Abstract
Therapy for chronic, nonspecific low back pain is mainly conservative: medication and/or exercise. Pharmacotherapy, however, has side effects, and the quantities of concomitant drugs in older persons require attention. Although exercise promises improved function, its use to alleviate pain is controversial. Thus, we compared the efficacy of pharmacotherapy versus exercise for treating chronic nonspecific low back pain. The pharmacotherapy group (n=18: 8 men, 10 women) were prescribed celecoxib monotherapy. The exercise group (n=22: 10 men, 12 women) undertook stretching exercises. Because of drop-outs, the NSAID group (n=15: 7 men, 8 women) and the exercise group (n =18: 8 men, 10 women) were finally analyzed. We applied a visual analog scale, Roland–Morris disability scores, and the 36-Item Short Form Health Survey. We used a paired t-test for within-group analyses and an unpaired t-test for between-group analyses. Pain relief was achieved after 3 months of pharmacotherapy or exercise. Quality of life improved only in the exercise group. Recovery outcomes for the two groups were not significantly different. Efficacy of exercise therapy for strictly defined low back pain was almost equivalent to that of pharmacotherapy and provided better quality of life.
Keywords: Chronic nonspecific low back pain, Exercise, 36-Item Short-Form Health Survey, Nonsteroidal anti-inflammatory drug, Roland Morris Disability Questionnaire score
Introduction
Nonspecific low back pain (NLBP) has become a major public health problem worldwide1). The lifetime prevalence of low back pain (LBP) is reported to be as high as 84%, and the prevalence of chronic LBP is about 23%, with 11%-12% of the population being disabled by it1). Mechanical factors, such as lifting and carrying, probably do not have a major pathogenic role, whereas an individual’s genetic constitution is important1). History-taking and clinical examination are included in most clinical practice guidelines for management of LBP2-5), but the use of clinical imaging for diagnosis may be restricted. NLBP manifests as tension, soreness, and/or stiffness in the lower back region, and it is not possible to identify a specific cause of the pain. Several structures in the back, including the joints, discs, and connective tissues, may contribute to the creation of the symptoms.
Generally, treatment for patients with chronic nonspecific low back pain (CNLBP) is conservative, using medication and/or exercise. Pharmacotherapy may be associated with side effects. Nonsteroidal anti-inflammatory drugs (NSAIDs) have been demonstrated to have therapeutic efficacy for CNLBP. It was reported, however, that 3-23% of patients using NSAIDs had to discontinue them because of side effects6). Also, with the increased numbers of medications available, the quantities of concomitant drugs given to older persons in regard to the risk of falling down should be particularly monitored. Another treatment─exercise─has well-established efficacy in patients with CNLBP and is popular. Many studies have demonstrated that therapeutic exercise can improve trunk flexibility, strength, endurance, aerobic capability, and stabilization7-12). Exercise promises improved function and activities of daily living (ADL). There are arguments, however, both for and against alleviating pain in this manner.
There has been little reported on comparisons between NSAID therapy and exercise therapy in regard to the outcomes, including (1) the degree of pain, (2) disability, and (3) quality of life (QOL) associated with LBP or health. The purpose of this study was to analyze the therapeutic efficacy of NSAID therapy compared with that of exercise therapy for patients with CNLBP.
Methods
We performed a prospective study of patients diagnosed with CNLBP. The ethics committee of the participating research institution, Aizu Medical Center Fukushima Medical University, approved this study. Written informed consent was obtained from all patients prior to their participation. Each of the patients agreed to the prescribed treatment in this study and to attend follow-up evaluations at our hospital for a minimum of 3 months.
The inclusion criteria for all patients are shown in Table 1. The study patients were randomly registered into two groups according to the treatment they received. Both groups were asked to maintain good posture10-12).
Table 1.
