Abstract
The C-reactive protein/albumin (CRP/Alb) ratio has been recently identified as a prognostic factor in various cancers, whereas its role remains unclear in metastatic nasopharyngeal carcinoma (NPC). The current study retrospectively analyzed 148 patients with metastatic NPC who underwent cisplatin-based chemotherapy and further evaluated the prognostic value of the CRP/Alb ratio and its association with clinical characteristics in these patients. The optimal cut-off value was 0.189 for the CRP/Alb ratio. The high CRP/Alb ratio was significantly associated with elevated NLR, platelet-to-lymphocyte ratio (PLR), and EBV-DNA levels and decreased haemoglobin level (all p < 0.05). The results of multivariate analysis showed that the CRP/Alb ratio was an independent prognostic factor of overall survival. Patients with a high CRP/Alb ratio (≥0.189) had a 1.867 times (p = 0.024, 95% CI = 1.085–3.210) greater risk of mortality compared with those with a low CRP/Alb ratio (<0.189). In addition, combining the CRP/Alb ratio with GPS could accurately discriminate the prognosis of our patients. Our results suggested that the CRP/Alb ratio is a feasible and inexpensive tool for predicting survival outcomes and is a valuable coadjutant for the GPS to further identify differences in survivals of patients with metastatic NPC.
1. Introduction
Nasopharyngeal carcinoma (NPC) is a distinct head and neck cancer with unique etiological and epidemiological feathers, with the incidence varying from 0.5–3/100,000 per year in North Africa to 20–30/100,000 in certain epidemic areas, such as South China and Southeast Asia [1–4]. Radiotherapy is the primary treatment of NPC, and the development of intensity-modulated radiotherapy technology (IMRT) and new chemotherapy agents significantly improves the cure rate [5–7]. However, more than 20% of NPC patients will ultimately develop distant metastasis after definitive chemoradiotherapy [8]. On the other hand, 5%–6% of NPC patients have disseminated disease at the time of initial diagnosis [8]. Hence, metastatic disease remains a critical problem and a leading cause of death among patients with NPC. For patients with metastatic NPC, systematic chemotherapy is the standard therapeutic option and has been well established [9]. Although multiple agents and regimens have been explored, the palliative chemotherapy for NPC has rarely been studied in any randomized trials yet. Cisplatin-based multiple chemotherapy was reported to reach a response rate of 50–80% in NPC patients; it has been recommended as the standard first-line regimen for metastatic NPC [10–12]. However, the clinical outcome varied significantly in patients with metastatic NPC. Therefore, it is critically important to find predictive factors for prognosis, which could help us to accurately select the patients who respond well to cisplatin-based chemotherapy.
Since the well-known tumor-node-metastasis (TNM) staging system could not provide prognostic information, a series of clinical characteristics and laboratory biomarkers associated with prognosis of patients with metastatic NPC have been explored in several studies [13–19]. Hemoglobin, performance status (PS), and disease-free interval (DFI) were first identified as prognostic factors for disseminated NPC by Toh et al. [20] in 2005. Then, lactate dehydrogenase (LDH) was also found to be significantly associated with survival of patients with metastatic NPC [21, 22]. Epstein-Barr virus (EBV) was a steady prognostic factor and predictor of treatment response for patients with metastatic NPC who underwent palliative chemotherapy [21, 23, 24]. In 2012, Jin et al. [21] built a prognostic model including EBV-DNA load, PS, LDH, alkaline phosphatase (ALP), and hemoglobin and successfully stratified the patients into different risk groups.
The role of systematic inflammatory response has been increasingly appreciated in multiple cancers; inflammatory biomarkers, such as C-reactive protein (CRP) level [25], Glasgow Prognostic Score (GPS) [26], modified Glasgow Prognostic Score (mGPS) [27], neutropil to lymphocyte ratio (NLR) [26], and monocyte to lymphocyte ratio (MLR) [28, 29], have already showed prognostic value in metastatic NPC. Xia et al. [25] retrospectively analyzed 116 patients with metastatic NPC who underwent palliative chemotherapy and found that baseline CRP level was significantly associated with survival. In 2014, Chen et al. [26] reviewed 211 Chinese patients with disseminated NPC who all received cisplatin-based chemotherapy and further validated the prognostic value of GPS and NLR. Recently, the CRP/albumin (CRP/Alb) ratio was recognized as a novel inflammatory index in infectious disease [30, 31]. In addition, the CRP/Alb ratio has showed promising prognostic value in gastric cancer [32], esophageal cancer [33], colorectal cancer [34], small cell lung cancer [35], and even in nonmetastatic NPC [36].
However, to the best of our knowledge, there is no data regarding the prognostic value of the CRP/Alb ratio in the metastatic NPC. Therefore, we conducted this study and aimed to examine the prognostic value of the CRP/Alb ratio and its association with clinical characteristics in patients with disseminated NPC.
2. Patients and Methods
2.1. Ethics Approval and Consent to Participate
All the patients offered informed consent for collection of clinical characteristics and pathologic information. The study was approved by the Bioethics Committee of the Sun Yat-Sen University Cancer Center for a retrospective analysis of the collected data. The study was undertaken in accordance with the ethical standards of the World Medical Association's Declaration of Helsinki.
