Abstract
Background
The association between C‐reactive protein (CRP) level, symptoms, and activities of daily living (ADL) in advanced cancer patients is unclear.
Methods
Secondary data analysis of a multicenter prospective cohort study consisted of 2426 advanced cancer patients referred to palliative care settings was conducted to examine the cross‐sectional relationships between CRP level, symptoms, and ADL disabilities. Laboratory data, symptoms, ADL, and manual muscle testing (MMT) results were obtained at baseline. Participants were divided into four groups: low (CRP < 1 mg/dl), moderate (1 = < CRP <5 mg/dl), high (5 = < CRP < 10 mg/dl), and very high CRP (10 mg/dl = < CRP). The proportions of eight symptoms, five ADL disabilities, and three categories of MMT according to the CRP groups were tested by chi‐square tests. Multiple‐adjusted odd ratios (ORs) were calculated by using ordinal logistic regression after adjustment for age, gender, site of primary cancer, metastatic disease, performance status, chemotherapy, and setting of care.
Results
A total of 1702 patients were analysed. Positive rates of symptoms and ADL disabilities increased with increasing CRP level. In the very high‐CRP group, rates of positivity for anorexia, fatigue, and weight loss were 89.8%, 81.0%, and 79.2%, respectively, and over 70% of patients received assistance for bathing, dressing, going to the toilet, and transfer. The grade of MMT also deteriorated with increasing CRP level. Adjusted ORs for the accumulated symptoms significantly increased with increasing CRP level in the moderate‐CRP, high‐CRP, and very high‐CRP groups [1.6 (95% confidence interval 1.2–2.0), P < 0.001; 2.5 (1.9–3.2), P < 0.001; 3.5 (2.7–4.6), P < 0.001, respectively]. Adjusted ORs for the accumulated ADL disabilities significantly increased in the very high‐CRP groups [2.1 (1.5–2.9), P < 0.001].
Conclusions
Associations between CRP level, symptoms, and ADL were observed in advanced cancer patients receiving palliative care.
Keywords: Advanced cancer patients, Palliative care, C‐reactive protein, Symptoms, Activities of daily living, Cancer cachexia
Introduction
The level of C‐reactive protein (CRP) has been reported to be a good prognostic marker in advanced cancer patients.1, 2, 3 It is elevated in advanced cancer patients as well as in those with chronic inflammatory disease1, 2, 3, 4, 5, 6 and has been shown to be related to an increased cancer risk,1, 2, 7 anorexia, weight loss, fatigue, and pain in cancer patients.8, 9, 10, 11
The synthesis of CRP in hepatocytes is induced by pro‐inflammatory cytokines, such as interleukin‐6 (IL‐6), IL‐1, and tumour necrosis factor α, as chronic systemic inflammation. These cytokines play major roles in the mechanism of cancer cachexia, and cancer cells may rely on them for growth, protection from apoptosis, and the promotion of angiogenesis/metastasis.4, 5 Cachexia, which is characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass), aggravates the symptoms and activities of daily living (ADL) of advanced cancer patients.4, 5 It is responsible for increased morbidity, reduced survival, and impinged quality of life in cancer patients.12, 13
To the best of our knowledge, there have been no large prospective studies investigating the clinical implications of CRP as a biomarker in advanced cancer patients in a variety of palliative settings including palliative care units, hospital palliative care teams, and home palliative care services, as well as in patients receiving chemotherapy. This cross‐sectional study was, therefore, designed to investigate the association between CRP level, symptoms, and ADL in advanced cancer patients receiving palliative care.
Methods
Sites and participants
This study involves a subanalysis of a multicenter prospective cohort study conducted in 58 palliative care services in Japan from September 2012 through April 2014.14, 15 The participating units included 16 palliative care units, 19 hospital palliative care teams, and 23 home palliative care services.
The consecutive eligible patients were enrolled in this study if they had been newly referred to the participating institutions during the study period. All institutions were asked to take a sample of data consecutively, up to the designated number of patients of 20, 40, 60, 80, or 100 according to the size of the institution. The inclusion criteria of this study included (i) adult patients, (ii) patients diagnosed with locally extensive or metastatic cancer (including haematological neoplasm), and (iii) patients admitted to palliative care units, receiving help from hospital palliative care teams or receiving home palliative care services.
This study was conducted in accordance with the ethical standards of the Helsinki Declaration and the ethical guidelines for epidemiological research presented by the Ministry of Health, Labour, and Welfare in Japan, and approved by the local Institutional Review Boards of all participating institutions.
Measurement
Patient demographics and clinical characteristics, such as gender, site of primary cancer, metastatic disease, and chemotherapy in the past 30 days, were obtained at baseline. The physician recorded patients' laboratory data analysed at a central laboratory of each institution and evaluated presence or absence of symptoms (i.e. anorexia, fatigue, dyspnea, dysphagia, weight loss within 1 month, edema, pressure ulcer, and jaundice) at baseline. The rationale for selecting these symptoms are that empirical studies demonstrated they were significantly related to patient survival, because this is secondary analysis of the original study with primary intent to explore prognostic measure scales.14, 15 Evaluation was also performed at baseline in terms of whether assistance for ADL was being received or not, using an adaptation of the Index of Independence in ADL16 (i.e. bathing, dressing, going to the toilet, transfer, and feeding) and manual muscle testing (MMT) for limbs, which is the most widely used clinical method of strength assessment and based on a system of grading movement against an examiner or resistance to the force due to gravity, as described below.17
- 5 (Normal)
The ability to move the body part into the test position and hold it up against the force due to gravity and maximum resistance.
