.
Sporadic epileptiform discharges are also statistically more common in patients with DCI (Figure 4C). This statistical separation becomes significant after post-SAH day 6.
Both lateralized and generalized periodic discharges are more common in patients with DCI (Figure 4D–E). As with epileptiform discharges, late-emerging periodic discharges are more common in patients with DCI, the separation becoming statistically significant after day 12 for lateralized and after day 5 for generalized discharges.
All but one of the patients with seizures also had DCI, as is evident in the cumulative distribution plot in Figure 4F (note that there is only one step in the no-DCI curve, vs five steps in the DCI group curve). The one patient without DCI had their first seizure late in the course, on post-SAH day 9. However, given the small number of patients with seizures, the differences in cumulative distribution curves for seizures between patients with and without DCI are not statistically significant. Additionally, unlike the other IICAs, the presence of GRDA is similar in both DCI and non-DCI groups (Figure 4A).
Overall, the presence of most IICAs is substantially higher in those who develop ischemic events than in those who do not. However, these results do not tell us whether IICAs generally precede and can therefore predict DCI. Therefore, we next investigate the relationship between the timing of IICAs and the time of DCI.
3.5 Relative appearance of first IICA compared to DCI event
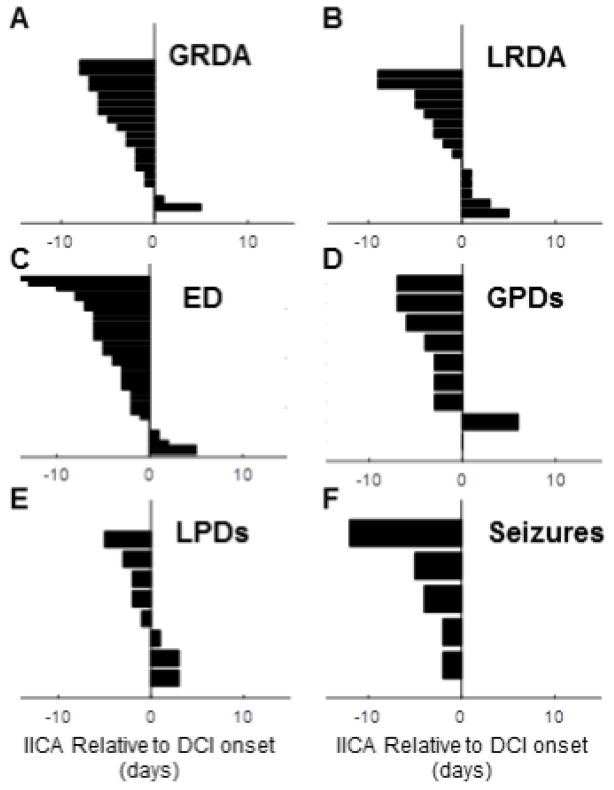
For patients with DCI events, we now compare the timing relationship between the onset of the IICA and the time at which DCI was diagnosed. For each IICA type we plot the appearance of the IICAs compared to the day of the DCI event (Figure 5A–F). If more than one DCI occurred, we use only the first event.
Figure 5.
Relationship of DCI event to first appearance IICA subtype in each patient. These plots show the relative onset time of the DCI event (time 0) and the onset of the IICA for each patient who had both a DCI and each respective IICA.
For the majority of cases, each IICA type occurs prior to DCI. However, only sporadic EDs, GPDs and seizures reach statistical significance based upon the one-tailed binomial test (p<0.05). For patients with seizures and DCI, the first seizure occurred before the DCI diagnosis in all cases (Figure 5F). For sporadic epileptiform discharges, there is a broad distribution of times from start of ED to the day of DCI (Figure 5C). Though the sample size is small, the onset of LPDs is generally closer to the time of DCI events compared to other IICA events. While rates of GRDA do not differ between patients with and without DCI, if a patient does have DCI and exhibits GRDA, the GRDA is also likely to occur prior to DCI (Figure 5A, p<0.05).
Taken together, the results shown in Figures 4 and 5 strongly suggest that, at least in the cases of sporadic EDs, GPDs, and seizures, IICAs are not only more common in patients who have DCI, but IICAs also generally precede and thus predict DCI. For example, consider the CDF in Figure 4C for sporadic EDs. If a patient develops EDs before day 4 and has not suffered a DCI event, this does not help us predict whether the patient is more likely to develop DCI, because up until day 4 the chances of having EDs for DCI and non-DCI groups are statistically indistinguishable. However, the situation is different if the EDs appear on day 5 or later. On the one hand, we see in Figure 5C that EDs tend to occur before DCI. At the same time, we see in Figure 4C that EDs have stopped accruing in the non-DCI group, whereas they continue accumulating in the DCI group up through day 10. We conclude that when EDs appear for the first time in a patient after a significant delay, beyond day 5, they indicate that the patient belongs to the group at higher risk for DCI. Similar arguments apply to patients with GPDs (Figures 4D, 5D) and seizures (Figures 4F, 5F). Thus, patients who develop EDs, GPDs, or seizures for the first time later in their course are more likely to be develop neurological deterioration and be diagnosed with delayed cerebral ischemia.
