Abstract
Scientists have been attracted by polymersomes as versatile drug delivery systems since the last two decades. Polymersomes have the potential to be versatile drug delivery systems because of their tunable membrane formulations, stabilities in vivo, various physicochemical properties, controlled release mechanisms, targeting abilities, and capacities to encapsulate a wide range of drugs and other molecules. Asymmetrical polymersomes are nano- to micro-sized polymeric capsules with asymmetrical membranes, which means, they have different outer and inner coronas so that they can exhibit better endocytosis rate and endosomal escape ability than other polymeric systems with symmetrical membranes. Hence, asymmetrical polymersomes are highly promising as self-assembled nano-delivery systems in the future for in vivo therapeutics delivery and diagnostic imaging applications. In this review, we prepared a summary about recent research progresses of asymmetrical polymersomes in the following aspects: synthesis, preparation, applications in drug delivery and others.
Keywords: asymmetrical polymer vesicle, drug delivery, self-assemble, nano-delivery vesicles
Introduction
Polymersomes, which also can be called polymeric vesicles, have attracted scientists' interests in recent years. Compared to other nanometer scale self-assembling delivery systems, such as polymer micelles, nanoparticles and nanogels, polymersomes provide a more stable condition for the storage of drugs, especially for nanoscopic emulsions (Asano et al., 2016). As compared to similar devices such as liposomes, which are comprised of low molecular weight lipids, polymersomes are composed of versatile macromolecular amphiphiles architectures, including dendritic copolymers (Yang et al., 2005), graft polymers (Dou et al., 2003; Lee et al., 2006), amphiphilic diblock polymers (Discher et al., 1999; Qin et al., 2006; Zhou et al., 2006), triblock polymers (Nardin et al., 2000; Napoli et al., 2004a) and so on. Because of this unique composition, polymersomes commonly exhibit much better colloidal stability, solidity, and protection of drugs, whereas illustrating very little chemical permeability. Composed of amphiphilic AB diblock or ABC triblock copolymers, polymersomes usually have relatively longer hydrophobic blocks to make up the dense core membranes, and shorter hydrophilic blocks in the facial membranes to protect the interior fluids in aqueous solutions. Hence, this advantage presents an excellent solution to the problem that no matter what hydrophilic or hydrophobic property a particular drug has, polymersomes can act as appropriate carriers for the drug. For example, both hydrophobic (e.g., doxorubicin, paclitaxel, quantum dots) and hydrophilic molecules (e.g., DNA, siRNA, therapeutic proteins, chelated Gd) can be encapsulated into polymersomes (Wang et al., 2012; Anajafi et al., 2017; Nomani et al., 2017).
As we all know, for a drug carrier, biodegradability is the first thing that must be considered carefully (Zhao et al., 2014). The alternative hydrophobic block-based drug carrier systems, such as liposomes, must be decorated with poly(ethylene glycol) (PEG) or polycarbonates in order to maintain a stealthy property. PEG is the most commonly used material for decorating drug carriers because it can maintain the carriers' stability in the plasma by prohibiting the adsorption of plasma proteins and has little toxicity to the cells (Kricheldorf, 2006). Hence, the carriers whose surfaces are decorated with PEG chains usually get longer blood circulation times (Gref et al., 2000; Ohya et al., 2011). However, this will not be a problem for polymersomes. Taking the advantage of the arbitrary structures, polymersomes can be intrinsically stealthy, because they present typical hydrophilic macromolecular amphiphilic parts (e.g., PEG and dextran) in the facial membranes. This non-fouling property offers scientists a great convenience to make further transformation. Though polymersomes have the above advantages over liposomes, polymersomes have relatively low loading levels and loading efficacies for hydrophilic drugs, including protein drugs and chemotherapeutics, which limits their applications as drug carriers, especially for anti-tumor therapy. To investigate this issue, great efforts have been made to improve the loading efficiency of polymersomes. It is a normal way to decorate polymersomes with specific ligands to create smart polymersomes, which selectively releases payloads to the sites of action in response to external stimulus (e.g., magnetic and photo) or internal signals (e.g., enzyme degradation, endo/lysosomal pH and cytoplasmic glutathione). Decorated materials usually include aptamers, lactoferrin, antibodies, peptides, and folate (Lu et al., 2015). Moreover, changing the permeability of the membranes, or controlling the degradability of the structures can also achieve the same effect (LoPresti et al., 2009). In addition, ingenious encapsulation procedures such as nano-precipitation method (Sanson et al., 2010) and transmembrane phosphate-, citrate-, sulfate-, or PH- gradient loading (Choucair et al., 2005; Ahmed et al., 2006a; Yin et al., 2009; Gubernator, 2011) are also smart ways to enhance the loading efficacy.
Lo Presti et al. have demonstrated that by exploiting endocytosis, pH-sensitive PMPC-PDPA polymersomes can deliver various drugs effectively to cells (Lomas et al., 2007, 2008; Massignani et al., 2009, 2010a; Murdoch et al., 2010). There exists different mechanisms that control the acidification of the internalized material within subcellular compartments (endosomes) (Doherty and McMahon, 2009). The authors have also found that after polymersomes are internalized by endocytosis, they will disassemble in a controllable manner when approaching the acidic endosomal lumen. This, conversely, leads to endosomal membrane perturbation in a short time, which will facilitate the polymersomes to escape from endosomes and get into the cell cytosol (Lomas et al., 2007, 2008; Massignani et al., 2009). The endocytosis efficiency is strongly dependent on the polymersome topology and surface chemistry, as well as the polymersome size (Massignani et al., 2009).
Although the difficulty can be conquered in various ways, it still remains a big challenge that symmetrical polymersomes exhibit inefficient intracellular drug delivery. First of all, as symmetrical polymersomes-based drug delivery systems show low endosomal escape ability, they may be trapped inside the endosomes. Besides, the side effects to healthy cells due to the slow endocytosis rate and drug diffusion can never be neglected. In recent years, asymmetric polymersomes have attracted a lot of attention in the drug delivery field. They have the following advantages: efficient drug loading capacity, fast endocytosis rate and endosomal escape ability, which can meet most requirements for drug delivery (Liu et al., 2014). Asymmetrical vesicles with different structures both inside and outside are self-assembled from asymmetric ABC triblock copolymers or AB and BC two diblock copolymers (Lu et al., 2015). In this review, we prepared a summary about recent progresses of asymmetrical polymersomes in the following aspects: synthesis, preparation, applications in drug delivery and others.
Synthesis and preparation of asymmetrical polymersomes
Synthesis of asymmetric triblock copolymers
There are two methods to synthesize the materials of polymersomes. One is mixing AB and BC diblock copolymers together, the other is directly using ABC triblocks to form asymmetrical polymersomes. Consisting of at least two homopolymer blocks, block copolymers are the ideal materials for forming self-assembling polymersomes. Normally, the homopolymer is designed to exhibit some specific physicochemical properties, and in consequence, the block copolymers will display versatile properties and utilization values (Lee and Feijen, 2012; Ge et al., 2014).
Sequential radical addition-fragmentation chain transfer (RAFT) polymerization is the most commonly used technique to synthesis triblock copolymers (Du et al., 2012). Ring-opening polymerization (ROP) is another feasible way to synthesize diblock or triblock copolymers (Kishimura et al., 2007). The properties of the diblock or triblock copolymers can determine the types and applications of the asymmetrical polymersomes by controlling the structures, compositions and molecular weights (Li et al., 2015). Therefore, we summarize some available diblock or triblock copolymers that constitute asymmetrical polymersomes, and the results are given in Table 1.