Inclusion and exclusion criteria for study subjects
| Inclusion criteria |
| ・Patients newly diagnosed with nonspecific low back pain at our initial examination |
| ・Presence of low back pain but no leg symptoms for more than 3 months |
| ・Exclusion of lumbar disc herniation and lumbar spinal stenosis (LSS) by a diagnostic support tool for LSS |
| ・No prior history of treatment for low back pain at our initial examination |
| ・Cognitive capability to respond to our inquiries |
| Exclusion criteria |
| ・Suspicion of serious spinal disease (e.g., tumor, infection, trauma) because of “red flags” |
| ・Specific low back pain caused by spinal disease, nerve disease, internal disease, vascular disease, mental disorder |
| ・Suspicion of neuromuscular disease |
| ・Known rheumatoid arthritis |
| ・Known renal insufficiency, diabetes, congestive heart failure, cardiac conduction abnormalities, thrombocytopenia |
| ・Known peripheral neuropathy |
| ・History of spinal surgery |
| ・History of workmen's compensation or disability issues |
| ・Chronic depression and use of antidepressant medication |
The NSAID group (18 participants:8 men, 10 women) took oral medications. They included celecoxib (100 mg) twice a day and a proton pump inhibitor once a day (to prevent adverse gastrointestinal reactions). The patients were also asked to visit the physician’s office at least once a week. At each visit, the dosage and side effects were reviewed using the patient’s NSAID diary (similar to the exercise diary) with daily notations by the patients, who were asked to record each day whether they took the NSAID. They were allowed to stop taking the NSAID but could resume taking it when they found it necessary to relieve their pain.
Each patient in the exercise group (22 participants:10 men, 12 women) was given a brochure describing therapeutic exercises and body mechanics. In addition, medical professionals at each outpatient clinic (an orthopedic surgeon and physical therapists) gave a practical lecture to the participants about the exercises. The exercise program, conducted at the patient’s home, consisted of two types of exercise:trunk muscle strengthening and stretching. For strengthening, a sit-up exercise was used for trunk flexor muscles and an extension exercise for trunk extensor muscles. For stretching, emphasis was on the abdominal and back muscles, iliopsoas, gluteal muscles, and hamstrings. The exercise focused mainly on increasing overall physical activity and spinal mobility10). Ten repetitions of each exercise were regarded as a set, and the patients were encouraged to perform at least two sets per day. Appropriate body mechanics was taught using the brochure with its figures. Based on the direction of the orthopedic surgeon, other disciplines could be instituted as well. Before implementing the program, the patients attended a 15- to 30-min class (the length depended on the participating facility) to receive instructions regarding the exercises and body mechanics. To encourage their compliance, the patients were asked to visit an orthopedic surgeon’s office at least once a week10). During these visits, it was determined whether they understood body mechanics properly and were performing the exercise as instructed.
The patients in the two groups were registered randomly (according to their medical examination) from among patients who had been newly diagnosed with possible NLBP at our hospital (using a diagnostic support tool designed for lumbar spinal stenosis13)). They also had to satisfy the inclusion criteria, did not meet any of the exclusion criteria, and agreed with the therapeutic approach of this study. They were attended at our hospital as outpatients by an orthopedic surgeon once a month for 3 months after starting treatment. The trial profile of this study is shown in Fig. 1. There were 18 participants allocated to the NSAID group and 22 patients to the exercise group. In all, 17 of the 18 participants received the NSAID (one did not). In contrast, all 22 patients in the exercise group performed the exercises. In the NSAID group, two patients were lost to follow-up because they had gastritis. In the exercise group, four were lost to follow-up because of a malignant tumor (n=1), heart disease (n=2), or Ménière’s disease (n=1). Finally, the NSAID group (n=15:7 men, 8 women) and the exercise group (n=18:8 men, 10 women) were analyzed. The demographic and clinical characteristics of the patients are shown in Table 2.
Fig. 1.
