Abstract
The efficacy of antibiotic monotherapy and combination therapy in the treatment of implant-associated infection by Staphylococcus aureus was evaluated in an animal study. The femoral medullary cavity of 66 male Wistar rats was contaminated with S. aureus (ATCC 29213) and a metal device was implanted, of which 61 could be evaluated. Six treatment groups were studied: flucloxacillin, flucloxacillin in combination with rifampin, moxifloxacin, moxifloxacin in combination with rifampin, rifampin, and a control group with aqua. The treatment was applied for 14 days. After euthanasia, the bacterial counts in the periprosthetic bone, the soft tissue, and the implant-associated biofilm were measured. Both antibiotic combination treatments (moxifloxacin plus rifampin and flucloxacillin plus rifampin) achieved a highly significant decrease in microbial counts in the bone and soft tissue and in the biofilm. Mono-antibiotic treatments with either moxifloxacin or flucloxacillin were unable to achieve a significant decrease in microbial counts in bone and soft tissue or the biofilm, whilst rifampin was able to reduce the counts significantly only in the biofilm. Antibiotic resistance was measured in 1/3 of the cases in the rifampin group, whereas no resistance was measured in all other groups. The results show that combinations of both moxifloxacin and flucloxacillin plus rifampin are adequate for the treatment of periprosthetic infections due to infections with S. aureus, whereas monotherapies are not effective or not applicable due to the rapid development of antibiotic resistance. Therefore, moxifloxacin is an effective alternative in combination with rifampin for the treatment of implant-associated infections.
Keywords: moxifloxacin, rifampin, flucloxacillin, implant-associated infection, prosthetic infection, Staphylococcus aureus
Introduction
Periprosthetic joint infections cause considerable mortality and morbidity in patients with joint arthroplasty. Due to the increasing number of interventions in primary joint replacement, the numbers of revision surgery following infection have been rising steadily. The choice of appropriate antibiotic therapy is still a challenge in the treatment of periprosthetic joint infection. In the treatment of staphylococcal infections, rifampin plays an important role.1 Due to the rapid development of resistance to rifampin in monotherapy, either a staphylococcal penicillin (ie, flucloxacillin) or a fluoroquinolone (ie, ciprofloxacin or levofloxacin) should be added for sufficient therapy. The antibiotic standard treatment of prosthesis infection by methicillin-sensitive staphylococcus currently consists of a combination of initial intravenous therapy with flucloxacillin and rifampin followed by a combination of an older quinolone (ie, ciprofloxacin or levofloxacin) with rifampin. Since many antibiotics that are able to penetrate into bone tissue, such as oxacillin, should be applied ideally parenterally, the treatment is often associated with a long hospitalization. Newer quinolones like moxifloxacin are possible combination partners, which were supposed to be examined more closely in this study due to their improved spectrum of activity against gram positive, gram negative, and anaerobic pathogens as well as their good oral bioavailability, activity, and safety.2 The superiority of moxifloxacin compared with vancomycin was demonstrated in the treatment of prosthetic infections.3 In addition, the oral bioavailability is almost as high as on parenteral administration.4 In numerous in vitro and in vivo studies, moxifloxacin was much more effective against staphylococci than older fluoroquinolones such as ciprofloxacin.5–10 Other studies demonstrated that moxifloxacin is capable of penetrating rapidly into infected soft and bone tissue.11–13
Our working group could successfully achieve bacterial contamination with intramedullary foreign body implantation in a minimally invasive technique and subsequent 14-day antibiotic treatment in rats in already completed animal studies; the local rate of infection in this animal model was 100%.3,14
To assess the antibiotic efficacy in implant-associated infections of the clinically frequently used moxifloxacin (group M), flucloxacillin (group F), rifampin (group R), and the combination of moxifloxacin plus rifampin (group MR) and flucloxacillin plus rifampin (group FR), compared to a placebo group (group A), the present controlled animal study was performed.
