Abstract
Rationale: Acute kidney injury may contribute to distant organ dysfunction. Few studies have examined kidney injury as a risk factor for delirium and coma.
Objectives: To examine whether acute kidney injury is associated with delirium and coma in critically ill adults.
Methods: In a prospective cohort study of intensive care unit patients with respiratory failure and/or shock, we examined the association between acute kidney injury and daily mental status using multinomial transition models adjusting for demographics, nonrenal organ failure, sepsis, prior mental status, and sedative exposure. Acute kidney injury was characterized daily using the difference between baseline and peak serum creatinine and staged according to Kidney Disease Improving Global Outcomes criteria. Mental status (normal vs. delirium vs. coma) was assessed daily with the Confusion Assessment Method for the ICU and Richmond Agitation-Sedation Scale.
Measurements and Main Results: Among 466 patients, stage 2 acute kidney injury was a risk factor for delirium (odds ratio [OR], 1.55; 95% confidence interval [CI], 1.07–2.26) and coma (OR, 2.04; 95% CI, 1.25–3.34) as was stage 3 injury (OR for delirium, 2.56; 95% CI, 1.57–4.16) (OR for coma, 3.34; 95% CI, 1.85–6.03). Daily peak serum creatinine (adjusted for baseline) values were also associated with delirium (OR, 1.35; 95% CI, 1.18–1.55) and coma (OR, 1.44; 95% CI, 1.20–1.74). Renal replacement therapy modified the association between stage 3 acute kidney injury and daily peak serum creatinine and both delirium and coma.
Conclusions: Acute kidney injury is a risk factor for delirium and coma during critical illness.
Keywords: acute kidney injury, delirium, coma, critical illness
At a Glance Commentary
Scientific Knowledge on the Subject
Acute kidney injury is common in the critically ill and may contribute to morbidity and mortality through effects on distant organ function. Delirium and coma are common manifestations of brain dysfunction in the intensive care unit whose mechanisms are poorly understood. Experimental evidence indicates that acute kidney injury contributes to brain dysfunction, but few studies have examined the association between acute kidney injury and brain dysfunction during critical illness.
What This Study Adds to the Field
Acute kidney injury is shown to be associated with delirium and coma during critical illness after adjusting for potential confounders. This suggests kidney injury is an underappreciated risk factor that is important in the pathogenesis of delirium and coma during critical illness.
The kidney and the brain are frequently injured during critical illness. Acute kidney injury, which affects up to half of critically ill patients (1), is strongly associated with short- and long-term morbidity and mortality (2–7). Similarly, acute brain dysfunction, which manifests as delirium or coma in the intensive care unit (ICU), is common during critical illness and associated with adverse short- and long-term outcomes. Delirium and coma both predict increased mortality and delirium has been associated with long-term cognitive impairment in survivors of critical illness (8–15).
One mechanism by which acute kidney injury may contribute to poor outcomes is through effects on distant organ function (16–22). Recent experimental data indicate that acute kidney injury can lead to inflammation in the brain and other remote organs (19, 21, 23). In addition, loss of kidney function can reduce the clearance of medications, metabolites, or other potential neurotoxins. To date, only one study has examined the association between kidney dysfunction and delirium during critical illness (24), which was limited by use of a single threshold for defining kidney dysfunction (i.e., serum creatinine >2 mg/dl), did not distinguish between acute and chronic disease, and was unable to describe the temporal relationship between acute kidney injury and delirium.
We hypothesized that acute kidney injury during critical illness is associated with delirium and coma in the ICU. We also hypothesized that renal replacement therapy would modify the association between an acute change in serum creatinine and delirium and coma. To test these hypotheses, we examined the relationship between acute kidney injury and cognitive dysfunction in the BRAIN-ICU (Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors) study (10), a multicenter, prospective cohort study of critically ill adults with frequent (i.e., daily) assessments of both kidney and neurologic function.
Methods
Study Population and Setting
This is a secondary analysis of the BRAIN-ICU study, a prospective cohort study of patients with acute respiratory failure and/or shock (cardiogenic or septic) enrolled at Vanderbilt University and Saint Thomas Hospital in Nashville, Tennessee between 2007 and 2010 (10). We excluded patients with recent critical illness, could not be assessed for delirium, unlikely to survive 24 hours, or at high risk for preexisting cognitive deficits. For the current study, we only included patients enrolled at Vanderbilt because this was the only site with daily serum creatinine data available. We also excluded patients without any serum creatinine measurements, sequential organ failure assessment (SOFA), or mental status assessments available; participants receiving chronic renal replacement therapy; or with a baseline estimated glomerular filtration rate less than 20 ml/min/1.73 m2. We obtained written informed consent from all participants or their surrogates, which allowed for additional data abstraction from the electronic health record, and the study protocol was approved by the Vanderbilt institutional review board.
