Abstract
Programmed cell clearance is a highly regulated physiological process of elimination of dying cells that occurs rapidly and efficiently in healthy organisms. It thus ensures proper development as well as homeostasis. Recent studies have disclosed a considerable degree of conservation of cell clearance pathways between nematodes and higher organisms. The externalization of the anionic phospholipid phosphatidylserine (PS) has emerged as an important “eat-me” signal for phagocytes and its exposition on apoptotic cells is controlled by phospholipid translocases and scramblases. However, there is mounting evidence that PS exposure occurs not only in apoptosis, but may also be actively expressed on the surface of cells undergoing other forms of cell death including necrosis; PS is also expressed on the surface of engulfing cells. Additionally, PS may act as a “save-me” signal during axonal regeneration. Here we discuss mechanisms of PS exposure and its recognition by phagocytes as well as the consequences of PS signaling in nematodes and in mammals.
Keywords: phosphatidylserine, engulfment, translocases, scramblases, axonal fusion, C. elegans
“The eagle picks my eye
The worm he licks my bone
I feel so suicidal
Just like Dylan’s Mr. Jones”
From: The Beatles (White Album) (1968)
1. Introduction
Programmed cell clearance – a term describing the mechanism and consequences of apoptotic cell clearance – is a well conserved process of apoptotic cell removal that is critical for development, tissue renewal and homeostasis [1]. In the adult organism it is important not only that the rate of cell division and cell death is balanced, but also that efficient clearance of dying cells takes place [2,3]. An imbalance of these processes is associated with inflammation and with the pathogenesis and progression of several diseases ranging from cancer to autoimmune diseases [4]. Dying cells signal their status to neighboring cells and the specific and efficient recognition of those signals by phagocytes is important to prevent further harm. In this scenario phagocytic cells are attracted towards the dying cell via the secretion of so called “find-me” signals which cause migration of the phagocytic cell [5]. In a second step recognition occurs via specific receptors expressed on the phagocytic cell and the corresponding ligands – or “eat-me” signals – on the dying cell [6]. This recognition can occur either directly or can be facilitated by so-called bridging molecules. After engulfment the phagocytic cell digests the dying cell via the endo-lysosomal pathway. The consequences of cell clearance are manifold; engulfment of dying cells is not merely a form of ‘waste disposal’, but also serves to instruct other neighboring cells and the immune system [7].
There are several different forms of (programmed) cell death which can be defined by specific morphological and/or molecular characteristics and corresponding biochemical processes (e.g. activation of caspases, activation of specific kinases). However, it is not fully understood how phagocytes recognize and distinguish between different types of cell death. This is especially interesting when considering that some signaling molecules feature prominently in more than one type of cell death. It is, however, likely that several “eat-me” signals cooperate and that a complex network of different ligands and receptors ensures efficient clearance and a proper immunological response to dying cells. Due to the high conservation of cell death and cell clearance pathways between nematodes and mammals, C. elegans has emerged as a model organism to study cell death and to help us understand cell clearance mechanisms as well as the cause of diseases associated with a deregulation of these pathways.
2. New skin for the old ceremony: definition of cell death
Dying cells are likely oblivious to the nature or molecular definition of their own demise. However, since 2005, the Nomenclature Committee on Cell Death (NCCD) has published several sets of recommendations for definitions of various cell death routines [8–11]. Interestingly, the approach taken by this expert committee has changed over the years. In the first report, it was noted that different cell death types were previously defined by morphological criteria and that mechanism-based definitions of cell death were largely missing [8]. Over the years, considerable emphasis has been placed on identifying measurable biochemical features which could serve as a basis for classification, instead of distinguishing between different forms of cell death based only on morphological criteria [9]. In the 2012 report, the number of potential subroutines had expanded to encompass more than one dozen different modes of “regulated” cell death [10]. Most recently, the NCCD has proposed the existence of two broad and mutually exclusive categories of cell death: accidental cell death and regulated cell death. Efforts were also made to define and to discriminate between essential and accessory aspects of cell death; in other words, whether cell death is actually occurring versus the biochemical (or morphological) manifestations of cell death [11].
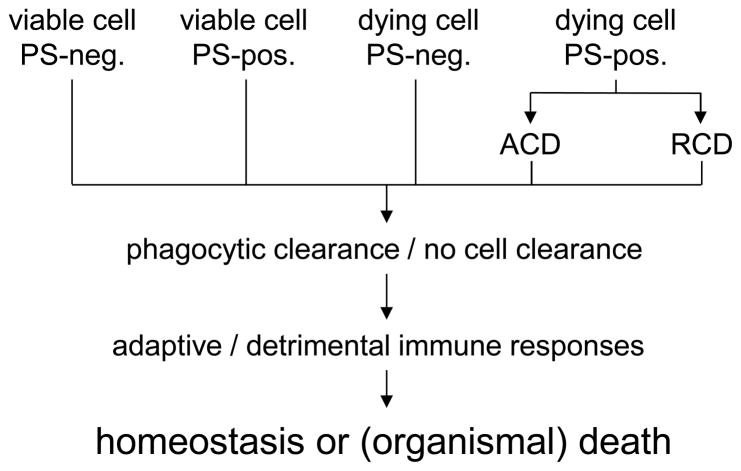
According to the 2015 iteration of the NCCD recommendations, accidental cell death (ACD) cannot be suppressed by pharmacological or genetic means while regulated cell death (RCD) can be inhibited [11]. RCD can either be initiated by environmental factors or can be part of embryonic development, tissue homeostasis, or the immune response. Importantly, different forms of cell death may share certain common features. Hence, blocking one cell death pathway may result in the cell undergoing another type of cell death. The cell death program is further divided into three stages - a reversible initiator phase that aims for repair and adaption to stress situations, an irreversible execution phase, and a propagation phase including the outcome and response to the RCD. Cytoprotection should therefore address the initiation of RCD and inhibit the propagation [11]. However, a problem with this approach to cell death classification is that the final outcome – whether dying cells are recognized and cleared or not – and the consequences of cell clearance, are not obviously in focus. The organism may not care whether cells are undergoing a particularly sophisticated subroutine of cell death: what ultimately matters at the organismal level is whether cell death will trigger tissue damage or repair and whether it will lead to homeostasis or disease (Fig. 1). It is interesting to note that several different subroutines of ACD and RCD all appear to share a common feature, namely the exposition of phosphatidylserine (PS) on the cell surface. In fact, while PS exposure was thought to be a near-universal marker of apoptotic cell death, studies in recent years have shown that PS is actively exposed on the surface of cells undergoing other forms of cell death as well. In the following section, some of the currently recognized forms of cell death are highlighted. We also discuss whether or not PS is exposed in these cell deaths.