Materials and methods
Animals
The controlled animal study was performed after approval by the local and state animal protection committee (Regierung der Oberpfalz, Bavaria, Germany; approval application no 54-2531.1-21/06). All animal experiments were carried out in accordance with the European (EU) Directive 2010/63/EU. The study used 66 male Wistar rats (Charles River, Sulzfeld, Germany), of which 61 were included in the analysis. At the beginning of the study, the animals were aged 12–14 weeks.
Bacterial strain
For experimental contamination, a bacterial strain of Staphylococcus aureus (ATCC 29213; American Type Culture Collection, Manassas, VA, USA) was used. This particular strain is known to be penicillin-resistant and oxacillin-sensitive. The bacterial suspensions prepared from logarithmically growing cultures contained 108 colony-forming units (CFU)/mL.
Experimental technique
On day 0, all experimental animals were anesthetized and a sterilized hollow steel needle 1.5 cm ×1.0 mm from an intravenous catheter 18G was implanted retrogradely into the left femur after parapatellar incision. Next, the bacterial suspension was introduced into the medullary cavity (100 µL with 108 CFU/mL of S. aureus). Afterward, the distal femur was sealed by bone wax, the joint was irrigated, and the wound was closed.
Antibiotic treatment
Following implantation, on day 7, antibiotic treatment was started. The rats were randomized into six groups (rifampin, flucloxacillin, moxifloxacin, rifampin plus moxifloxacin, flucloxacillin plus rifampin, aqua [control group], referred to as groups M, F, R, MR, FR, and A).
Antibiotics used were flucloxacillin 1 g (Delta Select GmbH, Dreieich/Pfullingen, Germany), moxifloxacin hydrochloride (Bayer HealthCare AG, Wuppertal, Germany), and rifampin 600 mg sodium (Fatol Arzneimittel GmbH, Schiffweiler, Germany). Intraperitoneal applied doses were 200 mg/kg body weight flucloxacillin three times/day, 20 mg/kg body weight rifampin once daily, 10 mg/kg body weight moxifloxacin two times/day, and 0.4 mL aqua two or three times/day without any change of doses in the above listed groups of combined therapy. The treatment was given until day 21.
Termination criteria included fracture of the operated femur with signs of instability, wound healing disorders, systemic septic reaction. In order to document the effects of surgery and antibiotic treatment, body weight was checked at day 0, 8, 15, and 23.
Microbiological analysis
On day 23 of the experiment, the animals were anesthetized and euthanized 48 hours after the last administration of antibiotics, and the contaminated legs were explanted under sterile conditions. The periarticular soft tissues, femur, and the implant with biofilm were separated and microbiologically analyzed directly. As already described in previous studies concerning microbial infection and antibiotic treatment of implants realized in our department,3 both bone and soft tissue were frozen after extraction with liquid nitrogen, then homogenized in a dismembranator (Braun, Melsungen, Germany), and resuspended mechanically with 4 mL of 0.9% saline at 250 rpm (Vortex Genie 2; Bender & Hobein, Zürich, Switzerland).
To remove the biofilm, samples were placed in an ultrasonic bath and then cleaned mechanically in the same way as before with the bone and soft tissue. Afterward, 50 µL suspensions of biofilm, bone, and soft tissue, respectively, were plated onto tryptic soy agar plates in dilution series using a semiautomatic spiral platter (Whitley Automatic Spiral platter; Don Whitley Scientific, Shipley, UK). After 48 hours of incubation at 36°C, CFU/mL were counted blinded to treatment. The detection limit of the culture systems is 20 CFU/mL and absence of S. aureus colonies was defined as sterile.
In order to evaluate systemically induced infection or contamination, the contralateral (right) hind leg was also examined in all experimental animals.
Decision criteria
In group FR, one animal had to be eliminated from the study because of postoperatively increasing systemic septic reactions, meeting termination criteria as defined above. Four animals died perioperatively or during the course of the experiment (one each in groups A, M, F, and FR). Thus, a total of 61 of the 66 animals were included in the final evaluation.
