Abstract
Purpose
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy among women at reproductive age. However, its etiology remains poorly understood. Recent studies indicated that telomere length was related to PCOS. However, the association between telomere length and PCOS has only been shown in leucocytes and remained controversial across different studies. To clarify the association between telomere length and PCOS, the current study interrogated telomere length not only in leucocytes, but also in follicular granulosa cells, which is essential for folliculogenesis and steroidogenesis.
Methods
Seventy-five patients with PCOS and 81 controls with mechanical infertility undergoing their first in vitro fertilization cycle were enrolled. Their peripheral blood and granulosa cells were collected on the oocyte retrieval day. Telomere length of both leucocytes in the blood and granulosa cells was assayed by quantitative polymerase chain reaction.
Results
No significant difference was found in the leucocyte telomere length between controls and PCOS patients (0.99 ± 0.44 vs. 1.00 ± 0.38, p = 0.93). Interestingly, when comparing telomere length in granulosa cells between controls and PCOS subjects, significantly lengthened telomere length was found in PCOS subjects (1.00 ± 0.37 vs. 1.57±0.67, p < 0.0001). After adjustments for age and body mass index, the p value remained significant (p < 0.0001).
Conclusions
This finding reinforced the association between telomere abnormalities and PCOS. Given the importance of telomere length in cellular proliferation, our findings provided novel insights into the pathophysiology of PCOS that abnormalities in telomere length possibly disturb folliculogenesis and subsequently result in PCOS.
Keywords: Telomere length, Polycystic ovary syndrome, Leucocyte, Granulosa cell
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy among women of reproductive age. Its prevalence is reported to be 6–8% in this age group [1, 2]. Features of PCOS include hyperandrogenemia, oligoovulation, and polycystic ovarian morphology; these commonly lead to anovulatory infertility and a series of metabolic complications, including metabolic syndrome, type 2 diabetes, and cardiovascular disease [3, 4]. PCOS jeopardizes a woman’s health throughout her entire lifespan, but the etiology and pathogenesis of this syndrome remain unclear. As a complex disease, the etiology of PCOS is thought to be multifaceted, including genetic factors and multiple environmental factors such as gestational environment and lifestyle. Notably, genetic factors are the major component of and predisposing factors to the etiology of PCOS [5]. Two large scale genome-wide association (GWA) studies of the Han Chinese population have identified 11 susceptibility loci for PCOS [6, 7]. However, other genetic components that were overlooked by GWA studies, such as telomeres, also play an essential role in gene expression and cellular proliferation. A prior study using a large cohort of PCOS subjects indicated that leukocyte telomere length (LTL) was strongly associated with PCOS. Individuals with PCOS exhibited significantly shorter LTL than controls (0.764 ± 0.016 vs. 0.876 ± 0.023, P = 0.001; OR = 1.403, 95% CI 1.150–1.712) [8]. These data were inspiring and suggested abnormalities in LTL might participate in the pathogenesis of PCOS. However, a recent study conducted in Brazilian women failed to detect the association between LTL and PCOS [9]. Considering the highly varied telomere length among different cell types, evidence from leucocytes has limited power to provide optimal evidence for the association between telomere length and PCOS. This is because leucocytes, though highly accessible, apparently do not directly participate in the pathophysiological process of PCOS. Moreover, previous existing studies focusing on ovarian insufficiency, another major ovulation disorder, discovered discrepant tendencies of telomere length abnormality in leucocytes and granulosa cells (GCs) [10, 11], which warrants the necessity of studying telomere length in cell types other than leucocytes. Importantly, GCs play a pivotal role in folliculogenesis and steroidogenesis. Thus, GCs is presumably an optimal cell type for telomere length studies of PCOS. Additionally, telomerase, a ribonucleoprotein enzyme capable of maintaining telomere length, is highly expressed in ovarian tissues. In vitro experiments have found telomerase activity in bovine follicles, suggesting that active telomerase permits the proliferation of granulosa cells required for proper follicle formation [12, 13].
Because of the importance of GCs in the pathogenesis of PCOS and the existence of telomere activity in GCs, in the current study, we proposed to investigate the association between GC telomere length (GCTL) and PCOS. To replicate prior discrepant results in leucocytes and prove our hypothesis, telomere length in both leucocytes and GCs was measured and compared between women with PCOS and controls. The association between GCTL and PCOS may reveal other important facets of the possible role of telomere length in the pathogenesis of PCOS.
