Abstract
To evaluate the risks of pesticides for pollinators, we must not only evaluate their toxicity but also understand how pollinators are exposed to these xenobiotics in the field. We focused on this last point and modeled honey bee exposure to pesticides at the landscape level. Pollen pellet samples (n = 60) from 40 Belgian apiaries were collected from late July to October 2011 and underwent palynological and pesticide residue analyses. Areas of various crops around each apiary were measured at 4 spatial scales. The most frequently detected pesticides were the fungicides boscalid (n = 19, 31.7%) and pyrimethanil (n = 10, 16.7%) and the insecticide dimethoate (n = 10, 16.7%). We were able to predict exposure probability for boscalid and dimethoate by using broad indicators of cropping intensity, but it remained difficult to identify the precise source of contamination (e.g. specific crops in which the use of the pesticide is authorized). For pyrimethanil, we were not able to build any convincing landscape model that could explain the contamination. Our results, combined with the late sampling period, strongly suggest that pesticides applied to crops unattractive to pollinators, and therefore considered of no risk for them, may be sources of exposure through weeds, drift to neighboring plants, or succeeding crops.
Introduction
Pollinators like bees cover very large areas every day, visiting numerous plants for nectar, pollen, or gum collection and water sources. So doing, they also unintentionally collect airborne particles or substances diluted in the air. This has lead to using honey bees, a species often used as a model, and beekeeping products as biological indicators for environmental monitoring1–18. Monitoring of exposure to various environmental contaminants has already been carried out; these contaminants include heavy metals2, 5, 14, 15, 17, pesticides3, 4, 11–13, polycyclic aromatic hydrocarbons6, 7, 9, 10, 18 and radioactivity16. Unfortunately, it is often not possible to identify the specific sources of contamination.
The exposure of honey bees to pesticides has been linked to increased probability of colony disorders and losses19–21, alone or in combination with other stress-creating factors like poor nutrition or pathogen and parasite loads22–24. For this reason, it is crucial to understand the possible exposure pathways of honey bees to pesticides once they are released in the environment. Pesticide risk assessment is not just about the evaluation of the toxicity of the products. Ideally, we should also be able to accurately estimate how living organisms will be exposed to these products in the environment.
Efforts to model the exposure of bees to pesticides have been carried out recently for risk assessment purposes. Some models aim to estimate direct contact exposure for spray applications25, while others have focused on contact exposure through dust26 or on estimating pesticide intake27–29. Several routes of exposure are today aggregated for a more comprehensive estimation of the exposure of the honey bee colony30. However, more quantitative data on residue levels and their impacts on bee and colony are still needed31.
On the other hand, models of honey bee colony dynamics already integrate a number of stressors, and are a promising tool for impact evaluation of land management or stressors like pesticides at the landscape level32, 33. The quality of these models will depend on their capacity to predict the sources of contamination. The aim of this study is to test if it is feasible to identify the contamination origin by modeling the exposure probability at the landscape level.
We study the relationship between pesticide contamination of pollen pellets and both the botanical origin of the pollen and the areas of grasslands and different crops at four spatial scales around the apiaries (n = 40). Three pesticides with different physico-chemical properties are examined in detail as case studies. The results are interpreted relative to the authorized uses of these pesticides in the different crops present in the potential foraging area of honey bees. The aim here is therefore not to evaluate the toxicity of these pesticides or the consequence of the contamination on honey bee health, but to explore the contamination pathways and to evaluate the methodology on these case studies.
Results
We consider three main data sets for the analyses: (1) pesticide load of pollen; (2) botanical origin of pollen and (3) landscape around the apiary.
Pesticides
About half of the analyzed samples contained at least one pesticide (n = 28/60). The most frequent were two fungicides, boscalid (n = 19, 31.7%) and pyrimethanil (n = 10, 16.7%), and one insecticide, dimethoate (n = 10, 16.7%). Boscalid residues ranged from 0.70 to 512 μg/kg, pyrimethanil residues ranged from 0.60 to 21.70 μg/kg and dimethoate residues ranged from 0.21 to 1.4 μg/kg (Supplementary Information 1 Table S1b). Four other active ingredients (a.i.) were detected with lower frequency (n = 1): trifloxystrobin, kresoxim-methyl, cyprodinil (fungicides) and thiamethoxam (a neonicotinoid insecticide) (Fig. 1). Eleven samples (18.3%) contained two or more a.i. simultaneously, reaching a maximum of three a.i. per sample and five a.i. per apiary. Pollen samples collected in July-August were more frequently contaminated than those from September-October (n = 18/29 and n = 10/31 respectively, binomial GLM, Likelihood Ratio (LR) = 5.7, df = 1, p = 0.017, Fig. 1). However, despite the small number of October samples analyzed, we were surprised to detect boscalid contaminations as late as 14 October.
Figure 1.
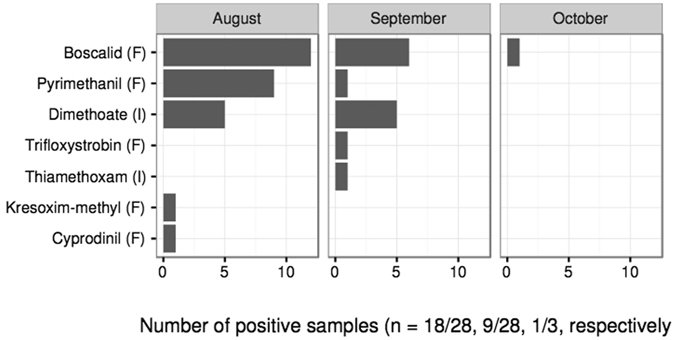
Frequency of pollen contamination per month and for each pesticide. Two samples from July are not shown. No pesticides were detected in these two samples. I = Insecticide, F = Fungicide.
Pollen botanical origin
During the period considered (late July-October) the most abundantly collected pollen grains belonged to Brassicaceae, Hedera elix (ivy), Trifolium spp., Phacelia tanacetifolia, Rosaceae and Asteraceae (Supplementary Information 1 Fig. S2b). The August samples were characterized by more diversified pollen resources with predominance of Trifolium spp., Rosaceae and Asteraceae (including Taraxacum spp.) pollen. The pollen collected in September and October was less diversified and characterized by a higher abundance of ivy and P. tanacetifolia pollen (Supplementary Information 1 Fig. S2a). Brassicaceae pollen was found to be used during the whole period under review. These most abundant pollen types were also collected by 35/40 (87.5%) of the apiaries with the exception of Rosaceae (24/40, 60%) and P. tanacetifolia (12/40, 30%). This indicates that P. tanacetifolia fields are less present around the apiaries, but that they are massively visited by bees when they are present.
