Abstract
New neurons are continuously integrated into adult hippocampal circuitry and play important roles in cognitive and affective functions. In this issue of Neuron, Bergami et al. (2015) report an experience-dependent remodeling of the afferent connectivity of adult-born dentate granule neurons.
If Ramón y Cajal were to live in 2015, he would probably be astonished by how plastic a mammalian brain can be and how little we really know about its true plastic nature. After Cajal’s famous statement, “In the adult centers the nerve paths are something fixed, ended and immutable. … It is for the science of the future to change, if possible, this harsh decree,” studies in past decades have led to discoveries of enormous self-remodeling of the adult mammalian brain under external and internal influences. It can occur by the modification, elimination or de novo generation of connections among neurons, homeostatic changes in the excitation or inhibition of neurons, or, even more dramatically, by the addition of newly generated neurons into the existing network. The sub-granular zone of the dentate gyrus in the hippocampus is one of the few known regions in which neurogenesis continues during mammalian adulthood (Ming and Song, 2011). Newborn dentate granule neurons arise from adult neural stem cells and become integrated into the existing neuronal network, exerting significant influence on cognition and mood regulation (Christian et al., 2014). While previous studies have delineated the basic connectivity of newborn neurons in the adult brain (Christian et al., 2014), whether, how, and when connections of adult-born neurons are influenced by experience remain largely unexplored. In this issue, Bergami et al. (2015) started to address these important questions concerning adult neurogenesis in the mouse hippocampus.
The current study is a continuum of an earlier piece of work from the same research group, in which a state-of-the-art dual virus-based monosynaptic tracing technique was applied to systematically map presynaptic partners of adult-born dentate granule neurons (Deshpande et al., 2013). The basic principle is the combination of the selective targeting of dividing cells by onco-retrovirus and the retrograde trans-synaptic spread of rabies virus (RABV) to their pre-synaptic partners. Briefly, retrovirus was engineered to express the fluorescent protein DsRed, the EnvA receptor (TVA) and the rabies virus glycoprotein (G). TVA restricts the primary RABV infection to newborn neurons, while G enables the spread of RABV to their presynaptic partners. When engineered retroviruses are injected into the adult mouse dentate gyrus, newly generated neurons can be selectively birth-dated and transformed into the “starter cells” for the monosynaptic spread of the GFP-encoding TVA pseudotyped ΔG RABV (Wickersham et al., 2007), which is later injected into the same region. One week following the transduction of RABV, the GFP+DsRed− cells could be identified as presynaptic partners of the GFP+DsRed+ newborn granule cells. The same group demonstrated previously that after their birth, adult-born granule neurons are first innervated by local interneurons in hippocampus, then by projection neurons in subcortical and finally cortical regions. Notably, similar findings of such a temporally ordered integration process have also been reported by other two independent groups using similar methods (Li et al., 2012; Vivar et al., 2012).
