Abstract
Engineered zinc oxide nanoparticles (ZnO-NPs) are currently being produced in high tonnage. Exposure to ZnO-NPs presents potential risks to cardiovascular system. Thus far, the toxicological effects of ZnO-NPs on cardiovascular system have not been well characterized. In this study, human coronary artery endothelial cells (HCAECs) were exposed to ZnO-NPs directly or indirectly using a transwell coculture system with human alveolar epithelial cell line A549 to mimic the lung/circulation interaction. It was shown that levels of proinflammatory mediators (interleukin-8 [IL-8] and tumor necrosis factor-α [TNF-α]) and biomarkers of atherosclerogenesis (heme oxygenase-1 [HO-1] and platelet endothelial cell adhesion molecules-1 [PECAM-1]) in the supernatants of culture media were significantly increased. Pretreatment of A549 cells on the apical side of the coculture system with the phagocytosis inhibitor cytochalasin B (CB) blocked ZnO-NP-induced HO-1 and PECAM-1 expression in HCAEC, indicating that endocytosis of ZnO-NPs by alveolar epithelial cells was involved in ZnO-NP-induced HO-1 or PECAM-1 expression in endothelial cells. Moreover, Wistar rats were intratracheally instilled with ZnO-NP suspension and high fat diet (positive control). ZnO-NP treatment induced lung and systemic inflammation, dyslipidemia, increased levels of serum HO-1 and PECAM-1, and aortic pathological damage. Taken together, exposure to ZnO-NPs could induce atherosclerotic alterations, which might involve phagocytosis of nanoparticles and inflammation in the lung.
Keywords: zinc oxide nanoparticles, atherosclerosis, lung inflammation, heme oxygenase-1, platelet endothelial cell adhesion molecules-1
Introduction
Zinc oxide (ZnO) nanoparticles (NPs) are widely applied in various commercial and industrial products such as food additives, cosmetics, drug delivery, and personal hygiene products, which are due to their UV light absorption, antimicrobial, and catalytic properties, among others.1,2 There are multiple exposure pathways to ZnO-NPs, such as occupational exposure to ZnO-NPs in manufacturing processes and environmental exposure to ZnO-NPs from air pollution.3,4
Inhalation of NPs is associated with pulmonary and cardiovascular responses.5,6 Inhaled nanoparticles can readily deposit in lung tissue and induce inflammatory response in lung alveoli.7,8 Translocation of inhaled nanoparticles into the systemic circulation has been detected and might be related to adverse cardiovascular effects. It has been recently assumed that the main mechanism for translocation of inhaled nanoscaled particles appears to be via endocytosis of alveolar epithelial cells.9 Recently, much attention has been focused on the link between nanoparticles and cardiovascular diseases. Existing evidence suggests that exposure to ultrafine particulate matter is linked to increased incidence of cardiovascular diseases.10,11 Silica nanoparticles induce autophagic activity in vascular endothelial cells (ECs) and pericytes and subsequently disturb the EC homeostasis and impair angiogenesis.12 A recent animal study has shown that a single intratracheal administration of saline solution containing TiO2 nanoparticles increases cardiac conduction velocity and tissue excitability, resulting in an enhanced propensity for inducible arrhythmias.13 In addition, both multiwalled carbon nanotubes and ZnO-NPs have been demonstrated to induce procoagulant effects.14 Thus, ZnO-NPs are possible to cause harmful effects on cardiovascular system,15 yet the mechanisms underlying this correlation remain unclear.
In this present study, we aimed to characterize the cardiovascular effects induced by ZnO-NPs in vitro and in vivo and possible mechanisms. In the in vitro study, human coronary artery endothelial cells (HCAECs) were exposed to ZnO-NPs in either monoculture or coculture with human alveolar type II epithelial cells (A549), then levels of interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), heme oxygenase-1 (HO-1), and atherosclerotic biomarkers including platelet endothelial cell adhesion molecules-1 (PECAM-1) were determined. Through these studies, whether and how the lung was involved in ZnO-NP-induced atherosclerotic alterations would be clarified. The animal study was designed to investigate the atherosclerotic disorders induced by intratracheally instilled ZnO-NPs.
Materials and methods
Preparation of ZnO-NP suspension
Uncoated ZnO-NPs (30 nm in diameter) were purchased from Nanjing High Technology of Nano Co. (Nanjing, People’s Republic of China) with the purity of 99.5%. The characteristics of ZnO-NPs were depicted previously.16 Stock suspension of ZnO-NPs (1 mg/mL) was prepared freshly in phosphate-buffered saline (PBS, pH 7.4) and sonicated for 30 s prior to exposure.
In vitro monoculture and coculture models
HCAECs were obtained from Lonza Inc (Basel, Switzerland) and A549 cells from Type Culture Collection of Chinese Academy of Sciences (Shanghai, People’s Republic of China). Cells were maintained in RPMI 1640 culture medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. Cells were passaged at 80%–90% confluency using trypsin-EDTA solution.
As for the coculture model, A549 cells were seeded into Transwell® inserts (1.12 cm2 for the 12-well polycarbonate plate, pore size 0.4 μm) precoated with collagen I separating the well into an apical and a basal compartment, and HCAECs were seeded into the basal tissue culture plates with 0.5 mL medium in the upper compartment (insert) and 1 mL in lower compartment (Scheme 1).
