Abstract
Purpose
Our study aimed to investigate the association of telomerase activity (TA) and telomere length (TL) in granulosa cells (GCs) with IVF outcomes of polycystic ovary syndrome (PCOS) patients, and the effects of oral contraceptive pill (OCP) pretreatment on these two parameters.
Methods
One hundred sixty-three infertile women were enrolled and divided into a PCOS group (n = 65) and a non-PCOS group (n = 98). The PCOS group was further divided into an OCP pretreatment group (n = 35) and a non-OCP pretreatment group (n = 30), a TA <0.070 group (n = 34) and a TA ≥0.070 group (n = 31), and a TL <1 group (n = 41) and a TL ≥1 group (n = 24), respectively.
Results
No obvious differences were observed in TA between these groups. The TL was 0.971 in PCOS group and 1.118 in non-PCOS group (P = 0.005). The patients with TL ≥1 accounted for 36.9% in PCOS group and 54.1% in non-PCOS group (P = 0.032). The average duration of infertility for PCOS patients was 5 years in TA <0.070 group and 4 years in TA ≥0.070 group (P = 0.038), and 5 years in TL <1 group and 3 years in TL ≥1 group (P = 0.006), respectively. No obvious differences were observed in IVF outcomes between these groups. No obvious differences were observed in TA, TL, or IVF outcomes between OCP pretreatment group and non-OCP pretreatment group in PCOS patients.
Conclusions
Shorter TL was found in PCOS patients. The TA levels did not change significantly in PCOS patients. PCOS patients with a lower TA level and shorter telomeres had an earlier onset of infertility symptoms. No predictive value was found for TA and TL in terms of embryo quality or IVF outcomes in PCOS patients, and no effect OCP pretreatment was observed on either TA and TL.
Keywords: Polycystic ovary syndrome, Granulosa cell, Telomerase activity, Telomere length, Oral contraceptive pill, In vitro fertilization-embryo transfer
Introduction
Approximately 74% of polycystic ovary syndrome (PCOS) patients are infertile [1]. Some PCOS patients can be pregnant after in vitro fertilization (IVF) [2]. But some patients are insensitive to drugs because of the abnormal basal hormone levels such as the high androgen level, which can affect the growth status of granulosa cells (GCs) [3, 4]. Follicular development is associated with the growth status of GCs [5], which is determined by the telomerase activity (TA) and telomere length (TL) [6]. Telomerase is the critical enzyme for maintaining TL [6].
The TA in GCs is highest in the preantral follicle and gradually decreases during the subsequent developmental stages. A reduction of TA can result in a decrease in serum follicle-stimulating hormone (FSH) and follicular atresia. An abnormal TL in GCs may lead to low quality and a low number of oocytes [5–7]. Longer TLs were found in cumulus cells of mature oocytes and good quality embryos, indicating that TL might be related to follicle development and embryo quality [8]. Chen H et al. showed that the TA in GCs of non-PCOS patients was positively correlated with IVF pregnancy outcomes [9]. Several research reports on the relationship between PCOS and TL found that TLs were shorter in the peripheral blood lymphocytes of patients with PCOS [10]. TL in peripheral blood lymphocytes was negatively correlated with inflammatory markers in PCOS patients [11]. Oral contraceptive pills (OCP) could reduce the basal luteinizing hormone (LH) and androgen levels [12] in PCOS patients and improve IVF outcomes [13]. Townsley et al. illuminated that danazol treatment caused telomere elongation in those with telomere diseases [14].
However, it is unknown whether the abnormal growth status of GCs in PCOS patients was associated with the TA or TL. Studies on the relationship between TL and PCOS have mainly focused on the peripheral blood lymphocytes of PCOS patients. Is the TL shortened in GCs of PCOS patients? We were also interested in several additional questions: Is the TA lower in GCs of PCOS patients? Are the TA and TL in GCs of PCOS patients associated with the outcomes of IVF? Given that OCP pretreatment can reduce the levels of androgen and LH in PCOS patients and improve their pregnancy outcomes, would OCP pretreatment affect the TA or TL in GCs of PCOS patients? Our study aimed to investigate the association of the TA and TL in GCs of PCOS patients with their IVF outcomes and to explore the effects of OCP pretreatment on the TA and TL in the same individuals.
Materials and methods
Study design and setting
One hundred sixty-three infertile women for IVF at the Reproductive Medicine Center of Sun Yat-sen Memorial Hospital between March 2011 and July 2011 were divided into a PCOS group (n = 65) and a non-PCOS group (n = 98), and all patients in this study provided written informed consent for the use of their medical records. The research was approved by the Ethics Committee of Reproductive Medicine, Sun Yat-sen Memorial Hospital.
The inclusion criteria for PCOS group were the PCOS Rotterdam diagnosis criteria [15] recommended by the European Society of Human Reproduction and Embryology. The inclusion criteria for non-PCOS group were a regular menstrual cycle (22–35 days) [16], normal ovulation, basal FSH <10 IU/L, and the main infertility factor was tubal factor infertility (obstruction of oviduct confirmed by hysterosalpingography or laparoscopy). The exclusion criteria were patients younger than 23 years old or older than 38 years old, endocrine diseases other than PCOS (adrenal gland disease, diabetes mellitus, thyroid disease, hyperprolactinemia, or endocrine diseases of other organs), other severe internal medicine or surgical diseases, chromosomal abnormalities in one or both spouses, congenital or acquired structural abnormalities of the reproductive tract, a history of benign or malignant ovarian cancer, ovarian endometriosis as indicated by B-type ultrasound or laparoscopy, and unexplained infertility. The PCOS group was further divided into an OCP pretreatment group (n = 35) and a non-OCP pretreatment group (n = 30).
OCP pretreatment
Before the IVF-ET treatment cycle, OCP pretreatment was performed for three cycles for the PCOS patients with hyperandrogenism and abnormal LH levels. One OCP was administered starting on days 3–5 of the menstrual cycle and 21 days was one cycle (Diane-35 Bayer, Leverkusen, Germany).