Landscape description
A high correlation exists between different crop areas (including grasslands), especially at the highest spatial scale (3000 m radius buffers) (Supplementary Information 1 Fig. S1c). These correlations are particularly high (0.78–0.93) for cereals, beet, potato and vegetables areas (Supplementary Information 1 Table S2a). Grassland areas are negatively correlated with most crop areas. The exploratory analysis showed a gradient in landscape composition around the apiaries: from landscapes dominated by crops to landscapes dominated by grasslands or with very little agricultural land use (urban or forest zones). The samples contaminated with pesticides are clearly more frequent in landscapes dominated by crops (Fig. 2). Cereals are present around all apiaries in 3000 m buffers. However, zooming into a radius of 500 m around the apiaries, one can divide the landscape into three groups: (1) dominance of cereals, beets and potatoes corresponding to the most intensive agricultural landscape; (2) dominance of cereals and grasslands (without beet and potato crops) corresponding to more extensive crop landscapes; and (3) areas dominated by grasslands and without cereals, beets and potatoes (clustering with heat map - see Supplementary Information 1 Fig. S1a and b).
Figure 2.
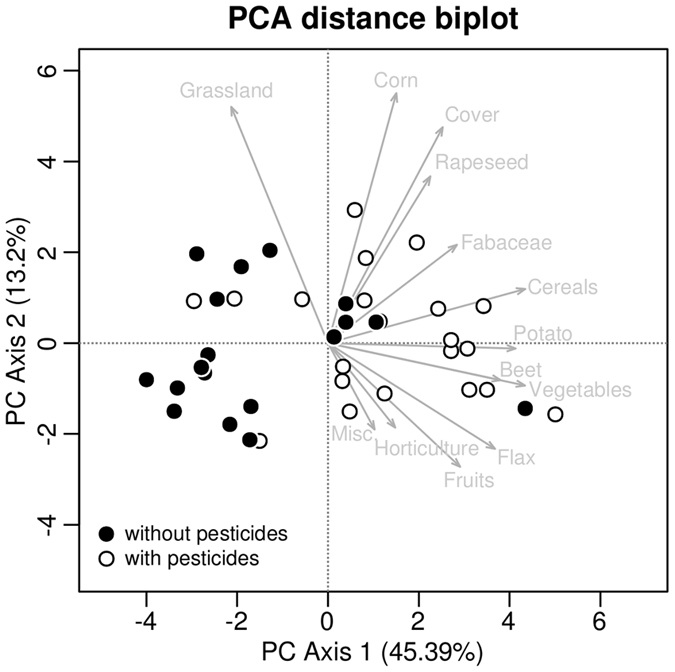
Principal Component Analysis distance biplot of the areas of crops and grasslands 3000 m around the apiaries. The areas were square root transformed and standardized before the analysis.
Case study 1 – Predicting bees’ exposure to boscalid
Boscalid is a carboxamid fungicide34 with systemic properties (octanol-water partition coefficient, log Kow = 2.96). In addition, this active substance is persistent in soil (DT50 typical = 200 days)35. In 2011, it was authorized in Belgium for the treatment of cereals, potatoes, rapeseed, many vegetables, fruits and horticulture.
We aimed to identify specific sources of boscalid contamination for bees. However, all crops have a predictive power with the exception of corn, rapeseed, cover crops and horticulture (AICc lower than the null model or with a difference <2, Table 1). The ten best models include as predictors: all crops and authorized crops areas at 3000 m, 1500 m and 1000 m and beet, potato and cereals at 3000 m (AICc differences > 18; Area Under the Receiver Operating Curve (AUC) 0.83–0.92). The models using 3000 m buffer data systematically have a better predictive performance, while the models using 500 m buffers systematically have the lowest one. Beet area in a 3000 m buffer is the best predictor of boscalid contamination despite the fact that the use of boscalid is not authorized in this crop. This predictor is four times better (ratio of AICc weights) than the second, using all crops as variable (i.e. areas of all crops combined) and more than 15 times better than the third, including potato and cereals areas in 3000 m (i.e. crops for which boscalid is authorized).
Table 1.
Results of univariate Binomial GLMs modeling the probability that a pollen sample would be contaminated by a given pesticide vs the areas of different (groups of) crops and grasslands at different spatial scales (Buffer column, in meters).
| Boscalid | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Buffer | Variable | AICc | Δ AICc | AICc w | AUC | Slope | LRT | p |
| 1 | 3000 | Beet | 40.01 | 0 | 0.604 | 0.894 | 0.33 | 29.14 | <0.0001 |
| 2 | 3000 | All Crops | 42.81 | 2.798 | 0.149 | 0.894 | 0.18 | 26.34 | <0.0001 |
| 3 | 3000 | Authorized Crops | 44.76 | 4.750 | 0.056 | 0.883 | 0.19 | 24.39 | <0.0001 |
| 4 | 3000 | Potato | 45.24 | 5.231 | 0.044 | 0.920 | 0.36 | 23.90 | <0.0001 |
| 5 | 3000 | Cereals | 45.74 | 5.734 | 0.034 | 0.863 | 0.21 | 23.40 | <0.0001 |
| 6 | 1000 | All Crops | 46.06 | 6.054 | 0.029 | 0.846 | 0.47 | 23.08 | <0.0001 |
| 7 | 1500 | All Crops | 46.40 | 6.386 | 0.025 | 0.851 | 0.31 | 22.75 | <0.0001 |
| 8 | 1500 | Authorized Crops | 47.03 | 7.016 | 0.018 | 0.854 | 0.34 | 22.12 | <0.0001 |
| 9 | 1000 | Authorized Crops | 47.35 | 7.344 | 0.015 | 0.840 | 0.53 | 21.79 | <0.0001 |
| 10 | 500 | All Crops | 48.79 | 8.779 | 0.007 | 0.834 | 0.70 | 20.36 | <0.0001 |
| 11–41 | (…) | ||||||||
| 42 | — | NULL MODEL | 66.92 | 26.91 | 0 | 0.500 | — | — | — |
| 43–55 | (…) | ||||||||
| Pyrimethanil | |||||||||
| 1 | 3000 | Rapeseed | 42.18 | 0 | 0.287 | 0.735 | 0.39 | 8.55 | 0.00346 |
| 2 | 1000 | Rapeseed | 43.69 | 1.515 | 0.134 | 0.756 | 0.61 | 7.03 | 0.00801 |