- 4 (Good)
The ability to move the body part into the test position and hold it up against the force due to gravity and moderate resistance.
- 3 (Fair)
The ability to move the body part into the test position and hold it up against the force due to gravity.
- 2 (Poor)
The ability to move the body part into the test position with the force due to gravity attenuated.
- 1 (Trace)
The ability to initiate weak contraction or flicker of muscle or tendon movement that is visible or palpable, but does not move the body part.
- 0 (Zero)
No ability to contract the muscle.
These six grades of MMT were divided into three categories: normal (MMT 5), slightly weak (MMT 3–4), and severely weak (MMT 0–2).
Statistical analyses
Patient characteristics were presented in mean ± standard deviation or number (%) as appropriate. Participants were divided into four groups: (i) low (CRP < 1 mg/dl), (ii) moderate (1 = < CRP <5 mg/dl), (iii) high (5 = < CRP < 10 mg/dl), and (iv) very high CRP (10 mg/dl = < CRP). We used approximate figures to quartile points as our previous study,3 which indicated that CRP could be useful in predicting prognoses in advanced cancer patients.
The proportions of eight symptoms, five ADL disabilities, and three categories of MMT according to the CRP groups were tested by chi‐square tests.
The number of symptoms and ADL disabilities in each CRP group was presented descriptively.
Because we assumed numbers of symptoms and ADL disabilities were ordinal (not interval) variables, that is patients with five or six symptoms are much worse than those with one or two symptoms, ordinal logistic regression models were used to examine the associations between CRP and symptoms or ADL disabilities. In the ordinal logistic models, adjusted odd ratios (ORs) were calculated after adjustment for age, gender, site of primary cancer, metastatic disease, Eastern Cooperative Oncology Group Performance Status (ECOG PS), chemotherapy, and setting of care. Eight patients were excluded from the final analysis model due to missing data in the confounding measures. Sufficiency of the sample size was tested by using the rules of thumb for ordinal response variables.18
All results were considered to be statistically significant if the P value was less than 0.05. All analyses were performed using the statistical package IBM SPSS (version 22.0, Chicago, IL, USA) and R software (version 3.2.3, R Foundation for Statistical Computing, Vienna, Austria).
Results
Among the original cohort of 2426 patients, the baseline CRP values were available in 1702 patients (70.2%), who were considered eligible for the current analysis. The availability of the CRP values was high in a hospital palliative care team setting and low in a home palliative care setting (availability: 86.0% in hospital palliative care teams, 69.5% in palliative care units, and 43.9% in home palliative care services; chi‐square P < 0.001).
We then classified the patients into four groups: (i) low (CRP < 1 mg/dl) (n = 366), (ii) moderate (1 = < CRP <5 mg/dl) (n = 565), (iii) high (5 = < CRP < 10 mg/dl) (n = 377), and (iv) very high CRP (10 mg/dl = < CRP) (n = 394). We confirmed that other cut‐off points achieved essentially the same results. The characteristics of the subjects are summarized in Table 1. Mean age was 68.4 ± 12.7 years old, and the proportion of patients aged 70 years or older was 50.2%. The top three sites of primary cancer were lung, upper and lower gastrointestinal tracts, and liver/biliary system/pancreas. The proportion of ECOG PS 0–1 was 10.6% and that of ECOG PS 4 was 35.1%. The proportion of hospital palliative care teams was 48.7%, palliative care units 36.9%, and home palliative care services 14.4%. Median CRP value was 4.3 (1.3–9.6) mg/dl.
Table 1.
Patient characteristics
| Values | |
|---|---|
| N | 1702 |
| Age | 68.4 ± 12.7 |
| Less than 70 years | 846 (49.7%) |
| 70 years or older | 855 (50.2%) |
| Male gender | 1003 (58.9%) |
| Site of primary cancer | |
| Lung | 388 (22.8%) |
| Upper and lower gastrointestinal tracts | 430 (25.3%) |
| Liver, biliary system, pancreas | 343 (20.2%) |
| Breast | 76 (4.5%) |
| Gynecologic | 93 (5.5%) |
| Urological | 103 (6.1%) |
| Others | 269 (15.8%) |
| Metastatic disease, yes | 1390 (81.7%) |
| ECOG PS | |
| 0–1 | 180 (10.6%) |
| 2 | 316 (18.6%) |
| 3 | 605 (35.5%) |
| 4 | 598 (35.1%) |
| Chemotherapy within 1 month, yes | 425 (25.0%) |
| Setting of care | |
| Hospital palliative care team | 829 (48.7%) |
| Palliative care unit | 628 (36.9%) |
| Home palliative care service | 245 (14.4%) |
| CRP (mg/dl) | 4.3 (1.3–9.6) |
Values represent mean ± SD, median (interquartile range), or n (%) where appropriate.