IVF treatment
All enrolled patients underwent the mid-luteal phase long protocol for IVF. Short-acting gonadotropin-releasing hormone agonist (GnRH-a) (Decapeptyl; Ferring GmbH, Oberhausen, Germany) was used for pituitary downregulation at 0.1 mg/day before recombinant human FSH (Gonal-F; Merck Serono, Geneva, Switzerland) and gonadotropin (Gn) stimulation. Oocyte retrieval was performed 34–36 h after 6000∼10,000 IU human chorionic gonadotropin (HCG; Lizhu Medical Company, Zhuhai, China) injection when the diameter of three follicles was ≥16 mm, the diameter of two follicles was ≥17 mm, or the diameter of one follicle was ≥18 mm, and when the serum E2 level reached or exceeded the level that corresponded to the size and number of follicles. Pronuclear formation was monitored 16–18 h after insemination. On day 3, embryonic quality was assessed [17]. Embryo transfer was performed 72 h after the oocyte retrieval. Conventional luteal support was performed after the transfer. The presentation of the gestational sac and fetal heart under a B-ultrasound 5 weeks after the transfer indicated clinical pregnancy [18].
Hormone testing
Blood samples of all patients were collected on days 2–4 of the menstrual cycle and on HCG day. And basal hormone levels, including FSH, LH, estrogen (E2), and testosterone (T) levels, as well as estrogen and FSH levels on HCG day, were determined by Beckman Coulter UniCel DxI 800 and the associated reagents (Beckman Coulter, CA, USA).
The baseline and clinical data of the patients we used to compare the results, including age, duration of infertility, body mass index (BMI), basal hormone levels including FSH, LH, E2, and T levels, were retrieved from the central database. The main outcome measures were the number of oocytes retrieved, metaphase II oocytes, available embryos, good quality embryos, pregnancy rate, and cumulative pregnancy rate.
Definitions
Mature oocytes: Corona radiata cells extended radially from the surroundings of the oocytes, with GCs extended into translucent uncertain bodies and visible first polar bodies.
Embryo assessment was performed according to the Istanbul consensus [17]. Good quality embryos: on day 2, the number of embryonic blastomeres ranged between two and four cells, with a uniform size and no. or <5% cell debris. On day 3, the number of embryonic blastomeres reached 7–9 cells, with a uniform size, <5–20% cell debris, and grade 1–2 embryos.
The pregnancy rate was the number of pregnant patients following fresh embryo transfer divided by the total number of patients undergoing fresh embryo transfer. The cumulative pregnancy rate was the total chances of being pregnant after one IVF cycle including fresh embryo transfer and frozen embryo transfer.
GC isolation and extract preparation
GC collection and extract preparation were performed according to the literature [19]. The follicular fluid obtained on oocyte retrieval day was centrifuged at 1500 rpm for 5 min. The precipitate was separated using lymphocyte separation medium (the interface between the two liquids was maintained) and then purified. GC samples with a live cell percentage <50% were excluded, and protein of GCs was determined [20].
Telomerase activity assay
The telomeric repeat amplification protocol enzyme-linked immunosorbent assay (TRAP-ELISA) was performed to determine TA using a commercial kit (Telo TAGGG Telomerase PCR ELISA PLUS; Roche Diagnostics GmbH, Mannheim, Germany). Based on the non-isotopic method introduced by Wen [21], the experiment was performed according to the manufacturer’s instructions. The values obtained for low control template should be in the range of 0.2–0.5 A450–A690nm units after 10-min substrate reaction. Values for negative controls were <0.1. All samples were analyzed three times. The TA was calculated as △A = A450 − A690nm (A450 and A690nm were absorbance values).
Telomere length assay
The DNA of GCs was extracted using the DNA extraction reagent in the kit (DNeasy Blood & Tissue kit; Qiagen, Hilden, Germany). The extracted samples were evaluated to determine the optical absorbance values at 260 and 280 nm using a spectrophotometer and using the A260/A280 formula to calculate the DNA concentration and purity. Samples with a value between 1.8 and 2.0 were selected. The TL of the DNA samples was measured using real-time fluorescence quantitative PCR according to the literature [22]. Primer sequences were determined based on the literature [23] and were synthesized by TaKaRa (TaKaRa, Kyoto, Japan). The materials included 2.8 nmol/OD forward telomere primer 1 (5′-CGG TTT GTT TGG GTT TGG GTT TGG GTT TGG GTT TGG GTT-3′), 3.1 nmol/OD reverse telomere primer 2 (5′-GGC TTG CCT TAC CCT TAC CCT TAC CCT TAC CCT TAC CCT-3′), 4.3 nmol/OD 36B4 upstream forward primer (5′-CAG CAA GTG GGA AGG TGT AATCC-3′), and 4.15 nmol/OD 36B4 downstream reverse primer (5′-CCC ATT CTA TCA TCA ACG GGT ACAA-3′). TL was calculated according to the formula of Cawthon [22] for the telomere to single copy gene ratio (T/S): .
Statistical analyses
All continuous variables were examined using the Kolmogorov-Smirnov test. Data that followed a normal distribution were expressed as mean ± standard deviation and were examined using the t test or ANOVA. Data that did not follow a normal distribution were described as median (IQR) and examined using a non-parametric test. Comparison of the percentages between two groups was performed using the χ 2 test or the Fisher exact probability test. The correlations of TA and TL with age and basal hormone levels, including FSH, LH, E2, and T levels, were determined using the Spearman correlation analysis. The correlations of TA and TL with BMI, total Gn dose, number of retrieved oocytes, metaphase II oocytes, available embryos, and good quality embryos were determined using the Pearson correlation analysis. The associations between TA, TL, age, BMI, basal hormone levels, including FSH, LH, E2, and T levels, and the total Gn dose were analyzed using multiple linear regression analysis. Statistical analysis was performed using SPSS 22.0 software. P < 0.05 was considered statistically significant.