Three animals showed extramedullary location of the implant. However, as they still showed an infection of both implant and adjacent femur, they were nonetheless included in the statistical analysis.
Statistical analysis
The study design of the main experiment is based on a placebo-controlled parallel trial with randomized experimental groups. The size of the study groups, number of animals within each group, was determined on the basis of previous studies.3 Analysis of a group size of n=9 showed a statistical power of 0.8 and a type I error alpha =0.05. Groups comprised 11 animals because possible complications during the experiment had to be taken into account.
For statistical analysis, a P-value <0.05 was considered to be statistically significant. The Mann–Whitney U-test for non normally distributed values was used (SigmaStat 3.1; SYSTAT Software Inc., Point Richmond, Richmond, CA, USA). For graphical representation, box plots were used.
Results
The body weight at the end of experiments did not significantly differ compared to the beginning of experiments.
In all infected knee joints, there were clear macroscopic signs of infection such as empyema, purulent arthritis with periarticular bone destruction and osteomyelitis with periprosthetic pus after explantation. In group A (control group), an aggravation especially of the bone defects could be seen. Samples from the right hind leg were sterile in all animals; the infection thus remained localized.
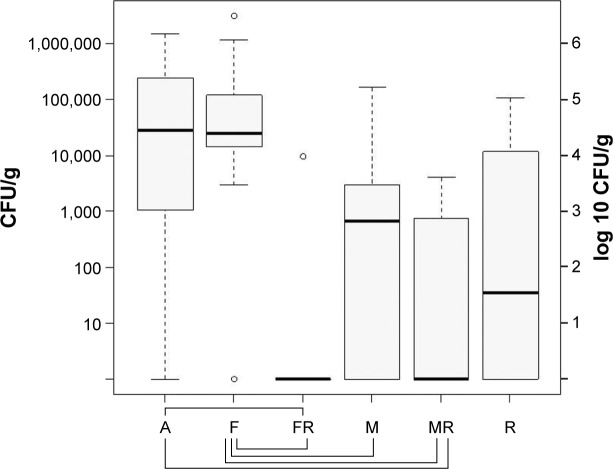
In the probes of the periarticular tissue, median bacterial counts of S. aureus were as follows: group A log 4.47 CFU/g (standard deviation [SD] log 5.73 CFU/g), group F log 4.42 CFU/g (SD log 6.02 CFU/g), group FR log ~0 CFU/g (SD log 3.51 CFU/g), group M log 2.83 CFU/g (SD log 4.75 CFU/g), group MR log ~0 CFU/g (SD log 3.12 CFU/g), group R log 2.81 CFU/g (SD log 4.59 CFU/g). The probes of the periarticular tissue showed negative results concerning the growth of bacteria in seven animals of group MR and eight animals of group FR. The highest bacterial counts were measured in group A, F, and R. As already mentioned, bacteria could be cultured only once in group FR; therefore, a significant reduction of bacterial count could be seen compared to group F (P=0.002) and group A (P=0.003), but no significant difference compared to group R (P=0.190). Group MR also showed a significant reduction of bacterial count in comparison to group F (P=0.002) and group A (P=0.006), but no significant reduction compared to group R (P=0.448). Furthermore, comparison between groups F and M showed that the difference of bacterial count was significant (P=0.037), favoring monotherapy using moxifloxacin. There was no significant difference upon comparing the combination therapy groups FR and MR (P=0.386) (Figure 1).
Figure 1.
Bacterial counts in the soft tissue of the knee joint after 14 days of therapy with aqua (control group A), flucloxacillin (group F), flucloxacillin in combination with rifampin (group FR), moxifloxacin (group M), moxifloxacin in combination with rifampin (group MR), and rifampin (group R).
Notes: The boundaries of the box indicate the 25th and 75th percentiles; whiskers above and below the box indicate the 95th and fifth percentiles. Significant bacterial count reduction is marked with braces below the graph. P<0.05.