Data Collection
Exposures
We identified acute kidney injury using serum creatinine values measured during clinical care, determining daily severity with the creatinine-based arm of the Kidney Disease Improving Global Outcomes (KDIGO) system (25), as follows:
Stage 1 injury: ≥0.3 mg/dl or 50% increase from baseline creatinine
Stage 2 injury: ≥100% increase in baseline serum creatinine
Stage 3 injury: ≥200% increase in baseline serum creatinine or receipt of renal replacement therapy
Because the KDIGO stage 1 definition is very sensitive, small, brief increases in creatinine are classified as acute kidney injury. Thus, we also conducted a sensitivity analysis that required sustained acute kidney injury in the definition of stage 1 injury. Specifically, we defined sustained stage 1 injury as an increase from baseline creatinine of greater than or equal to 0.3 mg/dl or 50% (and <100%) after a day of stage 1 or greater acute kidney injury.
We estimated baseline creatinine as the mean outpatient serum creatinine 7–365 days before admission (26). If baseline serum creatinine was greater than 0.5 mg/dl higher than the lowest creatinine during hospitalization (n = 13), two nephrologists (E.D.S. and W.H.F.) blinded to outcomes reviewed medical records and determined an adjudicated baseline creatinine. If preadmission creatinine was not available, we used the minimum serum creatinine during hospitalization. We verified the date and time of renal replacement therapy initiation and cessation with manual chart review and considered all days between initiation and cessation to be renal replacement therapy days unless renal replacement therapy was stopped greater than 72 hours. All creatinine data were collected retrospectively from the electronic medical record.
Outcomes
Trained research personnel used validated instruments to evaluate patients prospectively for delirium and coma twice daily until ICU discharge and then once daily until hospital discharge, study day 30, or death. Patients were assessed for delirium using the Confusion Assessment Method for the ICU (CAM-ICU) (27), and level of consciousness (coma) was assessed using the Richmond Agitation-Sedation Scale (RASS) (28, 29). We considered any day during which one or more CAM-ICU assessments were positive to be a day of delirium, and any day during which all RASS assessments were −4 (response only to physical stimulation) or −5 (no response to voice or physical stimulation) to be a day of coma (10). We classified days during which neither delirium nor coma were present to be normal mental status days.
Covariates
Covariates were collected prospectively and included age, sex, race, severe sepsis (30), modified SOFA score (31), previous day’s mental status, and sedative exposure during the previous day. We modified the SOFA score (which was calculated using organ failure data from the same day that the outcome was assessed) by subtracting out the neurologic and renal scores because these factors were already represented by our primary outcome and exposure, respectively. Points for respiratory, circulatory, hepatic, and coagulation systems were included for a total possible score of 0–16. Sedative exposure was examined both as daily dose (milligram of midazolam equivalents, microgram of fentanyl equivalents, and milligram of propofol) and as weight-based dose (daily dose by admission weight in kilogram).
Statistical Analyses
A detailed description of our statistical approach is provided in the online supplement. Briefly, we analyzed associations between acute kidney injury and mental status using first-order multinomial transition models that estimate the probability of each mental status outcome (normal, delirium, and coma) as a function of the previous day’s mental status, current acute kidney injury status, and all covariates. A priori, we chose to model acute kidney injury in two ways: one model uses KDIGO stage to represent severity of acute kidney injury, and another uses daily peak serum creatinine (adjusted for baseline creatinine) to represent acute kidney injury severity. As described in detail in the online supplement, we also examined whether the association between acute kidney injury and mental status was modified by (i.e., interacted with) sedative exposure or renal replacement therapy and conducted several subgroup analyses, including analyses of patient-days after days when no sedative was given, patients who never received benzodiazepines during the study period, patients with known preadmission serum creatinine values, and patients without delirium or coma on study day 1. We performed analyses with R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria) and Stata version 12 (StataCorp, College Station, TX).
Results
Population Characteristics
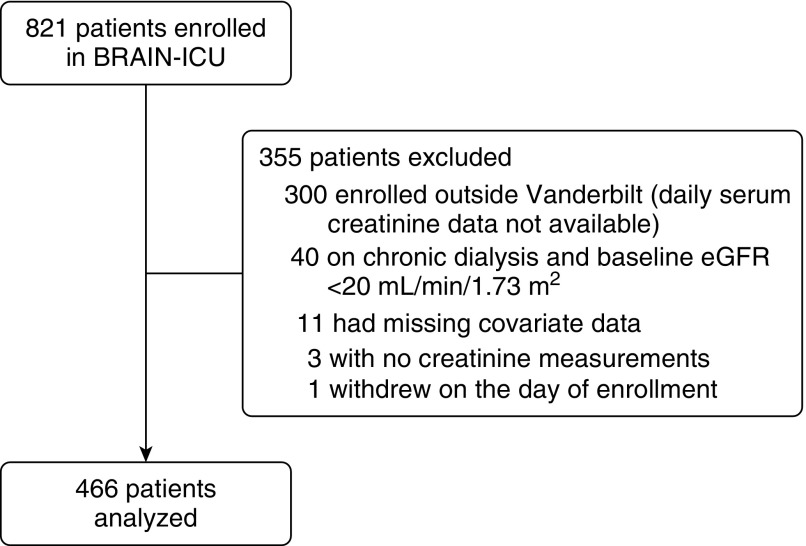
Of 521 patients enrolled in the BRAIN-ICU study at Vanderbilt University Medical Center, three had no creatinine values measured, 40 were excluded because of preexisting severe kidney disease, 11 were excluded because of missing outcome or covariate data, and one patient withdrew from the study such that 466 patients were included in the current study (Figure 1). Subject characteristics and hospital outcomes are shown in Table 1. Sepsis and acute respiratory distress syndrome were the most common admission diagnoses. Mechanical ventilation and sedation were common early during the study period but lasted only several days or less for most patients.