Results
Comparison of PCOS patients with and without OCP pretreatment
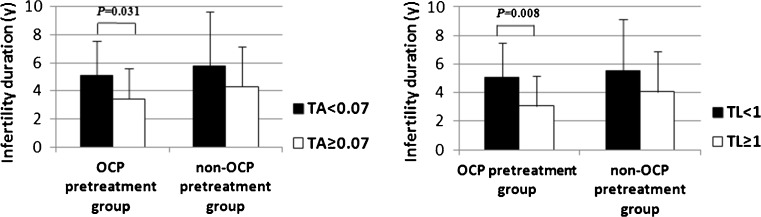
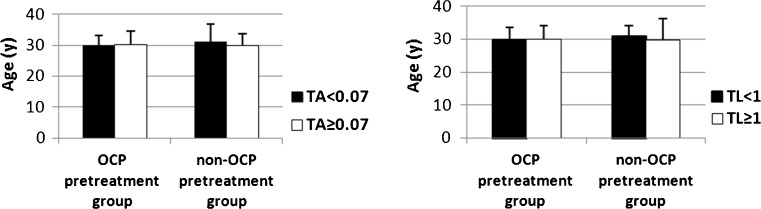
The TA and TL in the GCs of the PCOS patients did not significantly differ between the OCP pretreatment group and the non-OCP pretreatment group (P > 0.05). The percentages of PCOS patients with TA <0.070 and TA ≥0.070 did not significantly differ between these two groups (P > 0.05). No obvious differences were observed in the percentages of PCOS patients with TL <1 and TL ≥1 between these two groups (P > 0.05). In the OCP pretreatment group, the average duration of infertility for PCOS patients with TA <0.070 and TA ≥0.070 was 5.1 and 3.4 years, respectively (P = 0.031). And the average duration of infertility for PCOS patients with TL <1 and TL ≥1 was 5.1 and 3.1 years, respectively (P = 0.008). In non-OCP pretreatment group, the duration of infertility did not significantly differ in PCOS patients with TA <0.070 and TA ≥0.070 (P > 0.05). And no obvious differences were observed in the duration of infertility for PCOS patients with TL <1 and TL ≥1 (P > 0.05) (Fig. 1). Age did not significantly differ in PCOS patients with TA <0.070 and TA ≥0.070 (P > 0.05) or in PCOS patients with TL <1 and TL ≥1 (P > 0.05) between these groups (Fig. 2). The average basal serum testosterone levels in PCOS patients were 1.9 nmol/L in the OCP pretreatment group and 1.6 nmol/L in the non-OCP pretreatment group (P = 0.045). The age, BMI, basal hormone levels, total Gn dose, and IVF outcomes of PCOS patients did not significantly differ between these two groups (P > 0.05) (Table 1).
Fig 1.
The duration of infertility for PCOS patients with different TA and TL levels in the OCP pretreatment group and non-OCP pretreatment group
Fig 2.
The age of patients with different TA and TL levels in the OCP pretreatment group and non-OCP pretreatment group
Table 1.
TA, TL, clinical characteristics, and IVF laboratory parameters of PCOS patients between the OCP pretreatment group and non-OCP pretreatment group
| Characteristics | OCP pretreatment group (N = 35) | Non-OCP pretreatment group (N = 30) | P a |
|---|---|---|---|
| TA, OD × mm, median (IQR) | 0.066 (0.059–0.087) | 0.073 (0.059–0.096) | 0.540 |
| TA level | 0.180 | ||
| TA <0.070, n (%) | 21 (60.0) | 13 (43.3) | |
| TA ≥0.070, n (%) | 14 (40.0) | 17 (56.7) | |
| TL, T:S ratio, median (IQR) | 0.890 (0.785–1.149) | 0.937 (0.793–1.131) | 0.864 |
| TL level | 0.634 | ||
| TL <1, n (%) | 23 (65.7) | 18 (60.0) | |
| TL ≥1, n (%) | 12 (34.3) | 12 (40.0) | |
| Age, years, mean ± SD | 30.1 ± 3.9 | 30.4 ± 4.7 | 0.746 |
| BMI, kg/m2, median (IQR) | 21.7 (20.4–24.0) | 21.4 (18.3–23.3) | 0.216 |
| Basal serum FSH level, IU/L, mean ± SD | 6.6 ± 2.3 | 7.0 ± 1.5 | 0.443 |
| Basal serum LH level, IU/L, median (IQR) | 6.8 (4.2–11.7) | 5.4 (4.0–7.8) | 0.202 |
| Basal serum testosterone level, nmol/L, median (IQR) | 1.9 (1.5–2.4) | 1.6 (1.0–1.9) | 0.045* |
| FSH/LHb, median (IQR) | 1.0 (0.6–1.5) | 1.2 (0.8–1.8) | 0.076 |
| Retrieved oocytes, median (IQR) | 14 (9–18) | 16 (13–20) | 0.090 |
| Total Gn dose, IU, median (IQR) | 1650 (1275–2250) | 1500 (1219–2081) | 0.470 |
| Metaphase II oocytes, median (IQR) | 13 (8–17) | 14 (11–19) | 0.168 |
| Proportion of mature oocytesc, %, median (IQR) | 92.3 (83.3–100) | 92.3 (81.8–100) | 0.846 |
| Available embryos, median (IQR) | 9 (4–13) | 9 (6–12) | 0.989 |
| Good quality embryosd, median (IQR) | 3 (1–5) | 3 (1–5) | 0.785 |
| Rate of good quality embryose, %, median (IQR) | 33.3 (14.3–55.6) | 32.1 (10.8–58.8) | 0.884 |
| Pregnancy ratef, n (%) | 17 (63.0) | 13 (54.2) | 0.524 |
| Miscarriage rateg, n (%) | 1 (5.9) | 1 (7.7) | 1.000 |
| Cumulative pregnancy rateh, n (%) | 27 (79.4) | 20 (69.0) | 0.342 |
| Cumulative rate of taking baby homei, n (%) | 23 (67.6) | 17 (58.6) | 0.458 |
| Cumulative miscarriage ratej, n (%) | 4 (14.8) | 3 (15.0) | 1.000 |
TA telomerase activity, TL telomere length, PCOS polycystic ovary syndrome, OCP oral contraceptive pill, IQR interquartile range, SD standard deviation, BMI body mass index, FSH follicle-stimulating hormone, LH luteinizing hormone
*P < 0.05 was considered statistically significant
a P values: t test for age and basal serum FSH level, Mann–Whitney U test for other continuous variables, chi-square or Fisher’s exact test for categorical variables
bFSH/LH = basal serum FSH level/basal serum LH level
cProportion of mature oocytes = metaphase II oocytes/retrieved oocytes
dGood quality embryos were embryos identified as grade A (4 cells in day 2 or 7–8 cells in day 3) or grade B (4–5 cells in day 2 or 7–9 cells in day 3) [17]
eRate of good quality embryos = good quality embryos/available embryos
fPregnancy rate = the number of pregnant patients with fresh embryo transfer/the total number of patients with fresh embryo transfer
gMiscarriage rate = the number of patients with spontaneous abortion/the total number of patients with fresh embryo transfer
hCumulative pregnancy rate was total chances of being pregnant after one IVF cycle including fresh embryo transfer and frozen embryo transfer [24]
iCumulative rate of taking baby home was total chances of successfully giving birth to a healthy baby after one IVF cycle including fresh embryo transfer and frozen embryo transfer
jCumulative miscarriage rate was total chances of spontaneous abortion after one IVF cycle including fresh embryo transfer and frozen embryo transfer
Although the basal testosterone level was different between the two groups, correlation analysis revealed that the basal testosterone level was not associated with the TA (r = −0.011, P = 0.912) or TL (r = 0.160, P = 0.119) of GCs in our study population. Therefore, the OCP pretreatment group was included when we compared the PCOS patients to non-PCOS patients.