Scheme 1.
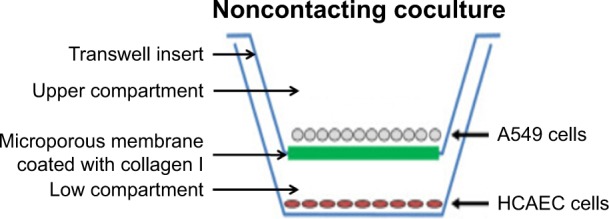
Schematic diagram of noncontacting coculture of HCAEC and A549 cells.
Abbreviations: HCAEC, human coronary artery endothelial cells; A549, human alveolar type II epithelial cells.
Cytotoxicity
HCAEC and A549 cells were incubated with ZnO-NPs (0, 12.5, 25, 50, 100, and 200 μg/mL) for 24 h, respectively. Cytotoxicity of ZnO-NPs to A549 and HCAEC was evaluated by MTT assay. Cell viability was expressed as percentage of control as described previously.16
Enzyme-linked immunosorbent assay (ELISA)
Protein levels of IL-8, TNF-α, HO-1, and PECAM-1 in the supernatants of culture media were measured with ELISA kits (Elabscience Biotechnology Co., Ltd., Cusabio, People’s Republic of China) according to the manufacturer’s recommendations. The experiment was performed in triplicate.
Measurement of ZnO-NPs solubility
The HCAEC in monoculture and coculture were collected and washed with PBS for five times after treatment of ZnO-NPs with indicated concentration for 24 h. The cells were disrupted by ultrasonication. The supernatant of cell lysate was collected and centrifuged at 13,000× g for 5 min. Zn levels in the supernatant were measured with C2II2 atomic absorption spectrophotometer.
Phagocytosis assay
HECAE and A549 cells were cocultured on 12-well plate. A549 cells in coculture were preincubated with 10 μg/mL cytochalasin B (CB; EMD, San Diego, CA, USA) and vehicle control (DMSO; Sigma-Aldrich Co., St Louis, MO, USA) for 30 min prior to ZnO-NPs (40 μg/mL) treatment for another 24 h. ZnO-NPs had the ability to interact with the epithelial barrier but did not appear to pass through the Transwell membrane to the endothelial layer. The supernatant in the basolateral chamber was harvested to measure the levels of HO-1 and PECAM-1 using ELISA.
Animal treatment
Male Wistar rats (body weight 180±10 g) were obtained from the Experimental Animal Center of Henan Province, China, and housed in a climate-controlled room with a 12-h light/dark cycle, fed with ad libitum diet and distilled water. After 1-week acclimation, 50 rats were randomized into five groups (n=10 rats per group). Four experimental groups were, respectively, intratracheally instilled with ZnO-NPs suspension with different concentrations (0, 1.25, 2.5, and 5.0 mg/kg) once a week for a total of 12 weeks and fed with normal diet. Meanwhile, the other group was instilled with PBS and raised with high fat diet containing 3% cholesterol, 0.5% sodium cholate, 0.2% propylthiouracil, 5% lard, and 91.3% basic diet. Animals were weighed each week. At the termination of experiment, fasting blood and bronchoalveolar lavage fluid (BALF) were collected. Epididymal adipose tissues were carefully removed and weighed, and its ratio to body weight was calculated.17 Aortas were separated for pathological examination at gross and microscopic levels, respectively. All the experimental procedures conducted on animals were approved by the Animal Care and Use Committee of Zhengzhou University and performed in strict accordance with the Guide for the Care and Use of Laboratory Animals of Zhengzhou University.
Measurement of total protein and lactate dehydrogenase (LDH) in BALF
Total protein content in BALF, a marker for vascular permeability,18 was assessed using a bicinchoninic acid assay kit (Beyotime, Jiangsu, People’s Republic of China) and expressed as μg/mL. LDH activity, a marker for cytotoxicity, was evaluated using an LDH assay kit (Beyotime) and expressed as unit per liter (U/L). Total protein and LDH activity in BALF were determined to evaluate the pulmonary inflammation.
Measurement of proinflammatory and atherosclerotic proteins
Blood was centrifuged at 1,500× g for 10 min at 4°C and serum separated, aliquoted, and stored at −80°C until analysis. Serum total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), IL-8, TNF-α, HO-1, and PECAM-1 were analyzed using ELISA kits (Elabscience Biotechnology Co., Ltd.) according to the manufacturer’s protocol.
Statistical analysis
All statistical analysis was performed using Statistical package for the Social Sciences (version 21.0; SPSS, Inc., Chicago, IL, USA). Data were presented as mean ± SD, and one-way analysis of variance (ANOVA) followed by post hoc LSD test was used for multiple comparisons except that body weight data were analyzed by repeated measures one-way ANOVA. Statistical significance was considered at P<0.05.