Table 1.
Examples of available diblock or triblock copolymers that constitute asymmetrical polymersomes.
| Polymers | Preparation method | Formation method | Copolymers | Targeting ligands | Drugs/stimulus for release | Pros and cons | References |
|---|---|---|---|---|---|---|---|
| PEO45-b-PS130-b-PDEA120 | Atrp | None reported | PEO45-b-PS130-b-PDEA120 | None reported | pH-induced | None reported | Giacomelli et al., 2007 |
| FA/DTPA-PGA-b-PCL | None reported | Solvent switching method | FA-PGA75-b-PCL30 and DTPA-PGA22-b-PCL30 | FA | DOX·HCl/pH-induced | Improve the sensitivity of a T2 MRI contrast agent | Oerlemans et al., 2010 |
| Anis-PEG-PTTMA-PAA | Raft | Solvent switching method | Anis-PEG-PTTMA-PAA and PEG-PTTMA-PAA | Anisamide | GrB/pH-induced | Targeting ability and prompt intracellular protein release | Lu et al., 2015 |
| PEG5K-P(CL-co-LA)11K-PEG2K | Rop | Film hydration method | mPEG-PCL or mPEG-P-(CL-co-LA) | None reported | Hb | Undamaged gas-binding capability and oxygen affinity, plus high stability and biocompatibility | Kishimura et al., 2007 |
| PEO113-b-PCL132-b-PAA15 | None reported | Solvent switching method | PEO113-b-PCL132-b-PAA15 | None reported | DOX·HCl/pH-induced | High DOX loading efficiency and good biodegradability, rapid endocytosis rate and endosomal escape ability | Liu et al., 2014 |
| Acupa-PEG-PTMBPEC-PSAC | Rop | Solvent switching method | Acupa-PEG-PTMBPEC-PSAC and PEG-PTMBPEC-PSAC | 2-[3-[5-amino-2-carboxypentyl]-ureido]-pentanedioic acid | GrB/pH-induced | Unimodal distribution, high protein loading contents, long circulation time | Du et al., 2012 |
| PEG-SS-PCL-PDEA | Rop | Solvent switching method | PEG-PCL-PDEA and PEG-SS-PCL | Galactose | GrB/reduction-induced | Unimodal distribution, highly efficient loading, high protein loading contents, long circulation time, target ability | Drummond et al., 1999 |
| PB-b-PS | Atrp | Blending in an oil-in-oil emulsion | polystyrene-b-poly(ethylene oxide) (SO) and polybutadiene-b-poly-(ethylene oxide) (BO) | None reported | None reported | Straightforward preparation method | Asano et al., 2016 |
| PEG-PTTMA-PAA | Raft | Solvent switching method | PEG-PTTMA-PAA | None reported | DOX·HCl/pH-induced | Conveniently prepared, high loading efficiency, excellent biocompatibility, quickly destabilized | Du et al., 2012 |
| PEG–PAA(SH)–PDEA) | Raft | Solvent switching method | PEG–PAA(SH)–PDEA) | None reported | FITC–CC/reduction- and pH–induced | Conveniently prepared, high loading content, excellent biocompatibility | Zupancich et al., 2006 |
| PEO-PAA-PNIPAM | Raft | Solvent switching method | PEO-PAA-PNIPAM | None reported | FITC–dextran/temperature-induced | Stability against high salt conditions and change of temperature | Du and O'Reilly, 2009 |
| PEO–PDPA–PDMA | Atrp | Solvent switching method | PEO–PDPA–PDMA | None reported | None reported | None reported | Zhang and Eisenberg, 1995 |
| PEG-PCL-PDEA | Raft | Film hydration method | PEG-PCL-PDEA | None reported | FITC-CC | High protein loading efficiencies and controlled release, able to simultaneously deliver and release hydrophobic anticancer drugs and proteins into cells | Liu et al., 2010 |
| PEG-PCL-DEX | Rop | Solvent switching method | PEG-PCL and DEX-PCL | None reported | PEO | A variety of chemically dynamic characteristics responding to biological pathways | Zhang et al., 2010 |
Preparation of asymmetrical polymersomes
There are two major methods to form self-assembling asymmetrical polymersomes: solvent change method (phase inversion) and direct hydration method (Zhang and Eisenberg, 1995; Blanazs et al., 2009). The first method is more widely used. Interestingly, Asano et al. first used the co-assembly method to generate asymmetric polymersomes. They put two distinct diblock copolymers PS-b-PEO (SO) and PB-b-PEO (BO) in an oil-in-oil emulsion PS/PB/CHCl3 (Asano et al., 2016).
Drug delivery by asymmetric polymersomes
As mentioned above, compared with liposomes, polymersomes have more robust membranes, thus can improve the circulation half-life, protect drugs and prevent uncontrolled drug release. However, the rate and spatial distribution cannot be controlled because of the restriction of the structures (Mecke et al., 2006). Therefore, polymersomes that can response to various stimuli are developed by changing the physical and chemical properties of the membranes of the polymersomes (Du and O'Reilly, 2009; Onaca et al., 2009; van Dongen et al., 2009). As a consequence, the side effects are reduced and the efficacy of drugs at the site of action is improved. The stimuli can be classified into two aspects: external stimuli (temperature change, UV light and magnetic field) and intracellular stimuli (pH, redox).
Temperature-responsive asymmetric polymersomes
Compared with UV stimuli for drug release, temperature stimuli are a more practicable way for building intelligent polymersomes, since natural temperature differences in tissues exist in human body. Tumor tissues have a higher temperature than normal tissues and the temperature can change easily in the external, such as hyperthermia (Xu et al., 2009). Furthermore, using the heating or cooling appliances for particular sections of the body can also achieve the effect of temperature differences (Christian et al., 2009).
The temperature-dependent mechanisms can be achieved by using the polymer poly(N-isopropylacrylamide) (PNIPAm) (Li et al., 2006; Qin et al., 2006). PNIPAm has a unique property that its conformation will change if the temperature is above the lower critical solution temperature (LCST) of PNIPAm (40°C). Above 40°C, the block will change its property from hydrophilic to hydrophobic. Thus, temperature-sensitive polymersomes are designed by using PNIPAm. Usually, PNIPAm chains are covalently combined with a hydrophilic block such as poly(N-(3-aminopropyl) methylacrylamide hydrochloride) (PAMPA) or PEO. The resulting copolymer will self-assemble to form polymersomes and have the capacity to load hydrophilic drugs. If the temperature decreases below 40°C, the polymersomes will disassemble to the former copolymer and release the encapsulated therapeutics. Cai et al. (2011) prepared asymmetric polymersomes based on poly(ethylene oxide)-b-poly(ethylene oxide-stat-butylene oxide)-b-poly(isoprene) (E-BE-I) ABC triblock copolymer, which presented the temperature dependent property and the LCST was 25°C. Compared to the usual temperature-responsive polymersomes using PNIPAm as responsive factors, these polymersomes had narrower molecular weight distribution and faster transformation rate because of the lacking of strong interchain hydrogen bonding (Figure 1).
Figure 1.