| 3 | 1500 | Rapeseed | 44.25 | 2.074 | 0.102 | 0.731 | 0.45 | 6.47 | 0.01095 |
| 4 | 3000 | Flax | 46.15 | 3.970 | 0.039 | 0.793 | 0.24 | 4.58 | 0.03240 |
| 5 | 3000 | Horticulture | 46.82 | 4.640 | 0.028 | 0.641 | −1.93 | 3.91 | 0.04807 |
| 6 | 500 | Beet | 47.09 | 4.908 | 0.025 | 0.683 | 0.47 | 3.64 | 0.05643 |
| 7 | 3000 | Cover | 47.36 | 5.178 | 0.022 | 0.630 | 0.48 | 3.37 | 0.06643 |
| 8 | 500 | Potato | 47.36 | 5.184 | 0.021 | 0.659 | 0.46 | 3.36 | 0.06666 |
| 9 | 1000 | Fabaceae | 47.66 | 5.482 | 0.018 | 0.619 | 0.52 | 3.07 | 0.07999 |
| 10 | 3000 | Cereals | 47.78 | 5.599 | 0.017 | 0.719 | 0.07 | 2.95 | 0.08600 |
| 11–12 | (…) | ||||||||
| 13–55 | — | NULL MODEL | 48.5 | 6.322 | 0.012 | 0.500 | — | — | — |
| 14–55 | (…) | ||||||||
| Dimethoate | |||||||||
| 1 | 1000 | Cereals | 16.52 | 0 | 0.989 | 0.991 | 3.32 | 37.55 | <0.0001 |
| 2 | 1000 | All Crops | 26.93 | 10.40 | 0.005 | 0.955 | 0.93 | 27.15 | <0.0001 |
| 3 | 1500 | Beet | 29.61 | 13.08 | 0.001 | 0.942 | 0.77 | 24.47 | <0.0001 |
| 4 | 1000 | Beet | 30.29 | 13.76 | 0.001 | 0.946 | 0.97 | 23.79 | <0.0001 |
| 5 | 1500 | Cereals | 30.68 | 14.15 | 0.001 | 0.906 | 0.62 | 23.40 | <0.0001 |
| 6 | 1500 | Authorized Crops | 31.37 | 14.85 | 0.001 | 0.920 | 0.67 | 22.70 | <0.0001 |
| 7 | 1500 | All Crops | 31.4 | 14.88 | 0.001 | 0.929 | 0.46 | 22.67 | <0.0001 |
| 8 | 1000 | Authorized Crops | 33.26 | 16.73 | 0 | 0.924 | 0.72 | 20.82 | <0.0001 |
| 9 | 3000 | Beet | 33.73 | 17.21 | 0 | 0.915 | 0.36 | 20.34 | <0.0001 |
| 10 | 3000 | Authorized Crops | 34.36 | 17.84 | 0 | 0.924 | 0.30 | 19.71 | <0.0001 |
| 11–37 | (…) | ||||||||
| 38 | NA | NULL MODEL | 51.85 | 35.32 | 0 | 0.500 | — | — | |
| 39–55 | (…) | ||||||||
Only the ten best models (lowest AICc) are shown along with the null model. LRT = Likelihood Ratio Test statistic (degrees of freedom = 1 for all models). Full tables available in the Supplementary Information 1.
The predicted probability of boscalid contamination is close to 0 when no boscalid-authorized crops are present in a radius of 3000 m around the apiary and rises to 0.9 for areas of boscalid-authorized crops of 1500 ha (Fig. 3).
Figure 3.
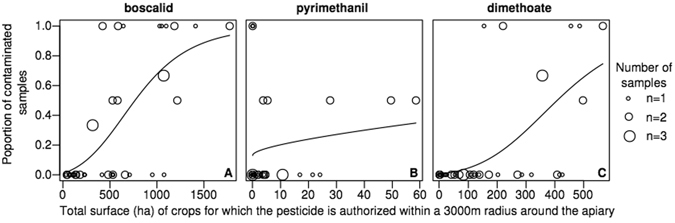
Observed proportion of samples contaminated for each pesticide and the corresponding predicted value (binomial GLM) relative to the areas of authorized crops around the apiary.
As for the botanical origin, Rosaceae and P. tanacetifolia pollen are systematically the most important predictors of boscalid contamination (AICc variable weight > 0.71- Table 2, positive relationship). There is no difference in boscalid contamination between July-August and September-October (AICc variable weight = 0.267).
Table 2.
Results of the model selection for the GLMs modeling the presence of pesticides in the pollen vs the abundance of different pollen taxa.
| Boscalid | Pyrimethanil | Dimethoate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| w | coef | se | w | coef | se | w | coef | se | |||
| Intcpt | 1 | −2.760 | 1.456 | Intcpt | 1 | −4.179 | 2.274 | Intcpt | 1 | −3.413 | 1.614 |
| ros | 0.834 | 0.323 | 0.158 | SepOct | 0.959 | −3.282 | 1.288 | vic | 0.996 | 0.822 | 0.269 |
| pha | 0.715 | 0.206 | 0.119 | bra | 0.952 | 0.747 | 0.341 | pha | 0.768 | 0.296 | 0.160 |
| tar | 0.538 | 0.173 | 0.132 | ivy | 0.250 | 0.002 | 0.064 | ros | 0.388 | 0.109 | 0.116 |
| bal | 0.433 | −0.108 | 0.102 | tar | 0.248 | 0.028 | 0.071 | tar | 0.280 | 0.054 | 0.087 |
| vic | 0.395 | 0.103 | 0.103 | api | 0.244 | −0.021 | 0.067 | SepOct | 0.263 | −0.124 | 0.374 |
| ivy | 0.350 | 0.062 | 0.071 | pha | 0.242 | −0.014 | 0.045 | api | 0.261 | −0.043 | 0.092 |
| bra | 0.332 | 0.056 | 0.066 | ast | 0.240 | 0.017 | 0.072 | ivy | 0.260 | −0.027 | 0.061 |
| api | 0.273 | 0.039 | 0.067 | tri | 0.240 | −0.016 | 0.065 | tri | 0.250 | 0.030 | 0.071 |
| SepOct | 0.267 | −0.019 | 0.321 | ros | 0.237 | 0.004 | 0.048 | ast | 0.246 | −0.029 | 0.084 |
| ast | 0.243 | −0.004 | 0.058 | vic | 0.232 | −0.011 | 0.068 | bra | 0.230 | 0.007 | 0.055 |
| tri | 0.231 | 0.002 | 0.042 | ||||||||
“w” = AICc variable weight, “coef” = models averaged coefficient, “se” = unconditional standard error. We interpreted only the explanatory variables with w > 0.60 (in bold). Intcpt = model intercept and SepOct = binary explanatory variable corresponding to the period: July/August or September/October. Abbreviation of the pollen types: api = Apiaceae, ast = Asteraceae, bal = Balsaminaceae, bra = Brassicaceae, ivy = Hedera elix, pha = Phacelia tanacetifolia, ros = Rosaceae, tar = Taraxacum spp., tri = Trifolium.