Results
Cytotoxicity of ZnO-NPs
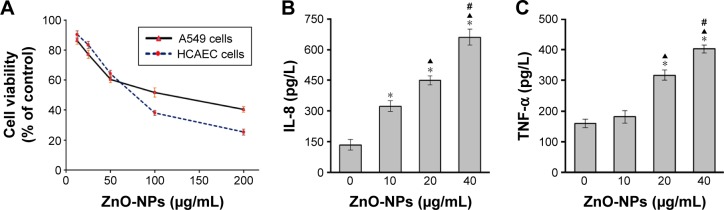
MTT assay has been widely used for detection of cytotoxicity induced by environmental toxicants. Herein, we used MTT assay to determine the injurious effect of ZnO-NPs on A549 and HCAEC. As shown in Figure 1A, ZnO-NP treatment induced a dose-dependent reduction of viability in both A549 and HCAEC. At the concentration of 100 μg/mL with the incubation time of 24 h, ZnO-NP exposure caused about 50% of cell death in A549 cells. As for HCAEC, it was about 80 μg/mL when the cell viability was 50%. These data indicated that ZnO-NPs could markedly induce cell toxicity in both A549 and HCAEC.
Figure 1.
Effect of ZnO-NPs on cell viability and expression of IL-8 and TNF-α in coculture.
Notes: (A) HCAEC and A549 cells were incubated with ZnO-NPs, respectively. Cell viability was assessed by MTT assay. A549 cells in coculture were exposed to ZnO-NPs for 24 h. The basolateral media were collected for analysis of IL-8 (B) and TNF-α (C) expression by ELISA. Data shown were representative of three separate experiments (*compared to control group, P<0.05; ▲compared to 10 μg/mL group, P<0.05; and #compared to 20 μg/mL group, P<0.05).
Abbreviations: ZnO-NPs, zinc oxide nanoparticles; IL-8, interleukin-8; TNF-α, tumor necrosis factor-α; HCAEC, human coronary artery endothelial cell; A549, human alveolar type II epithelial cells; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; ELISA, enzyme-linked immunosorbent assay.
Exposure to ZnO-NPs induced overexpression of IL-8 and TNF-α in coculture
To examine the effect of ZnO-NPs on inflammatory mediators in coculture, A549 cells in coculture system were exposed to ZnO-NPs for 24 h and ELISA was used to measure IL-8 and TNF-α protein levels. ZnO-NPs stimulation induced significant increases in both IL-8 (Figure 1B) and TNF-α (Figure 1C).
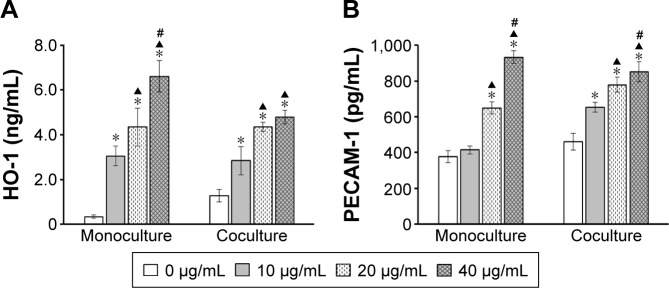
Exposure to ZnO-NPs induced HO-1 and PECAM-1 overexpression of HCAEC in monoculture and coculture
To determine the effect of ZnO-NPs on HO-1 and PECAM-1 expression in HCAEC, the monoculture and coculture system were exposed to ZnO-NPs for 24 h, and ELISA was used to measure the protein levels of HO-1 and PECAM-1 in HCAEC exposed to ZnO-NPs directly or indirectly. As shown in Figure 2, ZnO-NP direct stimulation induced a dose-dependent increase in both HO-1 and PECAM-1 expression in HCAEC monoculture. Similarly, the effect of ZnO-NPs on coculture system also presented a dose-dependent increase in HO-1 and PECAM-1 protein release from HCAEC. As HO-1 and PECAM-1 protein levels increased in the basolateral chamber after ZnO-NPs exposure, it was assumed that cellular mediators secreted by A549 cells were capable of passing through the transwell membrane to the basolateral chamber, and further resulting in HO-1 and PECAM-1 expressions in HCAEC. Taken together, these results indicated that ZnO-NP exposure upregulated HO-1 and PECAM-1 expression in HCAECs with direct contact or indirect action through the mediators released from alveolar epithelial cells.
Figure 2.
Effect of ZnO-NPs on HO-1 and PECAM-1 expression in HCAEC.
Notes: HCAECs were cultured in the presence (coculture) and in the absence (monoculture) of A549 cells on transwell membranes. HCAEC monoculture and A549 cells in coculture were incubated with ZnO-NPs for 24 h, respectively. The expression of HO-1 (A) and PECAM-1 (B) from HCAEC in the supernatant was measured using ELISA (*compared to control group, P<0.05; ▲compared to 10 μg/mL group, P<0.05; #compared to 20 μg/mL group, P<0.05).
Abbreviations: ZnO-NPs, zinc oxide nanoparticles; HO-1, heme oxygenase-1; PECAM-1, platelet endothelial cell adhesion molecule-1; HCAECs, human coronary artery endothelial cells; A549, human alveolar type II epithelial cells; ELISA, enzyme-linked immunosorbent assay.