2.2. Patients
We retrospectively analyzed the records of patients with NPC who were treated at the Sun Yat-Sen University Cancer Center between January 2008 and October 2011. All the patients met the following criteria: (a) the disease was pathologically diagnosed as NPC and evaluated clinically to be metastatic disease; (b) complete pretreatment data of CRP and albumin were available; (c) at least two cycles of first-line cisplatin-based palliative chemotherapy were administrated; (d) Karnofsky Performance Scores (KPS) were ≥60; and (e) hepatic and renal functions were normal. Exclusion criteria were as follows: (a) patients with brain metastases; (b) patients with other types of malignancy; or (c) patients with clinical evidence of infection or other inflammatory disease. Finally, a total of 148 patients were identified and originated the current study cohort.
2.3. Treatment and Follow-Up
The following three cisplatin-based chemotherapy regimens were administrated every 21 days:
TP regimen: paclitaxel (175 mg/m2 intravenously (IV) over 3 hours with standard premedication on day 1 of a 21-day cycle) plus cisplatin (25 mg/m2 IV on days 1–3 of a 21-day cycle)
PF regimen: cisplatin (25 mg/m2 IV on days 1–3 of a 21-day cycle) plus 5-fluorouracil (500 mg/m2 IV on days 1–5 of a 21-day cycle)
TPF regimen: paclitaxel (135 mg/m2 IV over 3 hours with standard premedication on day 1 of a 21-day cycle) plus cisplatin (25 mg/m2 IV on days 1–3 of a 21-day cycle) plus 5-fluorouracil (800 mg/m2, continuous IV infusion for 24 hours, on days 1–5 of a 21-day cycle).
After treatment, all patients were followed up every 3 months. Physical and radiological examinations were performed every 3 months or when clinical progression of disease was indicated. The last follow-up was performed on December 31, 2013. Overall survival (OS) was defined as the time interval from the initial diagnosis of metastasis to the date of death or the last follow-up.
2.4. Evaluation
Basic demographics (gender, age), baseline clinical characteristics (KPS, site of metastasis), and relevant laboratory data were collected. Hematological parameters, biochemical markers, and EBV-DNA load were examined within one week before chemotherapy. Smoking status was recorded as ever or never for all patients. Smoker ever was defined as ≥1 lifetime pack-year. The GPS, NLR, and platelet-to-lymphocyte ratio (PLR) were calculated. We calculated the CRP/Alb ratio as dividing the CRP level (mg/L) by the serum albumin level (g/L). The Response Evaluation Criteria in Solid Tumors (RECISTs) 1.0 was used to evaluate tumor response.
2.5. Statistical Analyses
Continuous variables, such as LDH and NLR, are presented as median value and range and were compared by using t-test or nonparametric test. Categorical variables, such as gender and GPS, were described as the numbers and percentages and were compared by using the chi-square or Fisher's exact test.
A web-based system, R software-engineered, designed by Budczies J et al. [37], was used to identify the optimal cutoff value of potential prognostic parameters (http://molpath.charite.de/cutoff/). The Kaplan-Meier method was used to estimate the OS. Univariate and multivariate survival analyses were performed based on the Cox proportional hazards regression methodology. Hazard ratios (HRs) with 95% CIs and two-sided p values were reported. An alpha value of p < 0.05 was considered statistically significant. The statistical analyses were performed using the Statistical Package for the Social Sciences version 19.0 (IBM, Armonk, NY, USA).
3. Results
3.1. Patient Characteristics
A total of 148 patients with metastatic NPC were included in our study. Of these, 124 (83.8%) were males and 24 (16.2%) were females; 68 (45.9%) were smokers and 80 (54.1%) were nonsmokers. The median age at initial diagnosis of metastatic NPC was 45 years (ranging 24–72 years). More than half (n = 79, 53.4%) of the patients had more than one metastasis site, and few of the patients (n = 43, 29.1%) developed synchronous metastasis. Liver metastasis, lung metastasis, and bone metastases were found in 56 patients (37.8%), 69 patients (46.6%), and 61 patients (41.2%), respectively. The baseline plasma EBV-DNA load ranged from 0 to 9.13 × 107 copies/mL, with a median value of 4.82 × 104 copies/mL. GPS was evaluated as 0 in 88 patients (59.5%), 1 in 46 patients (31.1%), and 2 in 14 patients (9.4%). The median values of the CRP/Alb, NLR, and PLR were 0.164, 3.3, and 181, respectively. Of the 148 patients, 12 (8.1%) were treated with the TP regimen, 45 (30.4%) with the PF regimen, and 91 (61.5%) with the TPF regimen (Table 1).
Table 1.
Baseline clinical features of 148 patients with metastatic nasopharyngeal carcinoma.