Fig. 1. Reflections on the definition of cell death.
According to the conventional view, cells undergoing apoptotic cell death display phosphatidylserine (PS) on the cell surface, while viable cells do not. PS exposure is thought to act as a unique signal for cell clearance by phagocytes. However, several studies have shown that PS exposure may also occur in cells undergoing other forms of cell death (e.g., necrosis); moreover, PS exposure appears to play a role also in non-cell death related processes such as axonal fusion. This diagram illustrates different (hypothetical) scenarios, i.e., viable cells, or cells undergoing accidental or regulated modes of cell death, with/without PS exposure, and the downstream consequences, namely cell clearance or other outcomes. Further research is needed to understand how phagocytes distinguish between viable cells and dying cells, and whether they “sense” different forms of cell death. This has ramifications for the definition of different modes of cell death; whether or not cells are cleared should be incorporated into any (functional) definition of cell death.
Necrotic cell death is viewed as an accidental form of cell death associated with rupture of the cell membrane. This causes the release of intracellular components – referred to as damage-associated molecular patterns (DAMPs) – which cause inflammation and tissue damage. Cells undergoing certain forms of necrosis were shown to display PS and to undergo PS-dependent clearance and evidence was provided that macrophages preferentially engulfed the necrotic cells versus certain apoptotic cells [12,13]. However, despite these early observations, the common notion has been that PS exposure is associated with apoptosis. Indeed, PS exposure is often used as a (surrogate) marker for apoptotic cell death, although the two events are not always linked [14].
Apoptosis is known as a physiological form of cell death taking place during embryonic development as well as in the adult organism and serves to control tissue homeostasis [15]. Traditionally, two major pathways of apoptosis induction are distinguished, extrinsic (death receptor-mediated) and intrinsic (mitochondria-mediated) apoptosis. The former is characterized by the binding of a death ligand to its receptor on the cell surface followed by caspase-8/caspase-3 activation while the latter pathway is defined by mitochondrial outer membrane permeabilization with release of pro-apoptotic factors, such as cytochrome c, and activation of caspase-9/caspase-3. Disruption of the asymmetric distribution of phospholipids in the plasma membrane and subsequent exposure of PS on the cell surface is commonly seen in cells undergoing apoptosis [16]. However, distinct modes of macrophage recognition of apoptotic and necrotic cells not determined only by PS exposure have been identified [17]. In other words, while PS is externalized on both apoptotic and necrotic cells, it is not a specific recognition ligand of either one. Thus, more research is needed to understand the signals for cell clearance. As has been emphasized before, cell clearance defines the “meaning” of cell death [3]. However, we also need to understand the “language” that gives meaning to cell death: the intra- and extracellular signals that drive programmed cell clearance.
Pyroptosis refers to a form of regulated cell death of infected macrophages characterized by the activation of caspase-1 and the production of the pro-inflammatory cytokines IL-1β and IL-18. Recent studies showed that externalized PS serves as an “eat-me” signal for pyroptotic cell clearance and that pyroptotic cells release macrophage attractant “find-me” signals [18]. In an interesting twist, a recent study has shown that macrophages undergoing pyroptosis are able to “trap” bacteria within the cellular debris and that neutrophils are responsible for clearance of the trapped bacteria in a scavenger receptor-dependent (and PS-independent) manner [19], suggesting that the mechanisms of clearance of dying cells and cell debris are different.
Necroptosis – a form of regulated necrosis – is defined by the activation of the RIPK1/3 signaling pathway [20]. Furthermore, MLKL has been shown to be critical for necroptotic cell membrane rupture. The loss of membrane integrity and the subsequent exposure of intracellular components to the extracellular environment causes inflammation and tissue damage. Caspase-independent PS exposure occurs in TNFα-triggered necroptosis [21]. However, the mechanism underlying necroptotic PS exposure is not yet known.
Ferroptosis is defined as an iron- and glutathione peroxidase 4 (GPX4)-dependent form of RCD that is morphologically, biochemically and genetically distinct from apoptosis and necrosis [22]. Lipid peroxidation and an iron-dependent increase of intracellular ROS are common features of ferroptosis. Whether or not cells undergoing ferroptosis display PS and/or undergo PS-dependent phagocytosis, or whether other lipid or protein signals are involved remains unknown, but this will be interesting to study.