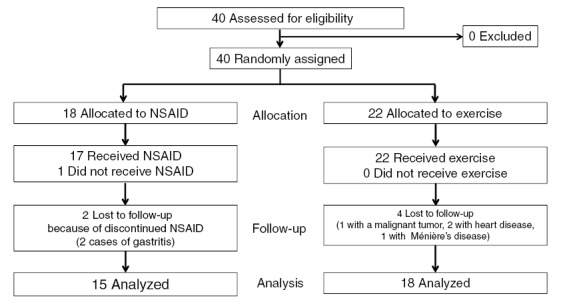
Flow chart of the progression of the trial. Finally, the diminished (owing to drop-outs) NSAID (n=15: 7 men, 8 women) and exercise (n =18: 8 men, 10 women) groups were compared.
Table 2.
Demographic and clinical characteristics of patients
| Characteristic |
NSAID therapy group (n = 15) |
Exercise therapy group (n = 18) |
P |
| Age (years) | 53.3 ± 5.57 | 57.6 ± 3.28 | 0.4774 |
| Sex (M/F) | 7/8 | 8/10 | 0.9023 |
| Current smoker | 3 | 5 | 0.6170 |
| Manual laborer | 11 | 9 | 0.1827 |
| Duration of pain (months) | 5.92 ± 0.883 | 7.63 ± 0.926 | 0.2056 |
| VAS before therapy | 69.2 ± 5.46 | 70.3 ± 4.07 | 0.8745 |
| RDQ before therapy | 8.92 ± 1.08 | 7.88 ± 1.35 | 0.5720 |
Data are the mean ± standard error or the number unless otherwise indicated.
RDQ, Roland Morris Disability Questionnaire scores; VAS, visual analog scale.
Outcomes were assessed at two time points: time zero (before treatment) and 3 months after starting treatment (final time point). The primary outcome was pain, which was assessed with a visual analog scale (VAS) that ranged from 0 (least pain) to 10 (most intense pain)14). The patients were asked to rate their LBP. Disability and QOL were assessed by a previously validated Japanese version of the Roland Morris Disability Questionnaire score (RDQ)15). It consisted of 24 items related to ADL. Scores ranged from 0 (no disability) to 24 (maximum disability). A patient-reported survey of his or her health was assessed using the 36-Item Short-Form Health Survey (SF-36)16-18), which measures health status. The SF-36 consists of eight scaled scores, which are the weighted sums of responses to the questions in their respective section. Each scale is directly transformed into a scale of 0-100 on the assumption that each question carries equal weight. The lower the score, the greater is the disability. Conversely, the higher the score, the less is the disability. That is, a score of zero is equivalent to maximum disability, and a score of 100 is equivalent to no disability. The eight sections of the SF-36 are (1) physical functioning, (2) role physical, (3) bodily pain, (4) general health, (5) vitality, (6) social functioning, (7) role emotional, and (8) mental health. We registered to use the SF-36 Japanese version 2 from iHope International, which holds the copyright, and calculated the eight scaled SF-36 scores using this exclusive scoring software.
The primary outcome was evaluated before treatment and 3 months after the start of treatment. We compared the efficacies of the NSAID therapy and the exercise therapy from before treatment to 3 months after the start of treatment using a paired t-test. We also assessed the recovery of the VAS, RDQ, and SF-36 scores at 3 months after starting treatment with the NSAID therapy versus the exercise therapy using an unpaired t-test. A value of P<0.05 was considered to indicate significance. The statistical analyses were performed using StatView 5.0 statistical software (SAS Inc., Cary, NC, USA). The statistical power analysis of this study was performed using G*Power 3.1 (Heinrich Heine Universität Düsseldorf)19). Its defined effect size (d) was 0.8, and the alpha error probability (prob) was 0.05.The sample size of the NSAID group was 18 and that of the exercise group was 22. Finally, power (1-beta error prob) was calculated to be 0.80.