| Characteristic | Number (%) | CRP/Alb < 0.189, number (%) | CRP/Alb ≥ 0.189, number (%) | p value |
|---|---|---|---|---|
| Gender (male/female) | 124/24 (83.8/16.2) | 63/15 (80.8/19.2) | 61/9 (87.1/12.9) | 0.373 |
| Age, years (median/range) | 45/24–72 | 45/26–70 | 43.5/24–72 | 0.765 |
| KPS (median/range) | 90/60–100 | 90/60–100 | 90/60–100 | 0.276 |
| Number of involved sites (one/multiple) | 69/79 (46.6/53.4) | 42/36 (53.8/46.2) | 27/43 (38.6/61.4) | 0.071 |
| Synchronous metastasis (yes/no) | 43/105 (29.1/70.9) | 20/58 (25.6/74.4) | 23/47 (32.9/67.1) | 0.368 |
| Liver metastasis (yes/no) | 56/92 (37.8/62.2) | 28/50 (35.9/64.1) | 28/42 (40/60) | 0.615 |
| Lung metastasis (yes/no) | 69/79 (46.6/53.4) | 38/40 (48.7/51.3) | 31/39 (44.3/55.7) | 0.623 |
| Bone metastasis (yes/no) | 61/87 (41.2/58.8) | 28/50 (35.9/64.1) | 33/37 (47.1/52.9) | 0.184 |
| Smoking (yes/no) | 68/80 (45.9/54.1) | 36/42 (42.1/53.8) | 32/38 (45.7/54.3) | 1.000 |
| GPS (0/1/2) | 88/46/14 (59.5/31.1/9.4) | 52/20/6 (66.7/25.6/7.7) | 36/26/8 (51.4/37.1/11.4) | 0.169 |
| Serum LDH, U/L (median/range) | 202.5/25–2975 | 179.5/122–2821 | 230/25–2975 | 0.069 |
| EBV-DNA, copies/mL (median/range) | 4.82 × 104/0–9.13 × 107 | 3.49 × 104/0–9.87 × 106 | 5.32 × 105/0–9.13 × 107 | 0.013∗ |
| Chemotherapy regimen (TP/PF/TPF) | 12/45/91 (8.1/30.4/61.5) | 7/26/45 (9/33.3/57.7) | 5/19/46 (7.2/27.1/65.7) | 0.605 |
| Treatment response (CR + PR/PD + SD) | 107/41 (72.3/27.7) | 59/19 (75.6/24.4) | 48/22 (68.6/31.4) | 0.363 |
| NLR (median/range) | 3.3/1–35.2 | 2.8/1–14 | 4/1–35.2 | 0.001∗ |
| PLR (median/range) | 181/56.4–820 | 161/88–735 | 231/56.4–820 | 0.001∗ |
| Hemoglobin, g/L (median/range) | 131/43–171 | 132/43–171 | 123/82–162 | 0.019∗ |
CRP/Alb: C-reactive protein/albumin ratio; KPS: Karnofsky Performance Score; GPS: Glasgow Prognostic Score; NLR: neutrophil to lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; ∗p < 0.05.
3.2. Survival
Using the Cutoff Finder, we determined 0.189 as the optimal cutoff value of the CRP/Alb ratio for OS. All participants were then divided into the high CRP/Alb ratio group (≥0.189, n = 70) and low CRP/Alb ratio group (<0.189, n = 78). Accordingly, binarization of other continuous data (LDH, NLR) by optimal cutoff value was performed for the subsequent analysis of OS (Table 2).
Table 2.
Univariate and multivariate analysis of OS in 148 patients with metastatic nasopharyngeal carcinoma.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| p value | HR (95% CI) | p value | HR (95% CI) | |
| Gender (male/female) | 0.570 | 0.83 (0.435–1.581) | ||
| Age, years (<50/≥50) | 0.773 | 1.078 (0.646–1.801) | ||
| KPS (≥90/<90) | 0.411 | 0.765 (0.403–1.451) | ||
| Number of involved sites (one/multiple) | 0.179 | 1.369 (0.866–2.164) | ||
| Synchronous metastasis (yes/no) | 0.168 | 0.704 (0.427–1.160) | ||
| Liver metastasis (yes/no) | 0.802 | 0.942 (0.593–1.498) | ||
| Lung metastasis (yes/no) | 0.389 | 1.219 (0.777–1.914) | ||
| Bone metastasis (yes/no) | 0.071 | 1.525 (0.965–2.411) | ||
| Smoking (yes/no) | 0.210 | 0.750 (0.478–1.177) | ||
| GPS (0/1/2) | <0.001∗ | 3.135 (2.309–4.256) | 0.001∗ | 2.137 (1.369–3.337) |
| CRP/Alb (<0.189/≥0.189) | 0.003∗ | 1.998 (1.253–3.185) | 0.024∗ | 1.867 (1.085–3.210) |
| Serum LDH, U/L (<212/≥212) | 0.006 | 1.880 (1.193–2.962) | 0.602 | 0.858 (0.483–1.524) |
| EBV-DNA, copies/mL (<4.82 × 104/≥4.82 × 104) | <0.001∗ | 4.554 (2.792–7.427) | 0.041∗ | 2.012 (1.027–3.941) |
| Chemotherapy regimen (PF/TP/TPF) | 0.644 | 0.922 (0.653–1.302) | ||
| Treatment response (CR + PR/PD + SD) | 0.005∗ | 1.984 (1.231–3.197) | 0.420 | 1.242 (0.734–2.101) |
| NLR (<5/≥5) | 0.017∗ | 2.039 (1.133–3.671) | 0.192 | 1.522 (0.810–2.858) |
| PLR (<152/≥152) | 0.002∗ | 2.450 (1.407–4.267) | 0.128 | 1.617 (0.870–3.003) |
| Hemoglobin, g/L (<11/≥11) | 0.585 | 1.172 (0.664–2.069) | ||
CRP/Alb: C-reactive protein/albumin ratio; KPS: Karnofsky Performance Score; GPS: Glasgow Prognostic Score; NLR: neutrophil to lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LDH: lactate dehydrogenase; ∗p < 0.05.
Median follow-up time was 15.3 months (ranging 1 month–66 months). Of the 148 patients, 77 (52.0%) died before the last follow-up. Median OS time for the entire patient group were 21.8 months, with the 1- and 2-year OS rates of 69.6% and 22.3%, respectively. Compared with the patients in the high CRP/Alb ratio group, patients in the low CRP/Alb ratio group had significantly longer overall survival (25.2 months versus 19.5 months, p = 0.003) (Figure 1).
Figure 1.
