Abstract
The severity and course of inflammatory processes differ between women and men, but the biochemical mechanisms underlying these sex differences are elusive. Prostaglandins (PG) and leukotrienes (LT) are lipid mediators linked to inflammation. We demonstrated superior LT biosynthesis in human neutrophils and monocytes, and in mouse macrophages from females, and we confirmed these sex differences in vivo where female mice produced more LTs during zymosan-induced peritonitis versus males. Here, we report sex differences in PG production in neutrophils during acute inflammation. In the late phase (4–8 hrs) of mouse zymosan-induced peritonitis and rat carrageenan-induced pleurisy, PG levels in males were higher versus females, seemingly due to higher PG production in infiltrated neutrophils. Accordingly, human neutrophils from males produced more PGE2 than cells from females. Increased PG biosynthesis in males was accompanied by elevated cyclooxygenase (COX)-2 expression connected to increased nuclear factor-kappa B activation, and was abolished when LT synthesis was pharmacologically blocked, suggesting that elevated PG production in males might be caused by increased COX-2 expression and by shunting phenomena due to suppressed LT formation. Conclusively, our data reveal that the biosynthesis of pro-inflammatory PGs and LTs is conversely regulated by sex with consequences for the inflammatory response.
Introduction
Sex has emerged as contributing factor in the incidence and progression of diseases associated with the immune system, in particular inflammation, with implications for outcomes and therapies. Most striking sex differences have been observed in asthma and autoimmune diseases (autoimmune thyroid diseases, scleroderma, rheumatoid arthritis etc)1, a spectrum of pathologies in which the patient population is prevalently female. On the other hand, other innate immune disorders such as sepsis2 and post-surgery infections as well as gout3 display a higher incidence and severity in males. The molecular and cellular basis underlying this sex dimorphism is still not completely elucidated and could present important implications for a sex-specific pharmacotherapy.
Sex differences in eicosanoid (leukotriene (LT) and prostaglandin (PG)) production may be responsible, at least in part, for the sex-dependent incidence of many diseases related to inflammation. In fact, we recently reported about a sex-dimorphism in LT biosynthesis (higher in female)4–6 which is of relevance in the light of the well-known sex-biased incidence of several immune diseases, providing a link to asthma pathology in humans7. In particular, androgens exert inhibitory effects on LT formation in human innate immune cells (isolated neutrophils or monocytes, and human whole blood) resulting in a substantial lower LT formation in male cells compared to females4, 5. We recently confirmed these sex differences also in vivo, making use of a well-established model of acute inflammation, the zymosan-induced peritonitis in mice6. In fact, LT biosynthesis as well as the inflammatory response were significantly greater in the inflamed peritoneum of female versus male mice. On the cellular level, differential 5-lipoxygenase (LO) subcellular compartmentalization in human leukocytes and in murine peritoneal macrophages (PMs) from males and females might be the basis for the observed differences4–6.
LTs and PGs are locally acting bioactive lipid mediators derived from arachidonic acid (AA) produced by 5-LO and cyclooxygenase (COX) as key enzymes, respectively. They are markedly biosynthesized by monocytes/macrophages, neutrophils, and mast cells and regulate a diverse set of homeostatic and inflammatory processes8 linked to numerous sex-dependent diseases. PGs are formed by the action of COX (COX-1 and COX-2) enzymes in a two-step conversion of AA. First, COXs convert AA to a cyclic endoperoxide (PGG2) and incorporate a 15-hydroperoxy group. This hydroperoxy group of PGG2 is reduced to a hydroxy moiety yielding PGH2 that is subsequently converted to the corresponding PGs by specific PG synthases, the nature of which is determined by the enzyme content of the respective cell. COX-1 is constitutively expressed in most cells, thus regarded as a housekeeping protein. On the other hand, the expression of COX-2 is inducible and remains undetectable in most mammalian tissues under basal conditions. Exposure of several types of cells including fibroblasts, neutrophils and monocytes to bacterial endotoxins, cytokines, and hormones induces activation of mitogen-activated protein kinases (MAPK) and nuclear factor-kappa B (NF-κB) which, in turn, induce COX-2 expression and PG production9. Inhibiting the formation of PGs by aspirin and other non-steroidal anti-inflammatory drugs during inflammation remains a classic and prevailing strategy to alleviate pain, swelling and fever. A sex-dependent efficacy of aspirin has been underlined in several randomized trials10, suggesting differential production or signalling of PGs in males and females. Moreover, as interrelations and crosstalk between the two branches of AA transformation (COX/PGs and 5-LO/LTs) exist11, imbalances in LT biosynthesis between genders may translate into differential PG formation.
Here, we show sex differences in PG production in neutrophils during acute inflammation using two different in vivo experimental models, that are, mouse zymosan-induced peritonitis and rat carrageenan-induced pleurisy, as well as in human neutrophils in vitro. Higher PG levels in males are seemingly caused by (i) increased COX-2 expression connected to elevated NF-κB activation versus females and (ii) by AA shunting phenomena due to lower LT production in males.
Results
Sex differences in PG biosynthesis during zymosan-induced peritonitis in mice
We have previously demonstrated that LT formation in zymosan-induced peritonitis in mice is higher in females compared to males, seemingly due to a divergent subcellular localization of 5-LO in LT-producing peritoneal macrophages6. Here, we investigated the temporal PG biosynthesis after intraperitoneal zymosan injection in male and female mice. Both sexes showed a similar time-course in the production of PGE2 with a peak after 2 hrs and continuous decrease until 8 hrs. Intriguingly, in male mice significant higher levels of PGE2 in the later phase of inflammation (p < 0.05 at 4 hrs; Fig. 1A) were evident in the peritoneal exudates after zymosan challenge, where the inflammatory cell population was mostly composed of neutrophils6, 12, 13. Moreover, in the exudates of male mice after 4 hrs, also higher levels of other PGs, such as 6-keto-PGF1α, the stable metabolite of PGI2, were evident as compared with females (p < 0.05, Fig. 1B). In contrast, the levels of LTC4 that peak after 15 min upon zymosan injection were higher in exudates from female versus male mice (Fig. 1C), which is in agreement with our previous report6.
