Abstract
Background
Pre‐operative weight loss has been consistently associated with increased post‐operative morbidity. The study aims to determine if pre‐operative oral nutritional supplements (ONSs) with dietary advice reduce post‐operative complications.
Methods
Single‐blinded randomized controlled trial. People with colorectal cancer scheduled for surgery with pre‐operative weight loss >1 kg/3–6 months were randomized by using stratified blocks (1:1 ratio) in six hospitals (1 November 2013–28 February 2015). Intervention group was given 250 mL/day ONS (10.1 KJ and 0.096 g protein per mL) and dietary advice. Control group received dietary advice alone. Oral nutritional supplements were administered from diagnosis to the day preceding surgery. Research team was masked to group allocation. Primary outcome was patients with one or more surgical site infection (SSI) or chest infection; secondary outcomes included percentage weight loss, total complications, and body composition measurements. Intention‐to‐treat analysis was performed with both unadjusted and adjusted analyses. A sample size of 88 was required.
Results
Of 101 participants, (55 ONS, 46 controls) 97 had surgery. In intention‐to‐treat analysis, there were 21/45 (47%) patients with an infection—either an SSI or chest infection in the control group vs. 17/55 (30%) in the ONS group. The odds ratio of a patient incurring either an SSI or chest infection was 0.532 (P = 0.135 confidence interval 0.232 to 1.218) in the unadjusted analysis and when adjusted for random differences at baseline (age, gender, percentage weight loss, and cancer staging) was 0.341 (P = 0.031, confidence interval 0.128 to 0.909). Pre‐operative percentage weight loss at the first time point after randomization was 4.1% [interquartile range (IQR) 1.7–7.0] in ONS group vs. 6.7% (IQR 2.6–10.8) in controls (Mann–Whitney U P = 0.021) and post‐operatively was 7.4% (IQR 4.3–10.0) in ONS group vs. 10.2% (IQR 5.1–18.5) in controls (P = 0.016).
Conclusions
Compared with dietary advice alone, ONS resulted in patients having fewer infections and less weight loss following surgery for colorectal cancer. We have demonstrated that pre‐operative oral nutritional supplementation can improve clinical outcome in weight losing patients with colorectal cancer.
Keywords: Colorectal, Supplements, Weight loss, Infections, RCT, Cancer
Introduction
Internationally, in terms of annual incidence, colorectal cancer is the third most common cancer in men and the second in women.1 Surgery, combined with either neo‐adjuvant or adjuvant chemotherapy and/or radiotherapy in selected patients, is the mainstay of curative treatment for colon and rectal malignancies.2 Malnutrition and weight loss have long been associated with an increased post‐operative morbidity and mortality.3, 4, 5 However, despite this, neither pre‐operative nutritional assessment nor nutritional screening is commonly practiced or integrated into the care pathways for patients with colorectal cancer.2
In pre‐operative patients with colorectal cancer, the prevalence of malnutrition has been reported as 36.4% by using subjective global assessment, whilst clinically significant or severe weight loss has been reported in 39%6 and any weight loss in 47% of people with colorectal cancer.7 The combination of a low muscle mass and impaired physical function referred to as sarcopenia8 has been identified in 12% of people with pre‐operative colorectal cancer by using computed tomography to measure muscle mass and is associated with older age, lower body mass index, and increased rate of post‐operative complications.9 A review of the literature on sarcopenia in abdominal malignancy concluded that sarcopenia is predictive of poorer clinical outcomes, increased morbidity, and increased hospital length of stay (LoS).10 Within an ‘enhanced recovery after surgery’ (ERAS) programme for colorectal cancer, patients who were malnourished were found to be at increased risk of post‐operative morbidity, delayed recovery of gastrointestinal function, and prolonged hospital stay.11 Thus, there is considerable evidence that pre‐operative malnutrition and sarcopenia are important prognostic factors for post‐operative complications.12 It is therefore reasonable to test whether or not nutritional interventions can decrease post‐operative morbidity to improve clinical endpoints. Enhanced recovery after surgery programmes is now widespread, and patients with colorectal cancer are now cared for in their homes for an increased length of time both pre‐operatively and post‐operatively because admission is often on the day of surgery and post‐surgical time to discharge has been drastically reduced.13 Decreased hospital LoS with ERAS programmes places more emphasis on pre‐operative and post‐operative supportive interventions that can be delivered in individuals' own homes.
Pre‐operative nutritional interventions in gastrointestinal surgery have been evaluated in a Cochrane review, which includes a number of studies on immune‐enhancing nutrition, oral nutritional supplements (ONSs), and parenteral nutrition.14 Studies have demonstrated a reduction in post‐operative complications with the use of pre‐operative immune‐enhancing nutrition.15 However, many of these studies include well‐nourished patients, excluding those on neo‐adjuvant anticancer therapy, and were conducted prior to the implementation of ERAS programmes.14 Studies that have looked at nutritional supplements in all participants undergoing colorectal surgery have demonstrated mixed results.16, 17
All trials of ONS compared with either standard care or dietary advice in those with colorectal cancer included a mixture of well‐nourished and malnourished participants.18 In one trial in people with colorectal cancer, a subgroup analysis of those who had lost weight demonstrated a reduction in surgical site infections (SSIs) in the ONS group compared with the controls.17 There is a paucity of evidence on ONS in people who have lost weight diagnosed with colorectal cancer during the pre‐operative period, although it has been repeatedly demonstrated in the evidence base that there is an association between a poor pre‐operative nutritional status and poor clinical outcomes.
The aim of this study was to determine if pre‐operative ONS with dietary advice, compared with dietary advice only, can reduce post‐operative infections in people prior to surgical resection for colorectal cancer who have previously lost weight.