Associations between TA, TL, and clinical characteristics and laboratory parameters
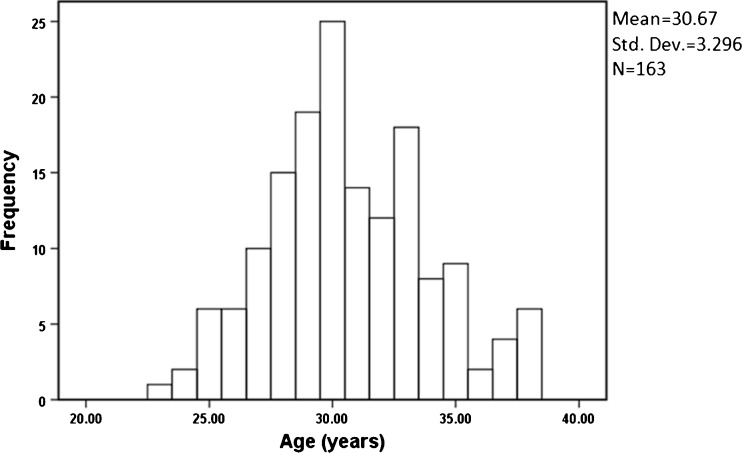
The age distribution of all participants is shown in Fig. 3. The age range of all participants was 23 to 38 years old. Multiple linear regression analysis revealed that the relationship between TL and the total Gn dose (IU) was determined by TL (T:S ratio) = −0.00017 × IU + 1.462 [R 2 = 0.077; multiple correlation coefficient (R) = 0.277; P = 0.008]. Age did not correlate with the TA (r = −0.185, P > 0.05) or TL (r = −0.083, P > 0.05). TA and TL did not correlate with other clinical characteristics and laboratory parameters (P > 0.05) (Table 2).
Fig 3.
Age distribution of all participants
Table 2.
Correlation analysis of clinical characteristics and laboratory parameters with the TL and TA
| TL | TA | |||
|---|---|---|---|---|
| r | P | r | P | |
| Age | −0.083 | 0.416 | −0.185 | 0.069 |
| BMI | −0.159 | 0.130 | 0.032 | 0.761 |
| Basal serum FSH level | 0.022 | 0.832 | −0.045 | 0.661 |
| Basal serum LH level | 0.129 | 0.207 | 0.011 | 0.917 |
| Basal serum estrogen level | −0.054 | 0.601 | −0.054 | 0.600 |
| Basal serum testosterone level | 0.160 | 0.119 | −0.011 | 0.912 |
| Total Gn dose | −0.250 | 0.013* | −0.029 | 0.780 |
| Retrieved oocytes | 0.020 | 0.847 | 0.048 | 0.641 |
| Metaphase II oocytes | 0.018 | 0.863 | 0.062 | 0.545 |
| Available embryos | −0.052 | 0.611 | 0.085 | 0.408 |
| Good quality embryos | 0.152 | 0.136 | 0.105 | 0.302 |
*P < 0.05 was considered statistically significant
TA, TL, clinical characteristics, and IVF outcomes between the PCOS group and the non-PCOS group
No obvious differences were observed in TA between PCOS patients and non-PCOS patients (P > 0.05). The average TL was 0.971 in PCOS group and 1.118 in non-PCOS group (P = 0.005). The patients with TL ≥1 accounted for 36.9% in PCOS group (24/65) and 54.1% in non-PCOS group (53/98) (P = 0.032).
The mean age of patients was 30.3 years old in PCOS group and 31.7 years old in non-PCOS group (P = 0.024). The mean basal serum testosterone level was 1.9 nmol/L in PCOS group and 1.3 nmol/L in non-PCOS group (P < 0.001). The average FSH/LH ratio of patients was 1.18 in PCOS group and 2.43 in non-PCOS group (P = 0.024). The mean total dose of Gn for patients was 1817 IU in PCOS group and 2063 IU in non-PCOS group (P = 0.045). The mean FSH level on the HCG day was 11.3 ng/L for patients in PCOS group and 15.9 ng/L in non-PCOS group (P < 0.001). The BMI, duration of infertility, basal E2 levels, E2 levels on the HCG day, IVF laboratory indicators, and IVF outcomes did not differ significantly between these two groups (P > 0.05) (Table 3).
Table 3.