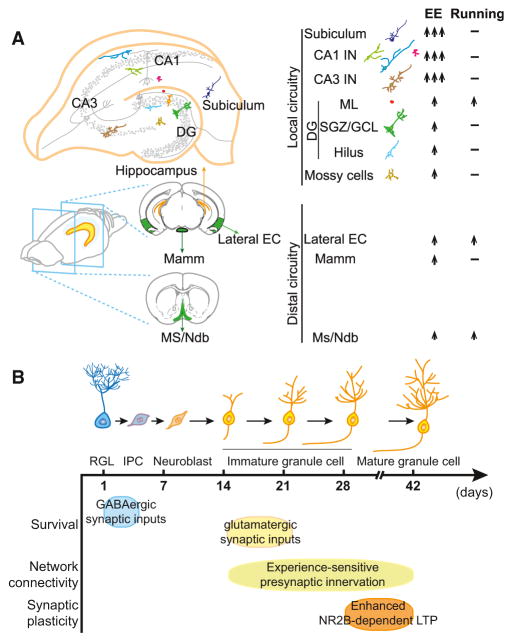
Knowing the presynaptic innervation pattern of adult-born neurons, Bergami and colleagues wanted to investigate, by using the same tracing technique, whether circuit integration is affected by an animal’s experience, like some other aspects of adult neurogenesis (Christian et al., 2014). The authors first confirmed in cultured neurons that altering neural activity does not directly affect the trans-synaptic spread of RABV, which empirically rules out the potential artifact caused by the virus tracing method and allows them to further examine the connectivity changes in response to different environmental regimes. Bergami et al. then subjected their experimental mice to a 4-week period of enriched environment (EE), which is known to promote adult neurogenesis in the dentate gyrus (Christian et al., 2014; Kempermann et al., 1997). Interestingly, they found when EE was presented 2 weeks after retrovirus transduction, both local and distal presynaptic innervation patterns of newly generated neurons were modified. They calculated the relative number of GFP+DsRed− cells in different regions as normalized to GFP+DsRed+ starter cells in the dentate gyrus. There was an approximate 2-fold increase in the number of GFP+DsRed− mossy cells and virtually all classes of local interneurons—in the hilus, the sub-granular zone (SGZ)/granule cell layer (GCL), and the molecular layer (ML)—suggesting increased innervation onto newborn neurons by mossy cells and local interneurons (Figure 1A). Moreover, a similar increase was found for distal projection neurons in the subcortical and cortical areas, such as cholinergic neurons in the medial septum/nucleus of diagonal band of Broca (MS/ Ndb), pyramidal neurons in the lateral entorhinal cortex (EC), and neurons in the mammillary body (Mamm) (Figure 1A). Consistent with this finding, newborn granule cells exhibited more mushroom dendritic spines. More strikingly, there was a dramatic increase in the number of GFP+DsRed− interneurons localized in CA1 and CA3 subregions of the hippocampus and projection neurons in subiculum, which were only occasionally detected in non-enriched animals (Figure 1A). These results suggest that EE not only modulates the quantity of synaptic inputs from different types of neurons but also recruits additional source of neurons. Do different experiences induce different remodeling of connectivity? Indeed, voluntary running, another paradigm that increases the number of adult-born dentate neurons (van Praag et al., 1999), also led to increased number of presynaptic partners, but with several differences as compared to EE mice. First, the seemingly de novo presynaptic innervations from CA1, CA3, and subiculum neurons were not found in running mice. Second, locally within the dentate gyrus, only an increase in the number of molecular layer interneurons was detected as compared to controls. Third, distal projections from Mamm were not altered (Figure 1A).
Figure 1. Experience-Dependent Regulation of Adult Hippocampal Neurogenesis.
(A) Summary of the connectivity changes in presynaptic innervations of adult-born neurons in the dentate gyrus. Left: Schematics showing major local (top) and distal (bottom) input origins. Right: Changes in response to enriched environment (EE) or voluntary running (Running) regimes. Up arrow represents increase; dash represents no change; multiple arrows indicate inputs that are largely undetectable under normal conditions. CA, Cornu Ammonis area; pp, perforant path; DG, dentate gyrus; M.L., molecular layer; SGZ/GCL, sub-granular zone/granule cell layer; MS/Ndb, medial septum/nucleus of diagonal band of Broca; EC, entorhinal cortex; Mamm, mammillary bodies. (B) Critical periods during adult hippocampal neurogenesis. RGL, radial glia-like neural stem cell; IPC, intermediate progenitor cell; LTP: long-term potentiation.