Materials and Methods
In this multi‐centre, single‐blinded, randomized controlled trial, we studied people with colorectal cancer who had lost weight pre‐operatively to determine the effectiveness of oral nutritional supplementation. The protocol was amended after commencement to incorporate the comprehensive complication index (CCI),19 an extension to the widely used grading system of post‐operative complications.20 The protocol (version 6 August 2013) is available at www.manchester.ac.uk/research/sorrel.burden/publications.
Study participants
The sample was recruited from colorectal surgical clinics in six hospitals in the northwest of England from 1 November 2013 to 28 February 2015. All hospitals had an ERAS protocol in colorectal surgery in place.
Participants were recruited at colorectal clinics. Data collection took place in the participant's residence for baseline and pre‐operative time points and either on a hospital ward or participant's residence for the post‐operative visit. At baseline, the participants' characteristics were recorded along with nutritional status measurements. Baseline visits occurred when the participants agreed to be in the study within a couple of days of surgical teams, informing the participants that they were suitable for an operation. Participants were included in the study if they had a primary colorectal tumour, were over 18 years old, listed for radical surgery, had capacity for informed consent, and reported unintentional weight loss over the previous 3–6 months (>1 kg). This weight loss was based on a subgroup analysis from a previous trial that demonstrated in all participants with colorectal cancer who had lost weight a significant reduction in wound infections in the group receiving ONS compared with controls.17 Participants were excluded if they were pregnant or had a pacemaker precluding the use of bioelectrical impedance analysis (BIA), already on a similar nutritional supplement, or had insulin dependent diabetes.
Ethical approval and trial registration
This study has been approved by the National Research Ethics Service Committee Northwest (12/NW/0208) and been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All study participants provided written informed consent prior to inclusion. The trial was pre‐registered ISRCTN: NCT24668100.
Randomization
The participants were randomly allocated on a 1:1 ratio by using blocks of two ensuring equal numbers in each group. Allocation was stratified according to tumour site (rectal vs. colon) and surgical approaches (open vs. laparoscopic). Four lists of random numbers were produced by a statistician, and an independent researcher set up the randomization procedure for each of the strata. Sequentially numbered opaque sealed envelopes were used, which allowed block randomization sequence allocation to be implemented and ensured sequence allocation concealment. The participants were randomized to either dietary advice alone (control) or ONS and dietary advice (intervention). Identification and recruitment of participants were undertaken by National Institute Health Research cancer nurses or colorectal specialist nurses at each site. Randomization was undertaken after participants consented prior to the baseline measurements.
Intervention
The intervention comprised oral supplementation (Fortisip Compact®, 10.1 KJ, 0.096 g of protein per mL, Nutricia UK) at a dose of 250 mL daily. Supplements were started at the point of allocation of an operation date. A minimum of 5 days pre‐operative treatment was given. A sealed box containing sufficient supplements for 7 days was left with the participants randomized to the intervention for each week prior to the planned operation date. A mixture of vanilla and strawberry flavours were provided. Dietary advice was given to all participants in the form of a leaflet (see Supplementary Material), which was left with the participant and which formed the basis of a structured discussion with the research assistant (DG), a nutritionist, at the baseline visit. The dietary advice given aimed to increase energy and protein intake through dietary means by increasing the amount of high fat, sugar, and protein‐rich foods in the diet. The leaflet also recommended the use of dietary supplements high in energy and protein that could be purchased from high street retailers. All participants were advised of potential side effects of the ONS and advised to discontinue the intervention if adverse effects were experienced.
Controls
Following randomization, procedures for control group participants were identical to those for the intervention group. This group received the dietary leaflet and discussion with the nutritionist, and for the purposes of blinding, control participants were given sealed cardboard boxes of identical weight and appearance as the ONS group at the time of group allocation. These boxes contained bottled water in 125 mL bottles. Thus, the same quantity of either bottled water or ONS (14 bottles) was in each cardboard box. Similar to the intervention group, further supplies of water in sealed boxes were delivered as required up to 24 h prior to admission date for surgery. The research team was blind to the intervention, but the participants were not.
Adherence
All participants were asked to keep a diary of the drinks they consumed for each week prior to surgery after recruitment. They were asked how much and how frequently they consumed the drinks. This diary was then given back to the researcher in a sealed envelope and was not opened until the unblinding at the end of the trial.
Data collection
The participants were seen three times by the research assistant (DG) (i) at baseline, (ii) 24–48 h pre‐operatively, and (iii) 5–7 days post operatively. Nutritional status measurements were recorded at each time point, and clinical outcomes were recorded from Day 1 up to 30 days post operatively.
Outcomes
The primary outcome was patients with one or more chest or SSI defined by using the US Centre for Disease Control definitions.21 Secondary outcome measures included post‐operative complications recorded prospectively from the participants' medical records using a standard classification.20 This classification was used to determine the CCI.19 Hospital LoS was recorded from the day of surgery until date of discharge. The participants received a telephone interview 30 days after surgery, and any self‐reported potential complications were recorded and followed up in the participants' medical records, although primary care records were not accessed due to permission restrictions.