Abbreviation: CFU, colony-forming units.
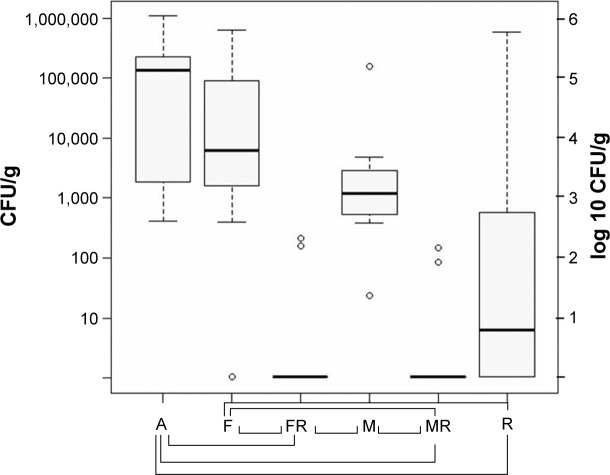
In the probes of the femur, median bacterial counts of S. aureus were as follows: group A log 5.14 CFU/g (SD log 5.54 CFU/g), group F log 3.79 CFU/g (SD log 5.31 CFU/g), group FR log ~0 CFU/g (SD log 1.92 CFU/g), group M log 3.09 CFU/g (SD log 4.73 CFU/g), group MR log ~0 CFU/g (SD log 1.70 CFU/g), group R log 1.27 CFU/g (SD log 5.31 CFU/g).
In the femur, in both groups MR and FR only two animals had positive cultures of S. aureus; all other groups had positives cultures in all animals. Considering this, bacterial counts in the femur of groups FR and MR were significantly lower than in group A, F, and M (P=0.001 each). Still, there was no significant difference compared to group R (FR/R P=0.357, MR/R P=0.263). The comparison between group F and group M showed no significant difference of bacterial count (P=0.168), similar to the comparison between FR and MR (P=0.836) (Figure 2).
Figure 2.
Bacterial counts in the femoral bone after 14 days of therapy with aqua (control group A), flucloxacillin (group F), flucloxacillin in combination with rifampin (group FR), moxifloxacin (group M), moxifloxacin in combination with rifampin (group MR), and rifampin (group R).
Notes: The boundaries of the box indicate the 25th and 75th percentiles; whiskers above and below the box indicate the 95th and fifth percentiles. Significant bacterial count reduction is marked with braces below the graph. P<0.05.
Abbreviation: CFU, colony-forming units.
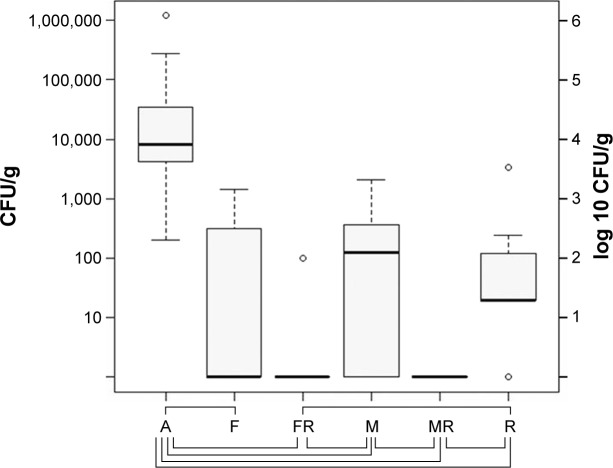
In samples obtained from biofilms, median bacterial counts of S. aureus were as follows: group A log 3.91 CFU/g (SD log 5.60 CFU/g), group F log ~0 CFU/g (SD log 2.70 CFU/g), group FR log ~0 CFU/g (SD log 1.52 CFU/g), group M log 2.15 CFU/g (SD log 2.82 CFU/g), group MR log ~0 CFU/g (SD log 0.00 CFU/g), group R log 1.3 CFU/g (SD log 3.05 CFU/g).