It has been proposed that adult-born neurons exhibit special properties during a critical period and are therefore capable of making unique contributions to brain functions (Ge et al., 2008). Initial studies have identified a critical period of 4 to 6 weeks after newborn neurons are born when they exhibit enhanced long-term potentiation (LTP) with higher potentiation amplitudes and a lower induction threshold (Ge et al., 2007; Schmidt-Hieber et al., 2004) (Figure 1B), a phenomenon also observed for adult-born neurons in the olfactory bulb (Nissant et al., 2009). Later studies showed that only a small proportion of newly generated neurons survive after birth, and their survival is regulated through distinct mechanisms during at least two critical periods. One is the first few days shortly after birth when the immature GABAergic synaptic inputs from parvalbumin-expressing interneurons are particularly beneficial for their survival (Song et al., 2013), while the other is between week 2 and 3, during which the NMDA (N-methyl-D-aspartate)-type glutamatergic receptor-mediated excitatory inputs become essential (Tashiro et al., 2006). Is there also a critical period for experience-dependent remodeling of the presynaptic connectivity of adult-born neurons? Bergami and colleagues’ results clearly demonstrate the existence of a window between 2 and 6 weeks after newborn neurons are born, only during which EE can impact the pre-synaptic inputs to newborn neurons but not during first 3 weeks or between 9 and 13 weeks (Figure 1B).
Bergami et al. have demonstrated once again the highly plastic nature of adult mammalian circuitry, including both de novo integration of newborn neurons and remodeling of mature neurons in different brain regions that synapse onto these newborn neurons. As in any interesting study, the current work raises important questions for future investigations. First, what is the functional impact of experience-induced changes in presynaptic partners of adult-born neurons? Bergami and colleagues showed that the altered number of local inter-neuron inputs returns to normal levels when animals are returned to a normal environment for 7 weeks after EE exposure, while the increase in cortical projections seems persistent. Does the transient change in presynaptic interneuron partners regulate development of adult-born neurons at immature stages? How will the permanently added cortical projections affect the existing circuitry and brain functions? In addition, local innervations from CA1, CA3 interneurons are only increased after EE exposure but not running. May the processing of increased spatial complexity in EE require more inhibitory feedback on newborn granule neurons from downstream regions? To address these questions, one certainly needs to first answer how adult-born neurons mechanistically contribute to brain functions, which remains the holy grail of the field and may require direct examination of newborn granule cells during behavior in vivo. Second, what is the mechanism underlying connectivity changes in response to experience? Does it depend on the activity of newborn neurons or presynaptic partners? Or is it simply a secondary effect of the altered systemic environment, for example, more secreted neurotrophic factors? Third, is the postsynaptic innervation of adult-born neurons to hilar neurons, CA3 neurons, and maybe others also experience dependent? The answer to these questions may facilitate a complete understanding of adult neurogenesis function. Forth, how do other experiences impact the integration process? For instance, does stress, which negatively regulates the generation and survival of newborn neurons (Christian et al., 2014), affect the number of presynaptic partners? It is of note that laboratory mice are typically housed in a non-enriched environment as compared to their natural habitats. Therefore, the observed pattern in EE mice may mimic the natural pattern in wild animals, while the laboratory environment renders it less complex. Other pathological conditions, such as epileptic seizure and disease-causing gene mutations (e.g., mutations in Fmrp or Disc1), all lead to aberrant development of adult-born neurons (Christian et al., 2014). Understanding how potential deficits in the connectivity of adult-born neurons under these conditions contribute to the brain dysfunctions will certainly be of broad interest to the society in general.
References
- Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Göbel J, Yang SM, Conzelmann K-K, Schinder AF, Götz M, et al. Neuron. 2015;85:710–717. doi: 10.1016/j.neuron.2015.01.001. this issue. [DOI] [PubMed] [Google Scholar]
- Christian KM, Song H, Ming GL. Annu Rev Neurosci. 2014;37:243–262. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A, Bergami M, Ghanem A, Conzelmann KK, Lepier A, Götz M, Berninger B. Proc Natl Acad Sci USA. 2013;110:E1152–E1161. doi: 10.1073/pnas.1218991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Sailor KA, Ming GL, Song H. J Physiol. 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Aimone JB, Xu X, Callaway EM, Gage FH. Proc Natl Acad Sci USA. 2012;109:4290–4295. doi: 10.1073/pnas.1120754109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissant A, Bardy C, Katagiri H, Murray K, Lledo PM. Nat Neurosci. 2009;12:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, Zhong C, Davoudi H, Christian KM, Toni N, et al. Nat Neurosci. 2013;16:1728–1730. doi: 10.1038/nn.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H. Nature communications. 2012;3:1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]