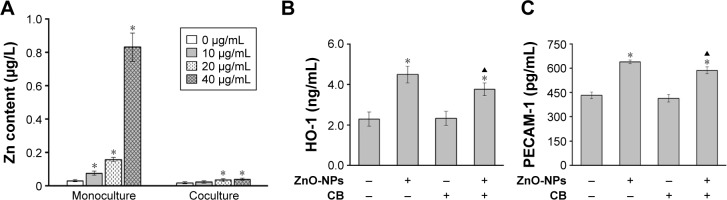
Involvement of phagocytosis in ZnO-NP-induced HO-1 and PECAM-1 expression of HCAEC in coculture
Exposure to ZnO-NPs could increase intracellular zinc levels of HCAEC in both monolayer and coculture models (Figure 3A), which suggested that ZnO-NPs might be internalized and penetrated through the alveolar epithelial cells. CB could restrict the access of exogenous particles to cells through inhibiting phagocytosis.19 Exposure to ZnO-NPs induced significant expressions of HO-1 and PECAM-1 in HCAEC either in the presence or in the absence of A549 cells. Notably, pretreatment of A549 with CB modestly inhibited ZnO-NP-induced HO-1 (Figure 3B) and PECAM-1 (Figure 3C) expression in HCAEC. In combination, these results indicated that ZnO-NPs could induce HO-1 and PECAM-1 expression in HCAEC through the mediators released from A549 cells.
Figure 3.
Effects of CB on ZnO-NP-induced HO-1 and PECAM-1 expression of HCAEC in coculture.
Notes: (A) HCAECs in monoculture and A549 cells in coculture were incubated with ZnO-NPs for 24 h. Levels of intracellular zinc contents in HEAEC were measured using flame atomic absorption spectrometry (*compared to control group, P<0.05). A549 cells in coculture were preincubated with CB or vehicle control, prior to ZnO-NP (40 μg/mL) treatment for 24 h. HO-1 (B) and PECAM-1 (C) expression of the supernatant in basolateral chamber were measured using ELISA (*compared to the vehicle control group, P<0.05; ▲compared to ZnO-NP-treated cells without CB treatment, P<0.05).
Abbreviations: CB, cytochalasin B; ZnO-NPs, zinc oxide nanoparticles; HO-1, heme oxygenase-1; PECAM-1, platelet endothelial cell adhesion molecules-1; HCAEC, human coronary artery endothelial cells; A549, human alveolar type II epithelial cells; ELISA, enzyme-linked immunosorbent assay.
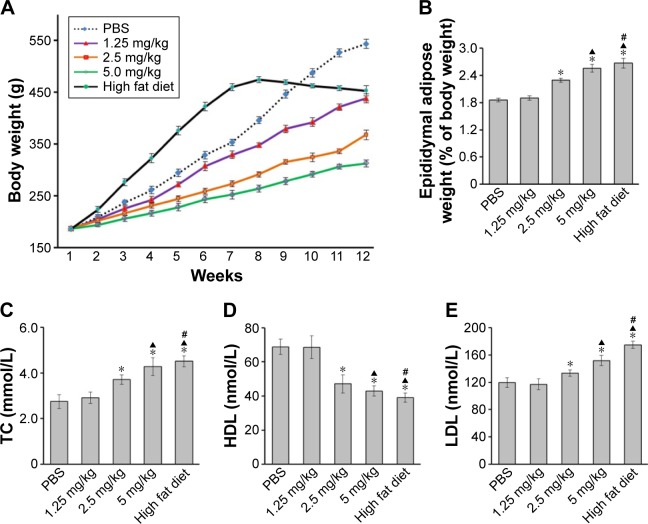
ZnO-NP treatment induced dyslipidemia in rats
Rats in all experimental groups had similar initial body weight. Compared to that of the rats instilled with PBS, the body weight of rats instilled with ZnO-NPs decreased from the fourth week (Figure 4A). Epididymal fat pads could be clearly isolated and often considered as the visceral fat. The ratio of epididymal fat pads vs body weight was calculated. As shown in Figure 4B, the ratios in high fat diet group and 2.5 or 5 mg/kg ZnO-NPs groups were all higher than that in PBS control group. The high fat diet group showed the highest ratio compared to other groups.
Figure 4.
ZnO-NPs treatment induced dyslipidemia in rats.
Notes: (A) Body mass of rats was measured weekly during the experiment. After last instillation, rats were anesthetized and sacrificed. (B) Epididymal adipose tissue was removed and weighed, and its ratio to body weight was calculated. The blood samples collected from heart were tested for levels of serum TC (C), HDL (D), and LDL (E) using ELISA (*compared to control group, P<0.05; ▲compared to 2.5 mg/kg group, P<0.05; #compared to 5 mg/kg group, P<0.05).
Abbreviations: ZnO-NPs, zinc oxide nanoparticles; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ELISA, enzyme-linked immunosorbent assay.
The effect of ZnO-NPs on serum lipid profile of rats was demonstrated in Figure 4C–E, which showed a significant increase in TC and LDL levels in ZnO-NP-treated rats. However, HDL levels were remarkably decreased compared with those of control rats, which was similar to the change in high fat diet group. Taken together, these results showed that exposure to ZnO-NPs could induce dyslipidemia, a premonitory alteration of atherosclerosis.