The biofilms removed from the implants were sterile in group MR, sterile with one exception in group FR, and sterile in six of 10 cases in group F. In the statistical analysis, group FR was significantly better in bacterial counts than group M (P=0.024), group MR was better than group M (P=0.009), and both groups FR and MR were superior to group R (P=0.021; P=0.005) with no significant difference between groups F and M (P=0.405), and groups FR and MR (P=0.707) (Figure 3).
Figure 3.
Bacterial counts in the implant-adherent biofilm after 14 days of therapy with aqua (control group A), flucloxacillin (group F), flucloxacillin in combination with rifampin (group FR), moxifloxacin (group M), moxifloxacin in combination with rifampin (group MR), and rifampin (group R).
Notes: The boundaries of the box indicate the 25th and 75th percentiles; whiskers above and below the box indicate the 95th and fifth percentiles. Significant bacterial count reduction is marked with braces below the graph. P<0.05.
Abbreviation: CFU, colony-forming units.
In 1/3 of group R cases, antibiotic resistance was measured, whereas no antibiotic resistance was measured in groups M, F, FR, and MR.
Discussion
Periprosthetic joint infection is a serious complication after artificial joint replacement, which is accompanied by a significant psychological and physical burden for the patient and represents a significant financial burden for health care.15 Due to age structure and the world’s growing number of prosthetic implants and prosthesis replacement operations, periprosthetic joint infection will continue to play an even greater role. By optimization of operative techniques, implants, and hygiene, the incidence of periprosthetic infections in primary arthroplasty has been reduced to 1%–2%,15–17 but especially in revision operations the incidence is still much higher.18
In addition to surgical revision and, if necessary, one- or two-stage prosthesis exchange, antibiotic treatment plays an important role for successful treatment in periprosthetic joint infection. To improve antibiotic treatment, considering increasing resistance rates as well, this efficacy investigating animal study of our already well established animal model was applied.3
One of the most important antibiotics in the treatment of periprosthetic joint infection is rifampin. Rifampin is a cornerstone in the treatment of prosthetic infection as it is active against staphylococci including methicillin-resistant S. aureus, can be given orally, has very good bioavailability, and is well tolerated.19 Due to the rapid development of resistance, it should not be used as monotherapy1 which has already been proven by O’Reilly in 199220 who showed that combination therapy is superior to monotherapy.
In clinical practice, rifampin is often initially combined with flucloxacillin, proven to have good activity and bioavailability against staphylococci. The initial treatment is given intravenously and then switched to oral antibiotic therapy after 2 weeks due to the simpler, outpatient treatment.
The most common combination partner nowadays is a quinolone (ie, ciprofloxacin) because it has a better spectrum of activity and good oral bioavailability, is well tolerated, and achieves high intracellular concentrations. It also demonstrates intracellular activity against staphylococci.19,21 Clinical trials with various quinolones confirmed the effectiveness of the combination therapy.1,6,22 New fluoroquinolones like levofloxacin or moxifloxacin differ from the quinolones of the first generation by a broader spectrum of activity including Gram positive bacteria, excellent pharmacokinetics with good tissue penetration, good oral bioavailability, and lesser side effects.23 The development of resistance during therapy with moxifloxacin appears to be smaller than with older quinolones.24–26
The current experimental animal study was supposed to evaluate the efficacy of newer quinolones such as moxifloxacin in the treatment of periprosthetic infection in order to determine its function as a valid alternative to treatments already in practice.
By choosing an intraosseous position of the foreign body, we were able to simulate a situation much closer to reality than previously published models such as the subcutaneous animal cage models Lucet,27 Chuard,28 and Zimmerli29 described or the intra-abdominal foreign body-associated abscess of Espersen30,31 or Gallimore,32 who could only evaluate the mechanisms and physiology of a foreign body infection.