Materials and methods
Subjects
Informed consent was obtained from all individual participants included in the study. Seventy-five women with PCOS undergoing their first IVF/ICSI cycle were recruited from January 2013 to May 2015. Eighty-one age-matched controls were enrolled at the same time. All of the participants were confirmed to have a normal karyotype. All PCOS cases were selected in strict accordance with the Revised 2003 Consensus on Diagnostic Criteria, which requires the presence of at least two of the following criteria for a PCOS diagnosis: oligo- and/or anovulation, clinical and/or biochemical signs of hyperandrogenism, and PCOS [14]. Diagnoses of PCOS were made after excluding other etiologies for hyperandrogenemia and ovulatory dysfunction (e.g., congenital adrenal hyperplasia, 21-hydroxylase deficiency, androgen-secreting tumors, Cushing’s syndrome, thyroid disease, and hyperprolactinemia). All subjects in the control group had regular menstrual cycles (26–35 days) and normal ovarian morphology with either male factor infertility or tubal factor infertility. Anthropometric variables, such as age, height, body weight, and menstrual cycle, and select endocrine and biochemical parameters were recorded. Total testosterone (T) and modified Ferriman–Gallwey scores were evaluated to exclude hyperandrogenism. Luteinizing hormone (LH), follicle stimulating hormone (FSH), and T were measured by chemiluminescence immunization (Beckman Access Health Company, MN).
Ovarian stimulation
All of the IVF/ICSI cycles were carried out according to the standard protocols of our IVF center. After gonadotropin-releasing hormone agonist (triptorelin acetate, Ferring AG, Switzerland) pituitary downregulation, the following ovarian stimulation by recombinant FSH (Gonal-F; Serono, Switzerland) was given. When three or more follicles reached 17 mm in diameter, 6000~10,000 IU human chorionic gonadotropin (hCG) (human Chorionic Gonadotrophin; Lizhu, China) was administered.
Blood and GCs collection
On the morning of the oocyte retrieval day 36 h after hCG trigger, peripheral blood was drawn and stored at −80 °C. Oocytes were routinely retrieved via transvaginal ultrasound-guided aspiration. All of the cumulus–corona oocyte complexes (COCs) were picked up by an embryologist. All the follicular fluid from one patient was pooled. The GC isolation protocol was modified slightly from the method introduced by Hirokazu et al. [15]. Briefly, after centrifugation (250×g, 10 min), hyaluronidase (SAGE IVF, Pasadena, CA) was added to the pellet. After incubation (5% CO2 and air, 37 °C for 30 min), cell masses were pipetted and then underlayered with Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) and were centrifuged (450×g, 20 min) for cell separation. Specifically, erythrocytes settled to the bottom. The ring-like layer containing GCs was collected, washed with phosphate-buffered saline (PBS), and stored at −80 °C.
Quantitative telomere length analysis
Relative telomere length was determined by the quantitative polymerase chain reaction (qPCR) method introduced by Cawthon [16, 17]. Briefly, this technique measures the factor by which the ratio of telomere repeat copy number to β-globin single-gene copy number differs between an unknown sample and a reference DNA sample. This method generates a relative telomere/single copy gene (T/S) ratio that is proportional to the average telomere length.
Genomic DNA was extracted from both GCs and leucocytes in blood using a DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA) and then diluted to approximately 10 ng/μl with Tris-EDTA buffer (TE). Genomic DNA was heated to 95 °C for 5 min and then cooled on ice to render it single-stranded. Telomere qPCR and single-copy gene (β-globin) qPCR were performed separately in two 384-well plates. The qPCRs for each sample were performed in triplicate, and three identical 20 ng aliquots of DNA samples were added. The well position of telomere qPCR for a certain sample on the first plate matched its well position of β-globin gene qPCR on the second plate. The qPCR were carried out on a LightCycler Roche 480 using LightCycler® 480 SYBR Green I Master kit (Roche Applied Science, Indianapolis, IN) in a total reaction volume of 20 μl/well. The composition of telomere and β-globin gene qPCR was identical except for the oligonucleotide primers. The sequence and final concentration of the primers were 100 nM of primer telomere 1 (5′ -CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′) and 900 nM of primer telomere 2 (5′ -GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′); 300 nM of β-globin 1 (5′ –GCTTCTGACACAACTGTGTTCACTAGC-3′); and 700 nM of β-globin 2 (5′ –CACCAACTTCATCCACGTTCACC -3′). PCR was performed by denaturing at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min for telomere qPCR, and 57 °C for 1 min for β-globin qPCRs, respectively, and extension at 72 °C for 15 s. To construct a standard curve, one reference DNA sample serially diluted in TE buffer by fivefolds per dilution to produce five concentrations from 50 to 0.08 ng/μl, and they were run in triplicate on each 384-well plate. Negative controls without DNA were also processed on each 384-well plate.