Lung inflammation and systemic inflammation induced by ZnO-NPs
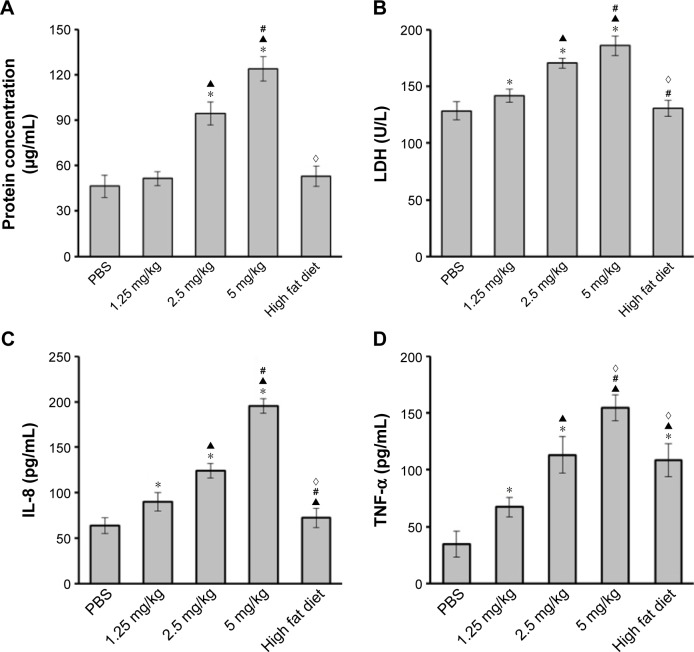
Protein contents (Figure 5A) in BALF from rats instilled with 2.5 or 5 mg/kg ZnO-NPs were increased compared with that in control group. There were no difference among 1.25 mg/kg ZnO-NPs group, high fat group, and control group. LDH activity (Figure 5B) in BALF of rats exposed to ZnO-NPs was increased in a dose-dependent manner and higher than those in control and high fat groups. Levels of serum IL-8 (Figure 5C) and TNF-α (Figure 5D) in rats exposed to ZnO-NPs were both increased in a dose-dependent manner. TNF-α expression in high fat diet group was higher than that in PBS control group, but there was no significant difference between high fat diet group and PBS control group in the levels of serum IL-8.
Figure 5.
ZnO-NP induces lung inflammation and systemic inflammation.
Notes: After last instillation, rats were anesthetized and sacrificed. BALF was collected for the measurement of total proteins (A) and LDH (B) activity to represent the lung inflammation. The blood samples collected from heart were tested for levels of serum IL-8 (C) and TNF-α (D) using ELISA (*compared to control group, P<0.05; ▲compared to 1.25 mg/kg group, P<0.05; #compared to 2.5 mg/kg group, P<0.05; ◊compared to 5 mg/kg group, P<0.05).
Abbreviations: ZnO-NPs, zinc oxide nanoparticles; BALF, bronchoalveolar lavage fluid; LDH, lactate dehydrogenase; IL-8, interleukin-8; TNF-α, tumor necrosis factor-α; ELISA, enzyme-linked immunosorbent assay.
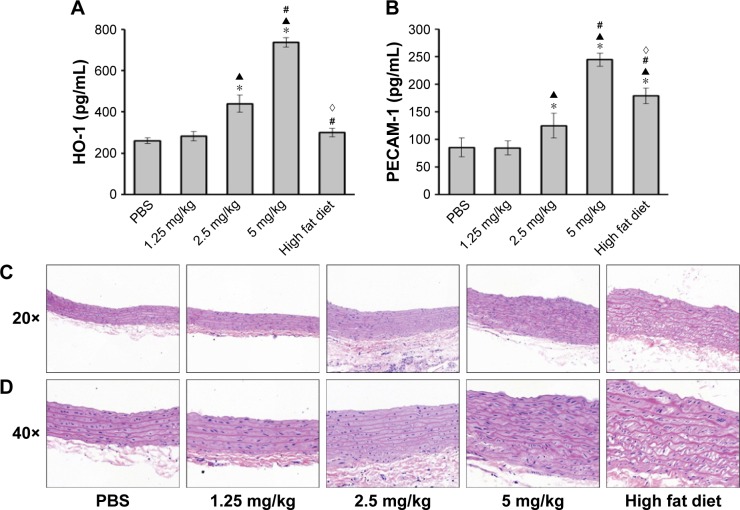
ZnO-NP-induced atherosclerotic alterations in rats
HO-1 and PECAM-1 have been associated with atherosclerotic pathogenesis.20–22 Levels of serum HO-1 (Figure 6A) and PECAM-1 (Figure 6B) in 2.5 and 5 mg/kg ZnO-NPs groups were higher than those in PBS control group. PECAM-1 expression in high fat diet group was higher than that in PBS control group, but there was no significant difference between high fat diet group and PBS control group in levels of serum HO-1.
Figure 6.
ZnO-NP-induced atherosclerotic alterations in rats.
Notes: After last instillation, rats were anesthetized and the blood samples collected from heart were tested for levels of serum HO-1 (A) and PECAM-1 (B) using ELISA. The aortas were separated from the anesthetized rats. Light microscopy was used to analyze the aortic sections stained with H&E at gross (C, 20×) and microscopic (D, 40×) levels (*compared to control group, P<0.05; ▲compared to 1.25 mg/kg group, P<0.05; #compared to 2.5 mg/kg group, P<0.05; ◊compared to 5 mg/kg group, P<0.05).
