Abstract
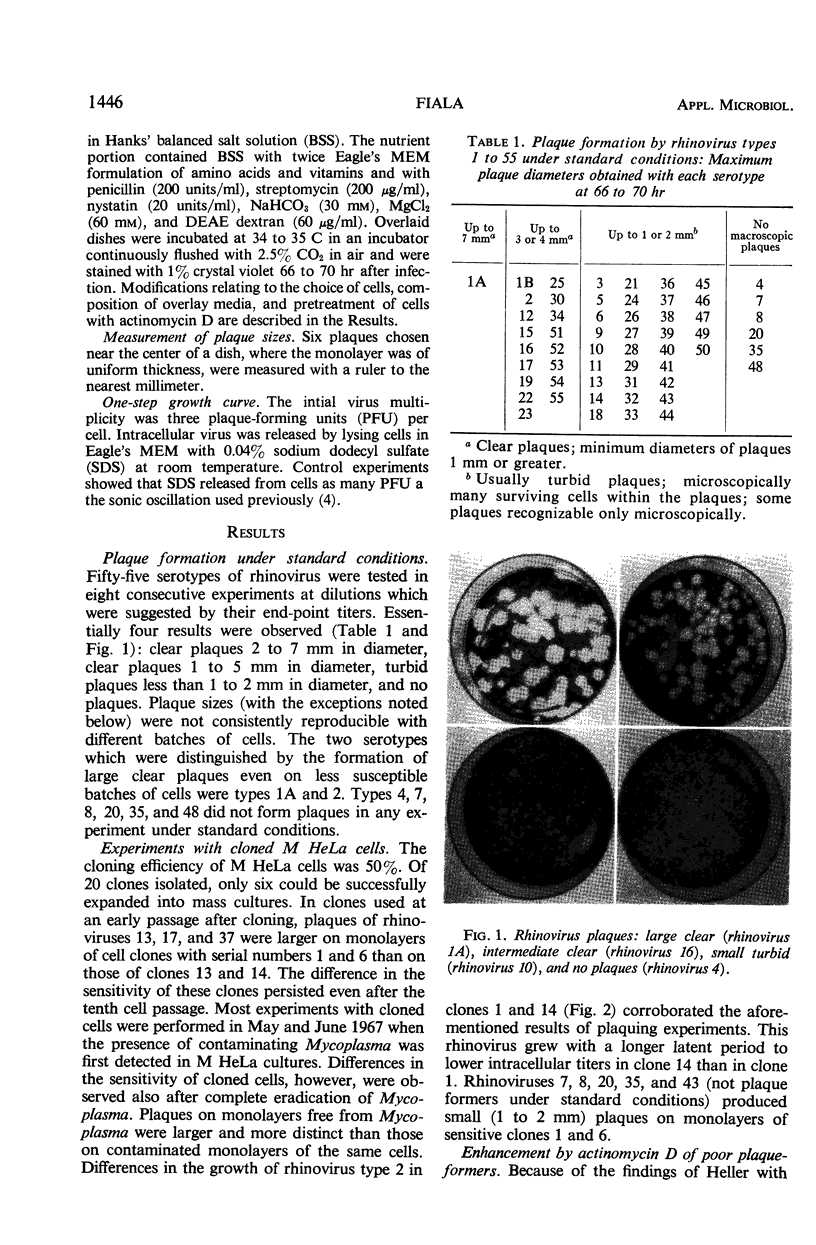
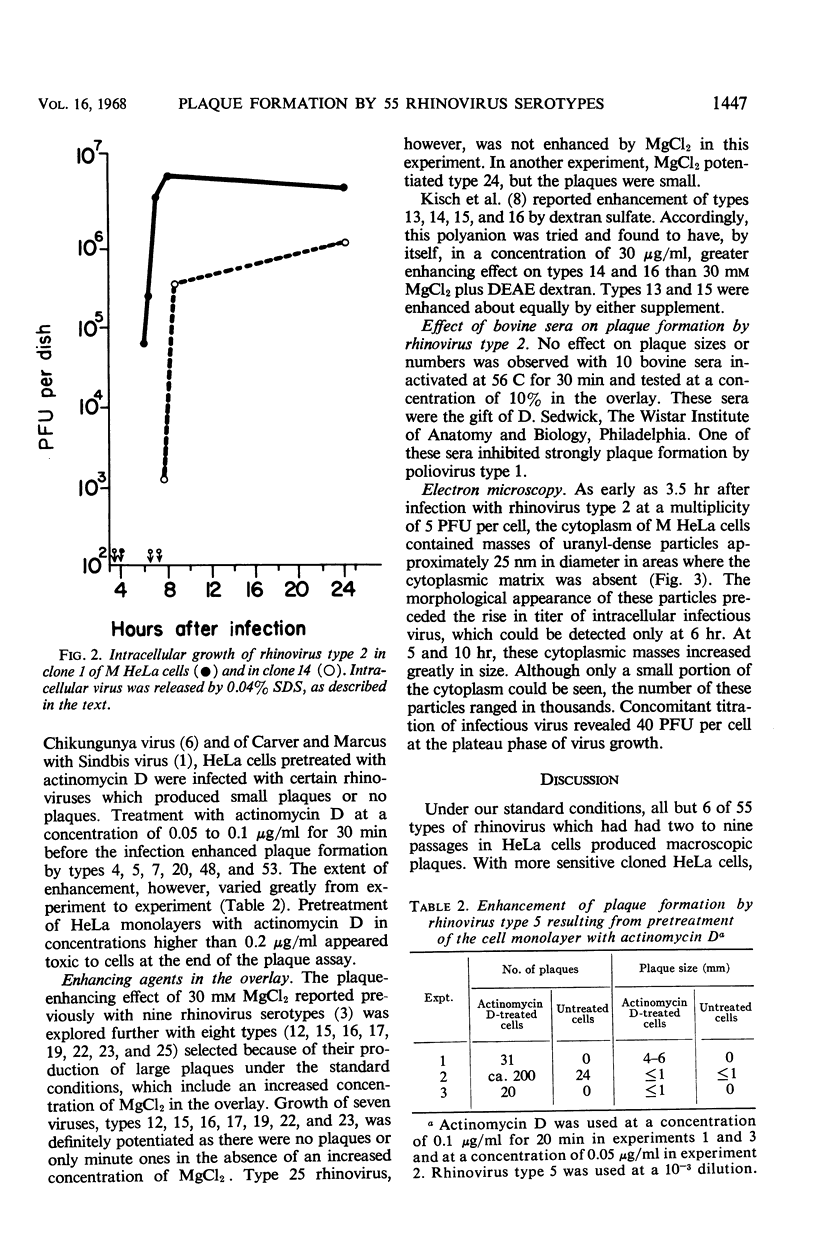
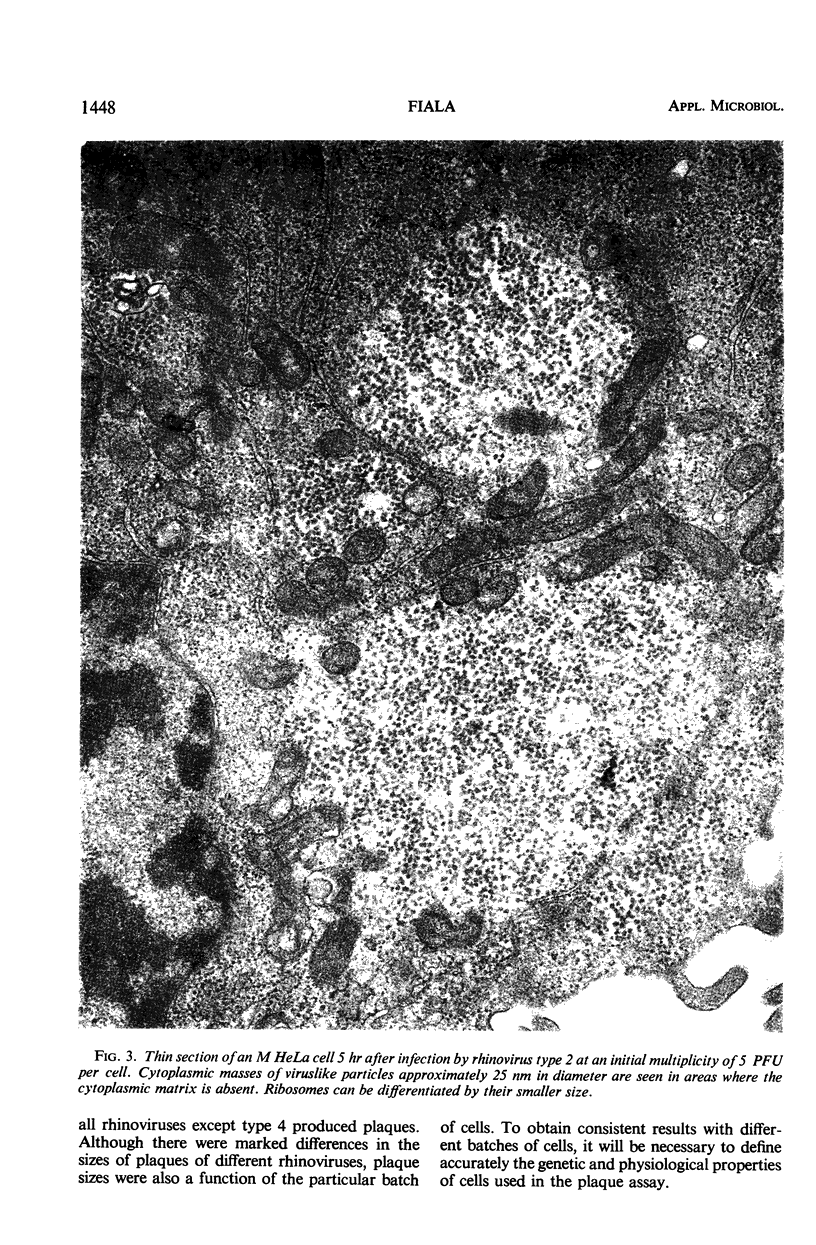
Plaque production by 55 rhinoviruses in several lines of HeLa cells is reported. Forty-nine types produced macroscopic plaques under the conditions described previously employing 30 mM MgCl2 and 30 μg/ml of DEAE-dextran in overlay and M HeLa cells. Basically three kinds of plaques were observed: clear large and intermediate sized plaques and small turbid plaques. Five rhinoviruses produced plaques only in a sensitive clonal line isolated from the M HeLa line. Rhinovirus type 4 produced plaques after the pretreatment of cells with actinomycin D. Enhancement of plaque formation by MgCl2 has been demonstrated to date with 17 rhinoviruses. Some rhinoviruses were enhanced either by DEAE-dextran or by dextran sulfate in the overlay. No inhibitors were found in any of 10 bovine sera tested. Rhinovirus type 2 was visualized inside HeLa cells by electron