The sums of some percentages do not add up to 100% due to missing values.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; CRP, C‐reactive protein; SD, standard deviation.
Positive rates of symptoms and ADL disabilities and proportions of three MMT categories in the four CRP groups are summarized in Table 2. Positive rates of symptoms and ADL disabilities increased with increasing CRP level. Anorexia, fatigue, and weight loss within 1 month were positive in about 70% of all patients, especially in the very high‐CRP group, being 89.8%, 81.0%, and 79.2%, respectively (Table 2). More than half of all patients received assistance for bathing, dressing, going to the toilet, and transfer, especially in the very high‐CRP group, for which the rate was over 70% (Table 2). Although 67.2% of the patients belonged to the slightly weak group (MMT 3–4), the number of patients in the normal group (MMT 5) decreased and that in the severely weak group (MMT 0–2) increased with increasing CRP level.
Table 2.
Levels of CRP, symptoms, ADL disabilities and MMT
| CRP levels (mg/dl) | ||||||
|---|---|---|---|---|---|---|
| Total (n = 1702) | CRP < 1 (n = 366) | 1 = < CRP < 5 (n = 565) | 5 = < CRP < 10 (n = 377) | 10 = < CRP (n = 394) | Chi‐square p | |
| Symptoms | ||||||
| Anorexia | 1279 (75.1%) | 223 (60.9%) | 404 (71.5%) | 298 (79.0%) | 354 (89.8%) | <0.001 |
| Fatigue | 1215 (71.4%) | 213 (58.2%) | 397 (70.4%) | 286 (75.9%) | 319 (81.0%) | <0.001 |
| Dyspnea | 492 (28.9%) | 68 (18.6%) | 152 (26.9%) | 112 (29.8%) | 160 (40.7%) | <0.001 |
| Dysphasia | 410 (24.1%) | 57 (15.6%) | 108 (19.2%) | 102 (27.1%) | 143 (36.4%) | <0.001 |
| Weight loss | 1129 (66.5%) | 201 (55.2%) | 351 (62.3%) | 265 (70.3%) | 312 (79.2%) | <0.001 |
| Edema | 665 (39.2%) | 89 (24.3%) | 199 (35.3%) | 177 (47.1%) | 200 (50.9%) | <0.001 |
| Pressure ulcer | 145 (8.5%) | 14 (3.8%) | 40 (7.1%) | 41 (10.9%) | 50 (12.7%) | <0.001 |
| Jaundice | 190 (11.2%) | 17 (4.6%) | 53 (9.4%) | 63 (16.8%) | 57 (14.5%) | <0.001 |
| ADL disabilities | ||||||
| Bathing | 1241 (72.9%) | 215 (58.7%) | 397 (70.3%) | 293 (77.7%) | 336 (85.3%) | <0.001 |
| Dressing | 967 (56.9%) | 146 (40.0%) | 300 (53.1%) | 236 (62.6%) | 285 (72.5%) | <0.001 |
| Going to the toilet | 936 (55.0%) | 141 (38.5%) | 286 (50.6%) | 226 (60.1%) | 283 (71.8%) | <0.001 |
| Transfer | 963 (56.6%) | 145 (39.8%) | 301 (53.3%) | 230 (61.0%) | 287 (72.8%) | <0.001 |
| Feeding | 656 (38.7%) | 86 (23.8%) | 193 (34.3%) | 155 (41.2%) | 222 (56.5%) | <0.001 |
| MMT (range 0–5) | ||||||
| Normal (5) | 350 (20.6%) | 106 (29.0%) | 146 (25.9%) | 52 (13.8%) | 46 (11.7%) | <0.001 |
| Slightly weak (3–4) | 1142 (67.2%) | 229 (62.6%) | 357 (63.3%) | 274 (72.9%) | 282 (71.6%) | |
| Severely weak (0–2) | 208 (12.2%) | 31 (8.5%) | 61 (10.8%) | 50 (13.3%) | 66 (16.8%) | |
CRP, C‐reactive protein; ADL: activities in daily living; MMT, manual muscle testing.
Rates of positivity of symptoms and ADL disabilities increased with increasing CRP level. Anorexia, fatigue, and weight loss within 1 month were positive in about 70% of all patients, especially in the very high‐CRP group (10 mg/dl = < CRP), in which the rates were 89.8%, 81.0% and 79.2%, respectively. More than half of all patients received assistance for bathing, dressing, going to the toilet, and transfer, especially in the very high‐CRP group, in which the rate was over 70%. Although 67.2% of the patients belonged to the slightly weak group (MMT 3–4), the number of patients in the normal group (MMT 5) decreased and that in the severely weak group (MMT 0–2) increased with increasing CRP level.
The distribution of symptoms (range 0–8) and ADL disabilities (range 0–5) in four CRP groups are shown in Tables 3 and 4. The accumulation of symptoms and ADL disabilities increased with increasing CRP level.
Table 3.