Figure 1.
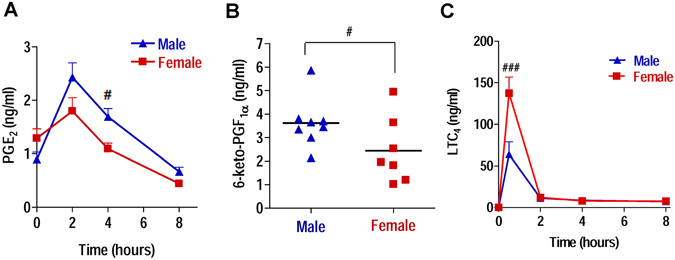
PGE2, 6-keto-PGF1α and LTC4 peritoneal levels in male and female mice after zymosan-induced peritonitis. Mice were treated with zymosan (i.p.), sacrificed at the indicated time points and (A) PGE2 and (C) LTC4 were measured in peritoneal exudates from male and female mice, and (B) 6-keto-PGF1α was measured in peritoneal exudates 4 hrs after zymosan injection. Time zero (T0) corresponded to untreated mice. Values represent means ± S.E.M; n = 10 mice/T0; n = 10 mice/2 hrs; n = 10 mice/4 hrs; n = 6 mice/8 hrs. ###p < 0.001 and #p < 0.05, male vs female mice; two-way ANOVA plus Bonferroni (A and C) and two-tailed Student’s t test (B).
Sex differences in eicosanoid biosynthesis in carrageenan-induced pleurisy in rats
Carrageenan-induced pleurisy in rats was chosen as another model of acute inflammation to investigate the sex-related regulation of eicosanoid biosynthesis. Two hrs after pleurisy induction, a peak of LTB4 was observed in both sexes, with a decrease at the later time points (4–8 hrs). In agreement with the data from the peritonitis model in mice6, higher levels of LTB4 were found in exudates of female versus male rats (p < 0.001, Fig. 2A) at the peak time (i.e., 2 hrs). The opposite was evident for PGE2 levels. Thus, a peak of PGE2 production was reached in both sexes 4 hrs after carrageenan injection and higher PGE2 levels were obvious in exudates of males, as observed in the murine peritonitis model. At 8 hrs, PGE2 levels dropped but the sex difference was still preserved (Fig. 2B). Moreover, a significant (p < 0.05) higher production of 6-keto-PGF1α was found in male exudates 4 hrs after carrageenan injection (Fig. 2C), correlating to elevated PGE2 levels. Taken together, sex differences exist in PG biosynthesis in vivo during acute inflammation in two different animal models (mice vs. rats, zymosan vs. carrageenan, peritonitis vs. pleurisy) where PG levels were significantly higher in males versus females.
Figure 2.
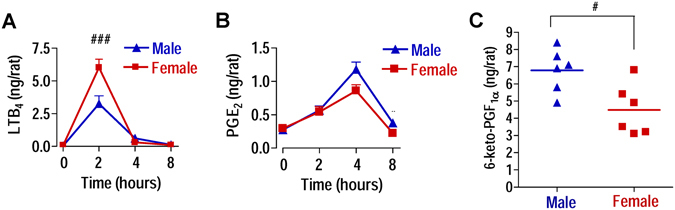
Sex differences in eicosanoid biosynthesis in carrageenan-induced pleurisy in rats. Rats were treated with carrageenan (intrathoracic injection), sacrificed at the given time points, and (A) LTB4 and (B) PGE2 in the thoracic exudates were measured by EIA and RIA, respectively. (C) 6-keto-PGF1α levels in thoracic exudates were measured 4 hrs after carrageenan injection by EIA. Time zero (T0) corresponds to untreated rats. Values represent means ± S.E.M; n = 6 rats/T0; n = 13 rats/2 hrs; n = 6 rats/4 hrs; n = 6 rats/8 hrs; #p < 0.05; ###p < 0.001, male vs female; two-way ANOVA plus Bonferroni (A and B) and two-tailed Student’s t test (C).
Cyclooxygenase-2 expression and NF-κB activation in thoracic exudates differ between male and female rats
Next, we investigated if the higher production of PGE2 in male animals during the inflammatory response was due to a higher number of PGE2-producing cells that infiltrate and mainly consist of neutrophils14. However, 4 hrs after pleurisy induction by carrageenan in rats, the cell number was not different between sexes (Fig. 3A). COX-1 and -2 play key roles in the biosynthesis of PGs, and the capacity of cells to produce PGs strongly depends on the amounts of COX enzymes expressed. Based on the sex difference in PG biosynthesis 4 hrs after pleurisy induction in rats, we analyzed the expression of COX-1 and -2 in the infiltrated cells by Western Blot (WB). No significant sex difference was observed for COX-1 expression, whereas COX-2 protein levels were higher in male cells (Fig. 3B,D), with respect to female counterparts. Interestingly, the sex difference in COX-2 protein expression was coupled to a significant higher activation status of NF-κB in male cells, visualized by elevated phospho-NF-κB p65 levels (Fig. 3E and F). Moreover, activation of p38 MAPK was more pronounced in cells from carrageenan-treated male rats as compared to cells from female animals (Fig. 3G and H), although the differences did not reach statistical significance.
Figure 3.