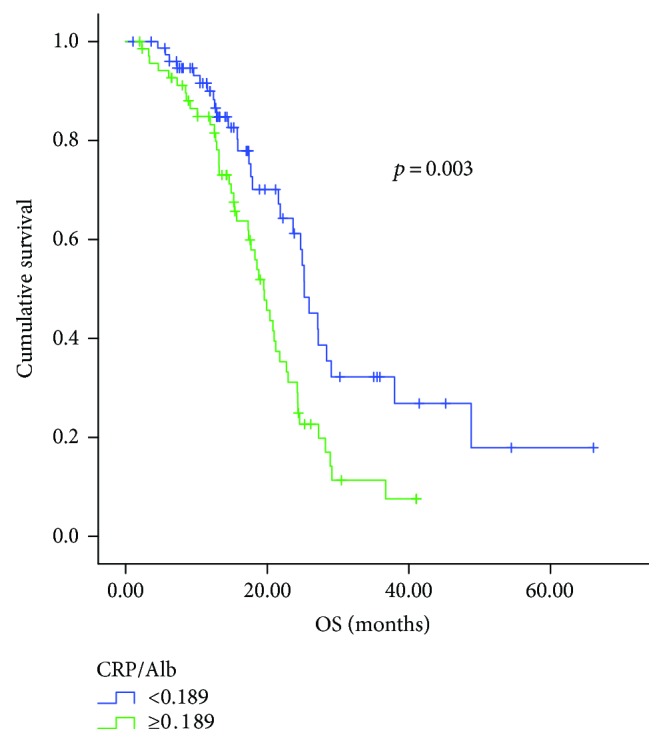
Kaplan-Meier curves for overall survival (OS) according to the CRP/Alb ratio.
Besides the CRP/Alb ratio, GPS (p < 0.001), serum LDH level (p = 0.006), plasma EBV-DNA load (p < 0.001), pretreatment NLR (p = 0.017), PLR (p = 0.002), and treatment response (p = 0.005) were associated with OS in the univariate analysis. After adjusting for other covariates in multivariate analysis, the CRP/Alb ratio (p = 0.024) was proved to be an independent prognostic factor of OS, as well as GPS (p = 0.001) and EBV-DNA load (p = 0.041). We found that patients in the high CRP/Alb ratio group had a 1.867 times (p = 0.024, 95% CI = 1.085–3.210) greater risk of mortality compared with those in the low CRP/Alb ratio group (Table 2).
3.3. Association of the CRP/Alb Ratio with Clinicopathologic Characteristics
Patient characteristics were compared between subgroups by the CRP/Alb ratio (Table 1). It was found that the high CRP/Alb ratio showed a trend to correlate with multiple metastasis (p = 0.071) and elevated LDH level (p = 0.069), with marginal statistical significance. Of note, the high CRP/Alb ratio was significantly associated with high NLR, PLR, and EBV-DNA level and low haemoglobin level (all p < 0.05).
3.4. Generation of a New Prognostic Score
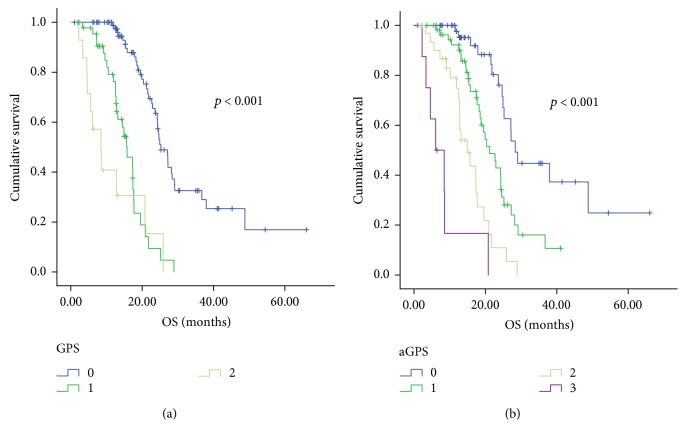
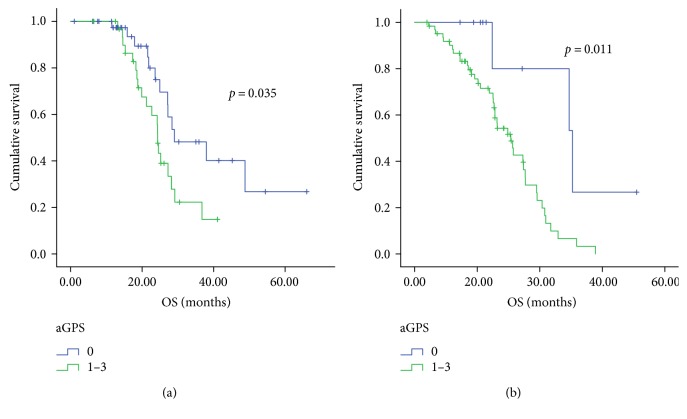
Although GPS was an independent prognostic factor in the Cox model, the difference in OS between patients with GPS 1 and those with GPS 2 was not statistically significant (15.8 months versus 8.5 months, p = 0.101, Figure 2(a)). Thus, we further added the CRP/Alb ratio to the GPS system and established a new prognostic score called aGPS. A score of 1 was assigned to CRP >10 mg/L, albumin <35 g/L, and the CRP/Alb ratio ≥ 0.189. Thus, of the 148 patients, 52 (35.1%) were evaluated as having an aGPS of 0, 56 (37.8%) had an aGPS of 1, 32 (21.6%) had an aGPS of 2, and 8 (5.4%) had an aGPS of 3; the median OS of these patients were 28.4 months, 21.2 months, 14.9 months, and 6.1 months, respectively (p < 0.001, Figure 2(b)). The OS curves of these subgroups were statistically separated from each other. Furthermore, aGPS (aGPS =0 versus aGPS >0) was also significantly associated with prognosis of NPC patients when stratified by plasma EBV-DNA level, whereas plasma EBV-DNA level (<4.82 × 104 copies/mL versus ≥4.82 × 104copies/mL) was not (Figure 3). Patients with aGPS 0 had longer OS than patients with aGPS >0 either in the high plasma EBV-DNA level group or in the low plasma EBV-DNA level group.