Case study 2 – Predicting bees’ exposure to pyrimethanil
The second most frequently detected pesticide is pyrimethanil, a fungicide with systemic properties (log Kow = 2.84) and moderate persistence in soil (typical DT50 = 55 days)36. In 2011 it was authorized in Belgium for fruit production and horticulture (including plant nurseries and Christmas trees).
It was impossible to predict pyrimethanil contamination based on its authorized uses (Table 1). We even found contaminated pollen samples coming from apiaries with no crops in a radius of 3000 m for which pyrimethanil use is authorized (Fig. 3). The only crop that could predict the frequency of pyrimethanil contamination to a certain extent was rapeseed, for which pyrimethanil use was not authorized in Belgium in 2011 (AICc difference with the null model: 6.3–4.4). However, the AUCs of these rapeseed models are quite low (0.73–0.75), meaning that their discrimination capability is not very good with this dataset and should be even lower with a different testing dataset. Areas of flax and horticulture in a radius of 3000 m are weak predictors of pyrimethanil contamination (AICc difference 3.3 and 1.7, respectively). Pyrimethanil use is authorized for horticulture, but the model slope is negative, which means that we tend to observe less pyrimethanil contamination when the horticulture areas increase.
Among the models considering the pollen types, both the sampling period and the Brassicaceae pollen are good predictors of the presence of pyrimethanil (AICc variable weight > 0.95, Table 2). The contamination is significantly more frequent in July-August than in September-October. After controlling for the period, we find a strong positive relationship between pyrimethanil and the abundance of Brassicaceae pollen. This pollen probably comes from wild plants or cover crops like mustard, since rapeseed does not bloom at this time of the year in Belgium. Hence these pollen results (pyrimethanil-Brassicaceae) do not particularly support the landscape results (pyrimethanil-rapeseed).
Case study 3 – Predicting bees’ exposure to dimethoate
Dimethoate is an organophosphorous insecticide, systemic (log Kow = 0.704), and non-persistent in soil (typical DT50 = 2.6 days)37. It was authorized in 2011 in Belgium to control insect pests in beets, peas, multi-annual fruit production, many vegetables, horticulture (incl. plant nurseries and Christmas trees), and until 2010 for potatoes.
Cereals, beet, all crops and authorized crops areas were the best predictors of pollen contamination by dimethoate (ten best models with differences of AICc relative to the null model between 35 and 17, AUC between 0.92 and 0.99, Table 1). Many other crop areas also had a good predictive power: vegetables, flax, grassland, horticulture, or potato. The models that consider the cropped areas at 1000 m and at 1500 m systematically had better predictive power, while the 500 m spatial scale systematically provided the worst models. Cereal areas in a 1000 m buffer were by far the best predictors of dimethoate contamination (AICc difference = 10.40), having a very high explanatory power (AUC = 0.99; AUC = 0.955 for the second best model).
As for the botanical origin, the probability of pollen contamination by dimethoate increases when pollen samples contain more Vicia spp. or P. tanacetifolia (AICc variable weight > 0.76, Table 2). There is no evidence for a difference of contamination between July-August and September-October.
Discussion
Contamination of pollen pellets provide a representative image of flower contamination at a moment in time in contrast with the pollen that is stored in the beehive as beebread. The variety and frequency of pesticides detected in pollen pellets of our study is surprising for two reasons. Firstly, we did not expect pollen pellets to be contaminated with such a variety of pesticides at the latitude of Belgium and at this period of the year (late July-October) because few pesticides are applied so late in the season. Secondly, most of the crops with authorized uses for the detected pesticides do not bloom at this time of the year, which is confirmed by the botanical origin of pollen samples, containing mainly pollen from wild flowers and crops grown as cover or catch crops. As a result, the current approach of pesticide risk assessment and authorization based on the attractiveness to bees of different crops proves to be erroneous. Our results entail that pesticides applied to crops unattractive to bees as food sources like cereals or sugar beets can in fact be a source of exposure, either through weed contamination, drift or by the mobilization of residues of systemic/persistent products by succeeding crops. These results provide strong evidence that the concept of “crop not attractive to bees” is irrelevant to evaluating the risk of pesticide exposure. This conclusion is further supported by our landscape analyses. Our results add support to an increasing body of evidence indicating that pesticides applied to a crop are much more mobile than expected. Contamination of pollen pellets collected by bees late in the season, outside the period of pesticide application, has also been observed in other studies21, 38, 39, and contamination of wild flowers of the field margins has also been described40–42.
Simon-Delso et al.19 described pesticides found in beebread collected during the same period of the year as the present study in 21 apiaries. Boscalid, the most frequently detected molecule in pollen pellets (present study), was observed in beebread as the third most frequent residue. Pyrimethanil was reported in ten samples in pollen pellets, and was the sixth most common contaminant in beebread, after the fungicide iprodione and the synergist piperonyl butoxide. The insecticide dimethoate was detected in pollen pellets but not in beebread. Of the active ingredients detected only once, only trifloxystrobin was also found in beebread. These different contamination profiles indicate the value of analyzing different beekeeping matrices. Pesticides with low persistence like dimethoate are more likely to be found in pollen pellets than in beebread because they are collected at the beehive entrance, while beebread is pollen processed and stored within the colony during longer periods. However, this also indicates how difficult it is to characterize the whole range of pathways of pesticide exposure for honey bees.
There are long debates about the foraging radius of honey bees around their colony, most likely due to the variability of and dependence on the resources available in the surroundings, the weather conditions and the needs of the colony43. In our study, based on pollen samples collected from August to October, the models with best predictive value for pollen contamination were almost systematically those that considered crops located in a radius of more than 500 m, and up to 3000 m from the colony. Future work could test if differences in foraging range are translated into differences of pesticide exposure. However, the higher predictive power of 3000 m models may be independent from the foraging distance of bees because crop surfaces at higher landscape scales could just be a better indicator of agricultural practices in the close surroundings of the apiary (crops rotations, more/less intensive agriculture, etc.; see below).