3. Conservation of cell clearance pathways: focus on PS exposure
The nematode C. elegans is a well-established model organism to study cell death [23]. The origin and fate of each cell has been described in detail and several key modulators of the cell clearance process have been shown to have homologs in mammals [24]. However, it is important to keep in mind that C. elegans does not have an immune system and that the clearance of dying cells is performed by neighboring cells rather than professional phagocytes. Notwithstanding, the worm has proven to be a ‘most valuable player’ in the field of cell death and cell clearance research.
Genetic studies defined two major signal transduction pathways that act in a partially redundant manner to control cell corpse engulfment [24]. The first pathway includes CED-6 and CED-7, and the phagocytosis receptor, CED-1. The second pathway involves CED-2, CED-5, CED-10, and CED-12, and these proteins, and their mammalian counterparts, constitute a Rac GTPase signaling pathway. The two pathways may converge on CED-10 to mediate the cytoskeletal rearrangements required for cell clearance [25]. PSR-1, the nematode homolog of the mammalian PS receptor, was shown to act as an upstream receptor in the signaling pathway containing CED-2/CED-5/CED-10/CED-12 [26]. The regulation of apoptotic PS exposure, and the conservation of the underlying pathways between nematodes and mammals, is discussed in further detail in this section.
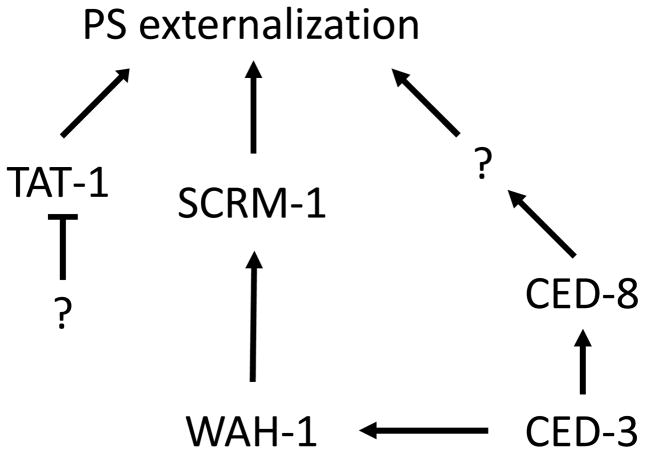
In viable cells, the anionic phospholipid, PS is restricted to the inner leaflet of the plasma membrane [16]. During apoptosis, PS is externalized from the inner leaflet of the plasma membrane to the surface of dying cells and serves as an “eat-me” signal to trigger clearance of apoptotic cells [27,28]. The exposure of PS during apoptosis is thought to result from the inactivation of aminophospholipid translocases that actively transport PS from the outer to the inner leaflet of the plasma membrane in a living cell, and the concomitant activation of phospholipid scramblases responsible for the bidirectional movement of phospholipids in the plasma membrane [29]. In addition, early work reported 20 years ago provided evidence for a role of proteases including caspases (or, ICE-like proteases, according to the nomenclature at the time) in PS externalization during apoptosis [30, 31]. However, the precise molecular regulation of cell surface exposure of PS during apoptosis has remained poorly understood. Recent studies in C. elegans suggest that two classes of lipid transporters, the phospholipid scramblases and the aminophospholipid translocases, could be involved in externalizing PS during apoptosis [32,33]. First, inactivation of scrm-1, which encodes the C. elegans phospholipid scramblase 1, reduces PS exposure on the surface of C. elegans apoptotic germ cells. Interestingly, WAH-1, a worm homolog of human apoptosis-inducing factor (AIF), is also important for PS exposure in C. elegans apoptotic germ cells [33]. WAH-1, a mitochondrial protein, is released from mitochondria during apoptosis through a CED-3 caspase-dependent mechanism and a portion of released WAH-1 likely relocates to the plasma membrane, where it binds to SCRM-1 and strongly activates the bidirectional phospholipid scrambling activity of SCRM-1 (Fig. 2), leading to PS externalization in dying cells [33]. However, loss of either scrm-1 or wah-1 only causes partial reduction of PS exposure in apoptotic germ cells and a mild engulfment defect, suggesting that additional genes are involved in PS externalization during apoptosis. Recently, two studies suggested that the C. elegans ced-8 gene and its human homologue Xkr8 (XK transporter related protein 8) play an important role in apoptotic PS externalization [34,35]. CED-8, a multi-pass transmembrane protein, is cleaved at its amino-terminus by the CED-3 caspase during apoptosis to generate an activated form of CED-8 (acCED-8) without its first 21 amino acids [34]. Notably, acCED-8 is both necessary and sufficient to promote PS externalization in C. elegans and may function by regulating the activity of other lipid-translocating proteins (Fig. 2). Interestingly, mammalian Xkr8 is cleaved by caspases during apoptosis at its carboxyl terminus to promote PS externalization, although it is unclear how the caspase cleavage activates Xkr8 or apoptotic PS externalization [35]. On the other hand, inactivation of the C. elegans aminophospholipid translocase TAT-1, a class IV P-type ATPase, results in PS externalization in every cell in C. elegans [32], indicating that TAT-1 plays an essential role in maintaining plasma membrane PS asymmetry. Consequently, loss of tat-1 causes random removal of living cells by neighboring phagocytes through phagocytic mechanisms that are dependent on two PS-recognizing receptors, CED-1 and PSR-1 [32]. A recent study in mammals confirms this finding and shows that inactivation of CDC50A, a co-factor of human TAT-1 homologue, ATP11C, causes ectopic exposure of PS in living cells and their removal through phagocytosis [36]. Interestingly, ATP11C is also cleaved and inactivated by caspases during apoptosis, which contributes to apoptotic PS externalization. Further evidence for the conservation of the signaling pathways leading to PS externalization is provided by the recent observation that AIF is released from mitochondria and promotes scramblase activation and subsequent PS exposure at the plasma membrane in mammalian cells undergoing Fas/APO-1/CD95-triggered apoptosis [37]. It could be argued, therefore, that AIF, or its worm homolog, WAH-1, is not only an “apoptosis-inducing factor”, but also a “phagocytosis-inducing factor”, as it plays a role in promoting PS-dependent cell clearance (Fig. 3). Indeed, depending on its subcellular localization – in mitochondria, in the nucleus, in lipid raft domains in the plasma membrane – AIF (WAH-1) may exert distinct functions related to the life and death of the cell.