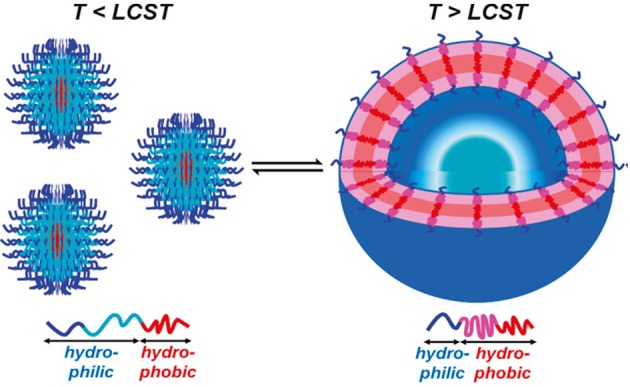
Schematic illustration of the thermally induced size change of assemblies of copolymers containing thermoresponsive blocks. Below the LCST, the central block (cyan) is hydrophilic; above the LCST, the central block (pink) becomes hydrophobic (Cai et al., 2011) (The figure has been obtained with copyright permission/license from ACS).
pH-responsive asymmetric polymersomes
As a matter of fact, in the tumor microenvironment there is an innate pH difference (pH 6.5−7.2) and the cancer cells' endosomes and lysosomes are acidic (pH 4.0−6.5) (Grabe and Oster, 2001; Rofstad et al., 2006; Meng et al., 2014). Ahmed et al. found that a number of polymersomes approached cellular endolysosomes by pinocytosis passageway (Ahmed et al., 2006b). Hence, this intracellular cue can be applied for pH-dependent asymmetric polymersomes. This release method has an advantage over external cues (temperature, UV) owing to the accessibility of such intracellular cues. Using polymersomes whose structures are susceptibility to this pH difference, targeted intracellular release of the specific encapsulated cargo can be achieved. Du et al. investigated endosomal pH-sensitive degradable asymmetric polymersomes constructed by ABC triblock poly(ethylene glycol)-b-poly(trimethoxybenzylidene tris (hydroxymethyl)ethane methacrylate)-b-poly(acrylic acid) (PEG-PTTMA-PAA). In this study, they demonstrated that PTTMA block could quickly destabilize due to the degradation of acetal groups in lower pH in the endo/lysosomal compartments of cancer cells, achieving targeted intracellular release of DOX·HCl and high anti-tumor therapeutic effects (Du et al., 2012).
Redox-responsive asymmetric polymersomes
Oxidation (Napoli et al., 2004b,a) or reduction-responsive (Cerritelli et al., 2007) reactions open up new spatial release mechanisms in the human body (Bodor, 1987; Corti et al., 2010). Oxidative environments exist at sites of inflammation, tumor tissues and in extracellular fluids, as well as intracellular compartments such as the endolysosomes (Grundl, 1994; Tew, 2007).
Wang et al. (2013) demonstrated reduction-responsive asymmetrical polymersomes based on PEG-SS-PCL diblock copolymer and asymmetric PEG-PCL-PDEA triblock copolymer. When exposed to reducing environments such as the nuclei and cytoplasm of cancer cells, this system will quickly rupture due to cleavage of the disulfide bonds between PEG and PCL blocks. This delivery system can protect encapsulated cargos in the extracellular environments (i.e., blood plasma) and induce efficient intracellular drug release, therefore it has the potential to be a versatile and multifunctional drug delivery platform by using intracellular stimuli (Krack et al., 2008).
Applications of asymmetric polymersomes
Polymersomes have higher stability than liposomes (Discher et al., 1999; Photos et al., 2003; Lee et al., 2011). Moreover, polymersomes not only can encapsulate hydrophilic drugs but also can hold hydrophobic or amphiphilic compounds into their lumina or membranes. This advantage can be used in the fields of drug delivery, medical imaging and disease diagnosis (Massignani et al., 2010b). It has been proved that anticancer drugs (i.e., PTX and DOX·HCl) (Ahmed et al., 2006a; Chen et al., 2010), membrane proteins (i.e., OmpF, LamB, and FhuA, Stoenescu et al., 2004), hydrophobic dyes (i.e., PKH26) (Photos et al., 2003) and amphiphilic dyes (i.e., octadecyl rhodamine B, Battaglia and Ryan, 2005; Lomas et al., 2008) can be incorporated into membranes of polymersomes.
Delivery of conventional drugs
Encapsulating drugs in asymmetric polymersomes can achieve efficient drug loading capacity, fast endocytosis rate and rapid endosomal escape ability. All of these advantages help to maintain the property of drugs in vivo or in vitro and control the release rate. With the development of smart delivery systems, many cancer-target chemotherapeutics drugs which have severe side effects now come to a renascence (Arrigo, 2000, 2005; Arrigo and Ducasse, 2002).
Liu et al. reported pH-sensitive asymmetric polymersomes based on PEO-b-PCL-b-PAA triblock copolymer. PEO chains constituted the outer corona of polymersomes because it showed well-behaved biocompatibility and was intrinsically stealthy to immune system. The pH-responsive PAA chains were designed in inner aqueous core since they could quickly destabilize at endosomal pH. Consequently, the release of encapsulated cargos in the cells was efficient and accurate (Liu et al., 2014).
Delivery of protein
Protein drugs have been recognized as one kind of the most potential leads for the growth of new therapeutics (Tan et al., 2010), and they are powerful antidotes toward many intractable diseases such as diabetes and cancers (Torchilin and Lukyanov, 2003; Futaki, 2006; Schrama et al., 2006). However, they are too sensitive to environmental conditions, which results in their short lives in vivo.
Polymersomes with huge aqueous compartments can encapsulate proteins and the membranes will protect proteins from degradation (Discher et al., 2007; Christian et al., 2009; Sun et al., 2009; Meng and Zhong, 2011; Lee and Feijen, 2012; Liu et al., 2012). So far, polymersomes have encapsulated a variety of proteins, including aquaporin Z (Kumar et al., 2007), cytochrome C (CC) (Hvasanov et al., 2011), hemoglobin (Rameez et al., 2008; Li et al., 2012), insulin (Xiong et al., 2007; Christian et al., 2009; Kim H. et al., 2012), immunoglobulin G (Fu et al., 2011), ovalbumin (Stano et al., 2013), and myoglobin (Kishimura et al., 2007). Recent investigations also have found that polymer vesicles with asymmetrical membranes exhibit much higher loading capacity of proteins than symmetrical polymersomes.
Li et al. reported self-aggregated vesicles with asymmetric membranes. These polymersomes can serve as hemoglobin (Hb)-based oxygen carriers with oxygen affinity and high Hb loading content and meanwhile no interference with blood cells. Further studies have shown that these vesicles have great stability and efficacy, which have the potential to be alternative blood substitutes (Li et al., 2014).
Theranostic vesicles for magnetic resonance imaging
Magnetic resonance imaging (MRI) is a widespread diagnosis apparatus that is clinically used for taking pictures of organs and structures inside the body to find blood vessels, bleeding, tumors or infection. Using asymmetric polymersomes to deliver contrast agents has better sensitivity and lower toxicity. Liu et al. (2015) reported asymmetrical polymersomes based on two kinds of diblock copolymer FA-PGA75-b-PCL30 and DTPA-PGA22-b-PCL30, which had diagnostic and therapeutic effects simultaneously. The highest drug DLE can reach 52.6% and the T1 relaxivity can be increased by 8-fold, meanwhile maintaining a low toxicity and remarkable positive contrast enhancement for tumor imaging.