Statistical analysis
Continuous variables were summarized with descriptive statistics (n, mean, standard deviation). Categorical variables were described with counts and percentages. For independent samples, Student’s t test was employed to analyze normally distributed quantitative variables, while the chi-square test or Fisher’s test was used to analyze nominal variables. Normality of variables was checked using a Kolmogorov-Smirnov test. The T/S ratios were not normally distributed, so they were log-transformed to employ Student’s t test for interpretation. Linear regression model was created to adjust covariates. P values less than 0.05 were considered statistically significant for all statistical tests. All of the statistical analysis was performed with SPSS software version 19 (IBM, Chicago).
Results
Clinical characteristics of subjects
The major anthropometric variables, endocrine parameters and IVF/ICSI cycle characteristics of all of the participants are displayed in Table 1. Women with PCOS and controls had comparable age. Subjects with PCOS had significantly elevated body mass index (BMI), LH, antral follicle count, and T compared with controls. Serum FSH level of women with PCOS was one unit higher than controls, which is probably due to our limited sample size. Though fewer starting Gn was administered, the PCOS subjects manifested better ovarian response because of their better ovarian reserve. PCOS patients had significantly elevated serum E2 level and more dominant follicles (≥14 mm) on hCG day, and more oocytes were obtained from them.
Table 1.
Comparison of clinical characteristics between PCOS subjects and controls
| PCOS | Controls | p value | |
|---|---|---|---|
| n | 75 | 81 | |
| Age (year) | 28.36 ± 2.55 | 28.09 ± 2.26 | 0.49 |
| BMI | 25.10 ± 4.32 | 22.80 ± 2.46 | <0.0001 |
| LH (IU/ml) | 10.01 ± 6.42 | 5.05 ± 2.29 | <0.0001 |
| FSH (IU/ml) | 5.65 ± 1.32 | 6.58 ± 1.361 | <0.0001 |
| T (ng/dl) | 45.62 ± 20.72 | 25.37 ± 10.89 | <0.0001 |
| AFC (n) | 33.25 ± 15.27 | 14.64 ± 4.02 | <0.0001 |
| Starting Gn dose (IU) | 154.08 ± 32.02 | 172.57 ± 36.57 | 0.001 |
| Gn stimulation duration (day) | 11.93 ± 2.46 | 10.89 ± 1.95 | 0.004 |
| Total Gn administrated (IU) | 1891.56 ± 738.98 | 1985.07 ± 787.27 | 0.57 |
| E2 level on hCG day (pg/ml) | 5733.27 ± 2827.69 | 4684.56 ± 1795.44 | 0.007 |
| Follicles ≥14 mm (n) | 15.25 ± 5.09 | 11.33 ± 4.00 | <0.0001 |
| Oocytes retrieved (n) | 18.16 ± 7.95 | 13.81 ± 5.40 | <0.0001 |
Relative telomere length and PCOS
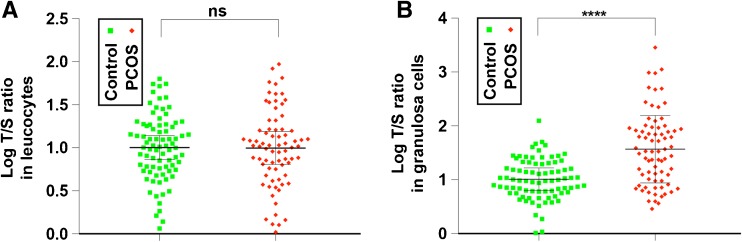
The telomere length distribution of leucocytes and GCs from PCOS subjects and controls is shown in Fig. 1. The telomere length values were not normally distributed by the Kolmogorov-Smirnov test; therefore, they were log-transformed to employ Student’s t test for interpretation. The t test indicated no significant difference in LTL between women with PCOS and controls (0.99 ± 0.44 vs. 1.00 ± 0.38, p = 0.93) (Fig. 1a). Interestingly, when comparing GCTL, women with PCOS exhibited significantly lengthened telomere length than controls (1.57 ± 0.67 vs. 1.00 ± 0.37, p < 0.0001) (Fig. 1b and Table 2). Linear regression was then used to adjust age and BMI. After adjustment, the p value remained significant (p < 0.0001). Logistic regression was then used to further determine the contribution of telomere length to the odd of PCOS. After adjusting for age and BMI, logistic regression showed that for every one unit increase in telomere length, the women were 2.29 times more likely to suffer from PCOS (Table 2).