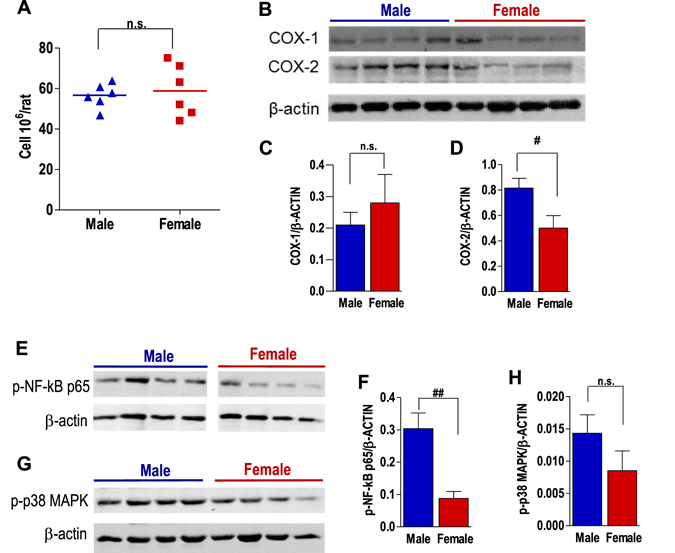
Cyclooxygenase expression and NF-κB activation in thoracic exudates differ between male and female cells. Rats were treated with carrageenan (intra thoracic injection) and sacrificed after 4 hrs. (A) Numbers of infiltrated cells (106/rat) were measured in a Burker chamber using light microscopy. (B) Analysis of COX-1/2, (E) phospho-NF-κB p65 and (G) phospho-p38 MAPK by Western blot in total cell lysates of thoracic exudates from male and female carrageenan-treated rats. Densitometric analysis of (C) COX-1, (D) COX-2, (F) phospho-NF-κB p65 and (H) phospho-p38 MAPK protein normalized to β-actin expression. Values represents means ± S.E.M., n = 4 rats. #p < 0.05; ##p < 0.01, male vs female; two-tailed Student’s t test.
Inhibition of 5-LO product formation abolishes the sex differences in PG formation in the thoracic exudates of carrageenan-treated rats
Based on the fact that LTB4 levels in the early phase (2 hrs) of the inflammatory response were lower in male versus female rats, we hypothesized that shunting phenomena from LTs to PGs might contribute to the opposite PG levels. Thus, we attempted to block 5-LO product formation by a pharmacological inhibitor (i.e. MK886)15 in order to investigate if the sex difference in PG biosynthesis at 4 hrs could be abolished. Rats were pre-treated with MK886 (1.5 mg/kg, i.p.) 30 min prior pleurisy induction, sacrificed after 4 hrs, and PGE2 levels were analyzed in the exudates. MK886 administration significantly (p < 0.05) abolished the sex bias in PGE2 (Fig. 4A) as well as in 6-keto-PGF1α production (Fig. 4B). Furthermore, pre-treatment of rats with 1.5 mg/kg MK886 efficiently suppressed LTB4 levels by 75 and 74% in exudates of male and female animals, respectively (Fig. 4C). In agreement with this finding, MK886 treatment reduced the number of infiltrated cells into the thoracic cavity without significant differences between male and female animals (Fig. 4D).
Figure 4.
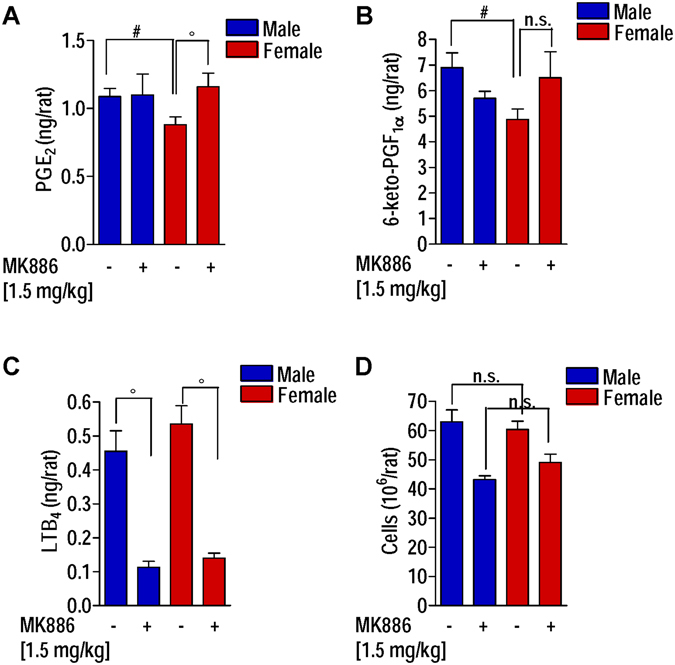
Inhibition of 5-LO product formation by MK886 abolishes the sex differences in PG formation in the thoracic exudates of carrageenan-treated rats. Rats were pre-treated with MK886 (1.5 mg/kg, i.p.) or vehicle (DMSO 2%, i.p.) 30 min prior to carrageenan intrathoracic injection and sacrificed after 4 hrs. (A) PGE2, (B) 6-keto-PGF1α, and (C) LTB4 levels in the exudates. (D) Number of infiltrated cells. Values represent means ± S.E.M; n = 6 rats; #p < 0.05, male vs female; °p < 0.05, female control rats vs female rats treated with MK886; two-tailed Student’s t test.
The capacity for PGE2 production in human neutrophils is sex-dependent
Neutrophils are immune cells involved in the inflammatory reaction, recruited by chemoattractants (i.e. LTB4)16 that are produced by resident cells17, 18. To investigate if the sex difference in PGE2 biosynthesis exists also in humans, neutrophils from blood of female and male donors were freshly isolated and immediately stimulated for PGE2 production by lipopolysaccharide (LPS), a receptor-coupled stimulus, or by A23187 that causes cell activation by substantial mobilization of intracellular Ca2+ in a receptor-independent manner. Formation of PGE2 upon stimulation with LPS (1 µg/ml), which is strongly upregulated over the time course of 20 hrs, was much more pronounced for neutrophils derived from male versus cells from female donors (Fig. 5A). Also in response to A23187 (0.5 µM), a trend of higher PGE2 biosynthesis from male cells was evident starting at 30 min post stimulation and reaching significance (p < 0.05) at 4 hrs (Fig. 5B). Increased availability of AA as substrate in male cells may account for higher PGE2 synthesis. However, in agreement with our previous findings4, we observed no differences in the release of AA upon neutrophil stimulation with 0.5 µM A23187 between cells from male and female donors (Fig. 5C).