We aimed to identify potential contemporaneous boscalid uses to explain direct pollen contamination. Boscalid-based products are authorized for a wide range of crops. They can still be used in orchards in August-September, a few weeks before the fruit harvest. They can also be used in August on beans. Consequently, direct exposure to treated crops like vegetables or orchards should be considered. However, the best predictor of boscalid contamination in pollen samples is sugar beet area in a radius of 3000 m, despite the fact that boscalid use is not authorized for this crop. Sugar beet is typically included in a crop rotation scheme with cereals and potatoes (sugar beet or potato/wheat/barley), in the most intensive agricultural areas of Belgium (Sandy-Loam Region). In contrast with cereals that are widely farmed even in less intensive areas, sugar beet may be a better indicator of cereals farmed in a more intensive way and hence potentially receiving more pesticides. After the beet model, all crops is the model with best predictive value and the most frequently found among the ten most predictive models (at all four landscape scales), followed by authorized crops. This, together with the fact that individual crops (i.e. potatoes and cereals) complete the list of the ten most predictive models, indicates that direct exposure to treated crops is unlikely to be the only source of contamination, as pesticides containing boscalid are typically applied to these crops much earlier in the season. Furthermore, with the exception of beans, none of the crops for which boscalid is authorized are attractive to honey bees for food collection at this time of the year. The high persistence of the molecule could possibly explain contamination later in the year or even during the next year. This may lead to the contamination of wild flowers or of succeeding crops like cover/catch crops. Contamination linked to cover/catch crops is supported by the positive relationship between boscalid presence and the abundance of P. tanacetifolia pollen observed in this study. P. tanacetifolia is not native in Belgium and is only used as a catch crop and rarely in gardens. Other persistent and systemic pesticides (e.g. neonicotinoids) can contaminate wild flowers in field margins41, 42 and succeeding crops44–47.
The case of pyrimethanil pollen contamination is difficult, because none of the authorized uses predict its presence. In addition, we found a number of positive samples (n = 4) with no crops for which this pesticide was authorized in a radius of 3000 m around the corresponding apiary. At this point, we considered the following hypotheses: (1) the contamination came from further than 3000 m; (2) the notification of crop areas for which this fungicide is authorized is not complete (e.g. Christmas trees, some horticultural or vegetable crops); (3) there is an illegal use of pyrimethanil in rapeseed earlier in the season (rapeseed is already harvested at this time of the year but the product is moderately persistent). The later hypothesis seems unlikely because there are many other efficient fungicides authorized for rapeseed and the model discriminatory power was quite low for this crop. Pyrimethanil-based products are also used in orchards at the beginning of the season, but we found no support for a pyrimethanil-Fruit areas relationship. These products could also be used for the production of some specific vegetables like peas, beans, and other legumes. However, in the year of the study, 2011, these uses were not authorized in Belgium. Pea fields that are typically harvested in July could be followed by mustard as cover/catch crop. This would match the link we found between the residues of pyrimethanil and the pollen of Brassicaceae in August. Models specifically using areas of cultivated peas and broad beans (instead of the grouped category fabaceae) showed better predictive value at 1500 m and 1000 m (difference of AICc = 3.60 and 2.85, Likelihood Ratio Test statistic (LRT p values = 0.016 and 0.024), but their discrimination power was not very high (AUC = 0.67 and 0.68 - see Supplementary Information 1 Table S6 for details). As a result, this hypothesis remains only a putative scenario that should be tested by specific sampling on peas in the field.
Dimethoate is not a persistent pesticide. We can therefore assume that pollen contamination came from an application during August-September. The best predictors of the presence of dimethoate in pollen were cereals (non-authorized use and crop already harvested at this time of the year), beet (authorized use, but unlikely to be applied because there are no insect pests at this time of the year), and all crops. At this time of the year, vegetables (e.g. carrots and Brussels sprouts) are the only crops possibly being treated with dimethoate for which the area can be used to predict the frequency of dimethoate in our models. As a result, the fact that cereals or sugar beets are the best predictors for dimethoate contamination could be because these large arable crops are good indicators of intensive, large scale, vegetable production, which may be included in crop rotation schemes: there is a strong correlation (R > 0.77) between vegetables and beet, cereals and potato areas (Supplementary Information 1 Fig. S1c). However, none of these vegetable crops are in bloom at the sampled period (except occasionally carrots), which made us wonder about the pertinence of this hypothesis. The palynological results show that pollen from Vicia spp. and P. tanacetifolia are positively linked with the presence of dimethoate. It is common agricultural practice in the region to include flowering strips in the borders of vegetable fields. Therefore, a possible explanation could be that Vicia spp. occur in field margins or that P. tanacetifolia is planted in flowering strips and that their flowers get contaminated by drift with dimethoate applied on the field. The abundance of Vicia spp. pollen is positively correlated to vegetables, beet, potato and cereals areas (see Supplementary Information 1 Fig. S3).
In conclusion, our findings show that the highest spatial scales (3 km) provide the best predictive power for pollen contamination. Pesticides applied to “non-bee-attractive” crops like cereals or sugar beets, generally considered of negligible risk for bees, can in fact be a source of exposure through weeds, through drift to neighboring plants or through succeeding crops. These results imply that the concept of “bee-attractive crop” (i.e. a crop visited by bees for nectar and/or pollen collection) is irrelevant for risk assessment and should not be used as a criterion for pesticide authorization. At the landscape level, honey bee exposure to pesticides depends on pesticide use level, physicochemical characteristics, period of the year and landscape composition. Our findings show that the task of modeling the exposure of bees to pesticides once released in the environment may be more complicated than expected. We were able to efficiently predict exposure for two pesticides by using very broad indicators of cropping intensity, but it remains difficult to track the direct source of contamination in the landscape. For the third pesticide, we were not able to find any convincing landscape model that could explain the contamination. On the other hand, our results have consequences for policies and agricultural practices intended to promote the multiplication of nutritional resources for pollinators, like flowering strips, buffer zones, catch crops with melliferous flowers, etc. These should be designed and applied in parallel to policies and practices leading to pesticide use reduction i.e. integrated pest management, organic farming or agro-ecological practices, precision farming and favoring non-persistent/non-systemic pesticide active ingredients. Without such considerations, instead of favoring pollinators through habitat improvement or food availability, we may transform these areas into highly risky zones or even ecological traps for pollinators.
Methods
Field work – sample collection
A group of voluntary beekeepers were requested to participate in the study, with a total of 40 apiaries. Pollen samples (n = 80) were taken from two random colonies per apiary with the help of a PVC pollen trap (Nicot®), placed during one or two days to collect a minimum of 20 g of pollen pellets. Samples from both colonies were pooled together. The samples were collected once, twice or four times per month from mid-July to mid-October 2011. Most of the pollen samples were collected in August and September 2011 (n = 32 and 36 respectively). Two pollen samples were collected in July and ten samples in October. The July samples were taken on 24 and 30 July, and were similar to the August samples in terms of botanical origin of the pollen (Supplementary Information 2 at https://figshare.com/s/86785808b5709331aa1c). One sample collected in April 2012 was removed from the dataset before analysis because this unique sample had a completely different pollen composition and was not comparable to the other samples. Samples were placed in hermetic plastic bags and stored at −20 °C until analysis.