Results
The age, sex distribution, smoking status, and proportion of patients performing manual labor were similar in the NSAID and exercise groups (Table 2). In both groups, the majority of patients were 40-50 years of age. The mean duration of pain was assessed, and the mean VAS and RDQ scores before therapy were measured, which were 69.2 ± 5.46 and 8.92 ± 1.08, respectively, in the NSAID therapy group and 70.3 ± 4.07 and 7.88 ± 1.35, respectively, in the exercise therapy group. They were similar in the two groups (p>0.05) (Table 2).
The NSAID group reported side effects, and the exercise group experienced complications of other diseases. Among the 17 patients on celecoxib therapy, two developed upper gastrointestinal disease (e.g., gastric ulcer) (Fig. 1). Among the 22 patients in the exercise group, 4 were lost to follow-up (a malignant tumor in 1, heart disease in 2, Meniere’s disease in 1) (Fig. 1).
There were statistically significant differences in the VAS for the NSAID group, from before starting therapy to after 3 months of it (p<0.05) (Table 3A). There were also statistically significant differences for the VAS in the exercise group from before starting therapy to after 3 months of therapy (Table 3B). After 3 months of therapy, however, the VAS scores were not significantly different between the two groups (Table 4).
Table 3A.
Therapeutic efficacy of NSAID therapy before and 3 months after starting treatment
| Parameter | Before |
3 months after starting treatment |
Significance |
| VAS | 69.3 ± 5.46 | 28.6 ± 11.3 | S.D (p = 0.0277) |
| RDQ | 8.92 ± 1.08 | 3.43 ± 1.49 | N.S (p = 0.0618) |
| SF-36 (eight scaled scores) | |||
| 1) physical functioning | 36.1 ± 5.87 | 43.1 ± 5.48 | N.S (p = 0.8413) |
| 2) role physical | 41.0 ± 2.95 | 40.2 ± 4.37 | N.S (p = 0.8781) |
| 3) bodily pain | 31.5 ± 1.75 | 37.4 ± 2.62 | N.S (p = 0.3341) |
| 4) general health | 41.5 ± 2.49 | 45.1 ± 2.33 | N.S (p = 0.5088) |
| 5) vitality | 42.7 ± 2.44 | 45.0 ± 3.54 | N.S (p = 0.8138) |
| 6) social functioning | 42.9 ± 2.37 | 47.7 ± 4.50 | N.S (p = 0.4554) |
| 7) role emotional | 44.4 ± 3.84 | 45.1 ± 4.97 | N.S (p = 0.7822) |
| 8) mental health | 46.0 ± 2.70 | 48.0 ± 3.61 | S.D (p = 0.0277) |
Data are the mean ± standard error or the number unless otherwise indicated.
N.S: Not significant
NSAID: Nonsteroidal anti-inflammatory drug
RDQ: Roland Morris Disability Questionnaire score
S.D: Significant difference
SF-36: 36-Item Short-Form Health Survey
VAS: Visual analog scale
Table 3B.
Therapeutic efficacy of exercise therapy before and 3 months after starting treatment
| Parameter | Before |
3 months after starting treatment |
Significance |
| VAS | 70.6 ± 3.86 | 30.4 ± 9.24 | S.D (p = 0.0011) |
| RDQ | 7.86 ± 1.35 | 3.92 ± 1.25 | S.D (p = 0.0426) |
| SF-36 (eight scaled scores) | |||
| 1) physical functioning | 34.8 ± 4.81 | 46.0 ± 2.51 | N.S (p = 0.1232) |
| 2) role physical | 33.4 ± 4.45 | 44.0 ± 4.07 | N.S (p = 0.1378) |
| 3) bodily pain | 35.1 ± 2.04 | 41.9 ± 2.75 | S.D (p = 0.0325) |
| 4) general health | 42.5 ± 2.98 | 44.9 ± 3.75 | N.S (p = 0.2243) |
| 5) vitality | 43.7 ± 2.81 | 48.4 ± 3.56 | N.S (p = 0.0655) |
| 6) social functioning | 45.6 ± 3.68 | 50.0 ± 2.86 | N.S (p = 0.1192) |
| 7) role emotional | 37.6 ± 4.23 | 48.8 ± 2.71 | N.S (p = 0.0675) |
| 8) mental health | 44.3 ± 3.05 | 50.9 ± 4.06 | N.S (p = 0.0617) |
Data are the mean ± standard error or the number unless otherwise indicated.