Figure 2.
Kaplan-Meier curves for overall survival (OS) according to the GPS (a) and to the aGPS (b).
Figure 3.
Kaplan-Meier curves for overall survival (OS) according to the aGPS in patients with pretreatment EBV DNA <4.82 × 104 copies/mL (a) and in those with pretreatment EBV DNA ≥4.82 × 104 copies/mL (b).
4. Discussion
Systematic inflammatory response has been well studied in the carcinogenesis and progression of malignancies during the past decade, and various inflammatory biomarkers have shown to be associated with cancer prognosis [38]. Serum CRP is an acute-phase protein produced by hepatocytes; it has been long recognized as a prognostic factor for many patients with malignancies including NPC [25, 39–42]. Meanwhile, hypoalbuminemia could predominantly reflect malnutrition as well as inflammatory condition and was found to be associated with impaired survival outcome of cancer patients [43]. GPS and mGPS were thus generalized from the combination of CRP and albumin; as new inflammatory markers, they have been proved to be useful prognostic factors to predict the risk of cancer.
More recently, a novel combination model of CRP and albumin—CRP/Alb ratio—has also been increasingly appreciated since it could serve as a predictor of a clinical outcome in patients with serious infectious disease and those with cancers [31, 32, 34, 35, 44]. He et al. [36] investigated the CRP/Alb ratio in nonmetastatic NPC. They found that the CRP/Alb ratio was a steady prognostic factor for the prognosis of patients with nonmetastatic NPC, and it could identify survival differences even if stratified by EBV-DNA level. However, most of previous studies on the CRP/Alb ratio limited the cohort to patients who were diagnosed with localized cancers and underwent curative therapy [32, 34, 44]. By contrast, our study focused on the patients with metastatic NPC and firstly demonstrated that the CRP/Alb ratio was an independent prognostic factor of overall survival.
Of note, the CRP/Alb ratio reflects a comprehensive state of both CRP and albumin rather than a simple addition of CRP and albumin. In contrast to GPS, the CRP/Alb ratio might indicate more comprehensive information, reflect dynamic change of systematic inflammation, and identify tiny difference among a large patient population. Although GPS was also found to be independently associated with OS (p = 0.001) as well as the CRP/Alb ratio, there were 59.3% of patients classified in the group of GPS 0, which implied that the GPS could not accurately predict the prognosis of a large proportion of our patients. Moreover, the GPS was not statistically different in the OS between the patients with GPS 1 and those with GPS 2. More interestingly, we could enrich the prognostic information of CRP-albumin-based model (aGPS) when we introduced the CRP/Alb ratio into the model along with GPS. Similar findings were also reported by Wei et al. [45], in which the patients with GPS 0 could be categorized into two risk subgroups by the CRP/Alb ratio. Furthermore, aGPS was significantly associated with OS when stratified by plasma EBV-DNA level in our cohort. From this point of view, aGPS which was consisted of three parameters (CRP, albumin, and CRP/Alb ratio) might serve as a more accurate prognostic factor than GPS and provide new insight into the inflammatory prognostic system. However, the prognostic efficacy of aGPS should be further explored in prospective studies and in other cancers other than in NPC.
The association of the CRP/Alb ratio with other clinical characteristics was also analyzed in our study. It was found that the high CRP/Alb ratio subgroup had significantly higher NLR, PLR, and pretreatment EBV-DNA level and lower hemoglobin level compared with the low CRP/Alb ratio subgroup. Based on these findings, it was suggested that the CRP/Alb ratio could not only reflect the individual's systematic inflammatory response but also perform as an indicator of nutritional status.
However, inconsistent with the results of Xu et al. [33] and Wei et al. [45] studies, our data did not show a significant association between GPS and the CRP/Alb ratio. From this point, it was suggested that the combination of GPS and the CRP/Alb ratio might be more informative on inflammation and nutrition than either one alone in NPC patients.
In contrast to localized NPC with curative treatment, the prognostic factors were less identified in metastatic NPC. Plasma EBV-DNA load has been long established as a strong indicator of tumor burden and as a reliable prognostic factor in both disseminated NPC and localized NPC [4, 21, 24]. In line with these data, high pretreatment EBV-DNA level was also found to be an unfavourable prognostic factor in our study. Besides EBV-DNA level, other prognostic factors such as LDH level [21, 22] and NLR [26] have been identified in metastatic NPC. Baseline LDH level, haemoglobin level, number of metastasis site, NLR, and PLR were also analyzed in the current study and showed a close association with the CRP/Alb ratio. However, they failed to independently predict prognosis in the multivariate analysis, indicating that they reflect tumor burden less sensitively and less precisely than the CRP/Alb ratio.
The optimal cutoff value for the CRP/Alb ratio varied in different studies. Even in the two studies of esophageal squamous cell carcinoma (ESCC), the cutoff values were different. In the study by Xu et al. [33], the cutoff was set as 0.50, which was much higher than that in the study by Wei et al. (0.095) [45], due to different methods and different patient population. In our study, we used the same method as Wei et al. [45] and determined the cutoff value as 0.189. In the study investigating the CRP/Alb ratio in nonmetastatic NPC, 0.064 was determined as the optimal cutoff value [36].