By implanting a foreign body into the femur and introducing a solution carrying a specific, penicillin-resistant, and oxacillin-sensitive specimen, the local infection rate obtained was 100% used in our predescribed model.3 As expected, the most serious infection appeared in the animals of the control group who did not receive antibiotic therapy.
It could be proven that moxifloxacin in combination with rifampin was as effective as the combination of flucloxacillin and rifampin. Only in the groups of combined antibiotic treatment the majority of samples were sterile. Neither monotherapy with moxifloxacin nor flucloxacillin was able to achieve a relevant reduction of germs. Nonetheless, in the biofilm of the implant, in contrast to soft tissue and bone samples, the monotherapies were superior to the control group which indicates the effectiveness of both antibiotics against bacteria in the biofilm. Rifampin monotherapy showed significant bacteria reduction in the biofilm and in the bone tissue compared to the control group. Still, it should be kept in mind that a rapid development of resistance has been seen under monotherapy with rifampin in 1/3 of the cases, as described in the literature.
Both moxifloxacin and flucloxacillin are especially effective against the most common bacterial strains in periprosthetic infection. In contrast to moxifloxacin, flucloxacillin is a penicillin only active against staphylococci and therefore has a very narrow spectrum of action. Due to the short half-life and the necessary parenteral administration to ensure high bioavailability it is inferior to quinolones. In summary, the most important advantage of quinolones against oxacillin in clinical practice is the good oral bioavailability with comparable tissue concentrations after both intravenous and oral administration. Therefore, it is possible to begin with a short intravenous therapy and convert into an oral therapy without changing the antibiotic which gives the possibility to both stay with an effective treatment, reduce development of resistance by unnecessary changes of antibiotics, and possibly reduce hospital length of stay, reduce costs, and enable an outpatient setting earlier. Furthermore, moxifloxacin can be given as a single dose of 400 mg/day due to long half-life and favorable pharmacokinetics.11,12,33,34 Reduction of the amount of pills to be taken daily is known to lead to a higher acceptance of the treatment and therefore compliance by patients.
Limitations
The limitation of this study is certainly the setting of an idealized animal experimental study with a small sample size of 11 animals per test group. Furthermore, the blood levels and therapeutic power of an agent administered intraperitoneally in animals might be different compared to intravenous or oral administration in human beings. Further clinical studies to assess these aspects are needed. Systemic antibiotic therapy is only one part in the effective treatment of periprosthetic joint infection, still probably the part that can and will be influenced most in the future. Effective and targeted use of antibiotics will improve the success of combined surgical and medical therapy.
Conclusion
In the present experimental animal study, both the combinations of flucloxacillin/rifampin and moxifloxacin/rifampin were found to be effective in the treatment of S. aureus causing periprosthetic joint infection. At equivalent effectiveness, due to many other advantages such as improved pharmacokinetics, the possibility of a single oral dose daily, extended activity spectrum compared to flucloxacillin and older quinolones, and currently better resistance profile, moxifloxacin combined with rifampin is a promising alternative in the treatment of periprosthetic joint infections, for example, in an outpatient setting. The central role of rifampin as one of the most potent antibiotics in combination therapy of periprosthetic joint infection is clearly confirmed by the present study.