Fig. 2. Multiple factors promote PS externalization during apoptosis.
Inhibition of the TAT-1 aminophospholipid translocase activity, activation of the SCRM-1 phospholipid scramblase activity by WAH-1, and cleavage and activation of the CED-8 protein by CED-3 all contribute to PS exposure on the cell surface during C. elegans apoptosis.
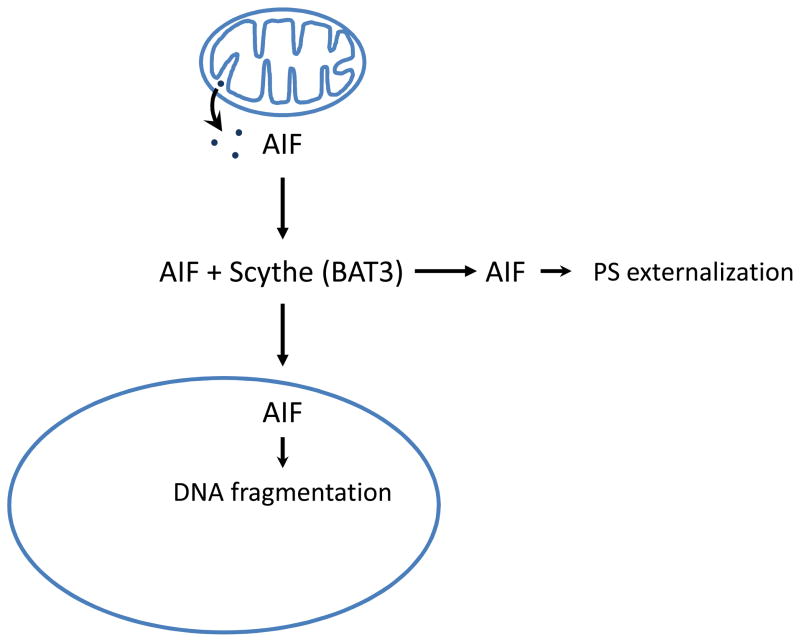
Fig. 3. Extramitochondrial roles for apoptosis-inducing factor, AIF.
AIF is an oxidoreductase residing in the intermembrane space of mitochondria. Upon apoptosis induction, AIF is released into the cytosol and translocates to the nucleus where it mediates large-scale DNA fragmentation. Recent studies have shown that a portion of AIF also translocates to lipid rafts in the plasma membrane where it promotes phospholipid scrambling and PS exposure, leading to engulfment by phagocytic cells. Scythe, released from the cell nucleus during apoptosis, appears to stabilize AIF in the cytosol [37].
Chung et al. [38] found that all ced genes known to be required for the engulfment of apoptotic cell corpses were also required for efficient elimination of cell corpses generated by necrosis-inducing stimuli, thus suggesting that a common set of engulfment genes mediate cell removal irrespective of the mode of cell death. More recent studies have provided evidence that PS exposure serves as a common “eat-me” signal for cells dying by necrosis and apoptosis [39]. Indeed, in contrast to common belief, PS was actively exposed on the outer surface of touch neurons dying by necrosis prior to the disintegration of the plasma membrane. Importantly, the authors identified two pathways for PS exposure in necrotic cells, of which one was shared with cells undergoing apoptosis, while the other was found to be unique to necrotic cells. The common pathway for PS exposure may involve CED-7, a member of the ATP-binding cassette (ABC) transporter family. The second pathway included anoctamin homolog-1 (ANOH-1) which promoted PS exposure in necrotic, but not apoptotic cells, in a calcium-dependent manner [39]. These data show that both shared and unique pathways exist for the presentation of a common “eat-me” signal. Notably, TMEM16F, a member of the mammalian family of anoctamins, promotes PS exposure in response to calcium ionophores [40], but not to apoptotic stimuli [41].
4. Recognition of apoptotic cells: from exposure to transfer of PS
Surface exposed PS is the most extensively studied “eat-me” signal [42,43] and represents a highly conserved signal for cell clearance [44]. PS is recognized by a broad range of receptors on macrophages and other phagocytes as well as by soluble bridging molecules that bind both to PS and to cell surface receptors on phagocytes (reviewed in [5,6]). Although PS exposure has commonly been viewed as a specific marker of apoptotic cells, recent research has revealed that PS is not only used as an “eat-me” signal by apoptotic cells, but is also exposed during other forms of cell death [45]. Despite the fact that both apoptotic and necrotic cells evidently utilize PS as an “eat-me” signal, their recognition and clearance appears to occur via distinct and noncompeting mechanisms, leading to different immunological responses [17]. Therefore, the question arises whether PS exposure is a unique signal for apoptotic cell clearance; and if this is not the case, then one may ask how does the immune system distinguish between different types of cell death? Consequently, it might be logical to assume that there are other signals involved – including the masking or unmasking of “eat-me” signals as well as “don’t-eat-me” signals – that help phagocytic cells to discriminate between different types of cell death. Multiple and redundant pathways and molecules may operate in parallel and with different specificity to facilitate recognition and cell clearance as well as an appropriate immune response. Moreover, it may be pertinent to recognize that different forms of cell death could partly overlap insofar as they might share elements of the same signaling pathways and/or express the same signals for cell clearance. The immune system, therefore, may have to decode a combination of signals emanating from cells undergoing different forms of cell death (sequentially or concurrently) and then “decide” how to respond.