Generally speaking, there were three main superiorities in this study. First, we explored the association of the CRP/Alb ratio with treatment response and chemotherapy regimen, which was not always discussed in previous studies. Second, all patients underwent at least two cycles of cisplatin-based chemotherapy, and the heterogeneity of therapeutic options might minimize the potential interference from the variation of different treatments. By contrast, most of previous studies included patients with noncisplatin chemotherapy. Xia et al. [25] enrolled two patients who underwent sorafenib, which was not recommended as standard first-line treatment. In the study by Jin et al. [21], 5% of the patients received monotherapy with capecitabine for their poor performance status. Toh et al. [20] did not describe the detailed treatment for their study cohorts, and 40% of the patients did not undergo any salvage chemotherapy.
Since it could be easily calculated from the routine clinical laboratory tests, testing the CRP/Alb ratio could be used as a simple, feasible, inexpensive method to predict prognosis and to monitor the dynamic change of tumor burden. It might be possible that early management of systematic inflammation and nutritional support could increase the survival of patients with disseminated NPC. Since the nonsteroidal anti-inflammatory drugs (NSAIDs) have showed promising effect on preventing carcinogenesis and reducing the risk of death in various cancers [46–49], NSAIDs might be a feasible and convenient drug to modulate the inflammation of NPC patients. Moreover, whether dynamic change of the CRP/Alb ratio during the treatment course could predict prognosis and treatment response remains unknown. Future prospective clinical trials and deeper basic research could address these questions and provide more reliable molecular and genetic mechanisms regarding the CRP/Alb ratio.
There were several limitations to be acknowledged in this study. First, this was a retrospective study limited to a single institute. Second, another independent cohort was not introduced to validate the novel prognostic index. Finally, we could not provide complete information on disease-free interval (DFI), which was previously established as an important prognostic factor and thus did not further discuss it in our study. All the inferiorities should be overcome by prospective, multicentre studies.
5. Conclusion
In summary, our study demonstrated that the CRP/Alb ratio was an independent prognostic factor in metastatic NPC. The CRP and Alb levels could be easily, feasibly, inexpensively examined and therefore it is convenient to use the CRP/Alb ratio in our clinical practice to evaluate systematic inflammation and predicate the survival outcome in metastatic NPC. In addition, we proposed a new prognostic score system based on both the GPS and the CRP/Alb ratio. Prospective multicentre studies are warranted to validate this model and to further explore the underlying mechanisms.
Acknowledgments
The authors feel deep gratitude to all the staff members in their department for their support and suggestion in this study.
Abbreviations
- ALP:
Alkaline phosphatase
- CRP/Alb:
The ratio of CRP/albumin
- CRP:
C-reactive protein
- DFI:
Disease-free interval
- EBV:
Epstein-Barr virus
- ESCC:
Esophageal squamous cell carcinoma
- GPS:
Glasgow Prognostic Score
- HRs:
Hazard ratios
- IMRT:
Intensity modulated radiotherapy technology
- KPS:
Karnofsky Performance Scores
- LDH:
Lactate dehydrogenase
- mGPS:
Modified Glasgow Prognostic Score
- MLR:
Monocyte to lymphocyte ratio
- NLR:
Neutropil to lymphocyte ratio
- NPC:
Nasopharyngeal carcinoma
- NSAIDs:
Nonsteroidal anti-inflammatory drugs
- PF:
Cisplatin plus 5-fluorouracil
- PLR:
Platelet-to-lymphocyte ratio
- PS:
Performance status
- RECIST:
Response Evaluation Criteria in Solid Tumors
- TNM:
Tumor-node-metastasis
- TP:
Paclitaxel plus cisplatin
- TPF:
Paclitaxel plus cisplatin plus 5-fluorouracil.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Peng Sun and Cui Chen collected clinical data and drafted the manuscript. Yi Xia, Xiwen Bi, and Fei Zhang participated in the design of the study. Hang Yang, Panpan Liu, and Xin An performed the statistical analysis. Fenghua Wang and Wenqi Jiang conceived the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript. Peng Sun, Cui Chen, and Yi Xia contributed equally to this work.