Telomerase activity, telomere length, clinical characteristics, and IVF outcomes between the PCOS group and non-PCOS group
| Characteristics | PCOS group (N = 65) | Non-PCOS group (N = 98) | P a |
|---|---|---|---|
| TA, OD × mm, mean ± SD | 0.094 ± 0.085 | 0.107 ± 0.119 | 0.424 |
| TA level | 0.184 | ||
| TA <0.070, n (%) | 34 (52.3) | 45 (45.9) | |
| TA ≥0.070, n (%) | 31 (47.7) | 53 (54.1) | |
| TL, T:S ratio, mean ± SD | 0.971 ± 0.289 | 1.118 ± 0.369 | 0.005* |
| TL level | 0.032* | ||
| TL <1, n (%) | 41 (63.1) | 45 (45.9) | |
| TL ≥1, n (%) | 24 (36.9) | 53 (54.1) | |
| Age, years, mean ± SD | 30.3 ± 4.3 | 31.7 ± 3.8 | 0.024* |
| BMI, kg/m2, mean ± SD | 21.8 ± 3.3 | 21.3 ± 3.4 | 0.365 |
| Duration of infertility, years, mean ± SD | 4.7 ± 3.0 | 5.4 ± 3.7 | 0.180 |
| Basal serum FSH level, IU/L, mean ± SD | 6.8 ± 2.0 | 9.0 ± 9.9 | 0.069 |
| Basal serum LH level, IU/L, mean ± SD | 7.4 ± 4.8 | 7.3 ± 28.5 | 0.967 |
| FSH/LHb, mean ± SD | 1.2 ± 0.6 | 2.4 ± 4.4 | 0.024* |
| Basal serum testosterone level, nmol/L, mean ± SD | 1.9 ± 0.8 | 1.3 ± 0.7 | <0.001* |
| Basal serum estrogen level, ng/L, mean ± SD | 45.6 ± 30.1 | 48.8 ± 59.4 | 0.692 |
| Total Gn dose, IU, mean ± SD | 1817 ± 911 | 2063 ± 636 | 0.045* |
| Serum FSH level on HCG day, ng/L, median (IQR) | 11.3 (8.0–14.6) | 15.9 (12.5–20.0) | <0.001* |
| Serum estrogen level on HCG day, ng/L, mean ± SD | 3501 ± 1484 | 4206 ± 7745 | 0.498 |
| Retrieved oocytes, mean ± SD | 16 ± 8 | 17 ± 7 | 0.510 |
| Metaphase II oocytes, mean ± SD | 14 ± 6 | 14 ± 6 | 0.663 |
| Proportion of mature oocytesc, %, mean ± SD | 89.1 ± 12.1 | 86.0 ± 13.8 | 0.139 |
| Available embryos, mean ± SD | 9 ± 5 | 9 ± 5 | 0.793 |
| Good quality embryosd, mean ± SD | 3 ± 3 | 3 ± 3 | 0.974 |
| Rate of good quality embryose, %, mean ± SD | 37.1 ± 30.7 | 35.5 ± 29.5 | 0.749 |
| Fertilization rate, %, mean ± SD | 82.7 ± 18.4 | 80.3 ± 19.9 | 0.437 |
| Cleavage rate, %, mean ± SD | 97.9 ± 5.4 | 95.5 ± 15.9 | 0.178 |
| Pregnancy ratef, n (%) | 30 (58.8) | 38 (52.8) | 0.506 |
| Miscarriage rateg, n (%) | 2 (6.7) | 2 (5.3) | 1.000 |
| Cumulative pregnancy rateh, n (%) | 47 (74.6) | 69 (74.2) | 0.954 |
| Cumulative rate of taking baby homei, n (%) | 40 (63.5) | 64 (68.8) | 0.489 |
| Cumulative miscarriage ratej, n (%) | 7 (14.9) | 5 (7.2) | 0.309 |
PCOS polycystic ovary syndrome, TA telomerase activity, SD standard deviation, TL telomere length, BMI body mass index, FSH follicle-stimulating hormone, LH luteinizing hormone, Gn gonadotropin, IQR interquartile range, HCG human chorionic gonadotropin
*P < 0.05 was considered statistically significant
a P values: Mann–Whitney U test for serum FSH level on HCG day, t test for other continuous variables, chi-square or Fisher’s exact test for categorical variables
bFSH/LH = basal serum FSH level/basal serum LH level
cProportion of mature oocytes = metaphase II oocytes/retrieved oocytes
dGood quality embryos were embryos identified as grade A (4 cells in day 2 or 7–8 cells in day 3) or grade B (4–5 cells in day 2 or 7–9 cells in day 3) [17]
eRate of good quality embryos = good quality embryos/available embryos
fPregnancy rate = the number of pregnant patients with fresh embryo transfer/the total number of patients with fresh embryo transfer
gMiscarriage rate = the number of patients with spontaneous abortion/the total number of patients with fresh embryo transfer
hCumulative pregnancy rate was total chances of being pregnant after one IVF cycle including fresh embryo transfer and frozen embryo transfer [24]
iCumulative rate of taking baby home was total chances of successfully giving birth to a healthy baby after one IVF cycle including fresh embryo transfer and frozen embryo transfer
jCumulative miscarriage rate was total chances of spontaneous abortion after one IVF cycle including fresh embryo transfer and frozen embryo transfer
Comparison of PCOS patients in different TA levels
The PCOS group was further divided into a TA <0.070 group (n = 34) and a TA ≥0.070 group (n = 31). The average duration of infertility for PCOS patients was 4 years in the TA ≥0.070 group and 5 years in the TA <0.070 group (P = 0.038). The number of retrieved oocytes, number of metaphase II oocytes, number of available embryos, number of good quality embryos, and IVF outcomes of PCOS patients did not differ significantly between these two groups (P > 0.05). In the fresh embryo transfer cycle, although the TA in PCOS patients without pregnancy was higher than that in pregnant PCOS patients and PCOS patients with persistent pregnancy, the difference was not significant (P > 0.05) (Table 4).
Table 4.
Clinical characteristics and IVF laboratory parameters of PCOS patients in different levels of TA
| Characteristics | TA <0.070 group (N = 34) | TA ≥0.070 group (N = 31) | P a |
|---|---|---|---|
| Age, years, mean ± SD | 30.3 ± 4.4 | 30.1 ± 4.2 | 0.880 |
| BMI, kg/m2, mean ± SD | 22.1 ± 2.9 | 21.5 ± 3.7 | 0.466 |
| Duration of infertility, y, median (IQR) | 5 (3–7) | 4 (2–4) | 0.038* |
| Basal serum FSH level, IU/L, mean ± SD | 7.0 ± 2.4 | 6.6 ± 1.4 | 0.425 |
| Basal serum LH level, IU/L, median (IQR) | 6.4 (4.3–11.3) | 5.3 (4.1–8.3) | 0.533 |
| Basal serum testosterone level, nmol/L, mean ± SD | 1.8 ± 0.8 | 1.9 ± 1.0 | 0.764 |
| FSH/LH, mean ± SD | 1.2 ± 0.6 | 1.2 ± 0.6 | 0.932 |
| Retrieved oocytes, mean ± SD | 16 ± 7 | 16 ± 8 | 0.676 |
| Metaphase II oocytes, mean ± SD | 14 ± 5 | 14 ± 6 | 0.992 |
| Proportion of mature oocytes, %, median (IQR) | 92.6 (86.6–100) | 92.3 (78.9–100) | 0.318 |
| Available embryos, mean ± SD | 9 ± 5 | 9 ± 5 | 0.856 |
| Good quality embryos, median (IQR) | 4 (1–5) | 2 (1–4) | 0.529 |
| Rate of good quality embryos, %, median (IQR) | 39.6 (13.2–58.8) | 33.3 (11.1–53.3) | 0.540 |
| Pregnancy rate, n (%) | 19 (67.9) | 11 (47.8) | 0.148 |
| Miscarriage rate, n (%) | 1 (5.3) | 1 (9.1) | 1.000 |
| Cumulative pregnancy rate, n (%) | 25 (73.5) | 22 (75.9) | 0.832 |
| Cumulative rate of taking baby home, n (%) | 21 (61.8) | 19 (65.5) | 0.758 |
| Cumulative miscarriage rate, n (%) | 4 (16.0) | 3 (13.6) | 1.000 |
IVF in vitro fertilization
*P < 0.05 was considered statistically significant
a P values: Mann–Whitney U test for the duration of infertility, basal serum LH level, proportion of mature oocytes and rate of good quality embryos; t test for other continuous variables; chi-square or Fisher’s exact test for categorical variables
Comparison of PCOS patients in different TL levels
The PCOS group was further divided into a TL <1 group (n = 41) and a TL ≥1 group (n = 24). The average duration of infertility was 5 years for PCOS patients in the TL <1 group and 3 years for PCOS patients in the TL ≥1 group (P = 0.006). In all cases with TL ≥1, the duration of infertility was 3 years in PCOS patients and 5 years in non-PCOS patients (P = 0.017). The mean E2 level of each mature oocyte on the HCG day in PCOS patients was 245.27 ng/L in the TL <1 group and 331.49 ng/L in the TL ≥1 group (P = 0.024). No obvious differences were observed in the age, BMI, basal hormone levels, total number of oocytes, number of metaphase II oocytes, number of available embryos, number of good quality embryos, and IVF outcomes between these two groups (P > 0.05) (Table 5).