Nutritional status measurements were recorded as secondary outcomes. Weight was measured to the nearest 0.1 kg on calibrated scales (Seca 875 flat scale) with shoes removed. Height was measured by using a portable stadiometer to the nearest 0.1 cm (Harpenden pocket stadiometer Practical Metrology, Sussex, UK) with participants looking straight ahead and shoes removed. Height and weight were required to calculate baseline body mass index, percentage weight loss, fat‐free mass index (FFMI), and fat mass index (FMI) so are not reported as standalone measures. To calculate percentage weight loss, the participants were asked to recall their previous weight (3–6 months ago); this was used with actual weight recorded at all time points to determine percentage weight loss. Percentage weight loss is included as it is part of the criteria that is used to define malnutrition22 and has universally been used as a prognostic variable for predicting post‐operative outcome.6 Handgrip strength was measured by using the non‐dominant hand (Takei 5001 Grip Dynamometer Analogue). Three measurements were taken and the mean recorded. Bioelectrical impedance analysis was measured (Bodystat 1500 machine, Isle of Man, Bodystat Ltd) with participant being adequately hydrated and in a supine position. Fat‐free mass and fat mass measured by BIA were standardized to FFMI and FMI by dividing by height squared.23
The nutritional screening and assessment tools used were (i) patient‐generated subjective global assessment (PG‐SGA)24 and (ii) the malnutrition universal screening tool,25 which were completed during the baseline visit to the participant's home. Only PG‐SGA was recorded at subsequent visits. Dietary intake was assessed at each time point by using 24 h semi‐structured dietary recall method which was self‐reported. For the dietary recall, the participants were asked semi‐structured questions on all food and drink eaten in the previous day, including quantities of food consumed by using household measures, volumes of fluids, cooking methods, and ingredients in any recipes. For the dietary recalls, food portions were converted to grams and analysed by using Microdiet (Version 2, Downlee Systems Limited, UK) to estimate total energy and protein intakes. The energy and protein content of the ONS consumed for the corresponding day from the diaries was added to the nutrient content assessed by 24 h recall for the pre‐operative time point to estimate daily total energy and protein intakes in the intervention group. Quality of life data, anthropometric measurements, and data on adherence to nutritional aspects of ERAS protocols are available in the Supplementary Material.
Power and sample size
We calculated that 88 patients needed to complete the trial (44 in each arm) to meet the number required to detect a difference in infective complications (chest and SSI defined by CDC definitions21) using 80% power, alpha = 0.05 (based on p1 = 0.26 and p2 = 0.54 on one‐sided significance using χ 2 test of equal proportions).17 We allowed for 12% dropout or non‐completion and recruited 101 patients.
Data analysis
Means and standard deviations are used to describe normally distributed interval data, and for non‐normally distributed data, median and interquartile ranges (IQRs) are displayed. Categorical data are displayed by using numbers and percentages. Confidence intervals (CIs) were calculated for primary and secondary outcomes where appropriate. To determine if there were any effects from random differences at baseline from prognostic variables, a logistic regression model was used. The dependent variable was infections, and the covariants were age, gender, baseline percentage weight loss, and cancer staging. Adjusted and unadjusted models were performed. Differences between groups were determined by using independent Student's t‐tests for normally distributed interval data, and for skewed data, Mann–Whitney U‐tests were performed. For dichotomous variables, χ 2 or Fisher's exact tests were used. For nominal data, Kruskal–Wallis test was used to determine the differences between groups. Data were analysed by using SPSS version 22.26 There were no interim stopping guidelines for this trial.
Results
CONSORT diagram
We recruited and randomly allocated 101 patients, of whom 96 completed the trial. The CONSORT flow diagram of the participants through the trial is shown in Figure 1. One participant withdrew consent from the control group prior to the pre‐operative visit. Participant characteristics and baseline measurements are shown in Table 1. Overall, there were more participants in the intervention group. At the point of recruiting participants, if it was undecided if surgery was open or laparoscopic, the default used was open‐surgery stratum for randomization. The two arms of the trial were well matched with similar proportions of participants within each stratum, site of cancer, and type of operation (laparoscopic or open). Data were complete for the majority of baseline assessments, and missing data were recorded (Table 1). The trial was discontinued when recruitment numbers were sufficient to meet the power equations for both arms.
Figure 1.
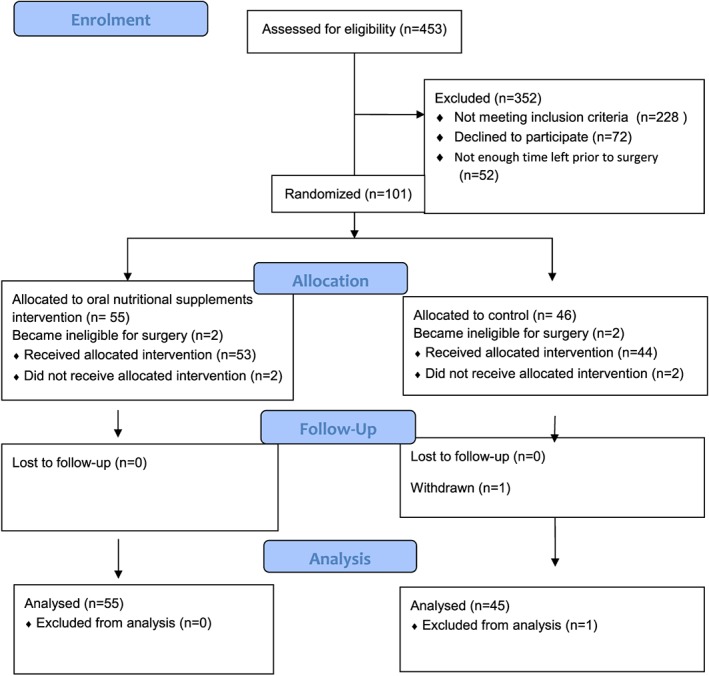
CONSORT flow diagram.
Table 1.