4. Discussion
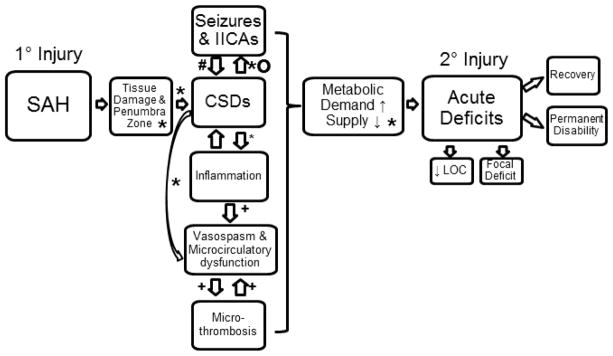
Our results provide new information about the relationship of IICAs to delayed cerebral ischemia in patients with SAH. We find that a large percentage of SAH patients exhibit at least one form of IICA following SAH onset. Each type of IICA event has a characteristic time course over which it is most likely to appear and then wane. Most importantly, we find that DCI is commonly associated with, and generally follows rather than precedes, an increase in any type of IICAs except GRDA. These findings suggest that the development of late-onset IICAs, particularly sporadic epileptiform discharges, generalized periodic discharges, or seizures might provide help identifying those at risk for delayed neurologic deterioration. Our data, combined with broader clinical experience suggests that same association might also prove statistically significant in a larger sample for other IICA patterns, namely lateralized periodic discharges and lateralized rhythmic delta activity. Taken in the context of prior literature and the current prevailing theory of DCI as the result of multiple causes leading to metabolic crisis, our findings establish an association between IICAs and DCI generation. Though not directly addressed in the present study, in the context of the metabolic supply-demand mismatch hypothesis for DCI, we can speculate that IICAs might contribute to the pathogenesis of DCI, e.g. by triggering CSDs, or to a lesser extent directly. This interpretation is illustrated in Figure 6.
Figure 6.
Flow diagram for possible contributing factors to the development of DCI. Legend refers to references as follows: “o” Eikoff et al. 2014, “#” Hartings et al. 2011, “*” Chung et al. 2016, “+” (Carr et al. 2013).
We have seen that there is a high prevalence of certain IICA subtypes in acute SAH. Sporadic epileptiform discharges are the most prevalent type of IICA, at a rate of 54.8%. Few prior studies quantify the prevalence of sporadic epileptiform discharges alone, as they are usually grouped with other epileptiform discharges including periodic discharges. Given that caveat, the prevalence we observed appears higher than the range previously reported in general patients undergoing EEG monitoring in the neurocritical care setting, 28–37% (Steinbaugh et al. 2012; Westover et al. 2015). Possible explanations for this intriguing difference are that perhaps sporadic epileptiform discharges are more common in SAH than in other conditions, or that the longer EEG period employed in monitoring for DCI provides a greater opportunity for detection, or both (our institutional protocol recommends 10 days; actual mean ± std dev time was 6.9±3.1 days, whereas in general EEG monitoring is performed for 24–48 hours when used solely for detecting electrographic seizures (Claassen et al. 2004b; Westover et al. 2015). Overall, the largest increases in cumulative probability for the first appearance of EDs occurs in the first 3 days after SAH. The first appearance of EDs in our cohort plateaus after day 10, indicating that those without epileptiform discharges by this time are unlikely to develop them later. The percentage of epileptiform discharges present in the population after day 10 also declines substantially (Figure 2), indicating that in most patients who initially developed them they subsequently resolve near the end of the high-risk period for DCI. Additionally, these EDs are more common in patients with DCI compared to those without. Our results suggest that late onset (after post-bleed day 6) EDs may indicate increased DCI risk.
Both lateralized and generalized periodic discharges appear in about 11% of our study population. Previously reported prevalence estimates for periodic discharges range widely, from <1%–37% (García-Morales et al. 2002; Claassen et al. 2006, 2007; Kramer et al. 2012; Ong et al. 2012; Ng et al. 2014). The wide range likely reflects the different populations tested and different inclusion criteria. Our results also suggest that differences in the timing of observation relative to time elapsed since onset of SAH in various studies could lead to variation in observed rates of epileptiform abnormalities.