Figure 5.
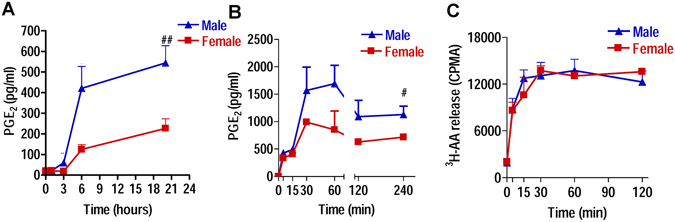
Capacity of human neutrophils for PGE2 production is sex-dependent. PGE2 production in human neutrophils from male and female donors after LPS or A23187 stimulation. Neutrophils from male and female donors were incubated with (A) LPS (1 µg/ml) for 0, 0.5, 3 and 20 hrs, or (B) with 0.5 µM A23187 for 0, 5, 15, 30, 60, 120, and 240 min. The reactions were stopped on ice, and PGE2 levels were measured by ELISA. (C) After selected time points (0, 5, 15, 30, 60, 120 min) upon stimulation with A23187 (0.5 µM), free 3H-AA content in the supernatant of 3H-AA-pre-labelled neutrophils was evaluated by scintillation counting. Values represents means ± S.E.M of n = 3–6 experiments each in duplicate. #p < 0.05, ##p < 0.01, male vs female cells; two-way ANOVA plus Bonferroni.
Cyclooxygenase-2 expression is higher in male human neutrophils
Next, we addressed if COX expression in human neutrophils is affected by the sex. Analysis of COX protein levels (normalized to β-actin) in freshly isolated neutrophils from male and female donors by Western blot revealed no sex difference in the expression of COX-1 (Fig. 6A,B), while a significant (p < 0.05) higher expression of COX-2 in male cells was observed (Fig. 6A,C). This suggests that elevated PGE2 formation in male neutrophils might be connected to higher amounts of COX-2.
Figure 6.
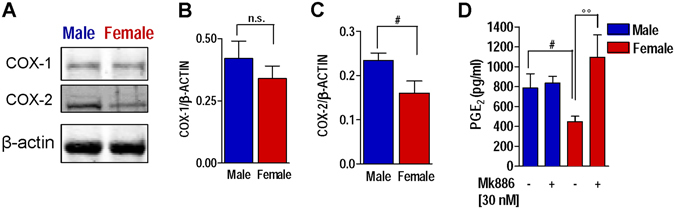
COX-2 expression is higher in neutrophils from male versus female donors; effects of 5-LO pathway inhibition on PGE2 production. (A–C) Western blot analysis of total cell lysates of neutrophils from male and female donors. (A) COX-1 and -2 protein expression. Densitometric analysis of (B) COX-1 and (C) COX-2 protein normalized to β-actin expression. Western blots are representatives of 11 independent experiments. Values represents means ± S.E.M of densitometric analyses of COX-1/-2 protein bands, normalized to β-actin. #p < 0.05, male vs female cells. (D) PGE2 production in human neutrophils from male and female donors after pre-treatment with MK886 (30 nM, 15 min) upon A23187 stimulation (0.5 µM, 4 hrs). Values represents means ± S.E.M of n = 5 experiments, each in duplicate. #p < 0.05, male vs female; °°p < 0.01, female cells with MK886 vs female control cells; two-tailed Student’s t test.
The sex difference in PGE2 production in human neutrophils is abolished by inhibition of 5-LO product synthesis
Human neutrophils from females produce significantly higher levels of LTs from AA than cells from males4, implying that in male neutrophils more AA might be available as substrate for COX to produce PGs. In line with the data obtained from the carrageenan-induced pleurisy in rats, pre-treatment of neutrophils with MK886 (30 nM, 15 min) to block 5-LO product formation did not affect PGE2 formation in A23187-stimulated male neutrophils, while it significantly (p < 0.01) increased PGE2 synthesis in female cells. Thus, suppression of 5-LO product biosynthesis abolishes the sex difference in PGE2 formation also in human neutrophils (Fig. 6D).
Discussion
A sex bias in inflammatory and immune diseases is clearly evident1, but the underlying biochemical or molecular mechanisms remain unclear. Here, we report about sex differences in PG production in human neutrophils in vitro and in rats and mice during acute inflammation in vivo. Our data suggest that the higher PG levels in neutrophils of males is due to AA substrate shunting phenomena because of lower LT production, but is also governed by higher amounts of COX-2 protein, as compared to neutrophils from females. The two major branches of biosynthetic pathways of pro-inflammatory lipid mediators produced from AA are the COX-mediated cascade leading to PGs and the 5-LO-mediated cascade yielding LTs19. Sex differences in the regulation of 5-LO in human leukocytes with consequences for LT formation were reported by us before4, 5, and we recently confirmed sex-biased LT biosynthesis in murine zymosan-induced peritonitis in vivo 6, a well-recognized model of acute inflammation12. Thus, we observed more pronounced LT formation and related inflammatory reactions (i.e., neutrophil infiltration and vascular permeability) in female mice as compared to male animals. The cells responsible for the observed sex difference were resident PMs, occupying the peritoneal cavity under normal physiological conditions, which are the first cells to respond to inflammatory stimuli. In analogy to human neutrophils4, the subcellular localization of 5-LO in female and male murine PMs differs, and the limited amount of mobile 5-LO in male PMs is seemingly responsible for lower LT biosynthesis6.