References
- 1.Chen W., Zheng R., Baade P. D., et al. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians. 2016;66(2):115–132. doi: 10.1016/j.ijscr.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 2.Chang E. T., Adami H. O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiology, Biomarkers & Prevention. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 3.Yu M. C., Yuan J. M. Epidemiology of nasopharyngeal carcinoma. Seminars in Cancer Biology. 2002;12(6):421–429. doi: 10.1016/S1044579X02000858. [DOI] [PubMed] [Google Scholar]
- 4.Sun P., Chen C., Cheng Y. K., et al. Serologic biomarkers of Epstein-Barr virus correlate with TNM classification according to the seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma. European Archives of Oto-Rhino-Laryngology. 2014;271(9):2545–2554. doi: 10.1007/s00405-013-2805-5. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y., Tang L. L., Chen L., et al. Promising treatment outcomes of intensity-modulated radiation therapy for nasopharyngeal carcinoma patients with N0 disease according to the seventh edition of the AJCC staging system. BMC Cancer. 2012;12(1):p. 68. doi: 10.1186/1471-2407-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L., Chen Q. Y., Liu H., Tang L. Q., Mai H. Q. Emerging treatment options for nasopharyngeal carcinoma. Drug Design, Development and Therapy. 2013;7(4):37–52. doi: 10.2147/DDDT.S30753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rottey S., Madani I., Deron P., Van Belle S. Modern treatment for nasopharyngeal carcinoma: current status and prospects. Current Opinion in Oncology. 2011;23(3):254–258. doi: 10.1097/CCO.0b013e328344f527. [DOI] [PubMed] [Google Scholar]
- 8.Bensouda Y., Kaikani W., Ahbeddou N., et al. Treatment for metastatic nasopharyngeal carcinoma. European Annals of Otorhinolaryngology, Head and Neck Diseases. 2011;128(2):79–85. doi: 10.1016/j.anorl.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Ma J., Wen Z. S., Lin P., Wang X., Xie F. Y. The results and prognosis of different treatment modalities for solitary metastatic lung tumor from nasopharyngeal carcinoma: a retrospective study of 105 cases. Chinese Journal of Cancer. 2010;29(9):787–795. doi: 10.5732/cjc.010.10098. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y., Shi Y. X., Cai X. Y., et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. Journal of Cancer Research and Clinical Oncology. 2012;138(10):1717–1725. doi: 10.1007/s00432-012-1219-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen C., Wang F. H., An X., et al. Triplet combination with paclitaxel, cisplatin and 5-FU is effective in metastatic and/or recurrent nasopharyngeal carcinoma. Cancer Chemotherapy and Pharmacology. 2013;71(2):371–378. doi: 10.1007/s00280-012-2020-x. [DOI] [PubMed] [Google Scholar]
- 12.Chen C., Wang F. H., Wang Z. Q., et al. Salvage gemcitabine-vinorelbine chemotherapy in patients with metastatic nasopharyngeal carcinoma pretreated with platinum-based chemotherapy. Oral Oncology. 2012;48(11):1146–1151. doi: 10.1016/j.oraloncology.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H., Xia W., Lu X., et al. A novel statistical prognostic score model that includes serum CXCL5 levels and clinical classification predicts risk of disease progression and survival of nasopharyngeal carcinoma patients. PLoS One. 2013;8(2, article e57830) doi: 10.1371/journal.pone.0057830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng S. H., Tsai S. Y., Horng C. F., et al. A prognostic scoring system for locoregional control in nasopharyngeal carcinoma following conformal radiotherapy. International Journal of Radiation Oncology, Biology, Physics. 2006;66(4):992–1003. doi: 10.1016/j.ijrobp.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Lee C. C., Huang T. T., Lee M. S., et al. Clinical application of tumor volume in advanced nasopharyngeal carcinoma to predict outcome. Radiation Oncology. 2010;5(1):p. 20. doi: 10.1186/1748-717X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong Y. K., Heng D. M., Chung B., et al. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. European Journal of Cancer. 2003;39(11):1535–1541. doi: 10.1016/S0959-8049(03)00310-1. [DOI] [PubMed] [Google Scholar]
- 17.Ruan H. L., Qin H. D., Shugart Y. Y., et al. Developing genetic epidemiological models to predict risk for nasopharyngeal carcinoma in high-risk population of China. PLoS One. 2013;8(2, article e56128) doi: 10.1371/journal.pone.0056128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L., Liu L. Z., Chen M., et al. Prognostic value of subclassification using MRI in the t4 classification nasopharyngeal carcinoma intensity-modulated radiotherapy treatment. International Journal of Radiation Oncology, Biology, Physics. 2012;84(1):196–202. doi: 10.1016/j.ijrobp.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Li J. X., Huang S. M., Wen B. X., Lu T. X. Prognostic factors on overall survival of newly diagnosed metastatic nasopharyngeal carcinoma. Asian Pacific Journal of Cancer Prevention. 2014;15(7):3169–3173. doi: 10.7314/APJCP.2014.15.7.3169. [DOI] [PubMed] [Google Scholar]
- 20.Toh C. K., Heng D., Ong Y. K., Leong S. S., Wee J., Tan E. H. Validation of a new prognostic index score for disseminated nasopharyngeal carcinoma. British Journal of Cancer. 2005;92(8):1382–1387. doi: 10.1038/sj.bjc.6602525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y., Cai X. Y., Cai Y. C., et al. To build a prognostic score model containing indispensible tumour markers for metastatic nasopharyngeal carcinoma in an epidemic area. European Journal of Cancer. 2012;48(6):882–888. doi: 10.1016/j.ejca.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Jin Y., Ye X., Shao L., et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. European Journal of Cancer. 2013;49(7):1619–1626. doi: 10.1016/j.ejca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Wang W. Y., Twu C. W., Chen H. H., et al. Plasma EBV DNA clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clinical Cancer Research. 2010;16(3):1016–1024. doi: 10.1158/1078-0432.CCR-09-2796. [DOI] [PubMed] [Google Scholar]
- 24.Hsu C. L., Chang K. P., Lin C. Y., et al. Plasma Epstein-Barr virus DNA concentration and clearance rate as novel prognostic factors for metastatic nasopharyngeal carcinoma. Head & Neck. 2012;34(8):1064–1070. doi: 10.1002/hed.21890. [DOI] [PubMed] [Google Scholar]