The presence of periodic discharges has been widely reported to correlate with increased risk for seizures (Pohlmann-Eden et al. 1996; Claassen et al. 2006; Ong et al. 2012; Punia et al. 2015). However, their relationship to DCI has not been previously investigated. Here, we find that both LPDs and GPDs are more common in patients with DCI compared to those without DCI. As with sporadic epileptiform discharges, LPDs and GPDs that are late onset after post-bleed day 12 and 5, respectively, correlate significantly with DCI. Interestingly, while LPDs have the shortest onset prior to the occurrence of DCI compared to all IICAs, they are also much more evenly distributed before and after DCI events compared to the other IICAs. GPDs almost exclusively precede DCI but their onset may be as far as seven days prior to DCI.
The presence of LRDA is well known to signify an increased risk for electrographic seizures (Brigo 2011; Gaspard et al. 2013), but has not been studied in relation to DCI. We found that LRDA is present in 18.5% of patients with SAH hemorrhage. We report a higher percentage of LRDA than documented in the above study by Gaspard et al. (2013) (4.7%), but their cohort included patients with multiple diagnoses rather than SAH alone. Additionally, the cumulative probability for LRDA in patients with DCI is three times that of patients without DCI. These results suggest that LRDA may also act as a marker for DCI risk, especially when it occurs late. However, the relationship of LRDA to DCI onset is variable, with 35% of patients with DCI and LRDA having LRDA onset after DCI. Thus, while the presence of LRDA may suggest an increased risk for DCI, it is not invariably antecedent.
Rates of GRDA are also highly variable in the literature ranging from 6–51% (Crepeau et al. 2013). In our SAH cohort GRDA appear in 43.5% of patients. Interestingly, unlike other IICAs, GRDA is not associated with increased DCI risk. This finding recalls prior studies showing that, unlike other IICA subtypes, GRDA appears not to confer increased risk for seizures (Accolla et al. 2011). Our results further reinforce the notion that GRDA is a nonspecific abnormality seen in a broad range of neurologic dysfunction without being itself predictive of future morbidities such as DCI or seizures.
While this study did not focus on seizures, we did measure seizure rates in our population. There is a broad range of seizure rates reported in SAH populations, from 7–19% (Claassen et al. 2005; O’Connor et al. 2014; Kondziella et al. 2015; De Marchis et al. 2016). Our rate of 5.6% is slightly lower than previous reports, though in concordance with previously recorded seizure rates using subdural electrodes (Dreier et al. 2012). This is likely due to our institutional guideline that makes EEG monitoring routine in SAH without the need for increased suspicion for subclinical seizures as an indication to monitor. Delayed ischemic events are present in all but one patient with seizures. However, this difference did not reach statistical significance, given the low prevalence of seizures in our cohort.
Taken together our data suggest that IICAs are correlated with DCI development and may be useful in identifying increased risk of delayed cerebral ischemia. The trends shown also suggest that it is late onset IICAs that are particularly telling. These observations lead us to hypothesize a possible causal link between epileptiform abnormalities and DCI. While vasospasm has long been thought to be the primary causal mechanism for DCI, multiple studies have shown that vasospasm is neither necessary nor sufficient for DCI (Strong and Macdonald 2012; Woitzik et al. 2012; Kramer et al. 2016). More recent evidence suggests cortical spreading depolarization (CSD) is very common in aneurysmal SAH and contributes ischemia-mediated secondary brain injury (Woitzik et al. 2012; Dreier et al. 2013a; Sánchez-Porras et al. 2013; Chung et al. 2016; Kramer et al. 2016). Multiple mechanisms by which this occurs have been thoroughly investigated, from animals (Leao 1947) to humans (Dohmen et al. 2008) demonstrating the clear translation of CSD-induced ischemia-mediated secondary brain injury. In the setting of SAH, normal vasodilatory responses are impaired. Abnormal vasoconstrictive responses and increased blood-brain-barrier permeability can be observed in the presence of CSDs (von Bornstädt et al. 2015; Kramer et al. 2016). Animal studies show that inducing CSD in models of aneurysmal SAH leads to increased infarct size (Hinzman et al. 2015; Hamming et al. 2016). Other studies suggest that CSDs cause increased oxygen use within activated cortex either in or near the ischemic zone, worsening supply-demand mismatch and leading to progression of ischemia (Bosche et al. 2010; Dreier et al. 2013a; von Bornstädt et al. 2015; Chung et al. 2016).