Figure 1.
Patient selection flow diagram. BRAIN-ICU = Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors; eGFR = estimated glomerular filtration rate.
Table 1.
Baseline and Hospital Characteristics of the Study Population*
| Characteristics | Total (n = 466) |
|---|---|
| Age, yr | 58 (47–68) |
| Male, % | 51 |
| White, % | 89 |
| Charlson Comorbidity Index | 2 (1–4) |
| Baseline creatinine, mg/dl | 0.95 (0.73–1.28) |
| Baseline eGFR, ml/min/1.73 m2 | 79 (54–99) |
| APACHE II at enrollment | 25 (19–31) |
| SOFA score at enrollment | 9 (7–12) |
| Admission diagnoses, % | |
| Sepsis/ARDS | 32 |
| Surgery† | 22 |
| CHF, myocardial infarction, arrhythmia | 16 |
| Asthma, COPD, other pulmonary‡ | 11 |
| Airway protection§ | 11 |
| Neurologic|| | 1 |
| Other diagnosis¶ | 6 |
| Mechanically ventilated at any time during study period, % | 91 |
| Median duration of ventilation, d | 3 (1–8) |
| Benzodiazepine use | |
| Duration of use, d | 3.0 (1.0–6.2) |
| 24-h dose on days of use, mg midazolam equivalents | 7.5 (2.5–32.0) |
| 24-h dose on days of use, mg/kg** midazolam equivalents | 0.1 (0.0–0.4) |
| Propofol use | |
| Duration of use, d | 1.5 (1.0–3.0) |
| 24-h dose on days of use, mg propofol | 972 (200–2,912) |
| 24-h dose on days of use, mg/kg** propofol | 12.4 (3.5–33.9) |
| Opiate use | |
| Duration of use, d | 4.0 (2.0–8.0) |
| 24-h dose on days of use, μg fentanyl equivalents | 875 (200–2,400) |
| 24-h dose on days of use, μg/kg** fentanyl equivalents | 11.4 (2.7–29.5) |
| Acute kidney injury, % | 65 |
| Maximum severity of acute kidney injury, % | |
| KDIGO stage 1 | 32 |
| KDIGO stage 2 | 16 |
| KDIGO stage 3 | 17 |
| Received renal replacement therapy during study period, % | 10 |
| Ever delirious during study period, % | 75 |
| Ever comatose during study period, % | 60 |
| ICU length of stay, d | 5 (2–11) |
| Hospital length of stay, d | 10 (6–17) |
| Hospital mortality, % | 16 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; ICU = intensive care unit; KDIGO = Kidney Disease Improving Global Outcome; SOFA = Sequential Organ Failure Assessment.
Results expressed as median (interquartile range) unless otherwise specified.
Including colonic, ears/nose/throat, gastric, gynecologic, hepatobiliary, orthopedic, transplant, urologic, and vascular surgeries.
Including respiratory failure caused by pulmonary edema, embolus, and fibrosis.
Including altered mental status and upper airway obstruction.
Including status epilepticus and other neurologic diagnoses.
Including gastrointestinal hemorrhage, hepatic failure, malignancy, and metabolic disorders.
Weight recorded by the ICU team at admission was used to calculate weight-based doses of sedatives.
Baseline kidney function was normal for most patients, with the median baseline estimated glomerular filtration rate being 79 (54–99) ml/min/1.73 m2. The overall incidence of acute kidney injury during the 30 days after enrollment was 65% with more than half of patients having moderate-to-severe kidney injury and 1 in 10 receiving renal replacement therapy. Of the 50 patients who received renal replacement therapy, only 34 (68%) received their initial renal replacement therapy during the study period, with 21 (62%) receiving continuous renal replacement therapy or slow low-efficiency dialysis and 13 (38%) starting with conventional intermittent hemodialysis.
Seventy-five percent of patients experienced delirium at least once during the follow-up period, 60% experienced coma at least once, and 47% experienced both. However, of 5,056 total patient-days included in the study, mental status was normal during 2,672 (52.8%), delirious during 1,655 (32.5%), and comatose during 740 (14.6%).
Association between Acute Kidney Injury and Delirium or Coma
Throughout the study period, the proportion of patients with acute kidney injury differed according to mental status. Acute kidney injury was present on 37% of study days when patients had a normal mental status, on 50% of days when patients were delirious, and on 65% of days when patients were comatose.