Participants' characteristics and baseline clinical details
| Control | Intervention | |
|---|---|---|
| Dietary advice only | ONS and dietary advice | |
| Randomization | n = 46 | n = 55 |
| Age mean (SD) | 68.9 (11.49) | 70.5 (11.66) |
| Gender n (%) | ||
| Male | 32 (70) | 35 (64) |
| Female | 14 (30) | 20 (36) |
| Occupation n (%) | ||
| Professional | 13 (28) | 19 (35) |
| Skilled | 16 (35) | 19 (35) |
| Unskilled | 14 (30) | 12 (22) |
| Unemployed | 0 (0) | 2 (4) |
| Missing | 3 (7) | 3 (5) |
| Site of surgery n (%) | ||
| Colon | 29 (63) | 35 (64) |
| Rectum | 17 (37) | 20 (36) |
| Type of surgery n (%) | ||
| Laparoscopic | 30 (65) | 37 (67) |
| Open | 16 (35) | 18 (33) |
| Smoking status n (%) | ||
| Never | 12 (26) | 27 (49) |
| Ex smoker | 21 (45) | 18 (33) |
| Current | 9 (20) | 10 (18) |
| Missing | 4 (9) | 0 (0) |
| Body mass index | ||
| Mean (SD) | 25.5 (4.54) | 25.9 (4.8) |
| Missing 0 | ||
| Percentage weight loss | ||
| Median (IQR) | 6.8 (3.4–12.1) | 4.90 (2.2–8.8) |
| Missing 0 | ||
| MUST n (%) | ||
| 0 | 13 (28) | 28 (51) |
| 1 | 16 (35) | 13 (24) |
| 2 | 14 (30) | 9 (16) |
| 3 | 1 (2) | 1 (2) |
| 4 | 0 (0) | 4 (7) |
| Missing 0 | 2 (4) | |
| Handgrip strength | ||
| mean (SD) | 24.9 (9.3) | 25.0 (10.6) |
| Missing | 4 | 0 |
| Patient‐generated SGA median (IQR) | 9 (4–12) | 6 (4–10) |
| Missing 0 | ||
| Cancer staging n (%) | ||
| I | 1 (2) | 2 (4) |
| II | 4 (9) | 9 (16) |
| III | 26 (56) | 26 (47) |
| IV | 12 (26) | 11 (20) |
| Villous adenoma | 1 (2) | 1 (2) |
| Missing | 1 (2) | 4 (7) |
| Did not have surgery | 2 (4) | 2 (3) |
| Anaesthetic risk score n (%) | ||
| Normal health | 5 (11) | 2 (4) |
| Mild systemic disease | 19 (41) | 24 (44) |
| Severe systemic disease | 8 (17) | 7 (13) |
| Missing data | 14 (30) | 22 (40) |
| Neo‐adjuvant treatment | ||
| Radiotherapy short course | 11 (23) | 15 (27) |
| Chemotherapy short course | 6 (13) | 7 (18) |
| Chemotherapy long course | 2 (4) | 3 (5) |
| Number of comorbidities n (%) | ||
| 0 | 6 (13) | 15 (27) |
| 1 | 7 (15) | 8 (15) |
| 2 | 12 (26) | 9 (16) |
| 3 | 4 (9) | 9 (16) |
| More than 4 | 8 (17) | 8(15) |
| Missing | 9 (20) | 6 (11) |
IQR, interquartile range; MUST, malnutrition universal screening tool; ONS, oral nutritional supplement; SGA, subjective global assessment.
Primary outcome
The data on the primary outcome of infectious complications on an intention‐to‐treat analysis are shown in Table 2. The odds of a patient having a chest or an SSI in the ONS group compared with the control group was 0.532 (P = 0.135, CI 0.232 to 1.218) with an unadjusted analysis. However, when adjusted for random effects at baseline, the odds of a patient in the ONS group compared with the controls of having an infection (either chest or surgical site) was 0.341 (P = 0.031 CI 0.128 to 0.909). Each type of infection was evaluated and shown in Table 3. A significant difference was demonstrated between the ONS group and controls for SSI (odds ratio 0.41, CI 0.16 to 1.00, χ 2 P = 0.044) with a lower rate of SSI in the intervention arm compared with the control (20 vs. 38%). However, there was no difference for chest infections (Fisher's exact P = 0.359).
Table 2.
Logistic regression showing adjusted and unadjusted analyses for primary outcome (patients with one or more infections either a chest or surgical site) as dependent variable and independent variables age, gender, cancer staging, baseline percentage weight loss, and treatment group
| Unadjusted odds ratio | P‐value | 95% CI | Adjusted odds ratio | P‐value | 95% CI | |
|---|---|---|---|---|---|---|
| Weight loss (%) | 0.952 | 0.182 | 0.885 to 1.024 | 0.922 | 0.059 | 0.848 to 1.003 |
| Age (years) | 0.998 | 0.928 | 0.964 to 1.034 | 0.998 | 0.920 | 0.956 to 1.041 |
| Gender | 1.500 | 0.366 | 0.623 to 3.613 | 0.976 | 0.963 | 0.347 to 2.747 |
| TNM staging | ||||||
| Stage 1 | 4.250 | 0.341 | 0.216 to 83.517 | 8.903 | 0.239 | 0.234 to 338.3 |
| Stage 2 | 8.500 | 0.112 | 0.609 to 118.637 | 11.113 | 0.079 | 0.760 to 162.5 |
| Stage 3 | 2.361 | 0.275 | 0.505 to 11.049 | 3.034 | 0.185 | 0.588 to 15.662 |
| Stage 4 | 4.250 | 0.020 | 1.252 to14.427 | 5.223 | 0.013 | 1.420 to 19.220 |
| Treatment group (ONS/control) | 0.532 | 0.135 | 0.232 to 1.218 | 0.341 | 0.031 | 0.128 to 0.909 |
CI, confidence interval; ONS, oral nutritional supplement; TNM, staging, tumour, nodal, metastases. Hosmer and Lemeshow test P = 0.792 for adjusted analysis.
Table 3.
Intention to treat analysis for number of participants with chest, surgical site, or urinary tract infections
| Control | Intervention | ||||
|---|---|---|---|---|---|
| n = 45(%) | 95% CI | n = 55(%) | 95% CI | P‐value | |
| Surgical site infection | 17 (38) | 25.1 to 52.4 | 11 (20) | 11.6 to 32.4 | a0.044 |
| Chest infection | 3 (7) | 2.3 to 17.9 | 5 (9) | 3.9 to 19.6 | b0.359 |
| Urinary tract infection | 6 (13) | 6.3 to 26.2 | 4 (7) | 2.9 to 17.3 | a0.315 |
CI, confidence interval.