We hypothesize that IICAs, too, cause a supply demand mismatch, though the mechanism by which this might occur has yet to be elucidated. Multiple animal studies show that CSDs increase metabolic demand (O’Connor et al. 1972; Hopwood et al. 2005; Suh et al. 2005; Ivanov et al. 2015). Metabolic imaging and microdialysis studies suggest a similar increased demand in humans, though the literature is limited (Theodore et al. 1983; Pohlmann-Eden et al. 1996; Parkin et al. 2005). While seizures and CSDs are biologically distinct phenomena, the co-occurrence of these phenomena is well documented (Van Harreveld and Stamm 1953; Fabricius et al. 2008). A variety of interlinked patterns between CSDs and seizures have been observed and show that CSDs occur in a majority of patients with electrographic seizures (Fabricius et al. 2008). Few studies have investigated the relationship between CSD and periodic discharges (Avoli et al. 1991; Hartings et al. 2011; Broberg et al. 2014; Eickhoff et al. 2014). Hartings et al. (2011) showed that prolonged direct current shifts, a model of CSD, were longer when preceded by periodic epileptiform discharges, which concurs with previously documented spike-triggered spreading depolarization waves in rats (Koroleva and Bures 1983). It is possible that IICAs trigger CSDs, which in turn worsen metabolic demand and lead to DCI (Figure 6), but this has yet to be proven. A reverse phenomena has also been described, in which CSDs themselves can trigger epileptiform discharges (Eickhoff et al. 2014), similar to the phenomenon previously observed in animal models (Van Harreveld and Stamm 1953). Thus another possibility is that IICAs may represent a surface EEG surrogate downstream from CSDs, which themselves are the primary mediators of increased risk for DCI.
Just as the CSD literature has benefited from the translational work of animal to human mechanistic exploration, developing animal models of IICAs in SAH may help us to more directly investigate the mechanism(s) by which IICAs may contribute to DCI. The relationship between EDs, CSDs and delayed ischemia in SAH is ripe for further investigation.
An important limitation of the present study is that, while it describes each IICA pattern as present or absent on each day, we did not directly quantify each IICA waveform. Further investigation using more detailed IICA quantification, including analysis of frequency, morphology and spatial extent, may be important in determining how much metabolic demand each IICA type places on injured tissue, thereby identifying the features placing susceptible tissue most at risk. Indeed, recent work by Claassen et al (2013) has shown that increased discharge frequency is associated with a trend in delayed regional cerebral blood flow. Detailed quantitative studies like this are technically challenging, but are needed to gain deeper mechanistic insights.
A second limitation which should be addressed in future studies is how the various IICA patterns the predict DCI might relate to long-term outcomes. This is a difficult issue to study, as DCI is a potentially modifiable outcome for which clinical interventions are routinely attempted (e.g. induced hypertension, intravascular rescue procedures). Nevertheless, DeMarchis et al. (2016) recently showed that seizure burden correlated with outcomes in SAH; it will be important to study whether the same holds true for IICAs.
A third limitation is that while exclusion of alternative explanations for neurological declines is required as a part of the diagnosis of DCI, clinical confounders such as sepsis, cardiac stress and drug exposure were not accounted for in detail in the present study. In particular, their possible effects on the EEG were not analyzed. These issues become of eminent importance as we hope to develop risk algorithms in the future using IICA burden in the presence of these variables.
A fourth limitation is that we did not correlate DCI with the presence of ischemic stroke on imaging. This was in part due to the variability in timing and modality by which patients in our cohort proceeded to imaging after DCI was clinically identified. However, it would be helpful in the future to better correlate the development of IICAs with changes detectable using serial imaging protocols. It may be that certain IICA characteristics are correlated with clinical decline alone whereas others may be associated with combined clinical-radiographic decline.
Another limitation of our present study is variability in timing and duration of cEEG monitoring. Because data were generated under a clinical guideline rather than a strictly enforced protocol, uniform initiation times and duration of cEEG monitoring was not possible. Our estimates of the temporal course of risk for epileptiform abnormalities could be made more precise with more complete EEG coverage of the entire DCI risk period. Nevertheless, we use principled statistical methodology from survival analysis to account for censored data to address these limitations.
5. Conclusions
Here, we demonstrate that IICAs are associated with increased DCI risk, and speculate that this might occur via increasing metabolic demand, either directly or by triggering CSDs. Our findings show that the presence of IICAs, particularly when they emerge late in the acute period following SAH, portend development of delayed cerebral ischemic ischemia. Given this finding, prolonged cEEG monitoring is warranted in the SAH population, and particular vigilance should be made towards those patients with late onset IICAs. Our findings also raise the possibility for future investigation that anticonvulsant drugs or other interventions to reduce epileptiform abnormalities may ultimately play a role not only in preventing seizures but also in preventing DCI.
Highlights.
Ictal-interictal continuum abnormalities (IICAs) are common after subarachnoid hemorrhage (SAH).
IICAs are associated with higher risk for delayed cerebral ischemia (DCI), especially if they emerge late in the acute period after SAH.
Quantification of IICA features may assist in the development of an algorithm to predict DCI risk.