The distribution of symptoms
| Number of symptoms | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CRP levels (mg/dl) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total |
| CRP < 1 | 63 (17.2%) | 47 (12.8%) | 67 (18.3%) | 100 (27.3%) | 54 (14.8%) | 26 (7.1%) | 8 (2.2%) | 1 (0.3%) | 0 (0%) | 366 (100%) |
| 1 = < CRP < 5 | 46 (8.1%) | 65 (11.5%) | 90 (15.9%) | 140 (24.8%) | 132 (23.4%) | 55 (9.7%) | 23 (4.1%) | 14 (2.5%) | 0 (0%) | 565 (100%) |
| 5 = < CRP < 10 | 22 (5.8%) | 26 (6.9%) | 40 (10.6%) | 83 (22.0%) | 96 (25.5%) | 64 (17.0%) | 37 (9.8%) | 9 (2.4%) | 0 (0%) | 377 (100%) |
| 10 = < CRP | 6 (1.5%) | 18 (4.6%) | 33 (8.4%) | 85 (21.6%) | 102 (25.9%) | 77 (19.5%) | 51 (12.9%) | 19 (4.8%) | 3 (0.8%) | 394 (100%) |
| Total | 137 (8.0%) | 156 (9.2%) | 230 (13.5%) | 408 (24.0%) | 384 (22.6%) | 222 (13.0%) | 119 (7.0%) | 43 (2.5%) | 3 (0.2%) | 1702 (100%) |
CRP, C‐reactive protein.
Table 4.
The distribution of ADL disabilities
| Number of ADL disabilities | |||||||
|---|---|---|---|---|---|---|---|
| CRP levels (mg/dl) | 0 | 1 | 2 | 3 | 4 | 5 | Total |
| CRP < 1 | 149 (40.7%) | 50 (13.7%) | 20 (5.5%) | 24 (6.6%) | 44 (12.0%) | 79 (21.6%) | 366 (100%) |
| 1 = < CRP < 5 | 161 (28.5%) | 69 (12.2%) | 38 (6.7%) | 32 (5.7%) | 89 (15.8%) | 176 (31.2%) | 565 (100%) |
| 5 = < CRP < 10 | 80 (21.2%) | 40 (10.6%) | 24 (6.4%) | 27 (7.2%) | 59 (15.6%) | 147 (39.0%) | 377 (100%) |
| 10 = < CRP | 54 (13.7%) | 30 (7.6%) | 28 (7.1%) | 19 (4.8%) | 45 (11.4%) | 218 (55.3%) | 394 (100%) |
| Total | 444 (26.1%) | 189 (11.1%) | 110 (6.5%) | 102 (6.0%) | 237 (13.9%) | 620 (36.4%) | 1702 (100%) |
CRP, C‐reactive protein; ADL: activities in daily living.
The multiple‐adjusted associations between CRP level, symptoms, and ADL disabilities are summarized in Table 5. In the ordinal logistic regression model, regarding symptoms, significantly higher adjusted ORs than in the low‐CRP group were observed in the moderate‐CRP, high‐CRP, and very high‐CRP groups {1.6 [95% confidence interval (CI) 1.2–2.0], P < 0.001; 2.5 (95% CI 1.9–3.2), P < 0.001; 3.5 (95% CI 2.7–4.6), P < 0.001, respectively} (Table 5). Regarding ADL disabilities, a significantly higher adjusted OR than in the low‐CRP group was not observed in the moderate‐CRP or high‐CRP group but was in the very high‐CRP group [1.3 (95% CI 1.0–1.7), P = 0.73; 1.3 (95% CI 1.0–1.8), P = 0.76; 2.1 (95% CI 1.5–2.9), P < 0.001, respectively] (Table 5). That the probability for change in ADL disabilities was 740 times higher in the patients with ECOG PS 4 (Table 5) might reflect sudden deterioration of ADL due to imminent death. The rules of thumb indicated that sample size was enough to estimate multiple‐adjusted ORs in the ordinal logistic regression model.
Table 5.