microscopy and there appeared to be a very high ratio of physical particles per infectious unit of virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOPER P. D. The plaque assay of animal viruses. Adv Virus Res. 1961;8:319–378. doi: 10.1016/s0065-3527(08)60689-2. [DOI] [PubMed] [Google Scholar]
- Fiala M., Kenny G. E. Effect of magnesium on replication of rhinovirus HGP. J Virol. 1967 Jun;1(3):489–493. doi: 10.1128/jvi.1.3.489-493.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M., Kenny G. E. Enhancement of rhinovirus plaque formation in human heteroploid cell cultures by magnesium and calcium. J Bacteriol. 1966 Dec;92(6):1710–1715. doi: 10.1128/jb.92.6.1710-1715.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLER E. ENHANCEMENT OF CHIKUNGUNYA VIRUS REPLICATION AND INHIBITION OF INTERFERON PRODUCTION BY ACTINOMYCIN D. Virology. 1963 Dec;21:652–656. doi: 10.1016/0042-6822(63)90239-3. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- KISCH A. L., WEBB P. A., JOHNSON K. M. FURTHER PROPERTIES OF FIVE NEWLY RECOGNIZED PICORNAVIRUSES (RHINOVIRUSES). Am J Hyg. 1964 Mar;79:125–133. doi: 10.1093/oxfordjournals.aje.a120368. [DOI] [PubMed] [Google Scholar]
- Rhinoviruses: a numbering system. Nature. 1967 Feb 25;213(5078):761–762. doi: 10.1038/213761a0. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., KIRSCHSTEIN R. L. DEXTRAN SULFATE PLAQUE VARIANTS OF ATTENUATED TYPE 1 POLIOVIRUS. RELATIONSHIP TO IN VITRO AND IN VIVO MARKERS. J Immunol. 1964 Feb;92:329–333. [PubMed] [Google Scholar]
- TAKEMOTO K. K., LIEBHABER H. Virus-polysaccharide interactions. II. Enhancement of plague formation and the detection of variants of poliovirus with dextran sulfate. Virology. 1962 Jul;17:499–501. doi: 10.1016/0042-6822(62)90148-4. [DOI] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L. Magnesium chloride enhancement of cell susceptibility to poliovirus. Virology. 1962 Feb;16:122–132. doi: 10.1016/0042-6822(62)90287-8. [DOI] [PubMed] [Google Scholar]