Abbreviations: ZnO-NPs, zinc oxide nanoparticles; HO-1, heme oxygenase-1; PECAM-1, platelet endothelial cell adhesion molecules-1; ELISA, enzyme-linked immuno-sorbent assay; H&E, hematoxylin and eosin.
Histopathological examination of the aortic ECs in rats instilled with PBS using hematoxylin and eosin (H&E) stain (Figure 6C and D) presented a complete continuous and smooth surface under light microscopy. In contrast, in the 1.25 and 2.5 mg/kg ZnO-NPs groups, the arterial intimal surface appeared smooth. However, the ECs were damaged and smooth muscle cell overgrowth to some extent. Remarkably, in the rats with high fat diet and 5 mg/kg ZnO-NPs, thickened vessel wall, damaged smooth endometrial ECs, discontinued, or fragmented intimal surface were observed. Overgrowth and derangement of medial superficial smooth muscle cells were also observed, and subendothelial space was enlarged. Importantly, the medial smooth muscle cells were seen in the intima layer, indicative of their migration to the surface. In summary, ZnO-NP exposure could induce atherosclerotic alterations in rats.
Discussion
A large number of studies have demonstrated that nanoparticles in the respiratory tract can cross the blood–air barrier, enter into bloodstream, and induce inflammation which is critical to the initiation of adverse pulmonary and cardiovascular reactions.9,13,23 There are two main mechanisms to explain how pulmonary NPs exposure can initiate cardiovascular responses. One hypothesis states that NPs deposited in the lung elicit local inflammatory responses via oxidative stress that further develop into systemic oxidative stress/inflammation.24 On the other hand, NPs deposited in the lung can translocate into the systemic circulation and directly interact with cardiovascular tissues to induce injury or inflammation.25,26
ECs, which form the inner cellular lining of the entire cardiovascular system, have direct contact with NPs.27 ZnO-NPs direct stimulation could markedly induce cell toxicity and upregulate cardiovascular mediators, HO-1 and PECAM-1, in HCAEC. HO-1 is considered as an endogenous cytoprotective enzyme with an antioxidant role in cells including the ECs in response to various cellular stressors.28–30 Human and rodent experimental data also suggest that HO-1 activity is antiatherogenic.29 PECAM-1, also called CD31, as a cell adhesion molecule, is mainly expressed on the surface of ECs, circulating platelets, monocytes, neutrophils, and certain T-cell subsets. PECAM-1 is thought to play a role in the pathological mechanisms of atherosclerosis.22,31 Therefore, HO-1 and PECAM-1 were known to have a role in the pathogenesis of atherosclerosis. This study was also to determine whether the interaction of lung alveolar epithelial cells with ZnO-NPs in vitro could affect the underlying vascular ECs. We examined nanoparticle indirect effects using an in vitro coculture model. Coculture is a promising alternative to monoculture and provides a more in vivo-like environment for the determination of toxicological results.32 In this system, levels of inflammatory cytokines, IL-8, and TNF-α were induced by ZnO-NPs in a dose-dependent manner. Meanwhile, expression of HO-1 and PECAM-1 was increased significantly after ZnO-NPs exposure in coculture model. Interestingly, differential background value of HO-1 expression was observed between monoculture and coculture cells, that is, HO-1 expression was very low in monoculture of HCAEC, but high in coculture, which is not consistent with the observation previous work showing that HO-1 expression and activity were only present in ECs from advanced atherosclerotic lesions.33 Although basal level of HO-1 protein in untreated HCAEC was typically low, a significant induction was seen following exposure to ZnO-NPs, and the change trend was in concert with the previous report, that is, atherosclerotic stimuli could upregulate the expression of HO-1.
ZnO-NPs were previously shown to enter the lung epithelium in vivo and also be taken up by epithelial cells in vitro.16 Documented evidence has shown that NPs can be taken up by cells via well-known pathways of endocytosis.5 In order to explore the indirect effect of ZnO-NPs on ECs, CB was used to block the phagocytosis of nanoparticles. Pretreatment of A549 with CB could markedly suppress ZnO-NP-induced expressions of HO-1 and PECAM-1 in HCEAC in coculture. CB could decrease the access of ZnO-NP to A549, which might release less cellular mediators so as to induce low HO-1 and PECAM-1 expressions in HCAEC. The results indicated that ZnO-NPs could be internalized and penetrated through the alveolar epithelial cells and further induced HO-1 and PECAM-1 expressions. Taken together, the in vitro studies showed that ZnO-NPs exposure induced the expression of inflammatory mediators and upregulated the regulators of atherosclerosis, such as HO-1 and PECAM-1, by means of endocytosis. This study also demonstrated that direct exposure of ECs to ZnO-NPs increased HO-1 and PECAM-1 expression in a dose-dependent manner. Therefore, it is envisioned that ZnO-NPs could induce atherosclerotic alteration through direct contact with vascular ECs and indirect action through the released mediators from alveolar epithelial cells.