Applications of non-spherical shape asymmetric polymersomes
Polymersomes with non-spherical shapes play important roles in applications such as vaccine development. Non-spherical shape can cause different interaction to immune cells because the shapes determine the in vivo behavior of polymersomes. Actually, there are not comprehensive studies that investigated the influence of shape and topology on the in vivo behavior of polymersomes.
Researchers have found that tubular structures are one of the most promising morphologies rather than spheres. Owing to the resembling with bacterial topologies, tubular structures offer larger contact areas between particles and cells. Van Hest's group has developed a series of methods that reshaped spherical vesicles to tubular structures. The ability of tunable membrane gives these spherical structures a chance to apply to the immunology field (Williams et al., 2017). As for polymersomes, not only the copolymer composition can influence the morphology but also the response to various stimuli such as temperature, pH, magnetic fields, or osmotic pressure can further change the structures. In a recent example by van Hest et al. (Abdelmohsen et al., 2016a), the formation of functional nanotubes from PEG-PDLLA was demonstrated. Biodegradable polymersomes made by PEG-PDLLA copolymer transformed their structures from spheres to nanotubes upon dialysis under hypertonic conditions. Owing to the dialysis with the increasing concentration of NaCl, the size can be osmotically controlled by the enrichment and elongation of the structures. In this way, such well-defined nanoparticles can be easily prepared and functionalized. This method established a novel platform for biomedical research where nanoscopic control over size and shape is highly valuable. Another example of non-spherical vesicles is the stomatocytes, which can also be prepared by dialysis. Through shape transformation of spherical polymersomes into bowl-shaped stomatocytes, polymersomes will form an extra nanocavity. Due to the direct contact with the outside environment, these vesicles present the ability to convert chemical energy into kinetic energy when encapsulating enzymes or catalytic nanoparticles in the nanocavity. Further investigations showed that stomatocytes have the chemotactic ability toward certain cell types (Williams et al., 2017). Stomatocytes are promising candidates for further useful applications such as immunoassays, protein and DNA isolation, detection and biosensing (Abdelmohsen et al., 2016b).
Applications of targeting polymersomes
Much efforts have been made for targeting drugs and genes to specific sites. It is crucial for clinical therapeutics because drugs without targeting ability may cause low effect and high toxicity. Targeted delivery is categorized into two types: active targeting and passive targeting. Passive targeting aims at capturing nanoparticles that are smaller than the aperture gap of endothelial cells. These nanoparticles can go through the interstitium and therefore gather in tumor tissue (Danhier et al., 2009). Active targeting takes advantage of molecular recognition to transport drugs to specific sites. Attaching biological ligands or antibodies to the nanoparticles is the most widely applied method and it is usually carried out by chemically conjugating (Yang et al., 2002; Li et al., 2005; Rerat et al., 2010), mixing (Guo et al., 2009) and coating (Yang et al., 2002; Elloumi Hannachi et al., 2009). Targeting moieties are usually conjugated to the hydroxyl groups of their hydrophilic polymer blocks (e.g., PEO, PEG) (Torchilin et al., 2001; Velonia et al., 2002; Levine et al., 2008). In addition, polymersomes conjugated with targeting moieties may change their hydrophilic-block-to-total-mass ratio, resulting in the change of morphology. For example, from vesicles to micelles.
Lu et al. reported the modification of their triblock copolymer (PEG-PTTMA-PAA) polymersomes with anisamide (Anis) to target the cancer cell receptor sigma (Lu et al., 2015). Sigma receptor is an over-expressed membrane protein that appears in many human malignant diseases including lung cancer and prostate cancer (Lu et al., 2015). Anis ligands exhibit high affinity to sigma receptor and so far have been used as guides to deliver versatile drugs including proteins, siRNAs and doxorubicin (Della Rocca et al., 2011; Guo et al., 2012; Kim S. K. et al., 2012). Their results validated that Anis-chimaeric polymersomes (CPs) presented high targeting ability to H460 and when Anis contents increased, antitumor efficacy was improved. If competitive antagonist is used, the antitumor activity will be reduced drastically (Lu et al., 2015).
Applications of leukopolymersomes
Many polymersomes are easily internalized by endocytosis of macrophages and induce inflammatory responses. Therefore, polymersomes are often designed to be biodegradable and biocompatible and with reduced in vivo inflammatory responses.
Leukopolymersomes are a kind of polymersomes that have adhesive properties to leukocytes. It can be easily made by just functionalizing the terminal groups on the membranes of the vesicles. There are two typically adhesive ligands on leukocytes, which are named as selectins and integrin. These two ligands can mimic the adhesive properties of activated leukocytes (Hammer et al., 2008). Engineered to express the adhesion molecules, leukopolymersomes can achieve binding to inflammatory substrates in the shear fluid flow in blood vessels (Hammer et al., 2008). Moreover, by altering the membrane materials and ligand ratio, the rate and type of adhesive interaction can be tuned. Hammer et al. demonstrated that the adhesion was specific because the adhesive rate was the same as that of leukocytes (Hammer et al., 2008).
The adhesiveness of leukopolymersomes can be coupled with other properties of polymersomes, such as their ability to encapsulate drugs and image contrast agents. These particles will ultimately be useful for creating theranostic particles that can detect, image and deliver drugs to inflammatory sites, cancer cells, and cardiovascular lesions (Robbins et al., 2010).
Conclusions
In this review, we summed up the recent progresses of asymmetrical polymersomes from the aspects of preparation, delivery, applications and targeting of asymmetrical polymersomes. Asymmetrical polymersomes with asymmetrical membranes and large watery cores can encapsulate various therapeutic molecules including both hydrophilic and hydrophobic molecules. Their tunable membrane formulations, stability in vivo, various physicochemical properties, controlled release mechanisms, and targeting ability make asymmetrical polymersomes one of the most ideal platforms for drug delivery. According to many recent papers, we have found that a large number of examples showing polymersomes research has caught up with liposomal science in the past decade and in many cases, adding extra dimensions to what is possible. In the future, more research should be performed to further improve the following aspects of asymmetrical polymersomes: (1) increasing the loading capacity and encapsulation rate of drugs into asymmetrical polymersomes; (2) enhancing the efficiency of controlled release with the goal of achieving zero-order release and stimuli-responsive release; (3) improving the delivery efficiency of macromolecular therapeutics such as protein and nucleic acid drugs; (4) improving the in vivo circulation half-life and the efficiency for specifically targeted drug delivery.
Author contributions
YZ, XL, XZ, YY, HL, XZ, and WY: conceived and participated in its design, searched databases, extracted and assessed studies and helped to draft the manuscript. WY: conceived the initial idea and the conceptualization, participated in the data extraction and analysis, and revised the manuscript. XZ and HL: participated in the conceptualization and design of the study and analysis, and YZ: wrote the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The study was supported by the Projects of National Science Foundation of China (No. 81373366), the Projects of National Science Foundation of Shanghai, China (15ZR1432500), Projects of the Shanghai Committee of Science and Technology, China (No. 12XD1403800) and Funds for Interdisciplinary Projects of Medicine and Engineering of Shanghai JiaoTong University (No. YG2015MS06, YG2014QN06, and YG2016QN22), National Science and Technology Major Projects for Major New Drugs Innovation and Development (2017ZX09101005-008-002).