Sample processing
Samples collected from the same apiary during the same month were pooled together and thoroughly mixed. One gram of the blend was sampled for palynological analysis. Whenever the sample quantity allowed it, at least 42 grams of the monthly blend were shipped for pesticide residue analysis (n = 60). Frozen samples were sent for pesticide analysis in dry ice.
Pesticide analyses
The monthly samples of pollen pellets were sent to Floramo Corporation, Italy. A multi-residue analysis was used based on the methodology described by Wiest et al.48, and 45 pesticides/metabolites were analyzed in pollen pellets (Supplementary Information 1 Table S1). The extraction method was based on a modified “QuEChERS method”: two-step Solid/Liquid extraction with solvent and MSPD (Matrix Solid Phase Dispersion) purification as follows: 10 g of the pollen sample extracted with acetonitrile/water followed by liquid/liquid purification with hexane and combined with MSPD purification on PSA and salts. Finally, the purified extract was concentrated below 100 µl and injected into UPLC-MS/MS (Ultra Pressure Liquid Chromatography coupled with tandem mass spectrometry) and gas chromatography coupled with tandem mass spectrometry (GC-MS/MS) programmed in MRM (Multiple Reaction Monitor) mode with two transitions/a.i. Pesticide analysis was possible for 28 samples from August and September each, 1 sample from July and 3 samples from October.
Palynological analyses
The extraction and homogenization method was inspired by the harmonized method of pollen analysis with acetolysis developed by Erdtman49. A minimum of 1000 pollen grains were counted and identified per slide50 at 500x microscopic magnification as described by von der Ohe et al.51. Pollen grains were generally identified up to their taxonomical family due to the difficulty of differentiating plant species. In a few easy cases, identification was performed up to genus level (Taraxacum spp., Trifolium spp., etc.) and to species level for ivy (Hedera helix) and lacy phacelia (Phacelia tanacetifolia).
Landscape data
We measured the areas of different kinds of detailed agricultural land use (i.e. different crops and grasslands) in a circle (buffer) with a radius of 500, 1000, 1500 and 3000 m around the 40 apiaries. We used the official Land Parcel Identification System (SIGEC) used by the Walloon administration to distribute agricultural subsidies to the farmers. This land use information was not available for one of the apiaries located outside the Walloon region. These detailed land use categories (n > 50) were pooled into thirteen more general categories (“crops”) according to their agronomic similarities (see Supplementary Information 1 Table S2b). For each of the detailed land use categories, we determined whether the three most frequent pesticides observed in this study could be used by checking the official pesticide use authorizations in Belgium in 2011. This allowed us to calculate the areas of authorized crops for each of these pesticides around the apiaries (see Supplementary Information 1 Table S2a).
Statistical analyses
All analyses were performed in R52. All raw datasets and R scripts are provided as supplementary information (Supplementary Information 2 at https://figshare.com/s/86785808b5709331aa1c). For confidentiality reasons, we are not allowed to share the exact location of the apiaries nor the agricultural land use geodata.
We considered three main datasets for the analyses: pesticides, landscape (areas of different crops and grasslands) and pollen (taxonomical origin). For the pesticides we used only presence/absence information in each pollen sample. The pollen samples had a variable number of total pollen grains (around 1000). To make direct comparison possible, the number of pollen grain (x) was standardized as x * 1000/N (N = total grain number in the sample) and rounded to units.
Predicting bees’ exposure to different pesticides with crops and grassland areas
As crop areas are only available at the apiary level, we used binomial GLMs with the proportion of positive samples in a given apiary as the response variable for each pesticide. Preliminary analyses showed that the crop areas are strongly correlated, causing multicollinearity problems when they are used as predictors in multiple regression approaches. Grouping correlated predictors was not an option here because we wanted to keep them separated to interpret the results relative to the authorizations of pesticide use for each crop. Consequently, we decided to use binomial GLMs with only one explanatory variable at a time. We built separate univariate models for each grouped land use surface and for each of the four spatial scales. We also used two additional predictors (at four scales): 1) all crops: sum of the crop areas i.e. without grasslands and without taking into account the product authorizations; 2) authorized crops: sum of the crop areas for which the product is authorized. A “null model” was also built with no explanatory variable, i.e. this model estimates the mean proportion of samples contaminated in the dataset. All areas were square root transformed, because this improved the quality of the models (i.e. linearity and homogeneity of the residuals). At the lowest spatial scales (500 m and 1000 m) some of the minor crops were totally absent from all apiaries and the corresponding models were therefore not estimated.
These models were compared in terms of AICc and AICc weights53 between each other and more particularly with the “null model”. Models with lower AICc are considered to be better (good fit but not overly complex to allow extrapolation to other datasets), and a difference of AIC lower than 2 is often considered as negligible. In addition we computed for each single model a likelihood ratio test which compares the model to the null model. We also computed the Area Under the Receiver Operating Curve (AUC or AUROC) as a descriptive statistic of the capacity of each model to discriminate (in this dataset) between apiaries with the pesticide (frequency in the samples > 0) or without it (frequency = 0). An AUC = 1 indicates a perfect discrimination capability (all predicted presences are effective and none of the predicted presences are absences). If AUC = 0.5, the model predictions are as good as pure chance.
We checked the spatial correlation of the best model residuals for each pesticide with a spline correlogram. Spatial correlation was always low and not significantly different from 0.
Predicting bees’ exposure to different pesticides with pollen composition
The pollen data are available at the sample level and there were no multicollinearity problems with these data. Consequently, we computed binomial GLMs with the presence/absence of the pesticide in the sample as response variable and the ten most common pollen types as explanatory variables. We also added the period of the year (July-August or September-October) as explanatory variable. The pollen data were log(x + 1) transformed because this clearly improved the model fit. With 59 pollen samples from 39 apiaries, some pollen samples came from the same apiary and were therefore not independent. We first tried to use binomial Generalized Linear Mixed Models with the apiary as random effect to take this pseudo-replication into account, but most of these models did not converge, probably because most of the apiaries in the sample had only one or two replicates (rarely three). Our results with the simple GLMs are therefore probably slightly anti-conservative. We removed the explanatory variable Balsaminaceae because none of the pollen samples containing grains of this family were contaminated with dimethoate. This lack of variability posed problems in the statistical analyses.