Because our results suggest that renal replacement therapy modifies the association between acute kidney injury and mental status, we present the association between KDIGO stage and delirium and coma first among only those study days that did not involve renal replacement therapy. Only 10% of the cohort received renal replacement therapy during the study period. After adjusting for demographic factors, nonrenal organ failure, severe sepsis, and the previous day’s mental status and sedative exposure, acute kidney injury as measured by KDIGO stage was a risk factor for both delirium and coma during non–renal replacement therapy days (Table 2, Figures 2A–2C). Specifically, compared with no acute kidney injury, the odds of delirium was significantly increased in the setting of KDIGO stage 2 (odds ratio [OR], 1.55; 95% confidence interval [CI], 1.07–2.26) and KDIGO stage 3 (OR, 2.56; 95% CI, 1.57–4.16) acute kidney injury. Similarly, the adjusted odds of coma was also increased in the setting of KDIGO stage 2 and stage 3 acute kidney injury (OR, 2.04; 95% CI, 1.25–3.34) (OR, 3.34; 95% CI, 1.85–6.03). As shown in Table 3 and Figures 2D–2F, the model that included all study days (both non–renal replacement therapy and renal replacement therapy days) also found acute kidney injury was a significant risk factor for delirium and coma, but the associations between KDIGO stage 3 (which, by definition, included all renal replacement therapy days) and both delirium (OR, 1.48; 95% CI, 1.00–2.16) and coma (OR, 1.79; 95% CI, 1.11–2.91) were attenuated compared with those observed on non–renal replacement therapy days.
Table 2.
Associations between Acute Kidney Injury by KDIGO Stage and Delirium or Coma during Non–Renal Replacement Therapy Days
| Delirium |
Coma |
|||
|---|---|---|---|---|
| Independent Variable | OR | 95% CI | OR | 95% CI |
| Acute kidney injury* | ||||
| KDIGO stage 1 | 1.13 | 0.91–1.41 | 1.31 | 0.95–1.80 |
| KDIGO stage 2 | 1.55† | 1.07–2.26 | 2.04† | 1.25–3.34 |
| KDIGO stage 3 | 2.56† | 1.57–4.16 | 3.34† | 1.85–6.03 |
| Previous day’s mental status‡ | ||||
| Delirium | 27.03† | 21.00–34.8 | 15.42† | 10.18–23.37 |
| Coma | 48.97† | 31.72–75.59 | 207.35† | 107.69–399.24 |
| Age (per yr) | 1.03† | 1.02–1.04 | 1.03† | 1.02–1.04 |
| Female | 1.16 | 0.92–1.46 | 1.08 | 0.77–1.53 |
| African American | 0.59† | 0.35–0.99 | 0.74 | 0.41–1.32 |
| Sepsis | 1.09† | 1.06–1.11 | 1.09† | 1.06–1.13 |
| Modified SOFA (per unit) | 1.04 | 0.98–1.11 | 1.19† | 1.11–1.28 |
| Previous day’s sedative exposure | ||||
| Benzodiazepines (per 1-mg midazolam eq.) | 1.02† | 1.01–1.04 | 1.03† | 1.02–1.04 |
| Opiates (per 100-μg fentanyl eq.) | 1.03† | 1.02–1.04 | 1.04† | 1.03–1.05 |
| Propofol (per 100-mg propofol) | 1.01 | 0.99–1.02 | 1.01 | 0.99–1.02 |
Definition of abbreviations: CI = confidence interval; KDIGO = Kidney Disease Improving Global Outcomes; OR = odds ratio; SOFA = Sequential Organ Failure Assessment.
Compared with a reference (OR of 1.0) of KDIGO stage 0 (i.e., no acute kidney injury).
Denotes a statistically significant association.
Compared with a reference (OR of 1.0) of “normal” mental status the previous day.
Figure 2.
Predicted probabilities of normal mental status versus delirium versus coma according to acute kidney injury by Kidney Disease Improving Global Outcomes (KDIGO) stage, stratified by mental status on the previous day. After adjusting for demographic factors, nonrenal organ failure, severe sepsis, and the previous day’s mental status and sedative exposure, KDIGO stage was a significant risk factor for delirium and coma. The boxes show that as KDIGO stage rises (i.e., acute kidney injury severity increases), the predicted probability of normal mental status (green) falls, whereas the probabilities of delirium (blue) and coma (red) rise, with the overall probabilities of each mental state also depending on the mental state the previous day. Dashed lines indicate 95% confidence interval boundaries. (A–C) Excludes days where patients were receiving renal replacement therapy (RRT) from stage 3 acute kidney injury. (D–F) Includes all days with and without RRT in stage 3 acute kidney injury.
Table 3.