The association between CRP, symptoms and ADL disabilities
| Symptoms | ADL disabilities | |||
|---|---|---|---|---|
| Adjusted odd ratio (95% CI) | p | Adjusted odd ratio (95% CI) | p | |
| CRP (mg/dl) | ||||
| CRP < 1 | 1.0 | Reference | 1.0 | Reference |
| 1 = < CRP < 5 | 1.6 (1.2–2.0) | <0.001 | 1.3 (1.0–1.7) | 0.73 |
| 5 = < CRP < 10 | 2.5 (1.9–3.2) | <0.001 | 1.3 (1.0–1.8) | 0.76 |
| 10 = < CRP | 3.5 (2.7–4.6) | <0.001 | 2.1 (1.5–2.9) | <0.001 |
| Age | ||||
| Less than 70 years | 1.0 | Reference | 1.0 | Reference |
| 70 years or older | 0.9 (0.7–1.0) | 0.14 | 1.6 (1.3–2.0) | <0.001 |
| Gender | ||||
| Male | 1.0 | Reference | 1.0 | Reference |
| Female | 1.0 (0.8–1.2) | 0.93 | 1.1 (0.9–1.4) | 0.50 |
| Site of primary cancer | ||||
| Lung | 1.0 | Reference | 1.0 | Reference |
| Upper and lower gastrointestinal tracts | 0.9 (0.7–1.2) | 0.43 | 0.9 (0.7–1.2) | 0.43 |
| Liver, biliary system, pancreas | 1.1 (0.8–1.4) | 0.55 | 0.7 (0.5–1.0) | 0.46 |
| Breast | 0.8 (0.5–1.2) | 0.27 | 1.0 (0.6–1.8) | 0.89 |
| Gynecologic | 0.8 (0.5–1.3) | 0.38 | 0.7 (0.4–1.2) | 0.18 |
| Urological | 0.6 (0.4–0.8) | 0.004 | 0.9 (0.5–1.4) | 0.55 |
| Others | 0.9 (0.7–1.2) | 0.34 | 0.9 (0.7–1.3) | 0.63 |
| Metastatic disease | ||||
| No | 1.0 | Reference | 1.0 | Reference |
| Yes | 1.4 (1.1–1.8) | 0.002 | 1.4 (1.1–1.9) | 0.009 |
| ECOG PS | ||||
| 0–1 | 1.0 | Reference | 1.0 | Reference |
| 2 | 4.1 (2.9–5.8) | <0.001 | 7.2 (4.1–12.6) | <0.001 |
| 3 | 10.8 (7.7–15.1) | <0.001 | 44.3 (25.5–76.8) | <0.001 |
| 4 | 28.2 (19.7–40.3) | <0.001 | 740.0 (408.6–1340.0) | <0.001 |
| Chemotherapy | ||||
| Yes | 1.0 | Reference | 1.0 | Reference |
| No | 0.9 (0.7–1.1) | 0.16 | 0.6 (0.4–0.7) | <0.001 |
| Setting of care | ||||
| Hospital palliative care team | 1.0 | Reference | 1.0 | Reference |
| Palliative care unit | 1.0 (0.8–1.2) | 0.89 | 1.0 (0.8–1.3) | 0.70 |
| Home palliative care service | 1.1 (0.8–1.4) | 0.46 | 0.5 (0.4–0.7) | <0.001 |
CRP, C‐reactive protein; ADL, activities in daily living; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Adjusted variables are baseline CRP value, age, gender, site of primary cancer, ECOG PS, chemotherapy and setting of care.
In the ordinal logistic regression model, regarding symptoms, significantly higher adjusted odd ratios (ORs) than in the low‐CRP group were observed in the moderate‐CRP, high‐CRP and very high‐CRP groups [1.6 (95% CI 1.2–2.0), P < 0.001; 2.5 (95% CI 1.9–3.2), P < 0.001; 3.5 (95% CI 2.7–4.6), P < 0.001, respectively]. Regarding ADL disabilities, a significantly higher adjusted OR than in the low‐CRP group was not observed in the moderate‐CRP or high‐CRP group but was in the very high‐CRP group [1.3 (95% CI 1.0–1.7), P = 0.73; 1.3 (95% CI 1.0–1.8), P = 0.76; 2.1 (95% CI 1.5–2.9), P < 0.001, respectively].
The predicted probabilities of symptoms and ADL disabilities based on CRP or ECOG PS are shown in Figures 1 and 2. These figures indicated the sigmoidal relationship between symptoms or ADL disabilities and CRP or ECOG PS classes: a patient with CRP value of 10 mg/dl has 50% probability for having four or more symptoms and 10% probability for having six or more symptoms.
Figure 1.
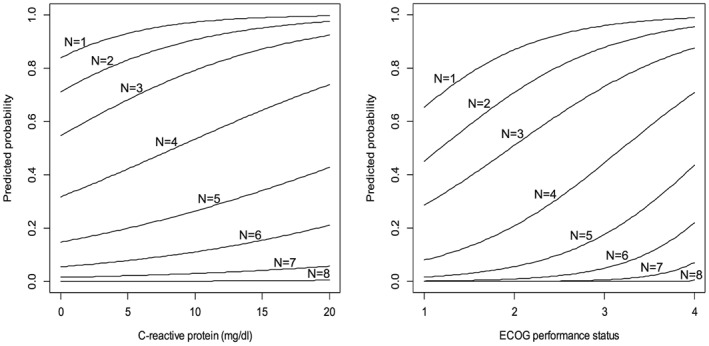
The predicted probabilities of symptoms based on C‐reactive protei or Eastern Cooperative Oncology Group (ECOG) performance status.
Figure 2.
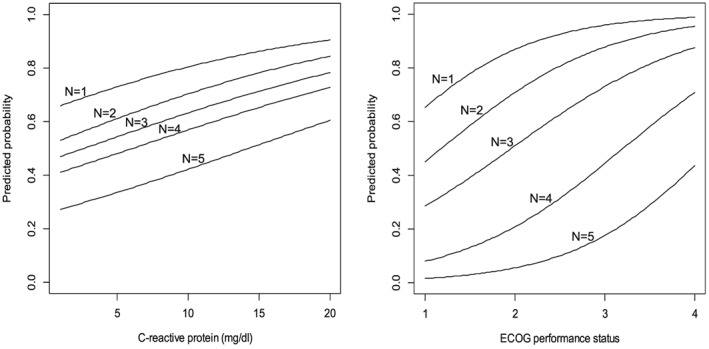
The predicted probabilities of activities in daily living (ADL) disabilities based on C‐reactive protein or Eastern Cooperative Oncology Group (ECOG) performance status.