- 25.Xia W. X., Ye Y. F., Lu X., et al. The impact of baseline serum C-reactive protein and C-reactive protein kinetics on the prognosis of metastatic nasopharyngeal carcinoma patients treated with palliative chemotherapy. PLoS One. 2013;8(10, article e76958) doi: 10.1371/journal.pone.0076958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C., Sun P., Dai Q. S., Weng H. W., Li H. P., Ye S. The Glasgow Prognostic Score predicts poor survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. PLoS One. 2014;9(11, article e112581) doi: 10.1371/journal.pone.0112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou T., Hong S., Hu Z., et al. A systemic inflammation-based prognostic scores (mGPS) predicts overall survival of patients with small-cell lung cancer. Tumour Biology. 2015;36(1):337–343. doi: 10.1007/s13277-014-2623-4. [DOI] [PubMed] [Google Scholar]
- 28.Lin G. N., Peng J. W., Liu D. Y., Xiao J. J., Chen Y. Q., Chen X. Q. Increased lymphocyte to monocyte ratio is associated with better prognosis in patients with newly diagnosed metastatic nasopharyngeal carcinoma receiving chemotherapy. Tumour Biology. 2014;35(11):10849–10854. doi: 10.1007/s13277-014-2362-6. [DOI] [PubMed] [Google Scholar]
- 29.Jiang R., Cai X. Y., Yang Z. H., et al. Elevated peripheral blood lymphocyte-to-monocyte ratio predicts a favorable prognosis in the patients with metastatic nasopharyngeal carcinoma. Chinese Journal of Cancer. 2015;34(6):237–246. doi: 10.1186/s40880-015-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra R., Marcelli D., von Gersdorff G., et al. Relationship of neutrophil-to-lymphocyte ratio and serum albumin levels with C-reactive protein in hemodialysis patients: results from 2 international cohort studies. Nephron. 2015;130(4):263–270. doi: 10.1159/000437005. [DOI] [PubMed] [Google Scholar]
- 31.Kim M. H., Ahn J. Y., Song J. E., et al. The C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PLoS One. 2015;10(7, article e0132109) doi: 10.1371/journal.pone.0132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X., Sun X., Liu J., et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Translational Oncology. 2015;8(4):339–345. doi: 10.1016/j.tranon.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X. L., Yu H. Q., Hu W., Song Q., Mao W. M. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One. 2015;10(9, artcle e0138657) doi: 10.1371/journal.pone.0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishizuka M., Nagata H., Takagi K., Iwasaki Y., Shibuya N., Kubota K. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Annals of Surgical Oncology. 2016;23(3):900–907. doi: 10.1245/s10434-015-4948-7. [DOI] [PubMed] [Google Scholar]
- 35.Zhou T., Zhan J., Hong S., et al. Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Scientific Reports. 2015;5:p. 10481. doi: 10.1038/srep10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He S., Wang Y., Chen H., et al. C-reactive protein/albumin ratio (CAR) as a prognostic factor in patients with non-metastatic nasopharyngeal carcinoma. Journal of Cancer. 2016;7(15):2360–2366. doi: 10.7150/jca.16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Budczies J., Klauschen F., Sinn B. V., et al. Cutoff Finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7(12, article e51862) doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 39.Xia W. X., Zhang H. B., Shi J. L., et al. A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment serum C-reactive protein and N-classification. European Journal of Cancer. 2013;49(9):2152–2160. doi: 10.1016/j.ejca.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Grimm T., Buchner A., Schneevoigt B., et al. Impact of preoperative hemoglobin and CRP levels on cancer-specific survival in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: results of a single-center study. World Journal of Urology. 2015;34(5):703–708. doi: 10.1007/s00345-015-1680-7. [DOI] [PubMed] [Google Scholar]
- 41.Haas M., Laubender R. P., Stieber P., et al. Prognostic relevance of CA 19-9, CEA, CRP, and LDH kinetics in patients treated with palliative second-line therapy for advanced pancreatic cancer. Tumour Biology. 2010;31(4):351–357. doi: 10.1007/s13277-010-0044-6. [DOI] [PubMed] [Google Scholar]
- 42.Koukourakis M. I., Kambouromiti G., Pitsiava D., Tsousou P., Tsiarkatsi M., Kartalis G. Serum C-reactive protein (CRP) levels in cancer patients are linked with tumor burden and are reduced by anti-hypertensive medication. Inflammation. 2009;32(3):169–175. doi: 10.1007/s10753-009-9116-4. [DOI] [PubMed] [Google Scholar]
- 43.Asher V., Lee J., Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Medical Oncology. 2012;29(3):2005–2009. doi: 10.1007/s12032-011-0019-5. [DOI] [PubMed] [Google Scholar]
- 44.Chen Z., Shao Y., Fan M., et al. Prognostic significance of preoperative C-reactive protein: albumin ratio in patients with clear cell renal cell carcinoma. International Journal of Clinical and Experimental Pathology. 2015;8(11):14893–14900. [PMC free article] [PubMed] [Google Scholar]
- 45.Wei X. L., Wang F. H., Zhang D. S., et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer. 2015;15:p. 350. doi: 10.1186/s12885-015-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nan H., Hutter C. M., Lin Y., et al. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. Jama. 2015;313(11):1133–1142. doi: 10.1001/jama.2015.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wender R. C. Aspirin and NSAID chemoprevention, gene-environment interactions, and risk of colorectal cancer. Jama. 2015;313(11):1111–1112. doi: 10.1001/jama.2015.1032. [DOI] [PubMed] [Google Scholar]
- 48.Johnson C. C., Jankowski M., Rolnick S., Yood M. U., Alford S. H. Influence of NSAID use among colorectal cancer survivors on cancer outcomes. American Journal of Clinical Oncology. 2014 doi: 10.1097/COC.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brasky T. M., Bonner M. R., Moysich K. B., et al. Non-steroidal anti-inflammatory drug (NSAID) use and breast cancer risk in the Western New York Exposures and Breast Cancer (WEB) study. Cancer Causes & Control. 2010;21(9):1503–1512. doi: 10.1007/s10552-010-9579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]