References
- Abdelmohsen L. K., Nijemeisland M., Pawar G. M., Janssen J. G. A., Nolte R. J., et al. (2016b). Dynamic loading and unloading of proteins in polymeric stomatocytes: formation of an enzyme-loaded supramolecular nanomotor. ACS Nano 10, 2652–2660. 10.1021/acsnano.5b07689 [DOI] [PubMed] [Google Scholar]
- Abdelmohsen L. K., Williams D. S., Pille J., Ozel S. G., Rikken R. S., Wilson D. A., et al. (2016a). Formation of well-defined, functional nanotubes via osmotically induced shape transformation of biodegradable polymersomes. J. Am. Chem. Soc. 138, 9353–9356. 10.1021/jacs.6b03984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F., Pakunlu R. I., Brannan A., Bates F., Minko T., Discher D. E. (2006a). Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J. Control. Release 116, 150–158. 10.1016/j.jconrel.2006.07.012 [DOI] [PubMed] [Google Scholar]
- Ahmed F., Pakunlu R. I., Srinivas G., Brannan A., Bates F., Klein M. L., et al. (2006b). Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation. Mol. Pharm. 3, 340–350. 10.1021/mp050103u [DOI] [PubMed] [Google Scholar]
- Anajafi T., Yu J., Sedigh A., Haldar M. K., Muhonen W. W., Oberlander S., et al. (2017). Nuclear localizing peptide-conjugated, redox-sensitive polymersomes for delivering curcumin and doxorubicin to pancreatic cancer microtumors. Mol. Pharm. 14, 1916–1928. 10.1021/acs.molpharmaceut.7b00014 [DOI] [PubMed] [Google Scholar]
- Arrigo A. (2000). sHsp as novel regulators of programmed cell death and tumorigenicity. Pathol. Biol. 48, 280–288. [PubMed] [Google Scholar]
- Arrigo A. P. (2005). In search of the molecular mechanism by which small stress proteins counteract apoptosis during cellular differentiation. J. Cell. Biochem. 94, 241–246. 10.1002/jcb.20349 [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Ducasse C. (2002). Expression of the anti-apoptotic protein Hsp27 during both the keratinocyte differentiation and dedifferentiation of HaCat cells: expression linked to changes in intracellular protein organization? Exp. Gerontol. 37, 1247–1255. 10.1016/S0531-5565(02)00131-6 [DOI] [PubMed] [Google Scholar]
- Asano I., So S., Lodge T. P. (2016). Oil-in-oil emulsions stabilized by asymmetric polymersomes formed by AC + BC block polymer co-assembly. J. Am. Chem. Soc. 138, 4714–4717. 10.1021/jacs.6b01697 [DOI] [PubMed] [Google Scholar]
- Battaglia G., Ryan A. J. (2005). Bilayers and interdigitation in block copolymer vesicles. J. Am. Chem. Soc. 127, 8757–8764. 10.1021/ja050742y [DOI] [PubMed] [Google Scholar]
- Blanazs A., Massignani M., Battaglia G., Armes S. P., Ryan A. J. (2009). Tailoring macromolecular expression at polymersome surfaces. Adv. Funct. Materials 19, 2906–2914. 10.1002/adfm.200900201 [DOI] [Google Scholar]
- Bodor N. (1987). Redox drug delivery systems for targeting drugs to the braina. Ann. N. Y. Acad. Sci. 507, 289–306. 10.1111/j.1749-6632.1987.tb45809.x [DOI] [PubMed] [Google Scholar]
- Cai Y., Aubrecht K. B., Grubbs R. B. (2011). Thermally induced changes in amphiphilicity drive reversible restructuring of assemblies of ABC triblock copolymers with statistical polyether blocks. J. Am. Chem. Soc. 133, 1058–1065. 10.1021/ja109262h [DOI] [PubMed] [Google Scholar]
- Cerritelli S., Velluto D., Hubbell J. A. (2007). PEG-SS-PPS: reduction-sensitive disulfide block copolymer vesicles for intracellular drug delivery. Biomacromolecules 8, 1966–1972. 10.1021/bm070085x [DOI] [PubMed] [Google Scholar]
- Chen W., Meng F., Cheng R., Zhong Z. (2010). pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: a comparative study with micelles. J. Control. Release. 142, 40–46. 10.1016/j.jconrel.2009.09.023 [DOI] [PubMed] [Google Scholar]
- Choucair A., Lim Soo P., Eisenberg A. (2005). Active loading and tunable release of doxorubicin from block copolymer vesicles. Langmuir 21, 9308–9313. 10.1021/la050710o [DOI] [PubMed] [Google Scholar]
- Christian D. A., Cai S., Bowen D. M., Kim Y., Pajerowski J. D., Discher D. E. (2009). Polymersome carriers: from self-assembly to siRNA and protein therapeutics. Eur. J. Pharm. Biopharm. 71, 463–474. 10.1016/j.ejpb.2008.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti A., Franzini M., Paolicchi A., Pompella A. (2010). Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 30, 1169–1181. [PubMed] [Google Scholar]
- Danhier F., Vroman B., Lecouturier N., Crokart N., Pourcelle V., Freichels H., et al. (2009). Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J. Control. Release 140, 166–173. 10.1016/j.jconrel.2009.08.011 [DOI] [PubMed] [Google Scholar]
- Della Rocca J., Huxford R. C., Comstock-Duggan E., Lin W. (2011). Polysilsesquioxane nanoparticles for targeted platin-based cancer chemotherapy by triggered release. Angew. Chem. Int. Ed. Engl. 50, 10330–10334. 10.1002/anie.201104510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher B. M., Won Y. Y., Ege D. S., Lee J. C., Bates F. S., Discher D. E., et al. (1999). Polymersomes: tough vesicles made from diblock copolymers. Science 284, 1143–1146. 10.1126/science.284.5417.1143 [DOI] [PubMed] [Google Scholar]
- Discher D. E., Ortiz V., Srinivas G., Klein M. L., Kim Y., Christian D., et al. (2007). Emerging applications of polymersomes in delivery: from molecular dynamics to shrinkage of tumors. Prog. Polym. Sci. 32, 838–857. 10.1016/j.progpolymsci.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty G. J., McMahon H. T. (2009). Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902. 10.1146/annurev.biochem.78.081307.110540 [DOI] [PubMed] [Google Scholar]
- Dou H., Jiang M., Peng H., Chen D., Hong Y. (2003). pH-dependent self-assembly: micellization and micelle-hollow-sphere transition of cellulose-based copolymers. Angew. Chem. Int. Ed. Engl. 42, 1516–1519. 10.1002/anie.200250254 [DOI] [PubMed] [Google Scholar]
- Drummond D. C., Meyer O., Hong K., Kirpotin D. B., Papahadjopoulos D. (1999). Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol. Rev. 51, 691–744. [PubMed] [Google Scholar]
- Du J., O'Reilly R. K. (2009). Advances and challenges in smart and functional polymer vesicles. Soft Matter 5, 3544–3561. 10.1039/b905635a [DOI] [Google Scholar]
- Du Y., Chen W., Zheng M., Meng F., Zhong Z. (2012). pH-sensitive degradable chimaeric polymersomes for the intracellular release of doxorubicin hydrochloride. Biomaterials 33, 7291–7299. 10.1016/j.biomaterials.2012.06.034 [DOI] [PubMed] [Google Scholar]