Discussion
To the best of our knowledge, there have been no large prospective studies investigating the clinical implications of CRP as a biomarker in advanced cancer patients in palliative settings. We demonstrated that CRP level was associated with the accumulation of symptoms and ADL disabilities independently of performance status and disease stage.
Our study revealed that elevated CRP level was associated with symptoms in advanced cancer patients. Anorexia, fatigue, and weight loss may be closely linked in patients receiving palliative care because the present study also found significant associations between CRP level and fatigue in advanced cancer patients, as in previous studies.10, 11 The associations between elevated CRP level and common symptoms of advanced cancer including anorexia, weight loss, and pain were indicated,8, 9 while CRP was not related to pain, depression, or fatigue.19 Fatigue was independently associated with chemotherapy treatment and experiencing other symptoms such as pain and depression, and moderately associated with haemoglobin level in advanced cancer patients. However, there was no link to cachexia, albumin, or CRP.20 Other common symptoms, such as dyspnea, dysphagia, and edema, had been unclear, but the present study revealed that 20–40% of patients suffered from them. Our results imply the association between elevated CRP level and a variety of symptoms of cachexia in advanced cancer patients.
Furthermore, our study revealed that elevated CRP level was associated with poor ADL and MMT in advanced cancer patients. Our results, especially those of MMT, imply the association between CRP level, ongoing loss of skeletal muscle mass and its strength, specifically, the effects of cachexia, in advanced cancer patients. Several studies have reported that CRP distinguished cancer patients with weight loss from weight‐stable patients, while loss of muscle mass distinguished patients with weight loss from weight‐stable patients in some studies.21 In addition, grip strength was lower in patients with >10% vs. 5–10% weight loss,22 and patients who only lost fat mass (stable lean body mass) had an unchanged exercise capacity.23 A few studies reported that physical exercise in prostate cancer, breast cancer, and leukaemia patients alleviated undesirable effects of cachexia.24, 25, 26 It was implied that the positive influence of physical exercise on protein synthesis in skeletal muscle was induced by an up‐regulation of anti‐inflammatory cytokines. In other words, physical exercise in advanced cancer patients may not only lower CRP level but also improve ADL. However, the studies did not investigate the associations between CRP, skeletal muscle mass, and ADL. Thus, CRP may be associated with loss of muscle mass and declining ADL.
This study has several limitations to be considered. First, pro‐inflammatory cytokines and chronic systemic inflammation greatly affect CRP level, and these factors would differ among individuals in various ways. Therefore, when comparing groups of patients with advanced cancer, any factor other than CRP, such as IL‐6, IL‐1, and tumour necrosis factor α, might have to be taken into consideration. Second, very high‐CRP group (10 mg/dl = < CRP) could be affected by acute infections or acute medical conditions. We believe that the clinical implications of CRP do not change, because these factors also deteriorate symptoms and ADL disabilities. Third, this study could not prove causal relationships between CRP level, symptoms, and ADL disabilities because of its cross‐sectional nature. There is a need for further investigation into the association between CRP level and time course changes of declining ADL in advanced cancer patients. Fourth, we did not investigate the effects of anticancer treatment. Advanced cancer patients generally receive systemic anticancer treatment until a late stage in their disease trajectory and the interaction of it with CRP level and the development of cachexia is unknown.12 Fifth, we classified the patients into four groups using approximate figures to quartile points of CRP values as our previous study, which indicated that CRP could be useful in predicting prognoses in advanced cancer patients.3 We believe the validity of our method, because these cut‐off values are statistically and clinically relevant. Furthermore, this classification discriminates mortality risk among the advanced cancer patients, and it would be convenient for clinical use. Finally, symptoms were rated by physicians using yes–no format, and thus, there might be overestimation or underestimation. The findings should be confirmed using patient‐rating symptom measurements.