Table 5.
Clinical characteristics and IVF laboratory parameters of PCOS patients in different levels of TL
| Characteristics | TL <1 group (N = 41) | TL ≥1 group (N = 24) | P a |
|---|---|---|---|
| Age, years, mean ± SD | 30.3 ± 3.6 | 30.1 ± 5.3 | 0.834 |
| BMI, kg/m2, median (IQR) | 21.6 (20.2–23.7) | 21.6 (18.7–23.9) | 0.693 |
| Duration of infertility, y, median (IQR) | 5 (3–7) | 3 (2–4) | 0.006* |
| Basal serum FSH level, IU/L, mean ± SD | 6.9 ± 2.3 | 6.6 ± 1.4 | 0.505 |
| Basal serum LH level, IU/L, median (IQR) | 6.9 (4.3–11.9) | 5.2 (4.1–7.2) | 0.073 |
| FSH/LH, median (IQR) | 1.0 (0.6–1.5) | 1.2 (0.9–1.8) | 0.082 |
| Basal serum testosterone level, nmol/L, median (IQR) | 1.8 (1.1–2.3) | 1.8 (1.5–2.4) | 0.605 |
| Retrieved oocytes, median (IQR) | 15 (12–22) | 14 (10–17) | 0.273 |
| Metaphase II oocytes, mean ± SD | 15 ± 6 | 13 ± 5 | 0.202 |
| Proportion of mature oocytes, %, median (IQR) | 92.9 (86.2–100) | 91.3 (80.0–100) | 0.589 |
| Available embryos, mean ± SD | 10 ± 5 | 8 ± 4 | 0.206 |
| Good quality embryos, median (IQR) | 3 (1–5) | 3 (1–5) | 0.848 |
| Rate of good quality embryos, median (IQR) | 33.3 (7.8–54.4) | 33.3 (19.9–67.1) | 0.309 |
| Serum estrogen level on HCG day/retrieved oocytes, ng/L, mean ± SD | 219 ± 93 | 290 ± 144 | 0.043* |
| Serum estrogen level on HCG day/metaphase II oocytes, ng/L, mean ± SD | 245 ± 88 | 331 ± 159 | 0.024* |
| Pregnancy rate, n (%) | 18 (60.0) | 12 (57.1) | 0.838 |
| Miscarriage rate, n (%) | 0 (0) | 2 (16.7) | 0.152 |
| Cumulative pregnancy rate, n (%) | 31 (77.5) | 16 (69.6) | 0.486 |
| Cumulative rate of taking baby home, n (%) | 27 (67.5) | 13 (56.5) | 0.384 |
| Cumulative miscarriage rate, n (%) | 4 (12.9) | 3 (18.8) | 0.676 |
*P < 0.05 was considered statistically significant
a P values: t test for age, basal serum FSH level, metaphase II oocytes, available embryos, serum estrogen level on HCG day/retrieved oocytes and serum estrogen level on HCG day/metaphase II oocytes; Mann–Whitney U test for other continuous variables; chi-square or Fisher’s exact test for categorical variables
Discussion
Patients less than 38 years old were enrolled because fertility of women significantly decreased after 38 years old [25]. In our study, TA and TL did not correlate with age after restricting the cohort age range. Previous research in fields outside gynecology has reported that androgen levels were associated with TA and TL. Androgens might reduce the TA through inhibiting hTERT expression in prostate cancer cells [26]. A recent study published in the New England Journal of Medicine showed that danazol treatment could increase TL in patients with telomere diseases [14]. In our study, neither TA nor TL was related to testosterone levels. No obvious differences were observed in the TA in GCs between PCOS patients and non-PCOS patients. Although the PCOS patients in our study were younger than the non-PCOS patients, the baseline levels were consistent in these two groups. Given that the TA in the patients of our study did not correlate with age and testosterone levels, it was suggested that PCOS might not influence the TA in GCs. We hypothesized that the poor growth status of GCs in PCOS patients might not correlate with the TA in GCs but rather might be associated with the metabolic disorder and hormone abnormalities. Chen H et al. showed that the TA in GCs of non-PCOS patients was positively correlated with IVF pregnancy outcomes [9]. In PCOS patients, however, no predictive value was found for the TA in terms of embryo quality and IVF outcomes.
Several reports on the relationship between PCOS and TL found that TL was shorter in the peripheral blood lymphocytes of patients with PCOS [10]. TL in peripheral blood lymphocytes is negatively correlated with inflammatory markers in the peripheral blood of PCOS patients [11]. But these studies mainly focused on the peripheral blood lymphocytes of PCOS patients. In our study, PCOS patients had shorter telomeres in the GCs than non-PCOS patients. The total Gn dose was negatively correlated with the TL. In addition, the total Gn dose was significantly lower in the PCOS group. Given that the TL of all subjects did not correlate with age and other clinical characteristics, suggesting that the abnormal growth status of GCs in PCOS patients might be associated with the decreased TL. Some studies indicated that telomerase could maintain the TL [7]. The cell growth status changes when the telomere shortens to a certain level. However, we showed that the TA in PCOS patients was not significantly different from that in non-PCOS patients, but TL was shorter in PCOS, suggesting that other factors such as insulin resistance might influence the TL [27]. Wang W et al. demonstrated that no predictive ability was found in the TL in GCs in terms of IVF pregnancy outcome in non-PCOS patients [19]. Keefe et al. indicated that the TL of oocytes was associated with the development of embryos, and shorter telomeres suggested a poorer IVF outcome [28]. We are interested in whether the TL in GCs of patients with PCOS is related to their outcomes of IVF. However, no predictive value was found in the TL in GCs of patients with PCOS in terms of their IVF outcomes in our study. While in PCOS patients, abnormal follicular development [29], genetic factors, hormone regulation, regulatory factors in the ovary, and cell apoptosis could also affect their oocyte quality [30]. The TL in GCs in PCOS patients in our study might not be able to intuitively reflect the TL of oocyte cells and predict IVF outcomes.