Pulmonary inflammation can further lead to systemic inflammation and various cardiovascular diseases.25 Therefore, the lung and systemic inflammatory responses may play a vital role in the pathological mechanisms of atherosclerosis and cerebral vascular disease.31 Based on the results from published studies, we hypothesized that ZnO-NPs exert their inflammatory effects, which further drives the adverse cardiovascular effects. In this study, we investigated the atherosclerotic effect of ZnO-NPs in vivo. High fat diet was thought to be a risk of cardiovascular diseases,34 therefore, the rats fed with high fat diet were served as the positive control. The present study demonstrated that ZnO-NPs exposure could influence the blood lipid by increasing TC, LDL, and visceral fat while lowering HDL, which indicated that ZnO-NPs could potentially elevate the risk of atherosclerosis. Meanwhile, ZnO-NP exposure obviously elicited pulmonary and systemic inflammation and cardiovascular toxicity through increased HO-1 and PECAM-1. Finally, the cardiovascular toxicity of ZnO-NPs was reflected as aortic morphological changes. Microscopic alterations in rats instilled with ZnO-NPs (5 mg/kg) were obvious, which were similar to the rats with high fat diet. However, it should also be noted that there was some difference between the effect of ZnO-NPs and high fat diet, for example, high fat diet did not induce the increase in total protein, LDH, IL-8, and HO-1, except TNF-α.
Conclusion
The current study has demonstrated that exposure to ZnO-NPs could induce atherosclerotic alterations via direct and indirect actions. This process may involve oxidative stress, lipid metabolism disorders, and inflammation. Nanoparticle-induced pulmonary and systemic inflammation and accelerated atherosclerosis might be part of the pathophysiological pathways, linking nanoparticles with cardiovascular morbidity. Further research about endothelial dysfunction and mechanisms was needed to investigate the cardiovascular toxic effects of ZnO-NPs.
Acknowledgments
This project was supported by the grants from National Natural Science Foundation of China (81573112), Outstanding Young Talent Research Fund of Zhengzhou University (1521329034), and Basic and Frontier Technology Research Program of Henan Province (144300510055).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cho WS, Duffin R, Howie SE, et al. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part Fibre Toxicol. 2011;8:27. doi: 10.1186/1743-8977-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chupani L, Zuskova E, Niksirat H, et al. Effects of chronic dietary exposure of zinc oxide nanoparticles on the serum protein profile of juvenile common carp (Cyprinus carpio L.) Sci Total Environ. 2017;579:1504–1511. doi: 10.1016/j.scitotenv.2016.11.154. [DOI] [PubMed] [Google Scholar]
- 3.Wu WD, Samet JM, Peden DB, Bromberg PA. Phosphorylation of p65 is required for zinc oxide nanoparticle-induced interleukin 8 expression in human bronchial epithelial cells. Environ Health Perspect. 2010;118(7):982–987. doi: 10.1289/ehp.0901635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan CH, Chuang KJ, Chen JK, et al. Characterization of pulmonary protein profiles in response to zinc oxide nanoparticles in mice: a 24-hour and 28-day follow-up study. Int J Nanomedicine. 2015;10:4705–4716. doi: 10.2147/IJN.S82979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhlfeld C, Gehr P, Rothen-Rutishauser B. Translocation and cellular entering mechanisms of nanoparticles in the respiratory tract. Swiss Med Wkly. 2008;138(27–28):387–391. doi: 10.4414/smw.2008.12153. [DOI] [PubMed] [Google Scholar]
- 6.McKinney W, Jackson M, Sager TM, et al. Pulmonary and cardiovascular responses of rats to inhalation of a commercial antimicrobial spray containing titanium dioxide nanoparticles. Inhal Toxicol. 2012;24(7):447–457. doi: 10.3109/08958378.2012.685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song W, Wang JX, Liu ML, et al. Titanium dioxide nanoparticles induced proinflammation of primary cultured cardiac myocytes of rat. J Nanomater. 2014;13(43):7246–7254. [Google Scholar]
- 8.Warheit DB, Brock WJ, Lee KP, Webb TR, Reed KL. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact of surface treatments on particle toxicity. Toxicol Sci. 2005;88(2):514–524. doi: 10.1093/toxsci/kfi331. [DOI] [PubMed] [Google Scholar]
- 9.Elsaesser A, Howard CV. Toxicology of nanoparticles. Adv Drug Deliv Rev. 2012;64(2):129–137. doi: 10.1016/j.addr.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Peters A, Pope CA. Cardiopulmonary mortality and air pollution. Lancet. 2002;360(9341):1184–1185. doi: 10.1016/S0140-6736(02)11289-X. [DOI] [PubMed] [Google Scholar]