The results of the present report confirm higher LT formation in females but also reveal a sex-biased production of PG, in particular by neutrophils. However, while LT formation was higher in female cells and animals4–6, PG formation was higher in males. Our data are consistent with several observations made in vitro and in vivo studies observing a sex dimorphism in PG production, with higher levels in males or in ovariectomized female subjects20–23.
A time-course study of PGE2 biosynthesis in zymosan-induced peritonitis revealed higher PGE2 levels in exudates from male mice compared to female animals, 4 hrs or later after zymosan injection. Although PGE2 levels peaked at 2 hrs, the sex difference was significant only at 4 and 8 hrs, but not earlier. It should be noted that 4 hrs after peritonitis induction the cellular population of the peritoneal cavity is mostly composed of neutrophils as shown previously by us6 and others12, 13. Thus, we suggest that the sex difference is attributable to neutrophils, rather than to PMs that seem to equally generate PGs in the early phase (0–2 hrs) independently of the sex6. Surprisingly, despite the higher PGE2 production in males, the number of infiltrating cells were lower at 4 and 8 hrs as compared to female animals. This might be due to reduced levels of LTB4 in males6 that acts as potent chemotactic factor for neutrophils18, and based on its greater abundance in female mice may recruit neutrophils more efficiently. To confirm the general validity of sex differences in PG biosynthesis, we chose a different animal model of acute inflammation (i.e. pleurisy) with a different stimulus (i.e. carrageenan) and different species (i.e. rat) to support sex-dependent production of eicosanoids as general and model-independent phenomenon. Carrageenan-induced pleurisy in rats represents one of the most commonly used models to investigate eicosanoid biosynthesis and signaling during acute inflammation19, 24–26. In agreement with the results from the zymosan-induced peritonitis, significant higher LTB4 levels in thoracic exudates of female rats were evident 2 hrs after carrageenan-injection, while superior PGE2 levels were found in thoracic exudates of male animals at later time points (4–8 hrs). These data highlight the converse transformation of AA to PGs (higher in males) and LTs (higher in females), and suggest that sex differences in PGE2 production in these experimental models are most likely related to neutrophils. During acute inflammation, the mobilization and recruitment of blood leukocytes into the tissue are mediated by several factors27. Among them, LTB4 through the BLT1 receptor might be the major chemoattractant molecule responsible for neutrophil infiltration18. Since the LTB4 levels were higher in the thoracic exudates of female rats, we hypothesized that this would cause an increased neutrophil infiltration in the cavity of female animals. Notably, however, the number of infiltrated neutrophils did not differ between males and females at 4 hrs after carrageenan, and MK886 at 1.5 mg/kg that strongly repressed LTB4 formation and caused significant inhibition of cell infiltration in both sexes.
For PG production, AA is converted in two steps by the action of COX enzymes that catalyze the transformation of AA into the endoperoxide PGG2 containing a 15-hydroperoxy moiety. Reduction of the hydroperoxy group to a hydroxyl function then leads to PGH2 that is further converted by specific PG synthases to the respective bioactive PGs, including PGE2, PGF2, PGD2, PGI2 and thromboxane(s), depending on the tissue-selective expression of the PG synthases28. Since besides PGE2 also the 6-keto-PGF1α (a stable metabolite and marker of instable PGI2) was consistently higher in males, these data support that upstream COX enzymes might be affected by the sex rather than mPGES-1, the terminal enzyme in pro-inflammatory PGE2 biosynthesis29. Nevertheless, sex differences related to mPGES-1 were found in spontaneously hypertensive rats, where female rats had enhanced mPGES-1 protein expression in the renal inner medulla and greater COX-2 expression in the outer medulla versus males21. In our hands, COX-2 protein, but not COX-1, was more abundant in cells from thoracic exudates of male rats and in human neutrophils from males, and such dominance of COX-2 in males is in agreement with observations by others. Thus, lower COX-2 expression and activity has been noted in the macula densa of female rats compared to males, contributing to the major protection of female to the blood pressure increment and renal damage30. Moreover, the inferior susceptibility to traumatic brain injury of male versus female rats has been related to a robust higher expression of COX-2 in the brain of male rats31. In addition, long-term testosterone treatment augmented COX-2 levels in male rat brain blood vessels, whereas treatment of male rats with 17β-estradiol significantly impaired cerebrovascular COX-2 levels after an inflammatory stimulus21.
Expression of COX-2 is regulated by several transcription factors including NF-kB9, whose activation in thoracic cells from carrageenan-treated rats was sex-dependent in our present study. In fact, phospho-NF-κB p65 levels in male rats were significantly higher with respect to female animals. Our data are in line with previously reported sex differences in cerebrovascular pathophysiology that were due to activation of the NF-κB-mediated COX-2 pathway by the androgen 5α-DHT that results in a state of vascular inflammation32.
Our data reveal that activation of human neutrophils by LPS or by A23187 leads to significantly higher PGE2 levels in cells from males versus female counterparts. We showed before that stimulation of human neutrophils with LPS plus fMLP or with A23187 caused higher LTB4 production in female cells as compared to males4. Although A23187 preferentially activates the 5-LO pathway via receptor-independent, massive elevation of intracellular Ca2+, we believe that it represents a suitable stimulus to investigate the sex-regulation of eicosanoid biosynthesis, considering the fact that other stimuli (e.g., LPS) act through receptors that are strongly modulated by sex as well33, 34. We hypothesized that the blockade of 5-LO product formation by using MK886 would redirect AA conversion by COX enzymes. In fact, the sex difference in PGE2 formation in human neutrophils in vitro as well as in carrageenan-treated rats in vivo was abolished by interruption of 5-LO product formation using MK886 that significantly increased PGE2 in females without any alterations in males. In parallel, MK886 strongly reduced LTB4 levels in both test systems. On the other hand, blockade of PGE2 production may have the converse phenotype, however, the COX inhibitor indomethacin did not increase LTB4 levels in male rats during carrageenan-induced pleurisy35.