- Elloumi Hannachi I., Itoga K., Kumashiro Y., Kobayashi J., Yamato M., Okano T. (2009). Fabrication of transferable micropatterned-co-cultured cell sheets with microcontact printing. Biomaterials 30, 5427–5432. 10.1016/j.biomaterials.2009.06.033 [DOI] [PubMed] [Google Scholar]
- Fu Z., Ochsner M. A., de Hoog H. P., Tomczak N., Nallani M. (2011). Multicompartmentalized polymersomes for selective encapsulation of biomacromolecules. Chem. Commun. (Camb). 47, 2862–2864. 10.1039/c0cc03971c [DOI] [PubMed] [Google Scholar]
- Futaki S. (2006). Oligoarginine vectors for intracellular delivery: design and cellular-uptake mechanisms. Peptide Sci. 84, 241–249. 10.1002/bip.20421 [DOI] [PubMed] [Google Scholar]
- Ge X., Zhang Q., Cai Y., Duan S., Chen S., Lv N., et al. (2014). PEG-PCL-DEX Polymersome-protamine vector as an efficient gene delivery system via PEG- guided self-assembly. Nanomedicine 9, 1193–1207. 10.2217/nnm.13.83 [DOI] [PubMed] [Google Scholar]
- Giacomelli C., Schmidt V., Borsali R. (2007). Nanocontainers formed by self-assembly of poly (ethylene oxide)-b-poly (glycerol monomethacrylate)-drug conjugates. Macromolecules 40, 2148–2157. 10.1021/ma062562u [DOI] [Google Scholar]
- Grabe M., Oster G. (2001). Regulation of organelle acidity. J. Gen. Physiol. 117, 329–344. 10.1085/jgp.117.4.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gref R., Lück M., Quellec P., Marchand M., Dellacherie E., Harnisch S., et al. (2000). “Stealth”corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B. Biointerfaces 18, 301–313. 10.1016/S0927-7765(99)00156-3 [DOI] [PubMed] [Google Scholar]
- Grundl T. (1994). A review of the current understanding of redox capacity in natural, disequilibrium systems. Chemosphere 28, 613–626. 10.1016/0045-6535(94)90303-4 [DOI] [Google Scholar]
- Gubernator J. (2011). Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 8, 565–580. 10.1517/17425247.2011.566552 [DOI] [PubMed] [Google Scholar]
- Guo J., Ogier J. R., Desgranges S., Darcy R., Caitriona O. (2012). Anisamide-targeted cyclodextrin nanoparticles for siRNA delivery to prostate tumours in mice. Biomaterials 33, 7775–7784. 10.1016/j.biomaterials.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Guo X., Park H., Liu G., Liu W., Cao Y., Tabata Y., et al. (2009). In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials 30, 2741–2752. 10.1016/j.biomaterials.2009.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer D. A., Robbins G. P., Haun J. B., Lin J. J., Qi W., Smith L. A., et al. (2008). Leuko-polymersomes. Faraday Discuss. 139, 129–141. 10.1039/b717821b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvasanov D., Wiedenmann J., Braet F., Thordarson P. (2011). Induced polymersome formation from a diblock PS-b-PAA polymer via encapsulation of positively charged proteins and peptides. Chem. Commun. (Camb). 47, 6314–6316. 10.1039/c1cc11662b [DOI] [PubMed] [Google Scholar]
- Kim H., Kang Y. J., Kang S., Kim K. T. (2012). Monosaccharide-responsive release of insulin from polymersomes of polyboroxole block copolymers at neutral pH. J. Am. Chem. Soc. 134, 4030–4033. 10.1021/ja211728x [DOI] [PubMed] [Google Scholar]
- Kim S. K., Foote M. B., Huang L. (2012). The targeted intracellular delivery of cytochrome C protein to tumors using lipid-apolipoprotein nanoparticles. Biomaterials 33, 3959–3966. 10.1016/j.biomaterials.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimura A., Koide A., Osada K., Yamasaki Y., Kataoka K. (2007). Encapsulation of myoglobin in PEGylated polyion complex vesicles made from a pair of oppositely charged block ionomers: a physiologically available oxygen carrier. Angew. Chem. Int. Ed. 46, 6085–6088. 10.1002/anie.200701776 [DOI] [PubMed] [Google Scholar]
- Krack M., Hohenberg H., Kornowski A., Lindner P., Weller H., Förster S. (2008). Nanoparticle-loaded magnetophoretic vesicles. J. Am. Chem. Soc. 130, 7315–7320. 10.1021/ja077398k [DOI] [PubMed] [Google Scholar]
- Kricheldorf H. R. (2006). Polypeptides and 100 years of chemistry of α-amino acid N-carboxyanhydrides. Angew. Chem. Int. Ed. 45, 5752–5784. 10.1002/anie.200600693 [DOI] [PubMed] [Google Scholar]
- Kumar M., Grzelakowski M., Zilles J., Clark M., Meier W. (2007). Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc. Natl. Acad. Sci. U.S.A. 104, 20719–20724. 10.1073/pnas.0708762104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Yang S. R., An E. J., Kim D. J. (2006). Biodegradable polymersomes from poly (2-hydroxyethyl aspartamide) grafted with lactic acid oligomers in aqueous solution. Macromolecules 39, 4938–4940. 10.1021/ma060198t [DOI] [Google Scholar]
- Lee J. S., Ankone M., Pieters E., Schiffelers R. M., Hennink W. E., Feijen J. (2011). Circulation kinetics and biodistribution of dual-labeled polymersomes with modulated surface charge in tumor-bearing mice: comparison with stealth liposomes. J. Control. Release 155, 282–288. 10.1016/j.jconrel.2011.07.028 [DOI] [PubMed] [Google Scholar]
- Lee J. S., Feijen J. (2012). Polymersomes for drug delivery: design, formation and characterization. J. Control. Release 161, 473–483. 10.1016/j.jconrel.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Levine D. H., Ghoroghchian P. P., Freudenberg J., Zhang G., Therien M. J., Greene M. I., et al. (2008). Polymersomes: a new multi-functional tool for cancer diagnosis and therapy. Methods 46, 25–32. 10.1016/j.ymeth.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Chen G., Meng F., Li T., Yue J., Jing X., et al. (2012). A novel amphiphilic copolymer poly (ethylene oxide-co-allyl glycidyl ether)-graft-poly (ε-caprolactone): synthesis, self-assembly, and protein encapsulation behavior. Polym. Chem. 3, 2421–2429. 10.1039/c2py20253k [DOI] [Google Scholar]
- Li B., Qi Y., He S., Wang Y., Xie Z., Jing X., et al. (2014). Asymmetric copolymer vesicles to serve as a hemoglobin vector for ischemia therapy. Biomater. Sci. 2, 1254–1261. 10.1039/C4BM00123K [DOI] [PubMed] [Google Scholar]
- Li F., Griffith M., Li Z., Tanodekaew S., Sheardown H., Hakim M., et al. (2005). Recruitment of multiple cell lines by collagen-synthetic copolymer matrices in corneal regeneration. Biomaterials 26, 3093–3104. 10.1016/j.biomaterials.2004.07.063 [DOI] [PubMed] [Google Scholar]