Associations between Acute Kidney Injury by KDIGO Stage and Delirium or Coma during All Study Days (Including Days on Renal Replacement Therapy)
| Delirium |
Coma |
|||
|---|---|---|---|---|
| Independent Variable | OR | 95% CI | OR | 95% CI |
| Acute kidney injury* | ||||
| KDIGO stage 1 | 1.13 | 0.91–1.41 | 1.30 | 0.95–1.79 |
| KDIGO stage 2 | 1.55† | 1.07–2.25 | 2.01† | 1.22–3.29 |
| KDIGO stage 3 | 1.48† | 1.00–2.16 | 1.79† | 1.11–2.91 |
| Previous day’s mental status‡ | ||||
| Delirium | 28.35† | 22.40–35.89 | 16.32† | 11.01–24.18 |
| Coma | 48.18† | 32.03–72.50 | 217.87† | 116.93–405.93 |
| Age (per yr) | 1.03† | 1.02–1.03 | 1.03† | 1.02–1.04 |
| Female | 1.08 | 0.86–1.36 | 1.02 | 0.73–1.44 |
| African American | 0.61† | 0.40–0.92 | 0.78 | 0.47–1.31 |
| Sepsis | 1.08† | 1.05–1.10 | 1.08† | 1.04–1.11 |
| Modified SOFA (per unit) | 1.06† | 1.00–1.12 | 1.23† | 1.15–1.31 |
| Previous day’s sedative exposure | ||||
| Benzodiazepines (per 1-mg midazolam eq.) | 1.02† | 1.01–1.04 | 1.03† | 1.02–1.05 |
| Opiates (per 100-μg fentanyl eq.) | 1.03† | 1.02–1.03 | 1.04† | 1.02–1.05 |
| Propofol (per 100-mg propofol) | 1.01 | 0.99–1.02 | 1.01 | 0.99–1.02 |
Definition of abbreviations: CI = confidence interval; KDIGO = Kidney Disease Improving Global Outcomes; OR = odds ratio; SOFA = Sequential Organ Failure Assessment.
Compared with a reference (OR of 1.0) of KDIGO stage 0 (i.e., no acute kidney injury).
Denotes a statistically significant association.
Compared with a reference (OR of 1.0) of “normal” mental status the previous day.
Similar to the KDIGO analysis, acute kidney injury as measured by peak serum creatinine was a risk factor for delirium and coma after adjusting for covariates, including baseline creatinine, and the association differed according to whether or not the patient received renal replacement therapy on the date in question (Table 4, Figure 3 show this interaction). Among patients not on renal replacement therapy, an increase in daily peak serum creatinine of 1 mg/dl was significantly associated with an increased odds of delirium (OR, 1.35; 95% CI, 1.18–1.55) and coma (OR, 1.44; 95% CI, 1.20–1.74), after adjusting for covariates. Among patients receiving renal replacement therapy, daily peak serum creatinine was not associated with delirium (OR, 1.07; 95% CI, 0.87–1.31) or coma (OR, 1.16; 95% CI, 0.94–1.44).
Table 4.
Associations between Change in Serum Creatinine from Baseline and Delirium or Coma
| Delirium |
Coma |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Clinical condition of interest | ||||
| Associations with peak serum creatinine today according to RRT status (per mg/dl)* | ||||
| Not on RRT | 1.35† | 1.18–1.55 | 1.44† | 1.20–1.74 |
| On RRT | 1.07 | 0.87–1.31 | 1.16 | 0.94–1.44 |
| Regression model predictors (full regression model with interaction term) | ||||
| Peak serum creatinine today (per mg/dl)‡ | 1.35† | 1.18–1.55 | 1.44† | 1.20–1.74 |
| RRT today§ | 1.61 | 0.64–4.04 | 1.64 | 0.65–4.17 |
| Peak creatinine × RRT (interaction term) | 0.79† | 0.63–0.99 | 0.81 | 0.62–1.05 |
| Baseline serum creatinine (per mg/dl) | 0.78 | 0.60–1.01 | 0.53† | 0.35–0.80 |
| Previous day’s mental status|| | ||||
| Delirium | 28.09† | 22.13–35.65 | 16.23† | 10.9–24.16 |
| Coma | 48.55† | 32.40–72.76 | 221.35† | 119.57–409.75 |
| Age (per yr) | 1.03† | 1.02–1.03 | 1.03† | 1.02–1.04 |
| Female | 1.11 | 0.87–1.40 | 0.98 | 0.69–1.38 |
| African American | 0.61† | 0.40–0.93 | 0.83 | 0.49–1.40 |
| Sepsis | 1.08† | 1.06–1.10 | 1.08† | 1.05–1.11 |
| Modified SOFA (per unit) | 1.06† | 1.00–1.12 | 1.23† | 1.15–1.32 |
| Previous day’s sedative exposure | ||||
| Benzodiazepines (per 1-mg midazolam eq.) | 1.02† | 1.01–1.04 | 1.03† | 1.02–1.05 |
| Opiates (per 100-μg fentanyl eq.) | 1.03† | 1.02–1.04 | 1.04† | 1.02–1.05 |
| Propofol (per 100 mg) | 1.01 | 0.99–1.02 | 1.01 | 1.0–1.02 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; RRT = renal replacement therapy; SOFA = Sequential Organ Failure Assessment.
Because of a significant interaction between RRT and peak serum creatinine today, the association between peak creatinine and mental status (normal vs. delirium vs. coma) differed according to the use of RRT. The associations between peak creatinine and mental status (normal vs. delirium vs. coma) are therefore reported here stratified according to whether RRT was present or not and modeled using the regression model predictors below.