Fig. 1.
Comparison of relative telomere length in leucocytes and granulosa cells between PCOS subjects and controls. Relative telomere length of leucocytes (a) and granulosa cells (b) are compared between controls (green squares, n = 81) and subjects with PCOS (red diamonds, n = 75)
Table 2.
Relative GC telomere length of PCOS subjects and controls, and odd ratio for PCOS
| Relative telomere length (±SD) | p value | Adjusted p value | OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|---|
| Controls (n = 81) | 1.00 ± 0.37 | <0.0001 | <0.0001 | Reference | Reference |
| PCOS (n = 75) | 1.57 ± 0.67 | 2.38 (1.26 ± 4.51) | 2.29 (1.15 ± 4.53) |
Correlation between telomere length and clinical characteristics
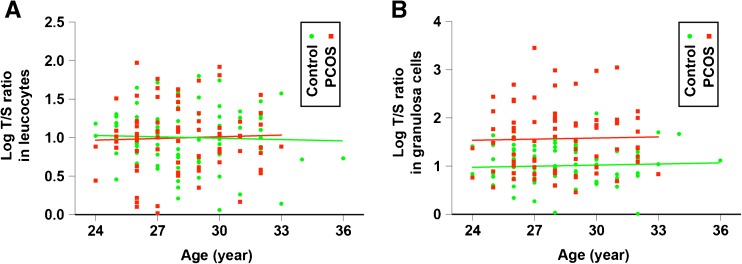
Linear regression models were used to determine whether there were significant associations between relative telomere length and the clinical characteristics in Table 1. The linear regression models were constructed in the PCOS group and the control group. There was no significant association between age and telomere length from leucocytes or GCs (Fig. 2). No clinical parameters were found to be significantly associated with relative telomere length.
Fig. 2.
Relative telomere length in leucocytes and granulosa cells stratified by age. Relative telomere length of leucocytes (a) and granulosa cells (b) are plotted according to age. Controls were shown as green circles (n = 81). Subjects with PCOS were shown as red squares (n = 75)
Discussion
The discovery of telomeres dates back to the early twentieth century. However, it was not until the late 1970s that a major breakthrough enlightened us that telomeres are the key structure protecting chromosomes from deleterious degradation [18]. In humans, the average telomere length typically ranges from 10 to 15 kb. Telomere DNA shortens at a rate of 50–200 bp with each round of chromosome replication in all replicating somatic cells. This unique mechanism determines the limited replications of somatic cells. If the continuously shortening telomere reaches a critical length, it will result in senescence or apoptotic cell death [19]. Due to the essential role of telomeres in cellular aging, since their discovery, researchers have devoted much attention to studying the relationship with human diseases. For example, telomeres are causally linked to dyskeratosis congenita, Werner syndrome, Bloom syndrome, Nijmegen breakage syndrome, Fanconi anemia, and cancer [20–25]. Telomeres have shown their implications in numerous metabolism related diseases, such as cardiovascular disease, atherosclerosis, and diabetes mellitus [26–28]. In the field of human reproduction, telomeres are found to be related to ovarian aging [10, 11]. Notably, a study discovered significantly shorter telomere length in leukocytes from women with PCOS [8], while a later study failed to replicate the association between telomere length in leukocytes and PCOS.