Acknowledgments
This work was supported by the NIH-NINDS (1K23NS090900, MBW), the Rappaport Foundation (MBW), the Andrew David Heitman Neuroendovascular Research Fund (MBW, ESR), and the NIH-NINDS (R25 R25NS065743, 2015; JAK).
Footnotes
Conflict of Interest: None of the authors have potential conflicts of interest to be disclosed.
References
- Accolla EA, Kaplan PW, Maeder-Ingvar M, Jukopila S, Rossetti AO. Clinical correlates of frontal intermittent rhythmic delta activity (FIRDA) Clin Neurophysiol. 2011;122:27–31. doi: 10.1016/j.clinph.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Avoli M, Drapeau C, Louvel J, Pumain R, Olivier A, Villemure JG. Epileptiform activity induced by low extracellular magnesium in the human cortex maintained in vitro. Ann Neurol. 1991;30:589–96. doi: 10.1002/ana.410300412. [DOI] [PubMed] [Google Scholar]
- Bere Z, Obrenovitch TP, Kozák G, Bari F, Farkas E. Imaging reveals the focal area of spreading depolarizations and a variety of hemodynamic responses in a rat microembolic stroke model. J Cereb Blood Flow Metab. 2014;34:1695–705. doi: 10.1038/jcbfm.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bornstädt D, Houben T, Seidel JL, Zheng Y, Dilekoz E, Qin T, et al. Supply-Demand Mismatch Transients in Susceptible Peri-infarct Hot Zones Explain the Origins of Spreading Injury Depolarizations. Neuron. 2015;85:1117–31. doi: 10.1016/j.neuron.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosche B, Graf R, Ernestus R-I, Dohmen C, Reithmeier T, Brinker G, et al. Recurrent spreading depolarizations after subarachnoid hemorrhage decreases oxygen availability in human cerebral cortex. Ann Neurol. 2010;67:607–17. doi: 10.1002/ana.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigo F. Intermittent rhythmic delta activity patterns. Epilepsy Behav. 2011;20:254–6. doi: 10.1016/j.yebeh.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Broberg M, Pope KJ, Olsson T, Shuttleworth CW, Willoughby JO. Spreading depression: evidence of five electroencephalogram phases. J Neurosci Res. 2014;92:1384–94. doi: 10.1002/jnr.23412. [DOI] [PubMed] [Google Scholar]
- Carr KR, Zuckerman SL, Mocco J. Inflammation, cerebral vasospasm, and evolving theories of delayed cerebral ischemia. Neurol Res Int. 2013;2013:506584. doi: 10.1155/2013/506584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong DJ, Hirsch LJ. Which EEG Patterns Warrant Treatment in the Critically Ill? Reviewing the Evidence for Treatment of Periodic Epileptiform Discharges and Related Patterns. J Clin Neurophysiol. 2005;22:79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- Chung DY, Oka F, Ayata C. Spreading Depolarizations: A Therapeutic Target Against Delayed Cerebral Ischemia After Subarachnoid Hemorrhage. J Clin Neurophysiol. 2016;33:196–202. doi: 10.1097/WNP.0000000000000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen J, Hirsch LJ, Frontera JA, Fernandez A, Schmidt M, Kapinos G, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103–12. doi: 10.1385/NCC:4:2:103. [DOI] [PubMed] [Google Scholar]
- Claassen J, Hirsch LJ, Kreiter KT, Du EY, Sander Connolly E, Emerson RG, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004a;115:2699–710. doi: 10.1016/j.clinph.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Claassen J, Jetté N, Chum F, Green R, Schmidt M, Choi H, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–65. doi: 10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- Claassen J, Mayer Sa, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004b;62:1743–8. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- Claassen J, Mayer SA, Hirsch LJ. Continuous EEG monitoring in patients with subarachnoid hemorrhage. J Clin Neurophysiol. 2005;22:92–8. doi: 10.1097/01.wnp.0000145006.02048.3a. [DOI] [PubMed] [Google Scholar]