In addition to asking whether PS exposure is specific or not for a certain form of cell death, one may also ask whether PS exposure is sufficient as a recognition signal for phagocytes. Previous studies using mammalian model systems have shown that masking of externalized PS on apoptotic cells by antibodies or by using PS-binding proteins resulted in reduced uptake by macrophages, proving that PS itself is a signal for engulfment [43]. Cells that undergo apoptosis without PS externalization are not efficiently engulfed by macrophages [46,47]. However, certain populations of viable cells constitutively expose PS yet they are not good targets for engulfment [48]. On the other hand, other studies have shown that the enrichment of the outer leaflet of the plasma membrane of non-apoptotic cells with PS promotes their engulfment by macrophages [47]. Taken together, it is possible that PS exposure may be required, but not sufficient for engulfment. It is also possible that discrepancies between different studies could depend on the use of different populations of phagocytes, with different repertoires of PS-binding receptors. Nonetheless, if we focus our attention on the target cell, there are several potential explanations for these opposing observations. First, it is possible that other “eat-me” signals are expressed during cell death and that the combination of different signals (on the cell surface or emitted as soluble factors) together with PS is required for efficient phagocytosis. For instance, the secretion of annexin I may serve as an auxiliary recognition signal for macrophages [49]. Furthermore, selective oxidation of PS has been demonstrated to occur in apoptosis and could serve to promote the selective recognition of these cells [47]. Second, it was shown that viable cells express “don’t-eat-me” signals such as CD31 or CD47 [50,51]. The downregulation (or masking) of “don’t-eat-me” signals, in combination with PS exposure, might be required for efficient phagocytosis by macrophages. Third, it is conceivable that macrophages have a sensitivity threshold for PS externalized on the cell surface that provides for the distinction between viable cells with low levels of externalized PS and apoptotic cells with elevated levels of PS [52]. Additionally, the mobility of PS in the plasma membrane and the resulting density of the signal on the cell surface may differ markedly between viable and apoptotic cells, and surface exposure of PS in the absence of clustering may prevent recognition by phagocytes [53].
In C. elegans, apoptotic cells are engulfed by their neighboring cells, which include hypodermal cells, muscle cells, intestinal cells, and gonadal sheath cells [54,55]. Interestingly, PS exposure is detected not only on the surface of apoptotic cells, but also on the surface of engulfing cells [56,57]. Genetic analyses have indicated that components in one of the two major engulfment pathways in C. elegans, comprising of ced-1, ced-7, ttr-52 and nrf-5, are important for PS exposure on the surface of the engulfing cell [56,57]. Electron microscopy analysis of C. elegans embryos revealed the presence of extracellular PS-containing vesicles between the apoptotic cell and the neighboring phagocytes and these PS vesicles were lost or greatly reduced in animals deficient in ced-7 and ttr-52, respectively [56], suggesting that these extracellular vesicles are involved in PS expression on the surface of phagocytes, possibly by transferring PS from apoptotic cells to phagocytes. Specifically, NRF-5, a lipid-binding protein, may act with TTR-52, a transthyretin-like protein that binds specifically to PS and is also present in PS vesicles [56,58], and CED-7, a homolog of the mammalian ABC transporter ABC1, to mediate the generation and transfer of PS-containing vesicles to neighboring cells expressing CED-1 [59]. CED-1, by binding to TTR-52, may provide a docking site for PS vesicles to unload PS or to promote fusion of TTR-52-containing PS vesicles with phagocytes. Alternatively, these extracellular PS-containing vesicles may trigger altered activities of lipid transporters, leading to PS expression on neighboring phagocytes. Importantly, PS exposure on the surface of phagocytes promotes clearance of apoptotic cells [56], either by changing the activities of phagocyte membrane proteins critical for the engulfment process, or by serving as a homotypic ligand to tether the apoptotic cell to the phagocytes through a bipartite PS-binding bridging molecule, such as annexin I. Previous studies have shown that PS is also expressed on the surface of mammalian macrophages, albeit at a lower level as compared to apoptotic cells, and is functionally significant for phagocytosis of PS-positive target cells [60]. Moreover, macrophages were shown to express annexin I and II on the cell surface, and the annexins, that bind PS in a calcium-dependent manner, were suggested to act both as ligand and receptor in promoting phagocytosis [61].
Recently, apoptotic cells were shown to transfer exposed PS to neighboring viable cells via intercellular membrane nanotubes. Transferred PS (along with oxidized PS) was shown to remain externalized at the cell surface of viable cells thus initiating phagocytosis [62]. This suggests a novel way for cells to communicate the ‘kiss of death’.
5. Death is not the end: consequences of PS exposure
Phagocytes play an important role in pathogen recognition and host defense; additionally, a major task of phagocytic cells is to recognize, engulf and remove dying cells from the organism and thereafter to elicit an appropriate immunological response [1]. Following the ingestion of dying cells, phagocytes secrete cytokines that are either pro-inflammatory or anti-inflammatory depending on the form of cell death of the engulfed cell [63]. Hence, phagocyte recognition of surface-exposed PS on apoptotic cells stimulates the production of the anti-inflammatory cytokine TGF-β, thus actively modulating and contributing to the resolution of inflammation [64–66].