Denotes a statistically significant association.
In the full regression model, the regression coefficients (and corresponding ORs) for the associations between peak serum creatinine today and delirium and coma indicate the associations among patients not on RRT.
Because of the significant interaction between RRT and peak serum creatinine today, the association between RRT and mental status (normal vs. delirium vs. coma) differed according to the change in creatinine. Results shown here assume a peak creatinine at the reference value (1 mg/dl).
Compared with a reference (OR of 1.0) of “normal” mental status the previous day.
Figure 3.
Predicted probabilities of normal mental status versus delirium versus coma according to change from baseline in serum creatinine, stratified by mental status on the previous day and by renal replacement therapy (RRT). After adjusting for demographic factors, baseline serum creatinine, nonrenal organ failure, severe sepsis, and the previous day’s mental status and sedative exposure, an increase in serum creatinine was a significant risk factor for delirium and coma. The solid lines show that as daily peak serum creatinine increases among patients not on RRT (A–C), the predicted probability of normal mental status (green) falls, whereas the probabilities of delirium (blue) and coma (red) rise, with the overall probabilities of each mental state also depending on the mental state the previous day. Alternatively, change in daily peak serum creatinine among patients on RRT (D–F) has no significant association with the predicted probability of mental status. Dashed lines indicate 95% confidence interval boundaries, and box plots on the x-axis indicate the distribution of peak serum creatinine (medians with interquartile range) according to previous day’s mental status and use of RRT.
Supporting Analyses
An analysis using weight-based sedative doses yielded results that were nearly identical to those of our primary analysis (see Table E1 in the online supplement). In addition, among the subgroup of 2,286 patient-days where sedatives were not administered the previous day, we observed dose-dependent associations between KDIGO stages 1, 2, and 3 acute kidney injury and both delirium (OR, 1.32; 95% CI, 0.93–1.89) (OR, 1.79; 95% CI, 0.96–3.34) (OR, 2.48; 95% CI, 1.26–4.90) and coma (OR, 1.46, 95% CI, 0.82–2.60) (OR, 2.71; 95% CI, 1.13–6.49) (OR, 3.60; 95% CI, 1.01–12.84) that were similar to those observed when analyzing all patient-days. The associations between acute kidney injury and delirium and coma observed among the 166 patients who never received benzodiazepines during the study period were also similar to those observed in the full cohort (see Table E2). Lastly, we did not find any evidence of an interaction between KDIGO and sedative doses as risk factors for delirium (P values for the interaction were 0.45 and 0.47 in models with and without renal replacement therapy days, respectively).
Details regarding the number of patients for whom baseline creatinine measurements were available versus estimated are provided in the online supplement as are the results of sensitivity analyses showing that the associations between acute kidney injury and mental status were similar among those with baseline creatinine measurements versus those with estimated baselines. According to a likelihood ratio test (P = 0.95), there was no evidence that cases requiring an imputation of baseline creatinine had any differential data patterns of consequence.
Requiring that stage 1 acute kidney injury be sustained (i.e., be present on the previous day and the day of outcome assessment) did not substantially change the results. We found no statistically significant association between sustained stage 1 acute kidney injury and delirium (OR, 1.11; 95% CI, 0.88–1.41) or coma (OR, 1.15; 95% CI, 0.83–1.59).
Lastly, in an exploratory subgroup analysis limited to 132 patients without delirium or coma on study day 1, the associations between acute kidney injury and delirium and coma were consistent with the primary analysis indicating that KDIGO stages 2 and 3 were associated with an increased risk for delirium and trended in a similar direction for coma (see Table E3).
Discussion
In this large, prospective cohort of critically ill adults, moderate-to-severe acute kidney injury was strongly associated with delirium and coma after adjusting for numerous potential confounders. Furthermore, renal replacement therapy modified the association between acute changes in serum creatinine and acute brain dysfunction. These data are consistent with the hypothesis that acute kidney injury contributes to distant organ dysfunction during critical illness and raise the question of what modifiable risk factors for delirium and coma may underlie these associations. The mechanisms underlying the effects of acute kidney injury on the brain and other contributors in this population should be examined in future studies.
The observation that patients who die with acute kidney injury often do so with concomitant nonrenal organ failure has led to the hypothesis that acute kidney injury contributes to poor outcomes via signals that promote dysfunction and failure of other organs (6, 32). Although not well studied in humans, experimental acute kidney injury leads to inflammation in the cerebral cortex and hippocampus that is associated with histologic changes and locomotor dysfunction (18, 19, 21, 23). Other animal model studies have shown lung inflammation increases and vascular capillary permeability worsens with experimentally induced acute kidney injury (18, 19, 23) and with bilateral nephrectomy (18, 23), suggesting that induction of inflammation and impaired cytokine clearance may each contribute to distant organ injury in the setting of acute kidney injury (33, 34).