Conclusions
The association between CRP level, symptoms, and ADL observed in advanced cancer patients receiving palliative care indicates that CRP level might be a good biomarker. Inflammatory processes and possible influences of different diseases as well as cancer cachexia should be taken into consideration to evaluate the clinical implications of CRP. Future research should assess the association between CRP level and advanced cancer patients and clarify whether the measurement of CRP, which is widely available and inexpensive, could improve the treatment allocation and survival of advanced cancer patients.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Acknowledgements
The authors have read and complied with the principles of ethical authorship of the Journal of Cachexia, Sarcopenia and Muscle.27
This study was performed in the Japan ProVal Study Group (Prognostic Scores Validation Study Group). The participating study sites and site investigators were as follows: Satoshi Inoue, MD (Seirei Hospice, Seirei Mikatahara General Hospital), Masayuki Ikenaga, MD (Children's Hospice Hospital, Yodogawa Christian Hospital), Yoshihisa Matsumoto, MD, PhD (Department of Palliative Medicine, National Cancer Center Hospital East), Mika Baba, MD (Department of Palliative Care, Saito Yukoukai Hospital), Ryuichi Sekine, MD (Department of Pain and Palliative Care, Kameda Medical Center), Takashi Yamaguchi, MD, PhD (Department of Palliative Medicine, Kobe University Graduate School of Medicine), Takeshi Hirohashi, MD (Department of Palliative Care, Mitui Memorial Hospital), Tsukasa Tajima, MD (Department of Palliative Medicine, Tohoku University Hospital), Ryohei Tatara, MD (Department of Palliative Medicine, Osaka City General Hospital), Hiroaki Watanabe, MD (Komaki City Hospital), Hiroyuki Otani, MD (Department of Palliative Care Team, and Palliative and Supportive Care, National Kyushu Cancer Center), Chizuko Takigawa, MD (Department of Palliative Care, KKR Sapporo Medical Center), Yoshinobu Matsuda, MD (Department of Psychosomatic Medicine, National Hospital), Hiroka Nagaoka, MD (Department of Medical Social Service Center for Palliative Care, University of Tsukuba), Masanori Mori, MD (Seirei Hamamatsu General Hospital), Yo Tei, MD (Seirei Hospice, Seirei Mikatahara General Hospital), Shuji Hiramoto, MD (Department of Oncology, Mitsubishi Kyoto Hospital), Akihiko Suga, MD (Department of Palliative Medicine, Shizuoka Saiseikai General Hospital), Takayuki Hisanaga, MD (Tsukuba Medical Center Foundation), Tatsuhiko Ishihara, MD (Palliative Care Department, Okayama Saiseikai General Hospital), Tomoyuki Iwashita, MD (Matsue City Hospital), Keisuke Kaneishi, MD, PhD (Department of Palliative Care Unit, JCHO Tokyo Shinjuku Medical Center), Shohei Kawagoe, MD (Aozora Clinic), Toshiyuki Kuriyama, MD, PhD (Wakayama Medical University Hospital Oncology Center), Takashi Maeda, MD (Department of Palliative Care, Tokyo Metropolitan Cancer and Infectious Disease Center Komagome Hospital), Ichiro Mori, MD (Gratia Hospital Hospice), Nobuhisa Nakajima, MD, PhD (Department of Palliative Medicine, Graduate School of Medicine, Tohoku University), Tomohiro Nishi, MD (Kawasaki Comprehensive Care Center, Kawasaki Municipal Ida Hospital), Hiroki Sakurai, MD (Department of Palliative Care, St. Luke's International Hospital, Tokyo), Satofumi Shimoyama, MD, PhD (Department of Palliative Care, Aichi Cancer Center Hospital), Takuya Shinjo, MD [Shinjo Clinic (T.S.), Kobe], Hiroto Shirayama, MD (Iryouhoujinn Takumikai Osaka Kita Homecare Clinic), Takeshi Yamada, MD, PhD (Department of Gastrointestinal and Hepato‐Biliary‐Pancreatic Surgery, Nippon Medical School), Shigeki Ono, MD (Division of Palliative Medicine, Shizuoka Cancer Center Hospital), Taketoshi Ozawa, MD, PhD (Megumi Zaitaku Clinic), Ryo Yamamoto, MD (Department of Palliative Medicine, Saku Central Hospital Advanced Care Center), Naoki Yamamoto, MD, PhD (Department of Primary Care Service, Shinsei Hospital), Hideki Shishido, MD (Shishido Internal Medicine Clinic), Mie Shimizu, MD (Saiseikai Matsusaka General Hospital), Masanori Kawahara, MD PhD (Soshukai Okabe Clinic), Shigeru Aoki, MD (Sakanoue Family Clinic), Akira Demizu, MD (Demizu Clinic), Masahiro Goshima, MD, PhD (Homecare Clinic Kobe), Keiji Goto, MD (Himawari Zaitaku Clinic), Yasuaki Gyoda, MD, PhD (Kanamecyo Home Care Clinic), Jun Hamano, MD (Division of Clinical Medicine, Faculty of Medicine, University of Tsukuba), Kotaro Hashimoto, MD (Fukushima Home Palliative Care Clinic), Sen Otomo, MD (Shonan International Village Clinic), Masako Sekimoto, MD (Sekimoto Clinic), Takemi Shibata, MD (Kanwakea Clinic – Eniwa), Yuka Sugimoto, MD (Sugimoto Homecare Clinic), Mikako Matsunaga, MD (Senri Pain Clinic), Yukihiko Takeda, MD (Hidamari Clinic), Takeshi Sasara, MD (Yuuaikai Nanbu Hospital), and Jun Nagayama, MD (Peace Clinic Nakai).
This work was supported in part by The National Cancer Center Research and Development Fund (25‐A‐22).