Additionally, apoptotic cells actively suppress the production of pro-inflammatory mediators such as IL-1β and TNFα [67]. In the absence of efficient cell clearance, autoimmune responses against “self” antigen may occur (reviewed in [68]). Interestingly, apoptotic cells were also shown to play a role in promoting wound healing and tissue regeneration in mice through the release of growth signals that stimulated the proliferation of progenitor cells [69]. The authors were able to delineate a signaling pathway involving executioner caspases 3 and 7, calcium-independent phospholipase A2, and prostaglandin E2. Notably, in a much earlier study, engulfment of apoptotic cells by epithelial cells was shown to lead to the secretion of growth factors, including vascular endothelial growth factor (VEGF) which subsequently promoted the proliferation of endothelial cells [70]. This was not observed when necrotic cells had been engulfed. Recently, it was suggested that macrophages that have engulfed apoptotic cells are primed for inflammatory responses and that this is a form of molecular “memory” which would allow the phagocytic cells to rapidly respond to infections or tissue damage [71]. Using the fruit fly as a model, the authors found that macrophage clearance of apoptotic cells triggered intracellular calcium bursts that together with elevated JNK activity and expression of the CED-1 homolog, Draper, were required for the priming effect. While it may be an exaggeration to refer to this as immunological “memory”, these studies provided evidence for cell corpse-induced macrophage priming that could serve to augment host responses at sites of infection or inflammation with high numbers of dead cells [71]. Taken together, it seems clear that there is life after death and also that the mode of cell death determines subsequent outcomes.
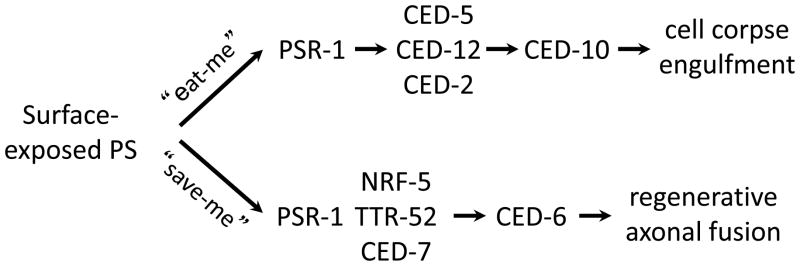
This review has focused on the role of PS exposure in cell clearance. However, PS externalization occurs in several other different (patho-)physiological processes such as platelet activation, where externalized PS acts as a scaffold for blood clotting [16], and virus entry into the host cell [72]. The latter is a case of apoptotic ‘mimicry’ whereby viruses deploy PS as a means to gain entry into host cells. Externalization of PS was also demonstrated in a subset of non-apoptotic T cells and changes in PS distribution in the plasma membrane in these cells were shown to modulate a range of membrane proteins, including the ATP receptor P2X7, and the multidrug transporter P-glycoprotein [73]. In a more recent study, PS externalization in HaCaT keratinocytes was shown to be required for the function of ADAM17, a member of the disintegrin and metalloproteinase (ADAM) family that promotes cellular functions through cleavage of transmembrane substrates [74]. The authors speculated that externalized PS directed the protease to its target where it executed its function. Additionally, PS exposure plays a role in myoblast fusion and axonal repair, indicating that PS may serve a beneficial role in tissue development and repair [75,76]. Hence, surface-exposed PS may serve not only as an “eat-me” signal to trigger engulfment of cells, but also can function as a “save-me” signal to mediate fusion and regeneration of injured neuronal axons [76]. PSR-1, a C. elegans PS receptor [26,77], acts cell-autonomously in the regrowing neuron and with TTR-52, CED-7 and NRF-5 to promote fusion and repair of transected axons during the regeneration process [76]. Thus, conserved PS transfer and recognition pathways are used to promote both apoptotic and non-apoptotic processes (Fig. 4).
Fig. 4. Multiple roles for externalized PS and its receptor PSR-1.
Surface-exposed PS can serve as an “eat-me” signal to trigger engulfment of apoptotic cells both in nematodes and higher organisms. Recent studies have shown that PS also may act as a “save-me” signal to promote fusion and regeneration of injured axons. PSR-1 mediates these two different processes through two different signaling pathways [76].
6. Concluding remarks
Cell death is only the beginning of the end; cell clearance is also an important step, and the display of “eat-me” signals such as PS serve to facilitate phagocyte recognition and engulfment. PS exposure is commonly viewed as a specific marker of apoptosis, but this may not necessarily be true as work published in recent years has shown that PS is also externalized in other forms of cell death, including (regulated) necrosis.
Another common perception is that cell death by apoptosis is “silent” in the sense that cells dying by apoptosis do so without awakening the immune system and without signs of inflammation. However, the view is now emerging that cell death by apoptosis is “anything but silent” [78]. Indeed, apoptotic cells emit signals that serve to modulate immune responses and may also direct cell growth in neighboring cells thereby contributing to homeostasis. We are only beginning to understand the outcome of other forms of cell death including regulated necrosis (necroptosis) and more research is needed to decipher the “meaning” of all these different cell death modalities, whether they occur in isolation, or perhaps concurrently: a cacophony (or symphony) of cell death.
The NCCD publishes recommendations for the correct nomenclature of cell death subtypes [11]. We acknowledge that these guidelines provide a framework for the correct usage of cell death related terms and help to clarify and distinguish between subtypes of cell death. However, these definitions are mainly focused on molecular pathways that are involved in the initiation or progression of cell death. The final step of this process – cell clearance – has so far not been considered to be part of the definition of cell death. However, there could be substantial differences in immunological responses depending on the form of cell death and how the dying cell is perceived by the engulfing cell. We therefore suggest that any (functional) definition of cell death should also encompass cell clearance mechanisms and phagocytic responses.