- Claassen J, Perotte A, Albers D, Kleinberg S, Schmidt JM, Tu B, et al. Nonconvulsive seizures after subarachnoid hemorrhage: Multimodal detection and outcomes. Ann Neurol. 2013;74:53–64. doi: 10.1002/ana.23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepeau AZ, Kerrigan JF, Gerber P, Parikh G, Jahnke H, Nakaji P, et al. Rhythmical and periodic EEG patterns do not predict short-term outcome in critically ill patients with subarachnoid hemorrhage. J Clin Neurophysiol. 2013;30:247–54. doi: 10.1097/WNP.0b013e3182933d2f. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–67. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus R-I, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720–8. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- Dreier J, Drenckhahn C, Woitzik J, Major S, Offenhauser N, Weber-Carstens S, et al. Spreading ischemia after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2013a;115:125–9. doi: 10.1007/978-3-7091-1192-5_26. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Isele T, Reiffurth C, Offenhauser N, Kirov SA, Dahlem MA, et al. Is spreading depolarization characterized by an abrupt, massive release of gibbs free energy from the human brain cortex? Neuroscientist. 2013b;19:25–42. doi: 10.1177/1073858412453340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Körner K, Ebert N, Görner A, Rubin I, Back T, et al. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab. 1998;18:978–90. doi: 10.1097/00004647-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–81. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Major S, Pannek HW, Woitzik J, Scheel M, Wiesenthal D, et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain. 2012;135:259–75. doi: 10.1093/brain/awr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont Sa, Wijdicks EFM, Lanzino G, Rabinstein Aa. Aneurysmal subarachnoid hemorrhage: An overview for the practicing neurologist. Semin Neurol. 2010;30:545–54. doi: 10.1055/s-0030-1268862. [DOI] [PubMed] [Google Scholar]
- Eickhoff M, Kovac S, Shahabi P, Khaleghi Ghadiri M, Dreier JP, Stummer W, et al. Spreading depression triggers ictaform activity in partially disinhibited neuronal tissues. Exp Neurol. 2014;253:1–15. doi: 10.1016/j.expneurol.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Fuhr S, Willumsen L, Dreier JP, Bhatia R, Boutelle MG, et al. Association of seizures with cortical spreading depression and peri-infarct depolarisations in the acutely injured human brain. Clin Neurophysiol. 2008;119:1973–84. doi: 10.1016/j.clinph.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman B, Claassen J. Quantitative EEG for the detection of brain ischemia. Crit Care. 2012;16:216. doi: 10.1186/cc11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Morales I, García MT, Galán-Dávila L, Gómez-Escalonilla C, Saiz-Díaz R, Martínez-Salio A, et al. Periodic lateralized epileptiform discharges: etiology, clinical aspects, seizures, and evolution in 130 patients. J Clin Neurophysiol. 2002;19:172–7. doi: 10.1097/00004691-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Gaspard N, Manganas L, Rampal N, Petroff OaC, Hirsch LJ. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol. 2013;70:1288–95. doi: 10.1001/jamaneurol.2013.3475. [DOI] [PubMed] [Google Scholar]
- Gollwitzer S, Groemer T, Rampp S, Hagge M, Olmes D, Huttner HB, et al. Early prediction of delayed cerebral ischemia in subarachnoid hemorrhage based on quantitative EEG: A prospective study in adults. Clin Neurophysiol. 2015;126:1514–23. doi: 10.1016/j.clinph.2014.10.215. [DOI] [PubMed] [Google Scholar]
- Hamming AM, Wermer MJH, Umesh Rudrapatna S, Lanier C, van Os HJ, van den Bergh WM, et al. Spreading depolarizations increase delayed brain injury in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2016;36:1224–31. doi: 10.1177/0271678X15619189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harreveld A, Stamm J. Spreading cortical convulsions and depressions. J Neurophysiol. 1953;16:352–66. doi: 10.1152/jn.1953.16.4.352. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Watanabe T, Bullock MR, Okonkwo DO, Fabricius M, Woitzik J, et al. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain. 2011;134:1529–40. doi: 10.1093/brain/awr048. [DOI] [PubMed] [Google Scholar]
- Hijdra A, Van Gijn J, Stefanko S, Van Dongen KJ, Vermeulen M, Van Crevel H. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: Clinicoanatomic correlations DECREASE IN LEVEL. 1986:329–33. doi: 10.1212/wnl.36.3.329. [DOI] [PubMed] [Google Scholar]