χ 2.
Fisher's exact test.
Secondary outcomes
On intention to treat analysis for total complications, there were no significant differences demonstrated. A total of 48 participants had a complication: 23/55 (42%) in the intervention group and 25/45 (56%) in the control group (χ 2 P = 0.114). For complications graded I or II, there were 13/55 (24%) in the intervention group and 11/45 (24%) in the control group, and for grades III to IVb, there were 9 (16%) and 10 (22%) complications in the ONS and control groups, respectively. A total of five people died, one in the ONS and four in the control (P > 0.05). The median CCI score was 29.6 (IQR 20.9–47.3) and 29.6 (IQR 20.9–43.3) for the intervention and the control groups, respectively (Mann–Whitney U P = 0.984). Hospital LoS was recorded for 92 participants, and in the ONS group, the median LoS was 7 days (IQR 4.0–10.5) and in the controls group also 7 days (IQR 4.0–10.0; Mann–Whitney U P = 0.630). Multivariate analysis for complications and LoS are included in the Supplementary Material but showed no difference between groups with regard to infections, although unsurprisingly, there was a significant effect from disease staging (Table S13).
Nutritional status and body composition
Nutritional status measurements are shown in Table 4. There was a significant difference in the percentage of weight lost between groups both pre‐operatively (intervention 4.1% IQR 1.7–7.0 vs. controls 6.7% IQR 2.6–10, P = 0.021) and post‐operatively (intervention 7.4% IQR 4.3–10 vs. controls 10.2% IQR 5.1–18.5, P = 0.016). However, there was no significant difference observed for the other measures of nutritional status recorded nor handgrip strength or PG‐SGA (P > 0.05 in all cases). The changes in measurements among baseline, pre‐operative, and post‐operative time points for BIA are shown in Table 5, and descriptive statistics for BIA with between‐group comparisons are in the supplementary material (Table S12). There were no differences in BIA between groups at the pre‐operative or post‐operative time points. However, for the mean difference between the intervention and the control from baseline to pre‐operative time points for FFMI (ONS group −0.345 kg/m2, IQR −3.241 to 0.160 vs. control group 0.100 kg/m2, −0.520 to 0.3150 Mann–Whitney U P = 0.008), there was a significant difference but not for FMI (ONS group 0.105 kg/m2, IQR −0.170 to 0.315 vs. control group −0.150 kg/m2 IQR −0.310 to 0.135 P = 0.083).
Table 4.
Nutritional status measurements and screening and assessment tools in control and intervention groups
| 24–48 h pre‐operative | 5–7 days post‐operative | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Control | ONS | P‐value | n | Control | ONS | P‐value | |
| Handgrip mean (SD) | 70 | 25.0 (8.52) | 25.2 (10.07) | 0.723 | 70 | 23.2 (7.85) | 24.9 (9.89) | a0.394 |
| Percentage weight loss median (IQR) | 73 | 6.7 (2.6–10.8) | 4.1 (1.7–7.0) | 0.021 | 79 | 10.2 (5.1–18.5) | 7.4 (4.3–10.0) | b0.016 |
| PG‐SGA score median (IQR) | 69 | 6.5 (3.0–9.7) | 4.0 (2.0–9.0) | 0.215 | 72 | 12.0 (8.0–15.0) | 10.0 (6.5–13.5) | b0.062 |
IQR, interquartile range; ONS, oral nutritional supplement; PG‐SGA, patient‐generated subjective global assessment.
Independent Student's t‐tests.
Mann–Whitney U‐test.
Table 5.
Changes in bioelectrical impedance analysis between baseline and measurements at pre‐operative and post‐operative time points
| Difference between baseline and pre‐operative measurements (n = 69) | Difference between baseline and post‐operative measurements (n = 64) | |||||
|---|---|---|---|---|---|---|
| Controls | ONS | P‐value | controls | ONS | P‐value | |
| Fat‐free mass index kg/m2 median (IQR) | 0.100 (−0.520, 0.3150) | −0.345 (−3.241, 0.160) | 0.008a | 0.720 (−0.190, 1.865) | 0.080 (−1.010, 0.960) | 0.100 |
| Fat mass index kg/m2 median (IQR) | −0.150 (−0.310, 0.135) | 0.105 (−0.170, 0.315) | 0.083a | 0.200 (−0.220, 0.885) | 0.410 (−0.100, 1.090) | 0.242 |
IQR, interquartile range; ONS, oral nutritional supplement.
Mann–Whitney U‐test.
Dietary intake
Data for self‐report energy and protein intake by using 24‐h recall are shown in Table 6.
Table 6.
Dietary intake at each time point for energy and protein intakes, including additional nutrition from oral nutritional supplements at pre‐operative time point
| Energy (KJ) | Protein (g) | |||||
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | |||||
| Time point | Control | ONS | P‐value | Control | ONS | P‐value |
| n = participants | ||||||
| Baseline | 6085(4743–7493) | 6407 (4233–8193) | 0.760 | 68 (48–83) | 57 (41–76) | 0.271 |
| n = 93 | ||||||
| Pre‐operative | 6350 (4714–6350) | 8120 (6490–9831) | 0.001 | 63 (49–78) | 79 (67–97) | 0.018 |
| n = 70 | ||||||
| Post‐operative | 4499 (3218–6416) | 5302 (3973–7173) | 0.282 | 46 (31–70) | 60 (43–70) | 0.181 |
| n = 89 | ||||||
IQR, interquartile range; ONS, oral nutritional supplement.
Mann–Whitney U‐tests.