In the current study, we revisited the relationship between telomere length and PCOS in leucocytes and, more importantly, follicular GCs. This is because telomere length varies in different cell types, and previous studies focusing on premature ovary insufficiency revealed contradictory tendencies of telomere length abnormalities in leukocytes and GCs. Interestingly, though failed to detect the association between LTL and PCOS, the current study discovered significantly lengthened telomeres instead of shortened telomeres in GCs from women with PCOS. The absolute difference of telomere length is statistically significant.
The discrepant tendency of telomere length in different cell types deserves profound discussion. In addition to mitotic division, telomere length can also be affected by other factors, including genetics, oxidative stress, and inflammation [29–31]. In the previous study using leukocytes, the authors explained that the significantly shorter LTL is mainly due to excessive oxidative stress, which is also reasonably capable of shortening telomeres in GCs. The key difference between GCs and leukocytes is the existence of telomerase in GCs but not in leukocytes. Telomerase is an enzyme complex that binds telomere and maintains telomere length and integrity. It is absent from differentiated cells or aged cells, but it expresses in germline cells and granulosa cells in the ovary [32]. The activity of telomerase is another determinant of telomere length in GCs except for oxidative stress.
As suggested by prior findings, elevated T, a hallmark of PCOS, potentially increases telomerase activity [33]. Moreover, studies have consistently shown that high telomerase activity is present in the smallest preantral follicles [12, 13]. In biopsies of polycystic ovaries from PCOS women, the median density of small preantral follicles was sixfold greater than in normal ovaries [34]. The accumulated PCOS preantral follicles, which have high telomerase activity, potentially have a greater capability for telomere replenishment than normal follicles, which would accomplish the transition from primordial to antral stages sooner. On the other hand, in situ hybridization and in situ assay of telomerase activity demonstrate the association of high levels of telomerase activity with the highly proliferative GCs in growing follicles [12, 13]. Coincidently, increased GCs proliferation was also found in PCOS [35]; therefore, we can assume that the highly proliferative GCs in PCOS possibly have higher telomere activity.
The GCs collected in the current study is from large ovulatory follicles after hCG trigger and these follicles have finished the luteinizing transition. Telomerase activity is the highest in preantral follicles, and it decreases significantly as the follicles enlarge. Moreover, telomerase is absent from differentiated or aged cells; therefore, telomerase activity was not measured in the study. Our data did not show a correlation between age and telomere length, probably because of our narrow-age cohort design and limited sample size (Fig. 2). Particularly, the replication of GCs only occurs after follicle activation and lasts for months until oocyte retrieval, thus age has little impact on GCTL. Our telomere study in GCs provided supplementary insight to the relationship between telomere length and PCOS, but further data are required to testify whether the relationship is merely an association or a potential mechanism underlying susceptibility to PCOS.
Acknowledgments
This study is supported by the Natural Science Foundation of China (Grant Number 81401262 and 81571407) and the Key Science and Technology Research of Henan Province (Grant Number 152102310145).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Contributor Information
Cuilian Zhang, Phone: +86-037965897665, Email: luckyzcl@qq.com.
Yingpu Sun, Phone: +86-13503841888, Email: syp2008@vip.sina.com.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Broekmans F, Knauff E, Valkenburg O, Laven J, Eijkemans M, Fauser B. PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG Int J Obstet Gynaecol. 2006;113(10):1210–1217. doi: 10.1111/j.1471-0528.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 3.Hardiman P, Pillay OS, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361(9371):1810–1812. doi: 10.1016/S0140-6736(03)13409-5. [DOI] [PubMed] [Google Scholar]
- 4.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. Journal of Clinical Endocrinology & Metabolism. 2010;95(5):2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 5.Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373(1):29–38. doi: 10.1016/j.mce.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Li Z, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16. 3, 2p21 and 9q33. 3. Nat Genet. 2010;43(1):55–59. doi: 10.1038/ng.732. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Zhao H, Cao Y, Yang D, Li Z, Zhang B, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44(9):1020–1025. doi: 10.1038/ng.2384. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Du J, Feng R, Xu Y, Wang H, Sang Q, et al. A possible new mechanism in the pathophysiology of polycystic ovary syndrome: the discovery that leukocyte telomere length is strongly associated with PCOS. The Journal of Clinical Endocrinology & Metabolism. 2013; [DOI] [PubMed]