Taken together, we showed that male mice and rats produce higher levels of PGs in various acute models of inflammation in vivo under conditions where LT production is elevated in female animals at the sites of injury. Neutrophils are abundant innate immune cells in the human body taking part of the first line of defense against host injury, and are considered to be a major source of LTs33. Our findings imply that neutrophils from male subjects have higher capacities to produce PG seemingly due to elevated COX-2 expression and AA substrate availability. These sex differences are of relevance for PG-related functions and pathophysiology, supported also by experimental observations reported by others, and might help to explain, at least in part, the sex dimorphism in innate immune disorders such as sepsis2 and post-surgery infections as well as gout3.
Material and Methods
Materials
Enzyme immunoassay (EIA) kits were from Cayman Chemical Company (BertinPharma, Montigny Le Bretonneux, France) or from Biotrend (Cologne, Germany). 3H-labelled PGE2 and 3H-labelled AA were from PerkinElmer Life Sciences (Milan, Italy and Germany). Unless otherwise stated, all other reagents and compounds were obtained from Sigma-Aldrich (Milan, Italy).
Animals
The animal studies are reported in accordance with the ARRIVE guidelines for reporting animal research36. Age-matched male and female CD-1 mice (8–9 weeks old, 26–40 g Charles River, Calco, Italy) and Wistar male and female rats (200–300 g, Harlan, Milan, Italy) were housed in a controlled environment (21 ± 2 °C) and provided with standard rodent chow and water. All animals were allowed to acclimate for four days prior to experiments and were subjected to 12 h light–12 h dark schedule. Experiments were conducted during the light phase. The experimental protocols were approved by the Animal Care Committee of the University of Naples Federico II, in compliance with Italian regulations on protection of animals used for experimental and other scientific purpose (Ministerial Decree 26/2014) as well as with the European Economic Community regulations (Official Journal of E.C. L358/1 12/18/1986).
Induction of peritonitis in mice
Peritonitis was induced in mice as previously described6. In brief, a solution of 2 mg/ml of zymosan A (boiled and washed) was injected intraperitoneally (i.p., 0.5 ml) and at selected time points (0–2–4–8 hrs), mice were sacrificed in a saturated atmosphere with CO2. Peritoneal exudates were collected by washing the cavity with 2 ml of phosphate-buffered saline (PBS) and then centrifuged at 20,000 × g for 20 min at 4 °C and supernatants frozen at −80 °C for measurements of eicosanoids. PGE2 was evaluated by radioimmunoassay (RIA), 6-keto-PGF1α and LTC4 by EIA (Cayman chemicals BertinPharma, Montigny Le Bretonneux, France), according to manufacturer’s protocol. Results are expressed as ng/ml.
Induction of pleurisy in rats
Rats were anesthetized with 4% enflurane mixed with 0.5 l/min O2, 0.5 l/min N2O and submitted to a skin incision at the level of the left sixth intercostal space. The underlying muscle was dissected and 1% (w/v) λ-carrageenan type IV (0.2 ml) was injected into the thoracic cavity. The skin incision was closed with a suture and the animals were allowed to recover. At selected time points (0–2–4–8 hrs) after carrageenan injection, animals were sacrificed by CO2 inhalation. Thoracic exudate was collected by lavage of the cavity with 2 ml of saline solution, after centrifugation (800 × g for 10 min), the leukocyte number was determined by light microscopy using a Bürker chamber. The cells as well as the supernatants were frozen at −80 °C for WB analysis and eicosanoid measurement, respectively. In one set of experiments, rats were pre-treated with 1.5 mg/kg MK886 (Cayman Chemical, Bertin Pharma, Montigny Le Bretonneux, France) or vehicle (2% DMSO in saline) 30 min prior to pleurisy induction. Animals were then sacrificed 4 hrs after carrageenan injection. The amount of PGE2, LTB4 and 6-keto-PGF1α in the supernatant of centrifuged exudates was measured by RIA and by EIA, respectively. Results are expressed as the total amount of eicosanoid measured in the thoracic exudate of one rat (nanograms per rat).
Isolation and stimulation of human neutrophils
Leukocyte concentrates, prepared from freshly withdrawn peripheral blood of healthy adult human donors who had not taken any anti-inflammatory drugs for the last 10 days were obtained from the Institute of Transfusion Medicine at the University Hospital Jena, Germany. Informed consent was obtained from all subjects. The experimental protocol was approved by the local ethical committee at the University Hospital Jena. All methods were performed in accordance with the relevant guidelines and regulations. Neutrophils were isolated as previously described4, 37. In brief, neutrophils were obtained from leukocyte concentrates by a multi-step procedure: (1) dextran sedimentation; (2) centrifugation on Nycoprep (872 × g, 10 min); (3) hypotonic lysis of erythrocytes. Finally, cells were suspended in ice-cold PBS containing 0.1% glucose (PG buffer) and counted by Vi-CELL™ XR. For PG production, 5 × 106 neutrophils, from female and male donors, were resuspended in PG buffer containing 1 mM CaCl2 (PGC buffer) and stimulated with 1 µg/ml LPS for 0, 0.5, 3 and 20 hrs or with 0.5 μM A23187 for 0, 5, 15, 30, 60, 120, or 240 min. In one set of experiments, freshly isolated neutrophils from male and female donors were pre-treated with 30 nM MK886 or DMSO as vehicle (15 min., 37 °C), and then stimulated with 0.5 μM A23187 for 4 hrs. The reaction was stopped on ice and samples were centrifuged (12,000 × g, 5 min, 4 °C), PGE2 levels in the supernatants were measured with ELISA kit (Biotrend, Cologne, Germany).