- Hinzman JM, DiNapoli VA, Mahoney EJ, Gerhardt GA, Hartings JA. Spreading depolarizations mediate excitotoxicity in the development of acute cortical lesions. Exp Neurol. 2015;267:243–53. doi: 10.1016/j.expneurol.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- Hopwood SE, Parkin MC, Bezzina EL, Boutelle MG, Strong AJ. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J Cereb Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Bernard C, Turner DA. Metabolic responses differentiate between interictal, ictal and persistent epileptiform activity in intact, immature hippocampus in vitro. Neurobiol Dis. 2015;75:1–14. doi: 10.1016/j.nbd.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamchandani RR, Fletcher JJ, Pandey AS, Rajajee V. Incidence of delayed seizures, delayed cerebral ischemia and poor outcome with the use of levetiracetam versus phenytoin after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2014;21:1507–13. doi: 10.1016/j.jocn.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Friberg CK, Wellwood I, Reiffurth C, Fabricius M, Dreier JP. Continuous EEG Monitoring in Aneurysmal Subarachnoid Hemorrhage: A Systematic Review. Neurocrit Care. 2015;22:450–61. doi: 10.1007/s12028-014-0068-7. [DOI] [PubMed] [Google Scholar]
- Koroleva VI, Bures J. Cortical penicillin focus as a generator of repetitive spike-triggered waves of spreading depression in rats. Exp brain Res. 1983;51:291–7. doi: 10.1007/BF00237205. [DOI] [PubMed] [Google Scholar]
- Kramer AH, Jette N, Pillay N, Federico P, Zygun DA. Epileptiform activity in neurocritical care patients. Can J Neurol Sci. 2012;39:328–37. doi: 10.1017/s0317167100013469. [DOI] [PubMed] [Google Scholar]
- Kramer DR, Fujii T, Ohiorhenuan I, Liu CY. Cortical spreading depolarization: Pathophysiology, implications, and future directions. J Clin Neurosci. 2016;24:22–7. doi: 10.1016/j.jocn.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Leao AAP. Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiol. 1947;10:409–14. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Lin C-L, Dumont AS, Lieu A-S, Yen C-P, Hwang S-L, Kwan A-L, et al. Characterization of perioperative seizures and epilepsy following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;99:978–85. doi: 10.3171/jns.2003.99.6.0978. [DOI] [PubMed] [Google Scholar]
- Macdonald RL. Delayed neurological deterioration after subarachnoid hemorrhage. Nat Rev Neurol. 2014;10:44–58. doi: 10.1038/nrneurol.2013.246. [DOI] [PubMed] [Google Scholar]
- Maciel CB, Gilmore EJ. Seizures and Epileptiform Patterns in SAH and Their Relation to Outcomes. J Clin Neurophysiol. 2016;33:183–95. doi: 10.1097/WNP.0000000000000268. [DOI] [PubMed] [Google Scholar]
- Mani R, Arif H, Hirsch LJ, Gerard EE, LaRoche SM. Interrater reliability of ICU EEG research terminology. J Clin Neurophysiol. 2012;29:203–12. doi: 10.1097/WNP.0b013e3182570f83. [DOI] [PubMed] [Google Scholar]
- De Marchis GM, Pugin D, Meyers E, Velasquez A, Suwatcharangkoon S, Park S, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86:253–60. doi: 10.1212/WNL.0000000000002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi J, Yap KH, Ahmad G, Ghosh J. Transcranial Doppler ultrasound: a review of the physical principles and major applications in critical care. Int J Vasc Med. 2013;2013:629378. doi: 10.1155/2013/629378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KWP, Wong HC, Rathakrishnan R. Should we treat patients with impaired consciousness and periodic patterns on EEG? Seizure. 2014;23:622–8. doi: 10.1016/j.seizure.2014.04.015. [DOI] [PubMed] [Google Scholar]
- O’Connor KL, Westover MB, Phillips MT, Iftimia Na, Buckley Da, Ogilvy CS, et al. High Risk for Seizures Following Subarachnoid Hemorrhage Regardless of Referral Bias. Neurocrit Care. 2014;21:476–82. doi: 10.1007/s12028-014-9974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ, Herman CJ, Rosenthal M, Jöbsis FF. Intracellular redox changes preceding onset of epileptiform activity in intact cat hippocampus. J Neurophysiol. 1972;35:471–83. doi: 10.1152/jn.1972.35.4.471. [DOI] [PubMed] [Google Scholar]
- Ong C, Gilmore E, Claassen J, Foreman B, Mayer Sa. Impact of prolonged periodic epileptiform discharges on coma prognosis. Neurocrit Care. 2012;17:39–44. doi: 10.1007/s12028-012-9728-7. [DOI] [PubMed] [Google Scholar]
- Parkin M, Hopwood S, Jones DA, Hashemi P, Landolt H, Fabricius M, et al. Dynamic changes in brain glucose and lactate in pericontusional areas of the human cerebral cortex, monitored with rapid sampling on-line microdialysis: relationship with depolarisation-like events. J Cereb Blood Flow Metab. 2005;25:402–13. doi: 10.1038/sj.jcbfm.9600051. [DOI] [PubMed] [Google Scholar]
- Pohlmann-Eden B, Hoch DB, Cochius JI, Chiappa KH. Periodic lateralized epileptiform discharges--a critical review. J Clin Neurophysiol. 1996;13:519–30. doi: 10.1097/00004691-199611000-00007. [DOI] [PubMed] [Google Scholar]
- Punia V, Garcia CG, Hantus S. Incidence of recurrent seizures following hospital discharge in patients with LPDs (PLEDs) and nonconvulsive seizures recorded on continuous EEG in the critical care setting. Epilepsy Behav. 2015;49:250–4. doi: 10.1016/j.yebeh.2015.06.026. [DOI] [PubMed] [Google Scholar]