Provision and adherence of ONS
Oral nutritional supplements were provided to the participants for a median of 8 days (IQR 5–15). Of the 53 participants in the intervention arm, 39 (74%) returned a diary detailing their adherence to the ONS. For participants who returned a diary, 29 (74%) participants managed all the supplements (two cartons daily), 2 (5%) participants reported that they managed one and a half cartons, 3 (8%) participants reported that they managed one carton, 3 (8%) managed half a carton, and 2 (5%) participants reported that they did not consume any of the ONS. Intolerance of ONS was reported by seven participants who did not manage to follow the ONS regimen; these included nausea reported by four participants, abdominal discomfort reported by three participants, and diarrhoea reported by two (two participants reported more than one intolerance symptoms). Thus, seven participants reported that they did not tolerate the supplements due to unpalatability.
Blood loss and duration of operation were included as variables at baseline but were missing in the majority of instances in medical records, so therefore are not reported.
Discussion
This is the first single‐blind randomized controlled trial of pre‐operative ONS based on standard nutritional recommendations27 in people with colorectal cancer who have lost weight. We demonstrate on intention‐to‐treat analysis significantly fewer infections in the ONS and dietary advice group compared with controls who received dietary advice alone. Prior to surgery, the participants who received ONS with dietary advice lost significantly less weight, and this difference was maintained post‐operatively. Percentage weight loss has long been regarded as a prognostic indicator for post‐operative morbidity,28 and this study demonstrates that a nutritional intervention is clearly linked to fewer post‐operative infectious complications. The primary outcome was participants who incurred one or more infections (chest or surgical site) post operative.
It is notable in the analysis of BIA data that the participants who lost weight had less of a difference in muscle lost between baseline and pre‐operative time points. This suggests that when people with colorectal cancer lose weight, they are losing fat‐free mass. Fat‐free mass is commonly used as a surrogate marker for skeletal muscle mass.29 Reduction in skeletal muscle mass is linked to sarcopenia, which is known to be associated with reduced function and increased frailty in older people.28 This is important as sarcopenia has been consistently linked to a poorer post‐operative outcome and is also known to impact negatively on other cancer therapies.30 Preventing weight loss and subsequent skeletal muscle mass would therefore seem to be a logical therapeutic strategy, especially given that it can be achieved by using a relatively inexpensive nutritional intervention that is easy to administer in the community pre‐operatively.
The compliance rates for such an intervention in this trial are more than acceptable with two‐thirds of participants managing more than 75% of the recommended dose. Most participants who were randomized to the ONS were able to consume some of the drink, and we recorded a 71% adherence rate. This supports the argument that weight loss in colorectal cancer during the perioperative period is preventable, and the benefits of increasing nutritional intake pre‐operatively are sustained throughout the perioperative period.
Oral nutritional supplementation was effective at increasing the nutritional intake of the participants randomized to the intervention in relation to energy and protein. In this study, we did not look at the micronutrient profile of the participants' intake, although this may be important if subclinical levels or deficiencies of vitamins and minerals are present prior to surgery. The ONS used in this study contained a full profile of vitamins and minerals in amounts proportionate to the macronutrient composition. So, it is unclear if it were either macronutrients in ONS that are having a positive effect or indeed the mixture of nutrients within the substrate. Micronutrients have been shown to be important in the perioperative period,31 and there are some vitamins and minerals which may be deficient particularly in older people.32 It is also of note that ONSs supplemented with immune‐enhancing agents administered pre‐operatively have resulted in a reduction in infections in patients with colorectal cancer.33
People with colorectal cancer lose weight due to symptom load, psychological distress, and adjuvant treatment effects.34 Weight loss in the perioperative period influences people's lives during recovery and post‐operative rehabilitation.34 Other researchers have highlighted that nutritional information is a priority for individuals and their families.35 In a recent study of body composition, only 5% of people with colorectal cancer were found to be cachexic based on measurements of fat‐free mass pre‐operatively, suggesting that weight loss is a treatable consequence of colorectal cancer.36 In this study, the participants recruited had lost more than 1 kg of weight. This was based on a subgroup analysis of a previous trial, which showed that all weight‐losing participants who were randomized to receive ONS compared with controls had significantly fewer wound infections.
This is the first single‐blind trial that we are aware of with ONS based on standard nutritional requirements in people with colorectal cancer. Improvements to decrease bias in future would be to double blind the trial so both participants and researchers are unaware of the allocation. Whilst this design was considered for this trial, it was not possible to obtain a non‐active drink packaged as a placebo. The initial power equations indicated that there should be a 28% difference in chest and SSIs between groups, although the actual difference was 21%. This is most likely due to evolving practice in surgery‐improving perioperative management as there has been the introduction of ERAS programmes and also an increase in laparoscopic surgery, resulting in fewer complications overall.37, 38 Nutrition is a supportive therapy in surgical oncology, and studies evaluating supportive therapies are subject to confounding factors that include technological developments and procedural alterations to the primary therapy as well as patient variables. This trial was designed to determine effectiveness and therefore recruited participants at the time point that was appropriate for each individual within the care pathway. This meant that the participants were given ONS for different lengths of time pre‐operatively. Future trials could standardize the length of time participants received ONS but also account for other confounders such as neo‐adjuvant therapies by stratification because these factors also influence pre‐operative management. Interestingly, the regression analysis showed that disease staging had a significant effect on infections and also on patients with one or more complications. This would therefore be a factor to consider in future trials.
The limitations of the study include reliance on participant recall to determine weight loss over 3–6 months which may be subject to bias. However, there is no reason to believe that this potential source of bias was differentially distributed across groups. There were also random differences in baseline variables which included differences in percentage weight loss and staging of disease, albeit these were adjusted for in the analysis. Also, individuals were required to fill in diaries to assess adherence, again potentially subject to reporting bias. An alternate method of determining adherence is to collect the empty cartons, or it may be preferable to develop a medication event monitoring system, although such systems are costly and themselves have disadvantages.39 Dietary assessment was undertaken by 24 h recall which only allows dietary intake to be assessed over a short period. However, a reasonable level of validity has been demonstrated with trained nutritionists and with the assessment of macronutrients.40 Technological developments surrounding the use of smartphone applications and user‐friendly databases offer more options in future trials to directly record dietary intake for nutritional analyses.