Total protein extraction and Western blot analysis
Protein analysis of COX-1/2, phospho-NF-κB p65, phospho-p38 MAPK and β-actin by Western blot was performed in whole cell lysates. Cells in the thoracic exudates were collected 4 hrs after carrageenan administration and then immediately lysed in a buffer for protein extraction, mixed with sodium dodecyl sulphate (SDS) loading gel buffer and analysed by Western blot according to ref. 6 on a 10% SDS–polyacrylamide gel. The membranes were incubated overnight with rabbit monoclonal antibody anti-COX-2 (1:500, BD Transduction Laboratories, Aurogene, Rome, Italy), mouse monoclonal antibody anti-COX-1 (1:1000, Cell Signaling, Aurogene, Rome, Italy), rabbit monoclonal antibody anti-phospho-NF-kB p65 (Ser536) (1:1000, Cell Signalling Technology, Inc., Germany), rabbit polyclonal antibody anti-phospho-p38 MAPK (Thr180/Thr182) (1:1000, Cell Signalling Technology, Inc., Germany) or β-actin (1:2000, Santa Cruz Biotechnology, Aurogene, Rome, Italy). Membranes were washed six times with 0.1% PBS-Tween and were incubated for 1.5 hrs at room temperature with horseradish peroxidase-conjugated anti-rabbit and anti-mouse secondary antibodies diluted 1:10,000 in 0.1% PBS-Tween containing 5% non-fat dry milk. Protein bands were detected by an enhanced chemiluminescence system (Amersham Pharmacia, Aurogene, Rome, Italy). Densitometric analysis was performed by using the Fluor S quantitative imaging system.
Human neutrophils from female and male donors were isolated and rapidly processed for protein extraction as previously described4. Briefly, 3 × 107 cells/ml PBS plus 1 mM EDTA were sonicated (3 × 5 sec, 4 °C) and centrifuged (12,000 × g, 15 min, 4 °C). Aliquots of the resulting supernatants were mixed 1:1 with ice-cold 2× SDS/PAGE sample loading buffer (SDS-b) and heated for 6 min at 95 °C. Samples were loaded (15 µl) and electrophoresed on a 10% SDS–polyacrylamide gel, and transferred to nitrocellulose membranes. After the membranes were incubated with primary antibodies (rabbit anti-COX-1, 1:1000, Cell signaling; rabbit anti-COX-2, 1:1000, Santa Cruz; mouse anti-β-actin, 1:1000; Santa Cruz) they were subsequently detected using IRDye 800CW-labeled anti-rabbit and/or anti-mouse antibodies (1:10,000 each). The immunoreactive bands were visualized using an Odyssey infrared imager (Li-Cor Biosciences, Lincoln, NE).
Arachidonic acid release in human neutrophils
Arachidonic acid release in neutrophils from male and female donors was evaluated as reported previously38. Briefly, freshly isolated neutrophils (2 × 107 cells/ml) were re-suspended in RPMI 1640 without additives, 0.5 µCi 3H-labelled AA/ml were added to the cell suspension and incubated for 2 hrs at 37 °C. Cells were washed twice (320 × g, 10 min, 4 °C) with incubation buffer (PBS, containing 0.1% glucose and 2 mg/ml fatty acid free BSA). Cells were adjusted to a cell number of 1 × 107/0.5 ml and 1 mM CaCl2 was added to the incubation buffer. Cells were simulated with Ca2+-ionophore A23187 (0.5 µM) for 5 to 120 min, as indicated, at 37 °C. The reaction was stopped on ice and samples were centrifuged (500 × g, 10 min, 4 °C). Aliquots (300 µl) of the supernatants were combined with 2 ml Rotiszint® eco plus and assayed for radioactivity by scintillation counting (Micro Beta Trilux, Perkin Elmer, Waltham, MA).
Statistical analysis
Data are expressed as mean ± standard error of the mean (S.E.M.) of n observations, were n represents the number of animals, or the number of experiments (in vitro) performed with cells from different donors in duplicates. Statistical evaluation was performed by two-tailed Student t-test for single comparisons or by two-way ANOVA using GraphPad InStat (Graphpad Software Inc., San Diego, CA) followed by a Bonferroni post-hoc test for multiple comparisons, respectively. P-values < 0.05 were considered as significant.
Acknowledgements
This research was supported by the Deutsche Forschungsgemeisnchaft, SFB 1127 “ChemBioSys”, and by the State of Thuringa, ProExcellence Initiative 2 (“RegenerAging”).
Author Contributions
S.P., A.R., O.W. and L.S. designed research; S.P., A.R., V.K., F.D., F.T., R.B., C.W. and S.R. performed research; S.P., A.R., O.W. and L.S. analyzed data; and S.P., A.R., O.W. and L.S. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Simona Pace and Antonietta Rossi contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Antonietta Rossi, Email: antrossi@unina.it.
Oliver Werz, Email: oliver.werz@uni-jena.de.