Supplementary Material
HIGHLIGHTS.
Cell clearance pathways are conserved between nematodes and mammals.
Phosphatidylserine (PS) externalization is a common signal for cell clearance.
Translocases and scramblases are involved in PS exposure during apoptosis.
PS also functions as a “save-me” signal to mediate the fusion of injured axons.
Definitions of cell death should take into account whether/how cells are cleared.
Acknowledgments
The authors are supported, in part, by grants from the Swedish Research Council (to BF) and National Institutes of Health (NIH) (R35 GM118188) (to DX). This essay is dedicated to Prof. Em. Sten Orrenius, Karolinska Institutet, on the occasion of his 80th Birthday.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fadeel B, Xue D, Kagan V. Programmed cell clearance: molecular regulation of the elimination of apoptotic cell corpses and its role in the resolution of inflammation. Biochem Biophys Res Commun. 2010;396:7–10. doi: 10.1016/j.bbrc.2010.02.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 3.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 4.Fadeel B, Orrenius S, Zhivotovsky B. Apoptosis in human disease: a new skin for the old ceremony? Biochem Biophys Res Commun. 1999;266:699–717. doi: 10.1006/bbrc.1999.1888. [DOI] [PubMed] [Google Scholar]
- 5.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 6.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G, El-Deiry WS, Golstein P, et al. Nomenclature Committee on Cell, Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12(Suppl 2):1463–1467. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, Galluzzi L, Vandenabeele P, et al. Nomenclature Committee on Cell, Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluzzi L, Bravo-San Pedro JM, Vitale I, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirt UA, Leist M. Rapid, noninflammatory and PS-dependent phagocytic clearance of necrotic cells. Cell Death Differ. 2003;10:1156–1164. doi: 10.1038/sj.cdd.4401286. [DOI] [PubMed] [Google Scholar]
- 13.Brouckaert G, Kalai M, Krysko DV, et al. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol Biol Cell. 2004;15:1089–1100. doi: 10.1091/mbc.E03-09-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadeel B, Gleiss B, Högstrand K, et al. Phosphatidylserine exposure during apoptosis is a cell-type-specific event and does not correlate with plasma membrane phospholipid scramblase expression. Biochem Biophys Res Commun. 1999;266:504–511. doi: 10.1006/bbrc.1999.1820. [DOI] [PubMed] [Google Scholar]
- 15.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annu Rev Physiol. 2003;65:701–734. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 17.Cocco RE, Ucker DS. Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol Biol Cell. 2001;12:919–930. doi: 10.1091/mbc.12.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Imamura R, Motani K, Kushiyama H, Nagata S, Suda T. Pyroptotic cells externalize eat-me and release find-me signals and are efficiently engulfed by macrophages. Int Immunol. 2013;25:363–372. doi: 10.1093/intimm/dxs161. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213:2113–2128. doi: 10.1084/jem.20151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 21.Maeda A, Fadeel B. Mitochondria released by cells undergoing TNF-α-induced necroptosis act as danger signals. Cell Death Dis. 2014;5:e1312. doi: 10.1038/cddis.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conradt B, Wu YC, Xue D. Programmed cell death during Caenorhabditis elegans development. Genetics. 2016;203:1533–1562. doi: 10.1534/genetics.115.186247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddien PW, Horvitz HR. The engulfment process of programmed cell death in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2004;20:193–221. doi: 10.1146/annurev.cellbio.20.022003.114619. [DOI] [PubMed] [Google Scholar]
- 25.Kinchen JM, Cabello J, Klingele D, et al. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature. 2005;434:93–99. doi: 10.1038/nature03263. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Wu YC, Fadok VA, et al. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science. 2003;302:1563–1566. doi: 10.1126/science.1087641. [DOI] [PubMed] [Google Scholar]
- 27.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 28.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem. 1997;272:26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- 30.Martin SJ, Finucane DM, Amarante-Mendes GP, O’Brien GA, Green DR. Phosphatidylserine externalization during CD95-induced apoptosis of cells and cytoplasts requires ICE/CED-3 protease activity. J Biol Chem. 1996;271:28753–28756. doi: 10.1074/jbc.271.46.28753. [DOI] [PubMed] [Google Scholar]
- 31.Vanags DM, Pörn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271:31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- 32.Darland-Ransom M, Wang X, Sun CL, et al. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science. 2008;320:528–531. doi: 10.1126/science.1155847. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Wang J, Gengyo-Ando K, et al. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat Cell Biol. 2007;9:541–549. doi: 10.1038/ncb1574. [DOI] [PubMed] [Google Scholar]
- 34.Chen YZ, Mapes J, Lee ES, Skeen-Gaar RR, Xue D. Caspase-mediated activation of Caenorhabditis elegans CED-8 promotes apoptosis and phosphatidylserine externalization. Nat Commun. 2013;4:2726. doi: 10.1038/ncomms3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341:403–406. doi: 10.1126/science.1236758. [DOI] [PubMed] [Google Scholar]
- 36.Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–1168. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- 37.Preta G, Fadeel B. AIF and Scythe (Bat3) regulate phosphatidylserine exposure and macrophage clearance of cells undergoing Fas (APO-1)-mediated apoptosis. PLoS One. 2012;7:e47328. doi: 10.1371/journal.pone.0047328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung S, Gumienny TL, Hengartner MO, Driscoll M. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat Cell Biol. 2000;2:931–937. doi: 10.1038/35046585. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Venegas V, Nagaoka Y, et al. Necrotic cells actively attract phagocytes through the collaborative action of two distinct PS-exposure mechanisms. PLoS Genet. 2015;11:e1005285. doi: 10.1371/journal.pgen.1005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem. 2013;288:13305–13316. doi: 10.1074/jbc.M113.457937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krahling S, Callahan MK, Williamson P, Schlegel RA. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999;6:183–189. doi: 10.1038/sj.cdd.4400473. [DOI] [PubMed] [Google Scholar]