In our study, PCOS patients with lower TA and shorter TL had a longer duration of infertility. The duration of infertility might also be influenced by age and infertile factors. Given that age did not differ between these groups in our study, and the main infertile factors of PCOS patients in our study were ovulation dysfunction and tubal obstruction, suggesting that the TA and TL in GCs may be associated with the duration of infertility in PCOS patients. PCOS patients with a lower TA and shorter TL might exhibit infertility symptoms earlier. Therefore, the TA and TL might play an important role in the maintenance of ovarian function. Reduction in ovarian function might be associated with a reduction in the TA in GCs [31].
Long-term estrogen replacement therapy can delay telomere shortening in somatic cells [32]. When the amount of estrogen synthesis decreases, the TA of large follicular GCs also decreases accordingly [33]. Another study showed that exogenous estrogen could significantly improve telomerase activity and TERT mRNA expression in the heart, liver, and brain cells in an ovariectomized menopausal mouse model [34]. Estrogen could increase TA through the MAPK pathway in human endometrial cancer cells [35]. Townsley DM et al. indicated the telomere elongation in patients with telomere diseases after danazol treatment [14]. However in PCOS patients of our study, no effect of OCP pretreatment on the TA or TL in GCs was found. OCP pretreatment did reduce androgen levels in PCOS patients to improve the ovarian microenvironment, reduce the number of antral follicles, and improve the IVF outcomes [13]. We also found that PCOS patients in our study with OCP pretreatment had higher levels of serum testosterone, but their basal FSH and LH levels, and IVF outcomes did not significantly differ from patients without OCP pretreatment. These results suggested that OCP pretreatment might only improve the microenvironment in the ovary of PCOS patients through hormone levels but not improve the growth status of GCs through the TA or TL levels.
As a retrospective study, our study had limitations on the enrollment of PCOS patients with OCP pretreatment. Only PCOS patients with hyperandrogenism and abnormal LH level were treated with OCP for three cycles. The sample size of PCOS patients was not large enough in our study. A better design would be a 1:1 paired study comparing PCOS and non-PCOS groups to increase the power of the test. Whether extension of the OCP pretreatment time affects the TA and TL in GCs of PCOS patients still needs further exploration.
Conclusions
Our study indicated that telomeres were shortened in the GCs of PCOS patients compared with non-PCOS patients. PCOS patients with a lower TA level and shorter telomeres had an earlier onset of infertility symptoms. No predictive value was found for the TA and TL in terms of embryo quality and IVF outcomes in PCOS patients. No effect was found for the OCP pretreatment on the TA and TL in GCs of PCOS patients.
E2, estrogen; FSH, follicle-stimulating hormone; GCs, granulosa cells; Gn, gonadotropin; GnRH-a, gonadotropin-releasing hormone agonist; HCG, human chorionic gonadotropin; IVF, in vitro fertilization; LH, luteinizing hormone; OCP, oral contraceptive pills; PCOS, polycystic ovary syndrome; T, testosterone; TA, telomerase activity; TL, telomere length; TRAP-ELISA, telomeric repeat amplification protocol enzyme-linked immunosorbent assay
Acknowledgements
The authors thank Huang Baoyun, Reproductive Medicine Centre, Sun Yat-Sen Memorial Hospital, for data collection.
Compliance with ethical standards
Informed consent
Informed consent was obtained from all the patients involved in the study.
Ethical approval
All procedures performed were in accordance with the ethical standards of the Ethics Committees of Reproductive Medicine, Sun Yat-Sen Memorial Hospital, and with the 1964 Helsinki declaration and its subsequent amendments. This study was approved by the Ethics Committee of Reproductive Medicine, Sun Yat-sen Memorial Hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by the Medical Scientific Research Foundation of Guangdong Province (A2015143), the Natural Science Foundation of Guangdong Province (2015A030313086), and the Science and Technology Planning Project of Guangdong Province (2014A020213014) in Guangdong, China.
Footnotes
The abstract was presented at the 7th Congress of the Asia Pacific Initiative on Reproduction (ASPIRE 2017), held during 30 March–2 April 2017, at The Kuala Lumpur Convention Centre, Malaysia.
The abstract was accepted for poster presentation by the 33rd Annual Meeting of European Society of Human Reproduction and Embryology (ESHRE 2017), to be held during 2 to 5 July 2017, in Geneva, Switzerland.