For each pesticide, we computed models for all possible combinations of explanatory variables (2048 models) along with their AICc and AICc model weight. The AICc model weight was used to compute shrinkage model averaged coefficients, unconditional standard errors and AICc variable weights53. The AICc model weight is a measure of model selection uncertainty (probability that a model will have the lowest AICc if we resample the data, given a set of models). The AICc variable weights allow us to compare the relative importance of the explanatory variables (it gives the probability that a given variable will be in the best - lowest AICc - model if we resample the data). We interpreted only the explanatory variables with an AICc variable weight > 0.6. The model averaged regression coefficients are shrunk toward 0 when the corresponding explanatory variable is present only in “bad” models.
Data availability
Supplementary information is available in a public repository at https://figshare.com/s/86785808b5709331aa1c
Electronic supplementary material
Acknowledgements
We would like to thank the beekeepers and beekeeping technicians for their dedication and active contribution to our study. Likewise, we thank Szaniszlo Szöke, Martin Dermine and John Nganga for their critical and constructive review of the article. We would like also to thank the Walloon Region for their financial support and the Direction Générale Opérationnelle Agriculture, Ressources Naturelles et Environnement (DGO3), Département des Aides (D4), Direction des Surfaces (D42), Service 42/3 - LPIS (Land Parcel Identification System) - Service Public de Wallonie for the spatial data. We would also like to thank the team in Floramo Corporation.
Author Contributions
N.S. and G.S.M. wrote the main manuscript text. G.S.M. performed the analyses with the help of L.H. and N.S. N.S., L.H. and E.B. designed the experiment and N.S. collected the samples in the field. C.D. performed the palynological analyses. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03467-5
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Accorti M, Persano Oddo L. Un servizio di monitoraggio ambientale urbano: ‘Apincittà’. Inf. Agrar. 1986;42:39–41. [Google Scholar]
- 2.Balestra V, Celli G, Porrini C. Bees, honey, larvae and pollen in biomonitoring of atmospheric pollution. Aerobiologia. 1992;8:122–126. doi: 10.1007/BF02291339. [DOI] [Google Scholar]
- 3.Celli G, Porrini C, Raboni F. Monitoraggio con api della presenza dei ditiocarbammati nell’ambiente (1983–1986) Boll Dell’Istituto Entomol. Dell’Università Degli Studi Bologna. 1988;43:195–205. [Google Scholar]
- 4.Celli G, Porrini C. Apicidi e residui di pesticidi nelle api e nell’alveare in Italia (1983–1986) Boll. Dell’Istituto Entomol. Guido Gd. Dell’Università Degli Studi Bologna. 1987;42:75–86. [Google Scholar]
- 5.Celli, G., Porrini, C. & Tiraferri, S. Rapporti tra apicoltura. e ambiente. L’ape come indicatore biologico dei esticidi con articolare riferimento alla L’PP provincia di Forlì). (Nota preventiva) (1985).
- 6.Ciemniak A, Witczak A, Mocek K. Assessment of honey contamination with polycyclic aromatic hydrocarbons. J. Environ. Sci. Health Part B. 2013;48:993–998. doi: 10.1080/03601234.2013.816609. [DOI] [PubMed] [Google Scholar]
- 7.Lambert O, et al. Polycyclic aromatic hydrocarbons: bees, honey and pollen as sentinels for environmental chemical contaminants. Chemosphere. 2012;86:98–104. doi: 10.1016/j.chemosphere.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Mercuri AM, Porrini C. Melissopalynological analysis applied to air pollution studies in urban areas of Modena and Reggio Emilia (Italy) Aerobiologia. 1991;7:38–48. doi: 10.1007/BF02450016. [DOI] [Google Scholar]
- 9.Moret S, Purcaro G, Conte LS. Polycyclic aromatic hydrocarbons (PAHs) levels in propolis and propolis-based dietary supplements from the Italian market. Food Chem. 2010;122:333–338. doi: 10.1016/j.foodchem.2010.02.041. [DOI] [Google Scholar]
- 10.Perugini M, et al. Monitoring of polycyclic aromatic hydrocarbons in bees (Apis mellifera) and honey in urban areas and wildlife reserves. J. Agric. Food Chem. 2009;57:7440–7444. doi: 10.1021/jf9011054. [DOI] [PubMed] [Google Scholar]
- 11.Porrini C, Celli G, Radeghieri P. Monitoring of pesticides through the use of honeybees as bioindicators of the Emilia-Romagna coastline (1995–1996) Ann. Chim. 1998;88:243–252. [Google Scholar]
- 12.Porrini, C. et al. Use of honey bees as bioindicators of environmental pollution in Italy. Honey Bees Estim. Environ. Impact Chem. 186 (2002).
- 13.Porrini C, et al. The death of honey bees and environmental pollution by pesticides: the honey bees as biological indicators. Bull. Insectology. 2003;56:147–152. [Google Scholar]
- 14.Stein K, Umland F. Spurenbestimmung von Blei, Cadmium und Mangan in Honigen und Zuckern. Fresenius J. Anal. Chem. 1986;323:176–177. doi: 10.1007/BF01172914. [DOI] [Google Scholar]
- 15.Stein K, Umland F. Mobile und immobile Probensammlung mit Hilfe von Bienen und Birken. Fresenius Z. Für Anal. Chem. 1987;327:132–141. doi: 10.1007/BF00469806. [DOI] [Google Scholar]
- 16.Tonelli D, et al. Honey bees and their products as indicators of environmental radioactive pollution. J. Radioanal. Nucl. Chem. 1990;141:427–436. doi: 10.1007/BF02035809. [DOI] [Google Scholar]
- 17.Celli, G., Porrini, C., Frediani, D. & Pinzauti, M. Api e piombo in città (nota preventiva). Atti Convegno Qual. Dellaria Indic. Biol. Api E Piante Firenze Italy 11–45 (1987).