- 44.Fadeel B, Xue D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit Rev Biochem Mol Biol. 2009;44:264–277. doi: 10.1080/10409230903193307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krysko DV, D’Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- 46.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 47.Kagan VE, Gleiss B, Tyurina YY, et al. A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J Immunol. 2002;169:487–499. doi: 10.4049/jimmunol.169.1.487. [DOI] [PubMed] [Google Scholar]
- 48.Segawa K, Suzuki J, Nagata S. Constitutive exposure of phosphatidylserine on viable cells. Proc Natl Acad Sci U S A. 2011;108:19246–19251. doi: 10.1073/pnas.1114799108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jitkaew S, Witasp E, Zhang S, Kagan VE, Fadeel B. Induction of caspase- and reactive oxygen species-independent phosphatidylserine externalization in primary human neutrophils: role in macrophage recognition and engulfment. J Leukoc Biol. 2009;85:427–437. doi: 10.1189/jlb.0408232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 51.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 52.Borisenko GG, Matsura T, Liu SX, et al. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells--existence of a threshold. Arch Biochem Biophys. 2003;413:41–52. doi: 10.1016/s0003-9861(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 53.Appelt U, Sheriff A, Gaipl US, Kalden JR, Voll RE, Herrmann M. Viable, apoptotic and necrotic monocytes expose phosphatidylserine: cooperative binding of the ligand Annexin V to dying but not viable cells and implications for PS-dependent clearance. Cell Death Differ. 2005;12:194–196. doi: 10.1038/sj.cdd.4401527. [DOI] [PubMed] [Google Scholar]
- 54.Robertson AM, Thomson JN. Morphology of programmed cell death in the ventral nerve cord of Caenorhabditis elegans larvae. J Embryol Exp Morphol. 1982;67:89–100. [Google Scholar]
- 55.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 56.Mapes J, Chen YZ, Kim A, Mitani S, Kang BH, Xue D. CED-1, CED-7, and TTR-52 regulate surface phosphatidylserine expression on apoptotic and phagocytic cells. Curr Biol. 2012;22:1267–1275. doi: 10.1016/j.cub.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Wang H, Kage-Nakadai E, Mitani S, Wang X. C. elegans secreted lipid-binding protein NRF-5 mediates PS appearance on phagocytes for cell corpse engulfment. Curr Biol. 2012;22:1276–1284. doi: 10.1016/j.cub.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Li W, Zhao D, et al. Caenorhabditis elegans transthyretin-like protein TTR-52 mediates recognition of apoptotic cells by the CED-1 phagocyte receptor. Nat Cell Biol. 2010;12:655–664. doi: 10.1038/ncb2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Z, Hartwieg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 60.Callahan MK, Williamson P, Schlegel RA. Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ. 2000;7:645–653. doi: 10.1038/sj.cdd.4400690. [DOI] [PubMed] [Google Scholar]
- 61.Fan X, Krahling S, Smith D, Williamson P, Schlegel RA. Macrophage surface expression of annexins I and II in the phagocytosis of apoptotic lymphocytes. Mol Biol Cell. 2004;15:2863–2872. doi: 10.1091/mbc.E03-09-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bittins M, Wang X. TNT-induced phagocytosis: tunneling nanotubes mediate the transfer of pro-phagocytic signals from apoptotic to viable cells. J Cell Physiol. 2016 doi: 10.1002/jcp.25584. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 64.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol. 1999;163:6164–6172. [PubMed] [Google Scholar]
- 66.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 68.Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010 May;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 69.Li F, Huang Q, Chen J, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3:ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golpon HA, Fadok VA, Taraseviciene-Stewart L, et al. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 2004;18:1716–1718. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- 71.Weavers H, Evans IR, Martin P, Wood W. Corpse engulfment generates a molecular memory that primes the macrophage inflammatory response. Cell. 2016;165:1658–1671. doi: 10.1016/j.cell.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 73.Elliott JI, Surprenant A, Marelli-Berg FM, et al. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol. 2005;7:808–816. doi: 10.1038/ncb1279. [DOI] [PubMed] [Google Scholar]
- 74.Sommer A, Kordowski F, Büch J, et al. Phosphatidylserine exposure is required for ADAM17 sheddase function. Nat Commun. 2016;7:11523. doi: 10.1038/ncomms11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van den Eijnde SM, van den Hoff MJ, Reutelingsperger CP, et al. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114:3631–3642. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]
- 76.Neumann B, Coakley S, Giordano-Santini R, et al. EFF-1-mediated regenerative axonal fusion requires components of the apoptotic pathway. Nature. 2015;517:219–222. doi: 10.1038/nature14102. [DOI] [PubMed] [Google Scholar]
- 77.Yang H, Chen YZ, Zhang Y, et al. A lysine-rich motif in the phosphatidylserine receptor PSR-1 mediates recognition and removal of apoptotic cells. Nat Commun. 2015;6:5717. doi: 10.1038/ncomms6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fogarty CE, Bergmann A. The sound of silence: signaling by apoptotic cells. Curr Top Dev Biol. 2015;114:241–265. doi: 10.1016/bs.ctdb.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.