- 11.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan J, Yu Y, Li Y, et al. Silica nanoparticles enhance autophagic activity, disturb endothelial cell homeostasis and impair angiogenesis. Part Fibre Toxicol. 2014;11(1):50. doi: 10.1186/s12989-014-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savi M, Rossi S, Bocchi L, et al. Titanium dioxide nanoparticles promote arrhythmias via a direct interaction with rat cardiac tissue. Part Fibre Toxicol. 2014;11(1):63. doi: 10.1186/s12989-014-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luyts K, Smulders S, Napierska D, et al. Pulmonary and hemostatic toxicity of multi-walled carbon nanotubes and zinc oxide nanoparticles after pulmonary exposure in Bmal1 knockout mice. Part Fibre Toxicol. 2014;11(1):61. doi: 10.1186/s12989-014-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li CH, Liao PL, Shyu MK, et al. Zinc oxide nanoparticles-induced intercellular adhesion molecule 1 expression requires Rac1/Cdc42, mixed lineage kinase 3, and c-Jun N-terminal kinase activation in endothelial cells. Toxicol Sci. 2012;126(1):162–172. doi: 10.1093/toxsci/kfr331. [DOI] [PubMed] [Google Scholar]
- 16.Yan Z, Xu L, Han J, et al. Transcriptional and posttranscriptional regulation and endocytosis were involved in zinc oxide nanoparticle-induced interleukin-8 overexpression in human bronchial epithelial cells. Cell Biol Toxicol. 2014;30(2):79–88. doi: 10.1007/s10565-014-9270-9. [DOI] [PubMed] [Google Scholar]
- 17.Cottrell EC, Martin-Gronert MS, Fernandez-Twinn DS, Luan J, Berends LM, Ozanne SE. Leptin-independent programming of adult body weight and adiposity in mice. Endocrinology. 2011;152(2):476–482. doi: 10.1210/en.2010-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Hwang SH, Jeong J, et al. Nickel oxide nanoparticles can recruit eosinophils in the lungs of rats by the direct release of intracellular eotaxin. Part Fibre Toxicol. 2015;13(1):30. doi: 10.1186/s12989-016-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papageorgiou I, Brown C, Schins R, et al. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials. 2007;28(19):2946–2958. doi: 10.1016/j.biomaterials.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Harry BL, Sanders JM, Feaver RE, et al. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in apoe-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28(11):2003–2008. doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of heme oxygenase-1 in atherosclerotic lesions. Am J Pathol. 1998;152(3):711–720. [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens HY, Melchior B, Bell KS, Yun SJ, Yeh JC, Frangos JA. PECAM-1 is a critical mediator of atherosclerosis. Dis Model Mech. 2008;1(2–3):175–181. doi: 10.1242/dmm.000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mossman BT, Borm PJ, Castranova V, Costa DL, Donaldson K, Kleeberger SR. Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Part Fibre Toxicol. 2007;4(1):1–10. doi: 10.1186/1743-8977-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X, Zhu T, Chen C, Liu Y. Right or left: the role of nanoparticles in pulmonary diseases. Int J Mol Sci. 2014;15(10):17577–17600. doi: 10.3390/ijms151017577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang GS, Gillespie PA, Gunnison A, Moreira AL, Tchou-Wong KM, Chen LC. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environ Health Perspect. 2011;119(2):176–181. doi: 10.1289/ehp.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoet PH, Bruske-Hohlfeld I, Salata OV. Nanoparticles – known and unknown health risks. J Nanobiotechnology. 2004;2(1):12. doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Sun J. Potential proinflammatory effects of hydroxyapatite nanoparticles on endothelial cells in a monocyte-endothelial cell coculture model. Int J Nanomedicine. 2014;9:1261–1273. doi: 10.2147/IJN.S56298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang G, Li Y, Wu W, et al. Anti-oxidant effect of heme oxygenase-1 on cigarette smoke-induced vascular injury. Mol Med Rep. 2015;12(2):2481–2486. doi: 10.3892/mmr.2015.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mylroie H, Dumont O, Bauer A, et al. PKCepsilon-CREB-Nrf2 signalling induces HO-1 in the vascular endothelium and enhances resistance to inflammation and apoptosis. Cardiovasc Res. 2015;106(3):509–519. doi: 10.1093/cvr/cvv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pechlaner R, Willeit P, Summerer M, et al. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with progressive atherosclerosis and incident cardiovascular disease. Arterioscler Thromb Vasc Biol. 2015;35(1):229–236. doi: 10.1161/ATVBAHA.114.304729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y, Li Q, Long L, Zhang N, Liu Y. Asn563Ser polymorphism of CD31/PECAM-1 is associated with atherosclerotic cerebral infarction in a southern Han population. Neuropsychiatr Dis Treat. 2015;11:15–20. doi: 10.2147/NDT.S75065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder-Talkington BN, Schwegler-Berry D, Castranova V, Qian Y, Guo NL. Multi-walled carbon nanotubes induce human microvascular endothelial cellular effects in an alveolar-capillary co-culture with small airway epithelial cells. Part Fibre Toxicol. 2013;10:35. doi: 10.1186/1743-8977-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morsi WG, Shaker OG, Ismail EF, et al. HO-1 and VGEF gene expression in human arteries with advanced atherosclerosis. Clin Biochem. 2006;39(11):1057–1062. doi: 10.1016/j.clinbiochem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Attorri L, Di Biase A, Di Benedetto R, Rigato P, Di Virgilio A, Salvati S. Micronutrient-enriched rapeseed oils reduce cardiovascular disease risk factors in rats fed a high-fat diet. Atherosclerosis. 2010;213(2):422–428. doi: 10.1016/j.atherosclerosis.2010.07.003. [DOI] [PubMed] [Google Scholar]