- 9.Cristina Chielli Pedroso D, Libardi Miranda-Furtado C. Inflammatory biomarkers and telomere length in women with polycystic ovary syndrome 2015. [DOI] [PubMed]
- 10.Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP. Telomere length and reproductive aging. Hum Reprod. 2009;24(5):1206–1211. doi: 10.1093/humrep/dep007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butts S, Riethman H, Ratcliffe S, Shaunik A, Coutifaris C, Barnhart K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. Journal of Clinical Endocrinology & Metabolism. 2009;94(12):4835–4843. doi: 10.1210/jc.2008-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavranos TC, Mathis JM, Latham SE, Kalionis B, Shay JW, Rodgers RJ. Evidence for ovarian granulosa stem cells: telomerase activity and localization of the telomerase ribonucleic acid component in bovine ovarian follicles. Biol Reprod. 1999;61(2):358–366. doi: 10.1095/biolreprod61.2.358. [DOI] [PubMed] [Google Scholar]
- 13.Yamagata Y, Nakamura Y, Umayahara K, Harada A, Takayama H, Sugino N, et al. Changes in telomerase activity in experimentally induced atretic follicles of immature rats. Endocr J. 2002;49(6):589–595. doi: 10.1507/endocrj.49.589. [DOI] [PubMed] [Google Scholar]
- 14.Rotterdam E. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Human reproduction (Oxford, England) 2004;19(1):41. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 15.Matsubara H, Ikuta K, Ozaki Y, Suzuki Y, Suzuki N, Sato T, et al. Gonadotropins and cytokines affect luteal function through control of apoptosis in human luteinized granulosa cells. Journal of Clinical Endocrinology & Metabolism. 2000;85(4):1620–1626. doi: 10.1210/jcem.85.4.6509. [DOI] [PubMed] [Google Scholar]
- 16.Callicott RJ, Womack JE. Real-time PCR assay for measurement of mouse telomeres. Comparative medicine. 2006;56(1):17–22. [PubMed] [Google Scholar]
- 17.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47–e4e. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 19.Wong JM, Collins K. Telomere maintenance and disease. Lancet. 2003;362(9388):983–988. doi: 10.1016/S0140-6736(03)14369-3. [DOI] [PubMed] [Google Scholar]
- 20.Knight S, Heiss N, Vulliamy T, Greschner S, Stavrides G, Pai G, et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am J Hum Genet. 1999;65(1):50–58. doi: 10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goto M, Miller RW, Ishikawa Y, Sugano H. Excess of rare cancers in Werner syndrome (adult progeria) Cancer Epidemiol Biomark Prev. 1996;5(4):239–246. [PubMed] [Google Scholar]
- 22.Ghosh A, Rossi ML, Aulds J, Croteau D, Bohr VA. Telomeric D-loops containing 8-oxo-2′-deoxyguanosine are preferred substrates for Werner and Bloom syndrome helicases and are bound by POT1. J Biol Chem. 2009;284(45):31074–31084. doi: 10.1074/jbc.M109.027532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Group INBSS Nijmegen breakage syndrome. Arch Dis Child. 2000;82(5):400–406. doi: 10.1136/adc.82.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callén E, Samper E, Ramírez MJ, Creus A, Marcos R, Ortega JJ, et al. Breaks at telomeres and TRF2-independent end fusions in Fanconi anemia. Hum Mol Genet. 2002;11(4):439–444. doi: 10.1093/hmg/11.4.439. [DOI] [PubMed] [Google Scholar]
- 25.DePinho RA, Polyak K. Cancer chromosomes in crisis. Nature genetics 2004;36(9). [DOI] [PubMed]
- 26.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 27.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358(9280):472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 28.Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29(2):283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- 29.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–427. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6(5):639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 31.Campisi J. Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev. 2011;21(1):107–112. doi: 10.1016/j.gde.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J-P, Li H. Telomerase in the ovary. Reproduction. 2010;140(2):215–222. doi: 10.1530/REP-10-0008. [DOI] [PubMed] [Google Scholar]
- 33.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114(11):2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webber L, Stubbs S, Stark J, Trew G, Margara R, Hardy K, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017–1021. doi: 10.1016/S0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 35.Stubbs SA, Stark J, Dilworth SM, Franks S, Hardy K. Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. Journal of Clinical Endocrinology & Metabolism. 2007;92(11):4418–4426. doi: 10.1210/jc.2007-0729. [DOI] [PubMed] [Google Scholar]