We aimed to collect data pre‐operatively and post‐operatively, although the participants were reluctant in some instances to see the researcher immediately pre‐operatively, possibly due to anxiety levels about impending surgery. Likewise, some were reluctant post‐operatively as they were still recovering from surgery. This led to some missing data at these time points. Future research may need to consider dropout rates in the region of 20–25%. Alternative outcomes which are part of routine care may be used to determine nutritional status or body composition.36 This could include the use of computed tomography for body composition and using food diaries undertaken as part of ERAS programmes.
The findings of this RCT are encouraging in that we demonstrate a reduction of post‐operative infections by pre‐operative nutritional supplementation. This supports the use of ONS in people who have lost weight prior to surgery for colorectal cancer and concurs with other research promoting pre‐operative optimization or prehabilitation.41 Nutritional optimization of people with colorectal cancer should start with nutritional screening by using a validated screening tool25 then implementing nutrition intervention into pre‐operative assessment protocols. The opportunities for nutritional interventions should be recognized for patients having neo‐adjuvant treatments as their pre‐operative duration is longer than for patients proceeding directly to surgery.
On the basis of this trial, it seems likely that ONS increases energy and protein intakes, which results in less perioperative weight loss and preservation of skeletal muscle mass, resulting in a positive effect on clinical outcome in people who were otherwise losing weight prior to surgery for colorectal cancer.
Conflict of interest
Dr Sorrel Burden received a travel grant received to attend a scientific meeting from Nutricia UK in 2013.
All other authors have no conflict of interests to declare.
Supporting information
Supplementary Material
jcsm12170‐sup‐0002‐build yourself up patient booklet.pdf supporting info item
Supporting info item
Supporting info item
Acknowledgements
The authors would like to thank the participants for taking part and the oncology research nurse (J Allsop) and colorectal specialist nurses (D Hitchen, M Parker, H Ashby, and D West) for assistance with recruiting participants. The authors would like to thank Nutricia UK for the provision of supplements for the trial.
All authors contributed to the study design. Data collection was undertaken by SB and DG, and analysis was undertaken by SB supported by MP. All authors contributed to interpretation of results and approved the final manuscript. All authors approved the final manuscript.
ClinicalTrials.gov NCT24668100.
The trial was pre‐ registered ISRCTN: NCT24668100. This study has been approved by the National Research Ethics Service Committee Northwest (12/NW/0208) and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All study participants provided written informed consent prior to inclusion. The authors certify that they comply with the ethical guidelines for Publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015.42
This study was funded by Macmillan Cancer Support and British Dietetics Association.
Burden, S. T. , Gibson, D. J. , Lal, S. , Hill, J. , Pilling, M. , Soop, M. , Ramesh, A. , and Todd, C. (2017) Pre‐operative oral nutritional supplementation with dietary advice versus dietary advice alone in weight‐losing patients with colorectal cancer: single‐blind randomized controlled trial. Journal of Cachexia, Sarcopenia and Muscle, 8: 437–446. doi: 10.1002/jcsm.12170.
Reference
- 1. Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray, F . GLOBOCAN 2012 v1.0,Cancer Incidence and Mortality Worldwide. In: [Internet]. ICN, ed. Available from: http://globocan.iarc.fr,: accessed 27/11/2015 2013. [DOI] [PubMed]
- 2. National Institute for Clinical Excellence . Colorectal cancer: the diagnosis and management of colorectal cancer. NICE Guideline 131 guidance.nice.org.uk/cg131: Accessed 22 October 2014; 2011.
- 3. Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post‐operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet 2010;23:393–401. [DOI] [PubMed] [Google Scholar]
- 4. Hardy SE, Dubin JA, Holford TR, Gill TM. Transitions between states of disability and independence among older persons. Am J Epidemiol 2005;161:575–584. [DOI] [PubMed] [Google Scholar]
- 5. Schwegler I, von Holzen A, Gutzwiller JP, Schlumpf R, Muhlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg 2010;97:92–97. [DOI] [PubMed] [Google Scholar]
- 6. Barbosa LR, Lacerda‐Filho A, Barbosa LC. Immediate preoperative nutritional status of patients with colorectal cancer: a warning. Arq Gastroenterol; 51:331–336. [DOI] [PubMed] [Google Scholar]
- 7. Burden ST, Hill J, Shaffer JL, Todd C. Nutritional status of preoperative colorectal cancer patients. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association 2010;23:402–407. [DOI] [PubMed] [Google Scholar]
- 8. Muscaritoli M, Anker SD, Argil J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by special interest groups (SIG) “cachexia–anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 9. Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis 2015;17:O256–O264. [DOI] [PubMed] [Google Scholar]
- 10. Gibson DJ, Burden ST, Strauss BJ, Todd C, Lal S. The role of computed tomography in evaluating body composition and the influence of reduced muscle mass on clinical outcome in abdominal malignancy: a systematic review. Eur J Clin Nutr 2015;10:1079–1086. [DOI] [PubMed] [Google Scholar]
- 11. Lohsiriwat V. The influence of preoperative nutritional status on the outcomes of an enhanced recovery after surgery (ERAS) programme for colorectal cancer surgery. Tech Coloproctol 2014;18:1075–1080. [DOI] [PubMed] [Google Scholar]