References
- 1.Vitek W, Hoeger K, Legro RS. Treatment strategies for infertile women with polycystic ovary syndrome. Minerva Ginecol. 2016;68:450–457. [PubMed] [Google Scholar]
- 2.Goldzieher JW, Axelrod LR. Clinical and biochemical features of polycystic ovarian disease. Fertil Steril. 1963;14:631–653. doi: 10.1016/S0015-0282(16)35047-6. [DOI] [PubMed] [Google Scholar]
- 3.Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv. 2008;63:39–48. doi: 10.1097/OGX.0b013e31815e85fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang RJ, Cook-Andersen H. Disordered follicle development. Mol Cell Endocrinol. 2013;373:51–60. doi: 10.1016/j.mce.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canipari R. Oocyte–granulosa cell interactions. Hum Reprod Update. 2000;6:279–289. doi: 10.1093/humupd/6.3.279. [DOI] [PubMed] [Google Scholar]
- 6.Ozturk S, Sozen B, Demir N. Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Mol Hum Reprod. 2014;20:15–30. doi: 10.1093/molehr/gat055. [DOI] [PubMed] [Google Scholar]
- 7.Butts S, Riethman H, Ratcliffe S, Shaunik A, Coutifaris C, Barnhart K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab. 2009;94:4835–4843. doi: 10.1210/jc.2008-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng EH, Chen SU, Lee TH, Pai YP, Huang LS, Huang CC, Lee MS. Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Hum Reprod. 2013;28:929–936. doi: 10.1093/humrep/det004. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Wang W, Mo Y, Ma Y, Ouyang N, Li R, Mai M, He Y, Bodombossou-Djobo MM, Yang D. Women with high telomerase activity in luteinised granulosa cells have a higher pregnancy rate during in vitro fertilisation treatment. J Assist Reprod Genet. 2011;28:797–807. doi: 10.1007/s10815-011-9600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Du J, Feng R, Xu Y, Wang H, Sang Q, Xing Q, Zhao X, Jin L, He L, Wang L. A possible new mechanism in the pathophysiology of polycystic ovary syndrome (PCOS): the discovery that leukocyte telomere length is strongly associated with PCOS. J Clin Endocrinol Metab. 2014;99:E234–E240. doi: 10.1210/jc.2013-3685. [DOI] [PubMed] [Google Scholar]
- 11.Pedroso DC, Miranda-Furtado CL, Kogure GS, Meola J, Okuka M, Silva C, Calado RT, Ferriani RA, Keefe DL, Dos RR. Inflammatory biomarkers and telomere length in women with polycystic ovary syndrome. Fertil Steril. 2015;103:542–7.e2. doi: 10.1016/j.fertnstert.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Golland IM, Elstein ME. Results of an open one-year study with Diane-35 in women with polycystic ovarian syndrome. Ann N Y Acad Sci. 1993;687:263–271. doi: 10.1111/j.1749-6632.1993.tb43875.x. [DOI] [PubMed] [Google Scholar]
- 13.Pan JX, Liu Y, Ke ZH, Zhou CL, Meng Q, Ding GL, Xu GF, Sheng JZ, Huang HF. Successive and cyclic oral contraceptive pill pretreatment improves IVF/ICSI outcomes of PCOS patients and ameliorates hyperandrogenism and antral follicle excess. Gynecol Endocrinol. 2015;31:332–336. doi: 10.3109/09513590.2014.995621. [DOI] [PubMed] [Google Scholar]
- 14.Townsley DM, Dumitriu B, Liu D, Biancotto A, Weinstein B, Chen C, Hardy N, Mihalek AD, Lingala S, Kim YJ, Yao J, Jones E, Gochuico BR, Heller T, Wu CO, Calado RT, Scheinberg P, Young NS. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922–1931. doi: 10.1056/NEJMoa1515319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;9:41–7. [DOI] [PubMed]
- 16.Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3–13. doi: 10.1016/j.ijgo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. [DOI] [PubMed]
- 18.Diedrich K, van der Ven H, Al-Hasani S, Krebs D. Ovarian stimulation for in-vitro fertilization. Hum Reprod. 1988;3:39–44. doi: 10.1093/oxfordjournals.humrep.a136649. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Chen H, Li R, Ouyang N, Chen J, Huang L, Mai M, Zhang N, Zhang Q, Yang D. Telomerase activity is more significant for predicting the outcome of IVF treatment than telomere length in granulosa cells. Reproduction. 2014;147:649–657. doi: 10.1530/REP-13-0223. [DOI] [PubMed] [Google Scholar]
- 20.Krieg RC, Dong Y, Schwamborn K, Knuechel R. Protein quantification and its tolerance for different interfering reagents using the BCA-method with regard to 2D SDS PAGE. J Biochem Biophys Methods. 2005;65:13–19. doi: 10.1016/j.jbbm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Wen JM, Sun LB, Zhang M, Zheng MH. A non-isotopic method for the detection of telomerase activity in tumour tissues: TRAP-silver staining assay. Mol Pathol. 1998;51:110–112. doi: 10.1136/mp.51.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30 doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gil ME, Coetzer TL. Real-time quantitative PCR of telomere length. Mol Biotechnol. 2004;27:169–172. doi: 10.1385/MB:27:2:169. [DOI] [PubMed] [Google Scholar]
- 24.Wang JX. Life table (survival) analysis to generate cumulative pregnancy rates in assisted reproduction: an alternative method of calculating the cumulative pregnancy rate in assisted reproduction technology. Hum Reprod. 2006;21:1–2. doi: 10.1093/humrep/dei281. [DOI] [PubMed] [Google Scholar]
- 25.Balasch J, Gratacos E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol. 2012;24:187–193. doi: 10.1097/GCO.0b013e3283517908. [DOI] [PubMed] [Google Scholar]
- 26.Guo C, Armbruster BN, Price DT, Counter CM. In vivo regulation of hTERT expression and telomerase activity by androgen. J Urol. 2003;170:615–618. doi: 10.1097/01.ju.0000074653.22766.c8. [DOI] [PubMed] [Google Scholar]
- 27.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 28.Keefe DL, Marquard K, Liu L. The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol. 2006;18:280–285. doi: 10.1097/01.gco.0000193019.05686.49. [DOI] [PubMed] [Google Scholar]
- 29.Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:881–887. doi: 10.1210/jc.2007-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 31.Liu JP, Li H. Telomerase in the ovary. Reproduction. 2010;140:215–222. doi: 10.1530/REP-10-0008. [DOI] [PubMed] [Google Scholar]
- 32.Lee DC, Im JA, Kim JH, Lee HR, Shim JY. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med J. 2005;46:471–479. doi: 10.3349/ymj.2005.46.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamagata Y, Nakamura Y, Umayahara K, Harada A, Takayama H, Sugino N, Kato H. Changes in telomerase activity in experimentally induced atretic follicles of immature rats. Endocr J. 2002;49:589–595. doi: 10.1507/endocrj.49.589. [DOI] [PubMed] [Google Scholar]
- 34.Cen J, Zhang H, Liu Y, Deng M, Tang S, Liu W, Zhang Z. Anti-aging effect of estrogen on telomerase activity in ovariectomised rats–animal model for menopause. Gynecol Endocrinol. 2015;31:582–585. doi: 10.3109/09513590.2015.1065478. [DOI] [PubMed] [Google Scholar]
- 35.Zhou C, Steplowski TA, Dickens HK, Malloy KM, Gehrig PA, Boggess JF, Bae-Jump VL. Estrogen induction of telomerase activity through regulation of the mitogen-activated protein kinase (MAPK) dependent pathway in human endometrial cancer cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055730. [DOI] [PMC free article] [PubMed] [Google Scholar]