Acknowledgments
This study was funded by an unrestricted research grant from Bayer Vital GmbH, Germany. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537–1541. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 2.Keating GM, Scott LJ. Moxifloxacin: a review of its use in the management of bacterial infections. Drugs. 2004;64:2347–2377. doi: 10.2165/00003495-200464200-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kalteis T, Beckmann J, Schröder H, et al. Treatment of implant-associated infections with moxifloxacin: an animal study. Int J Antimicrob Agents. 2006;27:444–448. doi: 10.1016/j.ijantimicag.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Stass H, Dalhoff A, Kubitza D, Schühly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalhoff A. Pharmacodynamics of fluoroquinolones. J Antimicrob Chemother. 1999;43(Suppl B):51–59. doi: 10.1093/jac/43.suppl_2.51. [DOI] [PubMed] [Google Scholar]
- 6.Frippiat F, Meunier F, Derue G. Place of newer quinolones and rifampicin in the treatment of Gram-positive bone and joint infections. J Antimicrob Chemother. 2004;54:1158. doi: 10.1093/jac/dkh451. [DOI] [PubMed] [Google Scholar]
- 7.Hoogkamp-Korstanje JA, Roelofs-Willemse J. Comparative in vitro activity of moxifloxacin against Gram-positive clinical isolates. J Antimicrob Chemother. 2000;45:31–39. doi: 10.1093/jac/45.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Ince D, Zhang X, Hooper DC. Activity of and resistance to moxifloxacin in Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:1410–1415. doi: 10.1128/AAC.47.4.1410-1415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones ME, Visser MR, Klootwijk M, Heisig P, Verhoef J, Schmitz FJ. Comparative activities of clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, ofloxacin, sparfloxacin, and trovafloxacin and nonquinolones linozelid, quinupristin-dalfopristin, gentamicin, and vancomycin against clinical isolates of ciprofloxacin-resistant and -susceptible Staphylococcus aureus strains. Antimicrob Agents Chemother. 1999;43:421–423. doi: 10.1128/aac.43.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaatz GW, Moudgal VV, Seo SM. Identification and characterization of a novel efflux-related multidrug resistance phenotype in Staphylococcus aureus. J Antimicrob Chemother. 2002;50:833–838. doi: 10.1093/jac/dkf224. [DOI] [PubMed] [Google Scholar]
- 11.Joukhadar C, Stass H, Müller-Zellenberg U, et al. Penetration of moxifloxacin into healthy and inflamed subcutaneous adipose tissues in humans. Antimicrob Agents Chemother. 2003;47:3099–3103. doi: 10.1128/AAC.47.10.3099-3103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller M, Stass H, Brunner M, Möller JG, Lackner E, Eichler HG. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob Agents Chemother. 1999;43:2345–2349. doi: 10.1128/aac.43.10.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.San Juan R, Garcia-Reyne A, Caba P, et al. Safety and efficacy of moxifloxacin monotherapy for treatment of orthopedic implant-related staphylococcal infections. Antimicrob Agents Chemother. 2010;54:5161–5166. doi: 10.1128/AAC.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalteis T, Beckmann J, Schröder H, et al. Moxifloxacin superior to vancomycin for treatment of bone infections – a study in rats. Acta Orthop. 2006;77:315–319. doi: 10.1080/17453670610046082. [DOI] [PubMed] [Google Scholar]
- 15.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Eveillard M, Mertl P, Canarelli B, et al. Le risque d’infection profonde des prothèses totales de hanche réalisées en première intention. Evaluation sur une série continue de 790 cas. [The risk of deep infection of total hip arthroplasty in first-line treatment. Evaluation on a continuous series of 790 cases] Presse Med. 2001;30:1868–1875. French. [PubMed] [Google Scholar]
- 17.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. [PubMed] [Google Scholar]