N.S: Not significant
NSAID: Nonsteroidal anti-inflammatory drug
RDQ: Roland Morris Disability Questionnaire score
S.D: Significant difference
SF-36: 36-Item Short-Form Health Survey
VAS: Visual analog scale
Table 4.
Therapeutic efficacy of NSAID therapy versus exercise therapy during 3 months
| Parameter | NSAID group | Exercise group | Significance |
| VAS | -44.0 ± 15.3 | -35.8 ± 8.07 | N.S (p = 0.6138) |
| RDQ | -5.00 ± 2.78 | -3.20 ± 1.03 | N.S (p = 0.4750) |
| SF-36 (eight scaled scores) | |||
| 1) physical functioning | 1.01 ± 4.86 | 6.43 ± 3.09 | N.S (p = 0.3364) |
| 2) role physical | 16.3 ± 6.16 | 16.0 ± 4.63 | N.S (p = 0.4135) |
| 3) bodily pain | 4.27 ± 4.07 | 7.45 ± 3.05 | N.S (p = 0.5377) |
| 4) general health | 1.83 ± 4.67 | 3.77 ± 2.93 | N.S (p = 0.7147) |
| 5) vitality | 0.87 ± 3.54 | 5.39 ± 2.64 | N.S (p = 0.3168) |
| 6) social functioning | 3.24 ± 4.07 | 4.40 ± 2.60 | N.S (p = 0.8043) |
| 7) role emotional | 2.46 ± 8.50 | 7.98 ± 3.94 | N.S (p = 0.5105) |
| 8) mental health | 2.29 ± 4.32 | 5.77 ± 2.77 | N.S (p = 0.4864) |
Data are the mean ± standard error or the number unless otherwise indicated.
N.S: Not significant
VAS: Visual analog scale
RDQ: Roland Morris Disability Questionnaire score
S.D: Significant difference
SF-36: 36-Item Short-Form Health Survey
There were no statistically significant differences in the RDQ scores in the NSAID group from before starting therapy to after 3 months of it (Table 3A). However, there were statistically significant differences in the RDQ scores for the exercise group from before starting therapy to after 3 months of it (p<0.05) (Table 3B). The RDQ scores for the two groups after 3 months of therapy were not significantly different (Table 4).
There were no statistically significant differences in any of the scores of the eight SF-36 scales in the NSAID group from before starting therapy to after 3 months of it (Table 3A). In the exercise group, from before starting therapy to after 3 months of it, there were statistically significant differences in scores of one of the eight SF-36 scales (bodily pain) (p<0.05) but not in the scores for the other seven scales (Table 3B). None of the scores of the eight SF-36 scales after 3 months of therapy was significantly different in the two groups (Table 4).
Thus, all of the patients with CNLBP had experienced pain relief after 3 months of treatment, regardless of whether they were treated with an NSAID or exercise. However, the QOL was statistically improved in the exercise group but not in the NSAID group after 3 months of treatment. There were no statistically significant differences in recovery for any of the outcomes between the two groups.
Discussion
The present study demonstrated that patients with CNLBP felt substantially better after 3 months of NSAID therapy or exercise therapy compared with how they were before the treatment regarding two outcomes:the degree of pain and the disability/QOL associated with LBP. None of the scores for the eight SF-36 scales, which assess QOL associated with health, had changed in the NSAID group at 3 months. In contrast, in the exercise group, scores for disability/QOL associated with LBP and one of the SF-36 scales─bodily pain─had changed after 3 months of treatment compared with before treatment. There was no statistically significant difference regarding recovery between the two therapies in regard to the degree of pain, disability/QOL associated with LBP, or QOL associated with health.