- 18.Dobrinas S, Birghila S, Coatu V. Assessment of polycyclic aromatic hydrocarbons in honey and propolis produced from various flowering trees and plants in Romania. J. Food Compos. Anal. 2008;21:71–77. doi: 10.1016/j.jfca.2007.07.003. [DOI] [Google Scholar]
- 19.Simon-Delso N, et al. Honeybee Colony Disorder in Crop Areas: The Role of Pesticides and Viruses. PloS One. 2014;9:e103073. doi: 10.1371/journal.pone.0103073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.V D Zee R, Gray A, Pisa L, De Rijk T. An observational study of honey bee colony winter losses and their association with Varroa destructor, neonicotinoids and other risk factors. PloS One. 2015;10:e0131611. doi: 10.1371/journal.pone.0131611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porrini C, et al. The Status of Honey Bee Health in Italy: Results from the Nationwide Bee Monitoring Network. PloS One. 2016;11:e0155411. doi: 10.1371/journal.pone.0155411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doublet V, Labarussias M, Miranda JR, Moritz RF, Paxton RJ. Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2015;17:969–983. doi: 10.1111/1462-2920.12426. [DOI] [PubMed] [Google Scholar]
- 23.Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347:1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez-Bayo F, et al. Are bee diseases linked to pesticides? —A brief review. Environ. Int. 2016;89:7–11. doi: 10.1016/j.envint.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Poquet Y, et al. A pragmatic approach to assess the exposure of the honey bee (Apis mellifera) when subjected to pesticide spray. PloS One. 2014;9:e113728. doi: 10.1371/journal.pone.0113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biocca M, Fanigliulo R, Gallo P, Pulcini P, Pochi D. The assessment of dust drift from pneumatic drills using static tests and in-field validation. Crop Prot. 2015;71:109–115. doi: 10.1016/j.cropro.2015.02.006. [DOI] [Google Scholar]
- 27.EFSA. Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 11, 3295 [pp. 266] (2013). [DOI] [PMC free article] [PubMed]
- 28.European Food Safety Authority. A small application developed in R for the estimation of the residue intake rate for certain bee species under given conditions: the SHVAL tool. EFSA Support. Publ. EN–623, 15 pp (2014).
- 29.Traynor, K. S. et al. In-hive Pesticide Exposome: Assessing risks to migratory honey bees from in-hive pesticide contamination in the Eastern United States. Sci. Rep. 6, (2016). [DOI] [PMC free article] [PubMed]
- 30.Berenbaum MR. Does the Honey Bee ‘Risk Cup’ Runneth Over? Estimating Aggregate Exposures for Assessing Pesticide Risks to Honey Bees in Agroecosystems. J. Agric. Food Chem. 2016;64:13–20. doi: 10.1021/acs.jafc.5b01067. [DOI] [PubMed] [Google Scholar]
- 31.Easton AH, Goulson D. The neonicotinoid insecticide imidacloprid repels pollinating flies and beetles at field-realistic concentrations. PLoS One. 2013;8:e54819. doi: 10.1371/journal.pone.0054819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becher MA, et al. BEEHAVE: a systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J. Appl. Ecol. 2014;51:470–482. doi: 10.1111/1365-2664.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rumkee JC, Becher MA, Thorbek P, Kennedy PJ, Osborne JL. Predicting Honeybee Colony Failure: Using the BEEHAVE Model to Simulate Colony Responses to Pesticides. Environ. Sci. Technol. 2015;49:12879–12887. doi: 10.1021/acs.est.5b03593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stammler, G., Brix, H. D., Nave, B., Gold, R., Schoefl, U. & Others. Studies on the biological performance of boscalid and its mode of action. In: Modern fungicides and antifungal compounds V: 15th International Reinhardsbrunn Symposium, Friedrichroda, Germany, May 6–10, 2007. Deutsche Phytomedizinische Gesellschaft eV Verlag, p. 45–51 (2007).
- 35.IUPAC. Pesticide Properties Database (PPDB) - Boscalid (Ref: BAS 510F). Available at: http://sitem.herts.ac.uk/aeru/ppdb/en/86.htm. (Accessed: 3rd January 2016).
- 36.IUPAC. Pesticide Properties Database (PPDB) - Pyrimethanil (Ref: SN 100309). Available at: http://sitem.herts.ac.uk/aeru/ppdb/en/573.htm. (Accessed: 3rd January 2016).
- 37.IUPAC. Pesticide Properties Database (PPDB) - Dimethoate. Available at: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/244.htm. (Accessed: 3rd January 2016).
- 38.Chauzat M-P, et al. A survey of pesticide residues in pollen loads collected by honey bees in France. J. Econ. Entomol. 2006;99:253–262. doi: 10.1093/jee/99.2.253. [DOI] [PubMed] [Google Scholar]
- 39.Chauzat M-P, et al. An assessment of honeybee colony matrices, Apis mellifera (Hymenoptera: Apidae) to monitor pesticide presence in continental France. Environ. Toxicol. Chem. 2011;30:103–111. doi: 10.1002/etc.361. [DOI] [PubMed] [Google Scholar]
- 40.Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One. 2012;7:e29268. doi: 10.1371/journal.pone.0029268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Botías, C. et al. Neonicotinoid Residues in Wildflowers, a Potential Route of Chronic Exposure for Bees. Environ. Sci. Technol. (2015). [DOI] [PubMed]
- 42.David A, et al. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 2016;88:169–178. doi: 10.1016/j.envint.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Bonmatin JM, et al. A LC/APCI-MS/MS method for analysis of imidacloprid in soils, in plants, and in pollens. Anal. Chem. 2003;75:2027–33. doi: 10.1021/ac020600b. [DOI] [PubMed] [Google Scholar]
- 44.Couvillon MJ, Schürch R, Ratnieks FL. Waggle dance distances as integrative indicators of seasonal foraging challenges. PloS One. 2014;9:e93495. doi: 10.1371/journal.pone.0093495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonmatin, J.-M. et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 1–33, doi:10.1007/s11356-014-3332-7 (2014). [DOI] [PMC free article] [PubMed]
- 46.Germany. Initial risk assessment provided by the rapporteur Member State Germany for the existing active substance IMIDACLOPRID of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC. 1–159 (2006).
- 47.Germany. Final addendum to the Draft Assessment Report (DAR) Imidacloprid. 1–461 (2008).
- 48.Wiest L, et al. Multi-residue analysis of 80 environmental contaminants in honeys, honeybees and pollens by one extraction procedure followed by liquid and gas chromatography coupled with mass spectrometric detection. J. Chromatogr. A. 2011;1218:5743–5756. doi: 10.1016/j.chroma.2011.06.079. [DOI] [PubMed] [Google Scholar]
- 49.Erdtman, G. An introduction to pollen analysis. Introd. Pollen Anal (1943).
- 50.Behm F, Von Der Ohe K, Henrich W. Zuverlässigkeit der Pollenanalyse von Honig: bestimmung der Pollenhäufigkeit. Dtsch. Lebensm.-Rundsch. 1996;92:183–188. [Google Scholar]
- 51.Von Der Ohe W, Oddo LP, Piana ML, Morlot M, Martin P. Harmonized methods of melissopalynology. Apidologie. 2004;35:S18–S25. doi: 10.1051/apido:2004050. [DOI] [Google Scholar]
- 52.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (2016).
- 53.Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference. Springer. (2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary information is available in a public repository at https://figshare.com/s/86785808b5709331aa1c