Amano, K. , Maeda, I. , Morita, T. , Baba, M. , Miura, T. , Hama, T. , Mori, I. , Nakajima, N. , Nishi, T. , Sakurai, H. , Shimoyama, S. , Shinjo, T. , Shirayama, H. , Yamada, T. , Ono, S. , Ozawa, T. , Yamamoto, R. , Yamamoto, N. , Shishido, H. , and Kinoshita, H. (2017) C‐reactive protein, symptoms and activity of daily living in patients with advanced cancer receiving palliative care. Journal of Cachexia, Sarcopenia and Muscle, 8: 457–465. doi: 10.1002/jcsm.12184.
References
- 1. Allin KH, Bojesen SE, Nordestgaard BG. Baseline C‐reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol 2009;27:2217–2224. [DOI] [PubMed] [Google Scholar]
- 2. Allin KH, Nordestgaard BG. Elevated C‐reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci 2011;48:155–170. [DOI] [PubMed] [Google Scholar]
- 3. Amano K, Maeda I, Morita T, Miura T, Inoue S, Ikenaga M, et al. Clinical implications of C‐reactive protein as a prognostic marker in advanced cancer patients in palliative settings. J Pain Symptom Manage 2016;51:860–867. [DOI] [PubMed] [Google Scholar]
- 4. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. [DOI] [PubMed] [Google Scholar]
- 5. Fearon KC, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 6. Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health 2007;61:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siemes C, Visser LE, Coebergh JW, Splinter TA, Witteman JC, Uitterlinden AG, et al. C‐reactive protein levels, variation in the C‐reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol 2006;24:5216–5222. [DOI] [PubMed] [Google Scholar]
- 8. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223–226. [DOI] [PubMed] [Google Scholar]
- 9. Laird BJ, Scott AC, Colvin LA, McKeon AL, Murray GD, Fearon KC, et al. Cancer pain and its relationship to systemic inflammation: an exploratory study. Pain 2011;152:460–463. [DOI] [PubMed] [Google Scholar]
- 10. Alexander S, Minton O, Andrews P, Stone P, et al. A comparison of the characteristics of disease‐free breast cancer survivors with or without cancer‐related fatigue syndrome. Eur J Cancer 2009;45:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun 2007;21:413–427. [DOI] [PubMed] [Google Scholar]
- 12. Fearon KC. Cancer cachexia and fat‐muscle physiology. N Engl J Med 2011;365:565–567. [DOI] [PubMed] [Google Scholar]
- 13. Laviano A, Seelaender M, Rianda S, Silverio R, Rossi FF. Neuroinflammation: a contributing factor to the pathogenesis of cancer cachexia. Crit Rev Oncog 2012;17:247–251. [DOI] [PubMed] [Google Scholar]
- 14. Baba M, Maeda I, Morita T, Hisanaga T, Ishihara T, Iwashita T, et al. Independent validation of the modified Prognosis Palliative care Study (PiPS) predictor models throughout three palliative care settings. J Pain Sympt Manage 2015;doi:10.1016/j.jpainsymman.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 15. Baba M, Maeda I, Morita T, Inoue S, Ikenaga M, Matsumoto Y, et al. Survival prediction for advanced cancer patients in the real world: a comparison of the Palliative Prognostic Score, Delirium‐Palliative Prognostic Score, Palliative Prognostic Index and modified Prognosis in Palliative Care Study predictor model. Eur J Cancer 2015;51:1618–1629. [DOI] [PubMed] [Google Scholar]
- 16. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 17. Marino M, Nicholas JA, Gleim GW, Rosenthal P, Nicholas SJ. The efficacy of manual assessment of muscle strength using a new device. Am J Sports Med 1982;10:360–364. [DOI] [PubMed] [Google Scholar]
- 18. Harrell FE Jr. Regression Modeling Strategies: with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York: Springer; 2015. p 72–74. [Google Scholar]
- 19. Laird BJ, Scott AC, Colvin LA, McKeon AL, Murray GD, Fearon KC, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage 2011;42:1–11. [DOI] [PubMed] [Google Scholar]
- 20. Minton O, Strasser F, Radbruch L, Stone P. Identification of factors associated with fatigue in advanced cancer: a subset analysis of the European palliative care research collaborative computerized symptom assessment data set. J Pain Symptom Manage 2012;43:226–235. [DOI] [PubMed] [Google Scholar]
- 21. Blum D, Omlin A, Baracos VE, Solheim TS, Tan BH, Stone P, et al. Cancer cachexia: a systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit Rev Oncol Hematol 2011;80:114–144. [DOI] [PubMed] [Google Scholar]
- 22. Fearon KC, Voss AC, Hustead DS, Cancer Cachexia Study Group . Definition of cancer cachexia: effect of WL, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 2006;83:1345–1350. [DOI] [PubMed] [Google Scholar]
- 23. Fouladiun M, Korner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care—correlations with food intake, metabolism, exercise capacity, and hormones. Cancer 2005;103:2189–2198. [DOI] [PubMed] [Google Scholar]
- 24. Battaglini C, Hackney AC, Garcia R, et al. The effect of an exercise program in leukemia patients. Integr Cancer Ther 2009;8:130–138. [DOI] [PubMed] [Google Scholar]
- 25. Winters‐Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat 2011;127:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010;28:340–347. [DOI] [PubMed] [Google Scholar]
- 27. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