It has been generally demonstrated that NSAID therapy is a higher-risk treatment because of co-morbidities (e.g., heart disease, upper gastrointestinal disease), whereas exercise therapy is a low-risk treatment.
The therapeutic efficacy of pharmacotherapy with NSAIDs or acetaminophen for patients with CNLBP has been recommended in clinical practice guidelines for the management of LBP worldwide2-5). Some studies, however, have reported that NSAID therapy had to be discontinued in some patients because of NSAID-induced side effects6). In our study, the degree of pain was alleviated in the patients with CNLBP after only 3 months of celecoxib therapy. However, two of the 17 (11.7%) patients on celecoxib therapy developed upper gastrointestinal disease in this study. The degree of pain and the QOL associated with LBP for patients in both the NSAID and exercise groups were not sufficiently improved (Fig. 1).
Exercise therapy for patients with CNLBP should be recommended in the Japanese clinical practice guidelines for managing LBP5). One of the main purposes of therapeutic exercise for patients with CNLBP is to strengthen trunk muscles and improve trunk flexibility20). Shirado et al.12) demonstrated that the therapeutic efficacy of exercise therapy was more effective than NSAID therapy in a Japanese population. They suggested that the degree of pain diminished and QOL improved in patients with CNLBP after exercise therapy alone, which included good posture, stretching, and lumbar stabilization exercises for 3 months performed under supervision of a physiotherapist. In our study, four of the 22 (18.2%) patients in the exercise group were lost to follow-up because of other diseases (Fig. 1). Based on these results, we decided that unavoidable problems had occurred in both groups that interrupted our assessment of the therapeutic efficacy for the patients with CNLBP.
The results in this study demonstrated that NSAID and exercise could have different effects on CNLBP. NSAIDs might be effective for addressing pain, whereas exercise would be more appropriate for improving activity and QOL. It has been demonstrated that chronic LBP is sometimes accompanied by secondary psychological and social factors. It therefore may be necessary to treat such patients using a multifaceted approach.
Some clinical practice guidelines for LBP2-5) recommend other pharmacotherapy, including antidepressants (e.g., a serotonin norepinephrine reuptake inhibitor), antiepileptic drugs (e.g., gabapentin, an antiepileptic γ-aminobutyric acid analog), pregabalin, opioids, and tramadol. It is thought that these drugs affect the descending pain modulatory system21), which consists of the 5-hydroxytryptamine and noradrenergic systems. However, the analgesic effect of these drugs may not be enough to relieve chronic LBP. Therefore, we on the medical staff side may need to educate patients with CNLBP to help them accept their pain and attempt to improve their QOL while coexisting with the pain.
The present study has some limitations that require attention. First, the follow-up period was relatively short, and future studies are needed to evaluate the long-term therapeutic efficacy of NSAID and exercise therapy. Second, our study population was small, although the power might be enough. Future studies need to evaluate a larger population. Third, we used an NSAID alone in this study. Future studies may be required to compare and evaluate other pharmacotherapeutic agents such as antidepressants or antiepileptic drugs as well as exercise therapy.
Conclusions
In patients with strictly defined CNLBP (using a diagnostic support tool designed for lumbar spinal stenosis13)), the therapeutic efficacy of an NSAID and exercise seemed to be almost equivalent with regard to pain relief. However, the QOL of the patients was statistically improved in the exercise group but not in the NSAID group during the initial 3 months of this study. Furthermore, according to the results of this study, exercise therapy could also be used to alleviate pain.
Conflicts of interest and source of funding
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Ethics
This study was approved by the ethics committees of the participating research institution.