- 12. Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewe KW, Hoofwijk AG, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg; 261:345–352. [DOI] [PubMed] [Google Scholar]
- 13. Nygren J, Soop M, Thorell A, Hausel J, Ljungqvist O. An enhanced‐recovery protocol improves outcome after colorectal resection already during the first year: a single‐center experience in 168 consecutive patients. Journal of the colon and rectum 2009;52:978–985. [DOI] [PubMed] [Google Scholar]
- 14. Burden S, Todd C, Hill J, Lal S. Pre‐operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev 2012;11:CD008879. [DOI] [PubMed] [Google Scholar]
- 15. Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology 2002;122:1763–1767. [DOI] [PubMed] [Google Scholar]
- 16. Smedley F, Bowling T, James M, Stokes E, Goodger C, O'Connor O, et al. Randomized clinical trial of the effects of preoperative and postoperative oral nutritional supplements on clinical course and cost of care. Br J Surg 2004;91:983–990. [DOI] [PubMed] [Google Scholar]
- 17. Burden ST, Hill J, Shaffer JL, Campbell M, Todd C. An unblinded randomised controlled trial of preoperative oral supplements in colorectal cancer patients. J Hum Nutr Diet 2011;24:441–448. [DOI] [PubMed] [Google Scholar]
- 18. Burden S, Todd C, Hill J, Lal S. Preoperative nutrition in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev 2012;11:CD008879. [DOI] [PubMed] [Google Scholar]
- 19. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien P‐A. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1–7. [DOI] [PubMed] [Google Scholar]
- 20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 22. National Institute for Health and Clinical Excellence . Clinical Guideline 32 Nutrition support in Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. London: National Institute for Clinical Excellence; 2006. [PubMed] [Google Scholar]
- 23. VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height‐normalized indices of the body's fat‐free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr 1990;52:953–959. [DOI] [PubMed] [Google Scholar]
- 24. Bauer JCS, Ferguson M. Use of the scored patient‐generated subjective global assessment (PG‐SGA) as a nutrition assessment. Eur J Clin Nutr 2002;56:779–785. [DOI] [PubMed] [Google Scholar]
- 25. Elia M, Chairman of MAG. The MUST Report: Nutritional screening of adults Redditch Worcs: BAPEN 2003.
- 26. IBM.Corp . SPSS Statistics for Windows. New York Armonk Released 2011.
- 27. The Commission of the European Communities March 1999 on dietary foods for special medical purposes. Commission Directive 1999 21 EC of 25 Official Journal of the European Communities 1999.
- 28. Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr 2009;48:78–83. [DOI] [PubMed] [Google Scholar]
- 29. Schutz Y, Kyle UUG, Pichard C. Fat‐free mass index and fat mass index percentiles in Caucasians aged 18–98 y. International Journal of Obesity & Related Metabolic Disorders 2002;26:953–960. [DOI] [PubMed] [Google Scholar]
- 30. Baracos V, Kazemi‐Bajestani SMR. Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int J Biochem Cell Biol 2013;45:2302–2308. [DOI] [PubMed] [Google Scholar]
- 31. Sriram K, Perumal K, Alemzadeh G, Osei A, Voronov G. The relationship between immediate preoperative serum 25‐hydroxy‐vitamin D3 levels and cardiac function, dysglycemia, length of stay, and 30‐d readmissions in cardiac surgery patients. Nutrition 2015;31:820–826. [DOI] [PubMed] [Google Scholar]
- 32. ter Borg S, Verlaan S, Hemsworth J, Mijnarends DM, Schols JMGA, Luiking YC, et al. Micronutrient intakes and potential inadequacies of community‐dwelling older adults: a systematic review. Br J Nutr 2015;113:1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moya P, Miranda E, Soriano‐Irigaray L, Arroyo A, Aguilar M‐d‐M, Bellón M, et al. Perioperative immunonutrition in normo‐nourished patients undergoing laparoscopic colorectal resection. Surg Endosc 2016;1–8. [DOI] [PubMed] [Google Scholar]
- 34. Burden ST, Stamataki Z, Hill J, Molasiotis A, Todd C. An exploration of food and the lived experience of individuals after treatment for colorectal cancer using a phenomenological approach. J Hum Nutr Diet 2016;29:137–145. [DOI] [PubMed] [Google Scholar]
- 35. Short V, Atkinson C, Ness AR, Thomas S, Burden S, Sutton E. Patient experiences of perioperative nutrition within an enhanced recovery after surgery programme for colorectal surgery. Colorectal Dis 2016;18:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gibson DJ, Lal S, Strauss B, Todd C, Pilling M, Burden S. Computed tomography compared with standard clinical measurements to assess body composition, facilitating the identification of sarcopenia and cachexia in colorectal malignancy. J Cachexia Sarcopenia Muscle 2015;6:69. [Google Scholar]
- 37. Rawlinson A, Kang P, Evans J, Khanna A. A systematic review of enhanced recovery protocols in colorectal surgery. The Annals of The Royal College of Surgeons of England 2011;93:583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. The Colon cancer Laparoscopic or Open Resection Study Group . Laparoscopic surgery versus open surgery for colon cancer: short‐term outcomes of a randomised trial. Lancet Oncol 2005;6:477–484. [DOI] [PubMed] [Google Scholar]
- 39. Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 1999;21:1074–1090. [DOI] [PubMed] [Google Scholar]
- 40. Bingham SA, Gill C, Welch A, Day K, Cassidy A, Khaw KT, et al. Comparison of dietary assessment methods in nutritional epidemiology: weighed records v. 24 h recalls, food‐frequency questionnaires and estimated‐diet records. Br J Nutr 1994;72:619–643. [DOI] [PubMed] [Google Scholar]
- 41. Carli F, Scheede‐Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin 2015;33:17–33. [DOI] [PubMed] [Google Scholar]
- 42. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
jcsm12170‐sup‐0002‐build yourself up patient booklet.pdf supporting info item
Supporting info item
Supporting info item