- Li X., Yang W., Zou Y., Meng F., Deng C., Zhong Z. (2015). Efficacious delivery of protein drugs to prostate cancer cells by PSMA-targeted pH-responsive chimaeric polymersomes. J. Control. Release. 220, 704–714. 10.1016/j.jconrel.2015.08.058 [DOI] [PubMed] [Google Scholar]
- Li Y., Lokitz B. S., McCormick C. L. (2006). Thermally responsive vesicles and their structural “locking” through polyelectrolyte complex formation. Angew. Chem. Int. Ed. 45, 5792–5795. 10.1002/anie.200602168 [DOI] [PubMed] [Google Scholar]
- Liu G., Ma S., Li S., Cheng R., Meng F., Liu H., et al. (2010). The highly efficient delivery of exogenous proteins into cells mediated by biodegradable chimaeric polymersomes. Biomaterials 31, 7575–7585. 10.1016/j.biomaterials.2010.06.021 [DOI] [PubMed] [Google Scholar]
- Liu G. Y., Chen C. J., Ji J. (2012). Biocompatible and biodegradable polymersomes as delivery vehicles in biomedical applications. Soft Matter 8, 8811–8821. 10.1039/c2sm25721a [DOI] [Google Scholar]
- Liu Q., Chen J., Du J. (2014). Asymmetrical polymer vesicles with a “stealthy” outer corona and an endosomal-escape-accelerating inner corona for efficient intracellular anticancer drug delivery. Biomacromolecules 15, 3072–3082. 10.1021/bm500676e [DOI] [PubMed] [Google Scholar]
- Liu Q., Chen S., Chen J., Du J. (2015). An asymmetrical polymer vesicle strategy for significantly improving T 1 MRI sensitivity and cancer-targeted drug delivery. Macromolecules 48, 739–749. 10.1021/ma502255s [DOI] [Google Scholar]
- Lomas H., Canton I., MacNeil S., Du J., Armes S. P., Ryan A. J., et al. (2007). Biomimetic pH sensitive polymersomes for efficient DNA encapsulation and delivery. Adv. Materials 19, 4238–4243. 10.1002/adma.200700941 [DOI] [Google Scholar]
- Lomas H., Massignani M., Abdullah K. A., Canton I., Lo Presti C., MacNeil S., et al. (2008). Non-cytotoxic polymer vesicles for rapid and efficient intracellular delivery. Faraday Discuss. 139, 143–159; discussion 213–228, 419–420. 10.1039/b717431d [DOI] [PubMed] [Google Scholar]
- LoPresti C., Lomas H., Massignani M., Smart T., Battaglia G. (2009). Polymersomes: nature inspired nanometer sized compartments. J. Mater. Chem. 19, 3576–3590. 10.1039/b818869f [DOI] [Google Scholar]
- Lu L., Zou Y., Yang W., Meng F., Deng C., Cheng R., et al. (2015). Anisamide-decorated pH-sensitive degradable chimaeric polymersomes mediate potent and targeted protein delivery to lung cancer cells. Biomacromolecules 16, 1726–1735. 10.1021/acs.biomac.5b00193 [DOI] [PubMed] [Google Scholar]
- Massignani M., Canton I., Sun T., Hearnden V., MacNeil S., Blanazs A., et al. (2010a). Enhanced fluorescence imaging of live cells by effective cytosolic delivery of probes. PLoS ONE 5:e10459. 10.1371/journal.pone.0010459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massignani M., Lomas H., Battaglia G. (2010b). Polymersomes: a synthetic biological approach to encapsulation and delivery, in Modern Techniques for Nano-and Microreactors/-reactions, ed Caruso F. (Berlin; Heidelberg: Springer; ), 115–154. [Google Scholar]
- Massignani M., LoPresti C., Blanazs A., Madsen J., Armes S. P., Lewis A. L., et al. (2009). Controlling cellular uptake by surface chemistry, size, and surface topology at the nanoscale. Small 5, 2424–2432. 10.1002/smll.200900578 [DOI] [PubMed] [Google Scholar]
- Mecke A., Dittrich C., Meier W. (2006). Biomimetic membranes designed from amphiphilic block copolymers. Soft Matter 2, 751–759. 10.1039/b605165k [DOI] [PubMed] [Google Scholar]
- Meng F., Zhong Y., Cheng R., Deng C., Zhong Z. (2014). pH-sensitive polymeric nanoparticles for tumor-targeting doxorubicin delivery: concept and recent advances. Nanomedicine 9, 487–499. 10.2217/nnm.13.212 [DOI] [PubMed] [Google Scholar]
- Meng F., Zhong Z. (2011). Polymersomes spanning from nano-to microscales: advanced vehicles for controlled drug delivery and robust vesicles for virus and cell mimicking. J. Phys. Chem. Lett. 2, 1533–1539. 10.1021/jz200007h [DOI] [Google Scholar]
- Murdoch C., Reeves K. J., Hearnden V., Colley H., Massignani M., Canton I., et al. (2010). Internalization and biodistribution of polymersomes into oral squamous cell carcinoma cells in vitro and in vivo. Nanomedicine 5, 1025–1036. 10.2217/nnm.10.97 [DOI] [PubMed] [Google Scholar]
- Napoli A., Boerakker M. J., Tirelli N., Nolte R. J., Sommerdijk N. A., Hubbell J. A. (2004b). Glucose-oxidase based self-destructing polymeric vesicles. Langmuir 20, 3487–3491. 10.1021/la0357054 [DOI] [PubMed] [Google Scholar]
- Napoli A., Valentini M., Tirelli N., Müller M., Hubbell J. A. (2004a). Oxidation-responsive polymeric vesicles. Nat. Mater. 3, 183–189. 10.1038/nmat1081 [DOI] [PubMed] [Google Scholar]
- Nardin C., Hirt T., Leukel J., Meier W. (2000). Polymerized ABA triblock copolymer vesicles. Langmuir 16, 1035–1041. 10.1021/la990951u [DOI] [Google Scholar]
- Nomani A., Nosrati H., Manjili H. K., Khesalpour L., Danafar H. (2017). Preparation and characterization of copolymeric polymersomes for protein delivery. Drug Res. [Epub ahead of print]. 10.1055/s-0043-106051 [DOI] [PubMed] [Google Scholar]
- Oerlemans C., Bult W., Bos M., Storm G. J., Nijsen F. W., Hennink W. E. (2010). Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm. Res. 27, 2569–2589. 10.1007/s11095-010-0233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Takahashi A., Nagahama K. (2011). Biodegradable polymeric assemblies for biomedical materials, in Polymers in Nanomedicine, eds Kunugi S., Yamaoka T. (Berlin; Heidelberg: Springer; ), 65–114. [Google Scholar]
- Onaca O., Enea R., Hughes D. W., Meier W. (2009). Stimuli-responsive polymersomes as nanocarriers for drug and gene delivery. Macromol. Biosci. 9, 129–139. 10.1002/mabi.200800248 [DOI] [PubMed] [Google Scholar]
- Photos P. J., Bacakova L., Discher B., Bates F. S., Discher D. E. (2003). Polymer vesicles in vivo: correlations with PEG molecular weight. J. Control. Release 90, 323–334. 10.1016/S0168-3659(03)00201-3 [DOI] [PubMed] [Google Scholar]
- Qin S., Geng Y., Discher D. E., Yang S. (2006). Temperature-controlled assembly and release from polymer vesicles of poly (ethylene oxide)-block-poly (N-isopropylacrylamide). Adv. Materials 18, 2905–2909. 10.1002/adma.200601019 [DOI] [Google Scholar]