- 18.Nguyen LL, Nelson CL, Saccente M, Smeltzer MS, Wassell DL, McLaren SG. Detecting bacterial colonization of implanted orthopaedic devices by ultrasonication. Clin Orthop Relat Res. 2002;403:29–37. doi: 10.1097/00003086-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Darley ES, MacGowan AP. Antibiotic treatment of gram-positive bone and joint infections. J Antimicrob Chemother. 2004;53:928–935. doi: 10.1093/jac/dkh191. [DOI] [PubMed] [Google Scholar]
- 20.O’Reilly T, Kunz S, Sande E, Zak O, Sande MA, Täuber MG. Relationship between antibiotic concentration in bone and efficacy of treatment of staphylococcal osteomyelitis in rats: azithromycin compared with clindamycin and rifampin. Antimicrob Agents Chemother. 1992;36:2693–2697. doi: 10.1128/aac.36.12.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barberán J. Management of infections of osteoarticular prosthesis. Clin Microbiol Infect. 2006;12(Suppl 3):93–101. doi: 10.1111/j.1469-0691.2006.01400.x. [DOI] [PubMed] [Google Scholar]
- 22.Drancourt M, Stein A, Argenson JN, Zannier A, Curvale G, Raoult D. Oral rifampin plus ofloxacin for treatment of Staphylococcus-infected orthopedic implants. Antimicrob Agents Chemother. 1993;37:1214–1218. doi: 10.1128/aac.37.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blondeau JM. A review of the comparative in-vitro activities of 12 antimicrobial agents, with a focus on five new respiratory quinolones. J Antimicrob Chemother. 1999;43(Suppl B):1–11. doi: 10.1093/jac/43.suppl_2.1. [DOI] [PubMed] [Google Scholar]
- 24.Firsov AA, Vostrov SN, Lubenko IY, Drlica K, Portnoy YA, Zinner SH. In vitro pharmacodynamic evaluation of the mutant selection window hypothesis using four fluoroquinolones against Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:1604–1613. doi: 10.1128/AAC.47.5.1604-1613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lister PD. Pharmacodynamics of moxifloxacin and levofloxacin against Staphylococcus aureus and Staphylococcus epidermidis in an in vitro pharmacodynamic model. Clin Infect Dis. 2001;32(Suppl 1):S33–S38. doi: 10.1086/319374. [DOI] [PubMed] [Google Scholar]
- 26.Pong A, Thomson KS, Moland ES, Chartrand SA, Sanders CC. Activity of moxifloxacin against pathogens with decreased susceptibility to ciprofloxacin. J Antimicrob Chemother. 1999;44:621–627. doi: 10.1093/jac/44.5.621. [DOI] [PubMed] [Google Scholar]
- 27.Lucet JC, Herrmann M, Rohner P, Auckenthaler R, Waldvogel FA, Lew DP. Treatment of experimental foreign body infection caused by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1990;34:2312–2317. doi: 10.1128/aac.34.12.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuard C, Herrmann M, Vaudaux P, Waldvogel FA, Lew DP. Successful therapy of experimental chronic foreign-body infection due to methicillin-resistant Staphylococcus aureus by antimicrobial combinations. Antimicrob Agents Chemother. 1991;35:2611–2616. doi: 10.1128/aac.35.12.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146:487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]
- 30.Espersen F, Frimodt-Møller N, Corneliussen L, Riber U, Rosdahl VT, Skinhøj P. Effect of treatment with methicillin and gentamicin in a new experimental mouse model of foreign body infection. Antimicrob Agents Chemother. 1994;38:2047–2053. doi: 10.1128/aac.38.9.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espersen F, Frimodt-Møller N, Corneliussen L, Thamdrup Rosdahl V, Skinhøj P. Experimental foreign body infection in mice. J Antimicrob Chemother. 1993;31(Suppl D):103–111. doi: 10.1093/jac/31.suppl_d.103. [DOI] [PubMed] [Google Scholar]
- 32.Gallimore B, Gagnon RF, Subang R, Richards GK. Natural history of chronic Staphylococcus epidermidis foreign body infection in a mouse model. J Infect Dis. 1991;164:1220–1223. doi: 10.1093/infdis/164.6.1220. [DOI] [PubMed] [Google Scholar]
- 33.Rodvold KA, Neuhauser M. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Pharmacotherapy. 2001;21:233S–252S. doi: 10.1592/phco.21.16.233s.33992. [DOI] [PubMed] [Google Scholar]
- 34.Stass H, Kubitza D, Schühly U. Pharmacokinetics, safety and tolerability of moxifloxacin, a novel 8-methoxyfluoroquinolone, after repeated oral administration. Clin Pharmacokinet. 2001;40(Suppl 1):1–9. doi: 10.2165/00003088-200140001-00001. [DOI] [PubMed] [Google Scholar]