Our results extend those of the one previous study demonstrating that impaired kidney function, measured by an admission serum creatinine greater than 2 mg/dl, was associated with delirium during critical illness (24). Whereas that study did not distinguish between acute and chronic kidney disease or examine the entire spectrum of acute kidney injury severity, we determined and adjusted for baseline kidney function and analyzed acute kidney injury in two ways (KDIGO stage and peak serum creatinine). Unlike acute kidney injury, baseline kidney function itself was not associated with delirium or coma. However, we did exclude patients with advanced chronic kidney disease and cannot therefore rule out a relationship between advanced chronic kidney disease and delirium or coma.
In analyses of both KDIGO stage and peak daily serum creatinine as risk factors for acute brain dysfunction, renal replacement therapy seemed to diminish the association in patients with the most severe acute kidney injury. One potential explanation for this interaction is that renal replacement therapy itself lowers creatinine, making the latter a poor marker of acute kidney injury severity in these patients. Alternatively, the effects of renal replacement therapy may reduce the risk for delirium or coma due to acute kidney injury. One hypothesis, which needs to be tested, is that renal replacement therapy diminishes the effects of acute kidney injury on the brain by clearing sedatives, antibiotics, or other metabolites that may cause neurotoxicity (35–38). Another hypothesis to be examined is that preventing or reducing acute kidney injury, particularly when detected in its early stages, can attenuate the risk for abnormal mental status.
Indeed, our findings confirm numerous studies demonstrating associations between sedatives (especially benzodiazepines) and delirium. Importantly, acute kidney injury was a significant risk factor for delirium and coma even after adjusting for sedative exposure, and we found no evidence of a statistical interaction between the severity of acute kidney injury and sedative use on the risk for delirium or coma. One reason for this lack of an interaction between acute kidney injury and sedative exposure may be that sedatives were used for just a few days in most participants, so our study may not be powered to rule out an interaction between the effects of acute kidney injury and sedatives on mental status. Thus, the lack of an interaction should not falsely reassure providers, who should keep in mind that some opiates or their metabolites (e.g., meperidine, morphine) and weakly active metabolites of some benzodiazepines (e.g., midazolam) are cleared by the kidney, so these drugs should be used with caution or avoided entirely in patients with acute kidney injury. When managing patients with impaired kidney function, ICU clinicians might choose to use fentanyl alone given that many ICU patients do not require sedation if pain is well controlled. For patients who do need more than analgesia, recent evidence suggests that certain sedatives (e.g., propofol) may reduce the risk of acute kidney injury (39–42).
Our findings also indicate that the previous day’s mental status (which is influenced by all previously incurred risk factors for delirium and coma, including prior acute kidney injury, and can fluctuate often during critical illness) is strongly associated with current mental status. These results are intuitive and consistent with clinical experience and prior research (which, for example, has shown coma to be an important predictor of delirium). Notably, only a minority (14%) of observations were coma days given the short duration of coma for most patients, so the strong effect of previous coma did not influence most observations. Incorporating prior mental status added robustness to our examination of the association between acute kidney injury and mental status not performed in previous studies. More importantly, these findings highlight an association between acute brain dysfunction and acute kidney injury, a potentially modifiable risk factor, and suggest this association is of equal or greater impact than other than that seen with increasing age and sepsis, widely accepted as clinically relevant risk factors for delirium and coma.
Strengths of this study include the large sample with a diverse array of diagnoses, which improve generalizability; prospective, frequent assessment of mental status by highly trained staff using well-validated tools; use of a standardized, laboratory-based definition of acute kidney injury; collection and adjustment for detailed data on important potential confounders, including nonrenal organ failure and sedative use; and a statistical analysis that takes into account the temporal changes in exposure and outcomes. In addition, our findings persisted when we used an alternative definition for acute kidney injury (peak creatinine) and regardless of which approach we used to estimate baseline creatinine.
Limitations include the single-center population that excluded patients with overt neurologic disorders, which may limit generalizability, and the possibility of residual confounding because our study was observational and cannot prove causality. For example, we did not account for the possibility of altered clearance of concomitant medications that may alter the metabolism of sedatives in the setting of acute kidney injury. We also did not have preadmission kidney function measures on all patients, although results remained similar throughout multiple sensitivity analyses. Because the number of patients who received renal replacement therapy was limited, we also could not analyze associations between the dose, timing, or modality of renal replacement therapy and delirium or coma in acute kidney injury.
We are also limited by the precision of the assessments and definitions used to assess mental status and acute kidney injury, although these remain the current standard for clinical evaluation. Our transition model, for example, assumed that serum creatinine elevation observed on a given study day reflected a change in renal function during the prior 24 hours based on the well-established lag between injury and changes in serum creatinine (43, 44). Although most ICU patients do have creatinine assessed at least once daily, it is possible that some acute kidney injury may have occurred on the same day that delirium or coma was assessed. Also, because the Sepsis-3 definition (45) was published after our study was completed, we used an older definition of sepsis that included systemic inflammatory response syndrome criteria and may have been insensitive to sepsis without systemic inflammatory response syndrome. Finally, the CAM-ICU and RASS are not designed to determine the etiology of delirium and coma. We did not distinguish disease-induced coma from drug-induced coma, nor did we identify rapidly reversible sedation-related delirium (which, in one recent study, affected 12% of patients similar to those in our cohort) (46). Lack of precision in these measurements may have increased the noise in our dataset and biased our results toward the null hypothesis.