References
- 1.Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non-specific low back pain. Lancet, 379: 482-491, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Airaksinen O, Brox JI, Cedraschi C, et al. COST B13 Working Group on Guidance for Chronic Low Back Pain. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J, 15(Suppl 2): S192-300, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossignol M, Poitras S, Dionne C, et al. An interdisciplinary guideline development process:the Clinic on Low-back pain in Interdisciplinary Practice (CLIP) low-back guidelines. Implement Sci, 2:36, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain:a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med, 147: 478-491, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Japanese Orthopaedic Association (JOA). Clinical Practice Guideline for the Management of Low Back Pain. Nankodo, Tokyo, 2012. [Google Scholar]
- 6.Schnitzer TJ, Ferrarob A, Hunsche E, Kong SX. A comprehensive review of clinical trials on the efficacy and safety of drugs for the treatment of low back pain. J Pain Symptom Manage, 28: 72-95, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Kuukkanen T, Malkia E. Muscular performance after a 3 month progressive physical exercise program and 9 month follow-up in subjects with low back pain:a controlled study. Scand J Med Sci Sports, 6: 112-121, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Mannion AF, Taimela S, Muntener M, Dvorak J. Active therapy for chronic low back pain. Part 1. Effects on back muscle activation, fatigability, and strength. Spine, 29: 897-908, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Mooney V. Manual therapy and exercise therapy in patients with chronic low back pain:a randomized, controlled, trial with 1-year follow-up. Spine,29:107;author reply 107-108, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Shirado O, Ito T, Kikumoto T, Takeda N, Minami A, Strax TE. A novel back school using a multidisciplinary team approach featuring quantitative functional evaluation and therapeutic exercises for patients with chronic low back pain:the Japanese experience in the general setting. Spine, 30: 1219-1225, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Shirado O, Doi T, Akai M, Fujino K, Hoshino Y, Iwaya T. An outcome measure for Japanese people with chronic low back pain:an introduction and validation study of Japan Low Back Pain Evaluation Questionnaire. Spine, 32: 3052-3059, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Shirado O, Doi T, Akai M, et al. Multicenter randomized controlled trial to evaluate the effect of home-based exercise on patients with chronic low back pain:the Japan Low Back Pain Exercise Therapy Study. Spine, 35: E811-819, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Konno S, Kikuchi S, Tanaka Y, et al. A diagnostic support tool for lumbar spinal stenosis:a self-administered, self-reported history questionnaire. BMC Musculoskelet Disord, 8:102, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huskisson EC. Measurement of pain. Lancet, 2: 1127-1131, 1974. [DOI] [PubMed] [Google Scholar]
- 15.Suzukamo Y, Fukuhara S, Kikuchi S, et al. Committee on Science Project, Japanese Orthopaedic Association. Validation of the Japanese version of the Roland-Morris Disability Questionnaire. Spine, 8: 543-548, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE Jr, Sherbourne CD. The MOS 36 Item Short Form Health Survey (SF 36). 1. Conceptual framework and item selection. Medical Care, 30: 473-483, 1992. [PubMed] [Google Scholar]
- 17.McHorney CA, Ware JE Jr, Lu JFR, Sherbourne CD. The MOS 36 Item Short Form Health Survey (SF 36). 3. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care, 32: 40-66, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF 36 health profile and summary measures:summary of results from the Medical Outcomes Study. Med Care, 33(Suppl):AS264-279, 1995. [PubMed] [Google Scholar]
- 19.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3:A flexible statistical power analysis program for the social, behavior, and biomedical sciences. Behavior Research Methods, 39: 175-191, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Mayer TG, Gatchel RJ, Kishino N, et al. Objective assessment of spine function following industrial injury. A prospective study with comparison group and one-year follow-up. Spine, 10: 482-493, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Basbaum AI, Field HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat:further studies on the anatomy of pain modulation. J Comp Neurol, 187: 513-531, 1979. [DOI] [PubMed] [Google Scholar]