- Roos YBWEM, de Haan RJ, Beenen LFM, Groen RJM, Albrecht KW, Vermeulen M, et al. Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: a prospective hospital based cohort study in the Netherlands. J Neurol Neurosurg Psychiatry. 2000;68:337–41. doi: 10.1136/jnnp.68.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KTS. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth. 2012;109:315–29. doi: 10.1093/bja/aes264. [DOI] [PubMed] [Google Scholar]
- Sánchez-Porras R, Zheng Z, Santos E, Schöll M, Unterberg AW, Sakowitz OW. The role of spreading depolarization in subarachnoid hemorrhage. Eur J Neurol. 2013;20:1121–7. doi: 10.1111/ene.12139. [DOI] [PubMed] [Google Scholar]
- Schwartz MS, Prior PF, Scott DF. the Occurrence and Evolution in the Eeg of a Lateralized Periodic Phenomenon. Brain. 1973;96:613–22. doi: 10.1093/brain/96.3.613. [DOI] [PubMed] [Google Scholar]
- Shafi MM, Westover MB, Cole AJ, Kilbride RD, Hoch DB, Cash SS. Absence of early epileptiform abnormalities predicts lack of seizures on continuous EEG. Neurology. 2012;79:1796–801. doi: 10.1212/WNL.0b013e3182703fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–30. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- Sivaraju A, Gilmore EJ. Understanding and Managing the Ictal-Interictal Continuum in Neurocritical Care. Curr Treat Options Neurol. 2016;18:8. doi: 10.1007/s11940-015-0391-0. [DOI] [PubMed] [Google Scholar]
- Staley KJ, White A, Dudek FE. Interictal spikes: harbingers or causes of epilepsy? Neurosci Lett. 2011;497:247–50. doi: 10.1016/j.neulet.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaugh LA, Lindsell CJ, Shutter LA, Szaflarski JP. Initial EEG predicts outcomes in a trial of levetiracetam vs. fosphenytoin for seizure prevention. Epilepsy Behav. 2012;23:280–4. doi: 10.1016/j.yebeh.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, et al. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Macdonald RL. Cortical spreading ischemia in the absence of proximal vasospasm after aneurysmal subarachnoid hemorrhage: evidence for a dual mechanism of delayed cerebral ischemia. J Cereb Blood Flow Metab. 2012;32:201–2. doi: 10.1038/jcbfm.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck AF, Westover MB, Hall LT, Deck GM, Cole AJ, Rosenthal ES. Metabolic Correlates of the Ictal-Interictal Continuum: FDG-PET During Continuous EEG. Neurocrit Care. 2016;24:324–31. doi: 10.1007/s12028-016-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart RM, Waziri A, Weintraub D, Schmidt MJ, Fernandez L, Helbok R, et al. Intracortical EEG for the detection of vasospasm in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2010;13:355–8. doi: 10.1007/s12028-010-9414-6. [DOI] [PubMed] [Google Scholar]
- Suarez JI, Qureshi AI, Yahia AB, Parekh PD, Tamargo RJ, Williams MA, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002;30:1348–55. doi: 10.1097/00003246-200206000-00035. [DOI] [PubMed] [Google Scholar]
- Suh M, Bahar S, Mehta AD, Schwartz TH. Temporal Dependence in Uncoupling of Blood Volume and Oxygenation during Interictal Epileptiform Events in Rat Neocortex. J Neurosci. 2005;25:68–77. doi: 10.1523/JNEUROSCI.2823-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore WH, Newmark ME, Sato S, Brooks R, Patronas N, De La Paz R, et al. [18F]fluorodeoxyglucose positron emission tomography in refractory complex partial seizures. Ann Neurol. 1983;14:429–37. doi: 10.1002/ana.410140406. [DOI] [PubMed] [Google Scholar]
- Vergouwen MDI, Etminan N, Ilodigwe D, Macdonald RL. Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011;31:1545–53. doi: 10.1038/jcbfm.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- Vespa P, Tubi M, Claassen J, Buitrago-blanco M, McArthur D, Velazquez AG, et al. Metabolic Crisis occurs with Seizures and Periodic Discharges after Brain Trauma. Ann Neurol. 2016;79:579–90. doi: 10.1002/ana.24606. [DOI] [PubMed] [Google Scholar]
- Westover MB, Shafi MM, Bianchi MT, Moura LMVR, O’Rourke D, Rosenthal ES, et al. The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol. 2015;126:463–71. doi: 10.1016/j.clinph.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woitzik J, Dreier JP, Hecht N, Fiss I, Sandow N, Major S, et al. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2012;32:203–12. doi: 10.1038/jcbfm.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]