References
- 1.Whitacre CC. Sex differences in autoimmune disease. Nat. Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 2.Adrie C, et al. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132:1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 3.Weaver AL. Epidemiology of gout. Cleve. Clin. J. Med. 2008;75:S9–12. doi: 10.3949/ccjm.75.Suppl_5.S9. [DOI] [PubMed] [Google Scholar]
- 4.Pergola C, et al. ERK-mediated regulation of leukotriene biosynthesis by androgens: a molecular basis for gender differences in inflammation and asthma. Proc. Natl. Acad. Sci. USA. 2008;105:19881–19886. doi: 10.1073/pnas.0809120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pergola C, et al. Testosterone suppresses phospholipase D, causing sex differences in leukotriene biosynthesis in human monocytes. FASEB J. 2011;25:3377–3387. doi: 10.1096/fj.11-182758. [DOI] [PubMed] [Google Scholar]
- 6.Rossi A, et al. In vivo sex differences in leukotriene biosynthesis in zymosan-induced peritonitis. Pharmacol. Res. 2014;87:1–7. doi: 10.1016/j.phrs.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch. Dis. Child. 2003;88:587–590. doi: 10.1136/adc.88.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell. Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N. Engl. J. Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 11.Rossi A, et al. Up-regulation of prostaglandin biosynthesis by leukotriene C4 in elicited mice peritoneal macrophages activated with lipopolysaccharide/interferon-{gamma} J. Leukoc. Biol. 2005;78:985–91. doi: 10.1189/jlb.1004619. [DOI] [PubMed] [Google Scholar]
- 12.Rao TS, Currie JL, Shaffer AF, Isakson PC. In vivo characterization of zymosan-induced mouse peritoneal inflammation. J. Pharmacol. Exp. Ther. 1994;269:917–925. [PubMed] [Google Scholar]
- 13.Doherty NS, et al. Intraperitoneal injection of zymosan in mice induces pain, inflammation and the synthesis of peptidoleukotrienes and prostaglandin E2. Prostaglandins. 1985;30:769–89. doi: 10.1016/0090-6980(85)90006-1. [DOI] [PubMed] [Google Scholar]
- 14.Lopes RP, et al. The effects of fructose-1,6-bisphosphate and dexamethasone on acute inflammation and T-cell proliferation. Inflamm. Res. 2006;55:354–8. doi: 10.1007/s00011-006-6044-8. [DOI] [PubMed] [Google Scholar]
- 15.Rouzer CA, Ford-Hutchinson AW, Morton HE, Gillard JW. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J. Biol. Chem. 1990;265:1436–1442. [PubMed] [Google Scholar]
- 16.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 17.Dimasi D, Sun Wai Y, Bonder CS. Neutrophil interactions with the vascular endothelium. Int. Immunopharmacol. 2013;17:1167–1175. doi: 10.1016/j.intimp.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 18.Lämmermann T, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 20.Mallery SR, Zeligs BJ, Ramwell PW, Bellanti JA. Gender-related variations and interaction of human neutrophil cyclooxygenase and oxidative burst metabolites. J. Leukoc. Biol. 1986;40:133–146. doi: 10.1002/jlb.40.2.133. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan JC, Sasser JM, Pollock DM, Pollock JS. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension. 2005;45:406–411. doi: 10.1161/01.HYP.0000156879.83448.93. [DOI] [PubMed] [Google Scholar]
- 22.Francois H, et al. Role of microsomal prostaglandin E synthase 1 in the kidney. J. Am. Soc. Nephrol. 2007;18:1466–1475. doi: 10.1681/ASN.2006040343. [DOI] [PubMed] [Google Scholar]
- 23.Brito HO, et al. Female Sex Hormones Influence the Febrile Response Induced by Lipopolysaccharide, Cytokines and Prostaglandins but not by Interleukin-1β in Rats. J. Neuroendocrinol. 2016;28:10. doi: 10.1111/jne.12414. [DOI] [PubMed] [Google Scholar]
- 24.Di Rosa M. Biological properties of carrageenan. J. Pharm. Pharmacol. 1972;24:89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- 25.Gilroy DW, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 26.Rossi A, et al. The hallucinogenic diterpene salvinorin A inhibits leukotriene synthesis in experimental models of inflammation. Pharmacol. Res. 2016;106:64–71. doi: 10.1016/j.phrs.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 27.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 28.Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta. 2015;1851:414–421. doi: 10.1016/j.bbalip.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Rådmark O, Samuelsson B. Microsomal prostaglandin E synthase-1 and 5-lipoxygenase: potential drug targets in cancer. J. Intern. Med. 2010;268:5–14. doi: 10.1111/j.1365-2796.2010.02246.x. [DOI] [PubMed] [Google Scholar]
- 30.Saez F, et al. Sex-dependent hypertension and renal changes in aged rats with altered renal development. Am. J. Physiol. Renal. Physiol. 2014;307:F461–470. doi: 10.1152/ajprenal.00198.2014. [DOI] [PubMed] [Google Scholar]
- 31.Günther M, et al. COX-2 regulation and TUNEL-positive cell death differ between genders in the secondary inflammatory response following experimental penetrating focal brain injury in rats. Acta. Neurochir. (Wien) 2015;157:649–659. doi: 10.1007/s00701-014-2331-2. [DOI] [PubMed] [Google Scholar]
- 32.Gonzales RJ, Duckles SP, Krause DN. Dihydrotestosterone stimulates cerebrovascular inflammation through NFkappaB, modulating contractile function. J. Cereb. Blood Flow. Metab. 2009;29:244–253. doi: 10.1038/jcbfm.2008.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surette ME, Palmantier R, Gosselin J, Borgeat P. Lipopolysaccharides prime whole human blood and isolated neutrophils for the increased synthesis of 5-lipoxygenase products by enhancing arachidonic acid availability: involvement of the CD14 antigen. J. Exp. Med. 1993;178:1347–1355. doi: 10.1084/jem.178.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Card JW, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J. Immunol. 2006;177:621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koeberle A, et al. Discovery of benzo[g]indol-3-carboxylates as potent inhibitors of microsomal prostaglandin E(2) synthase-1. Bioorg. Med. Chem. 2009;17:7924–32. doi: 10.1016/j.bmc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pergola C, et al. The novel benzimidazole derivative BRP-7 inhibits leukotriene biosynthesis in vitro and in vivo by targeting 5-lipoxygenase-activating protein (FLAP) Br. J. Pharmacol. 2014;171:3051–3064. doi: 10.1111/bph.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pergola C, et al. On the inhibition of 5-lipoxygenase product formation by tryptanthrin: mechanistic studies and efficacy in vivo. Br. J. Pharmacol. 2012;165:765–776. doi: 10.1111/j.1476-5381.2011.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