- Rameez S., Alosta H., Palmer A. F. (2008). Biocompatible and biodegradable polymersome encapsulated hemoglobin: a potential oxygen carrier. Bioconjug. Chem. 19, 1025–1032. 10.1021/bc700465v [DOI] [PubMed] [Google Scholar]
- Rerat V., Pourcelle V., Devouge S., Nysten B., Marchand-Brynaert J. (2010). Surface grafting on poly (ethylene terephthalate) track-etched microporous membrane by activation with trifluorotriazine: application to the biofunctionalization with GRGDS peptide. J. Polym. Sci. 48, 195–208. 10.1002/pola.23778 [DOI] [Google Scholar]
- Robbins G. P., Saunders R. L., Haun J. B., Rawson J., Therien M. J., Hammer D. A. (2010). Tunable leuko-polymersomes that adhere specifically to inflammatory markers. Langmuir 26, 14089–14096. 10.1021/la1017032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofstad E. K., Mathiesen B., Kindem K., Galappathi K. (2006). Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 66, 6699–6707. 10.1158/0008-5472.CAN-06-0983 [DOI] [PubMed] [Google Scholar]
- Sanson C., Schatz C., Le Meins J. F., Soum A., Thévenot J., Garanger E., et al. (2010). A simple method to achieve high doxorubicin loading in biodegradable polymersomes. J. Control. Release 147, 428–435. 10.1016/j.jconrel.2010.07.123 [DOI] [PubMed] [Google Scholar]
- Schrama D., Reisfeld R. A., Becker J. C. (2006). Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 5, 147–159. 10.1038/nrd1957 [DOI] [PubMed] [Google Scholar]
- Stano A., Scott E. A., Dane K. Y., Swartz M. A., Hubbell J. A. (2013). Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials 34, 4339–4346. 10.1016/j.biomaterials.2013.02.024 [DOI] [PubMed] [Google Scholar]
- Stoenescu R., Graff A., Meier W. (2004). Asymmetric ABC-triblock copolymer membranes induce a directed insertion of membrane proteins. Macromol. Biosci. 4, 930–935. 10.1002/mabi.200400065 [DOI] [PubMed] [Google Scholar]
- Sun H., Guo B., Cheng R., Meng F., Liu H., Zhong Z. (2009). Biodegradable micelles with sheddable poly (ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials 30, 6358–6366. 10.1016/j.biomaterials.2009.07.051 [DOI] [PubMed] [Google Scholar]
- Tan M. L., Choong P. F., Dass C. R. (2010). Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides 31, 184–193. 10.1016/j.peptides.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Tew K. D. (2007). Redox pathways in cancer drug discovery. Curr. Opin. Pharmacol. 7:353. 10.1016/j.coph.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin V., Levchenko T., Lukyanov A., Khaw B., Klibanov A., Rammohan R., et al. (2001). p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim. Biophys. Acta 1511, 397–411. 10.1016/S0005-2728(01)00165-7 [DOI] [PubMed] [Google Scholar]
- Torchilin V. P., Lukyanov A. N. (2003). Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov. Today 8, 259–266. 10.1016/S1359-6446(03)02623-0 [DOI] [PubMed] [Google Scholar]
- van Dongen S. F., de Hoog H. P., Peters R. J., Nallani M., Nolte R. J., van Hest J. C. (2009). Biohybrid polymer capsules. Chem. Rev. 109, 6212–6274. 10.1021/cr900072y [DOI] [PubMed] [Google Scholar]
- Velonia K., Rowan A. E., Nolte R. J. (2002). Lipase polystyrene giant amphiphiles. J. Am. Chem. Soc. 124, 4224–4225. 10.1021/ja017809b [DOI] [PubMed] [Google Scholar]
- Wang L., Chierico L., Little D., Patikarnmonthon N., Yang Z., Azzouz M., et al. (2012). Encapsulation of biomacromolecules within polymersomes by electroporation. Angew. Chem. Int. Ed. 51, 11122–11125. 10.1002/anie.201204169 [DOI] [PubMed] [Google Scholar]
- Wang X., Sun H., Meng F., Cheng R., Deng C., Zhong Z. (2013). Galactose-decorated reduction-sensitive degradable chimaeric polymersomes as a multifunctional nanocarrier to efficiently chaperone apoptotic proteins into hepatoma cells. Biomacromolecules 14, 2873–2882. 10.1021/bm4007248 [DOI] [PubMed] [Google Scholar]
- Williams D., van der Weijden J., van Oers M., Abdelmohsen L., Peng F., Wilson D., et al. (2017). Functional polymersomes for application in nanomedicine, in 14th Annual UNESCO/IUPAC Conference on Macromolecules and Materials (Stellenbosch: ). [Google Scholar]
- Xiong X. Y., Li Y. P., Li Z. L., Zhou C. L., Tam K. C., Liu Z. Y., et al. (2007). Vesicles from Pluronic/poly (lactic acid) block copolymers as new carriers for oral insulin delivery. J. Control. Release 120, 11–17. 10.1016/j.jconrel.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Xu H., Meng F., Zhong Z. (2009). Reversibly crosslinked temperature-responsive nano-sized polymersomes: synthesis and triggered drug release. J. Mater. Chem. 19, 4183–4190. 10.1039/b901141b [DOI] [Google Scholar]
- Yang J., Bei J., Wang S. (2002). Enhanced cell affinity of poly (D,L-lactide) by combining plasma treatment with collagen anchorage. Biomaterials 23, 2607–2614. 10.1016/S0142-9612(01)00400-8 [DOI] [PubMed] [Google Scholar]
- Yang M., Wang W., Yuan F., Zhang X., Li J., Liang F., et al. (2005). Soft vesicles formed by diblock codendrimers of poly(benzyl ether) and poly(methallyl dichloride). J. Am. Chem. Soc. 127, 15107–15111. 10.1021/ja052713t [DOI] [PubMed] [Google Scholar]
- Yin H., Kang S. W., Bae Y. H. (2009). Polymersome formation from AB2 type 3-miktoarm star copolymers. Macromolecules 42, 7456–7464. 10.1021/ma901701w [DOI] [Google Scholar]
- Zhang L., Eisenberg A. (1995). Multiple morphologies of “Crew-Cut” aggregates of polystyrene-b-poly(acrylic acid) block copolymers. Science 268, 1728–1731. 10.1126/science.268.5218.1728 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wu F., Yuan W., Jin T. (2010). Polymersomes of asymmetric bilayer membrane formed by phase-guided assembly. J. Control. Release 147, 413–419. 10.1016/j.jconrel.2010.07.121 [DOI] [PubMed] [Google Scholar]
- Zhao L., Li N., Wang K., Shi C., Zhang L., Luan Y. (2014). A review of polypeptide-based polymersomes. Biomaterials 35, 1284–1301. 10.1016/j.biomaterials.2013.10.063 [DOI] [PubMed] [Google Scholar]
- Zhou W., Meng F., Engbers G. H., Feijen J. (2006). Biodegradable polymersomes for targeted ultrasound imaging. J. Control. Release 116, e62–64. 10.1016/j.jconrel.2006.09.051 [DOI] [PubMed] [Google Scholar]
- Zupancich J. A., Bates F. S., Hillmyer M. A. (2006). Aqueous dispersions of poly (ethylene oxide)-b-poly (γ-methyl-ε-caprolactone) block copolymers. Macromolecules 39, 4286–4288. 10.1021/ma060642s [DOI] [Google Scholar]