In conclusion, this large, prospective cohort study demonstrates that acute kidney injury is a significant risk factor for delirium and coma in adults with critical illness. Future studies are now needed to examine the mechanisms underlying these associations and the effects of processes of care (e.g., medications, renal replacement therapy) that may impact the risk of acute brain dysfunction in patients with acute kidney injury and the impact of reducing the incidence, severity, or duration of acute kidney injury on delirium or coma.
Footnotes
Supported by National Institutes of Health grant AG027472 (E.W.E.). E.D.S. was supported by the Vanderbilt Center for Kidney Disease and Veterans Affairs (VA) Health Services Research and Development Service grant IIR-13-073; P.P.P., E.W.E., and T.D.G. were supported by National Institutes of Health grants HL111111, AG035117, and AG034257, respectively; P.P.P. and E.W.E. were supported by the VA Career Development Award and VA Merit Review Award, respectively, from the VA Clinical Science Research and Development Service; and E.W.E. and T.D.G. were supported by the VA Tennessee Valley Geriatric Research, Education and Clinical Center.
Author Contributions: E.D.S., W.H.F., C.M.T., J.D.B., M.D.W., A.J.C., A.J.V., E.W.E., P.P.P., and T.D.G. contributed to study conception and design as well as acquisition, analysis, or interpretation of data. C.M.T. and J.D.B. conducted statistical analysis. E.D.S. and T.D.G. drafted the manuscript. All authors critically revised the manuscript and approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201603-0476OC on November 17, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 4.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089–1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 5.Amin AP, Salisbury AC, McCullough PA, Gosch K, Spertus JA, Venkitachalam L, Stolker JM, Parikh CR, Masoudi FA, Jones PG, et al. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch Intern Med. 2012;172:246–253. doi: 10.1001/archinternmed.2011.1202. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 7.Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, et al. NSARF Group. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 10.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, et al. BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, McArthur C, Seppelt IM, Webb S, Weisbrodt L Sedation Practice in Intensive Care Evaluation (SPICE) Study Investigators; ANZICS Clinical Trials Group. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 13.Shehabi Y, Chan L, Kadiman S, Alias A, Ismail WN, Tan MA, Khoo TM, Ali SB, Saman MA, Shaltut A, et al. Sedation Practice in Intensive Care Evaluation (SPICE) Study Group investigators. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. 2013;39:910–918. doi: 10.1007/s00134-013-2830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, Hinshaw LB The Veterans Administration Systemic Sepsis Cooperative Study Group. Impact of encephalopathy on mortality in the sepsis syndrome. Crit Care Med. 1990;18:801–806. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 16.Kelly KJ. Acute renal failure: much more than a kidney disease. Semin Nephrol. 2006;26:105–113. doi: 10.1016/j.semnephrol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron, Exp Nephrol. 2008;109:e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, Tao Y, Dursun B, Voelkel NF, Edelstein CL, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 19.Altmann C, Andres-Hernando A, McMahan RH, Ahuja N, He Z, Rivard CJ, Edelstein CL, Barthel L, Janssen WJ, Faubel S. Macrophages mediate lung inflammation in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F421–F432. doi: 10.1152/ajprenal.00559.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19:547–558. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–1370. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu R, Kiernan MC, Murray A, Rosner MH, Ronco C. Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephrol. 2015;11:707–719. doi: 10.1038/nrneph.2015.131. [DOI] [PubMed] [Google Scholar]
- 23.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74:901–909. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 24.Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167:1629–1634. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- 25.Kellum JA, Lameire N KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siew ED, Ikizler TA, Matheny ME, Shi Y, Schildcrout JS, Danciu I, Dwyer JP, Srichai M, Hung AM, Smith JP, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7:712–719. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 28.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 29.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 30.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 31.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 32.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: a cohort analysis. JAMA. 1996;275:1489–1494. [PubMed] [Google Scholar]
- 33.Salama M, Farrag SM, abulasrar Sa, Amin MM, Ali AA, Sheashaa H, Sobh M, Arias-Carrión O. Up-regulation of TLR-4 in the brain after ischemic kidney-induced encephalopathy in the rat. CNS Neurol Disord Drug Targets. 2013;12:583–586. doi: 10.2174/1871527311312050006. [DOI] [PubMed] [Google Scholar]
- 34.Himmelfarb J, McMonagle E, Freedman S, Klenzak J, McMenamin E, Le P, Pupim LB, Ikizler TA The PICARD Group. Oxidative stress is increased in critically ill patients with acute renal failure. J Am Soc Nephrol. 2004;15:2449–2456. doi: 10.1097/01.ASN.0000138232.68452.3B. [DOI] [PubMed] [Google Scholar]
- 35.Grill MF, Maganti R. Cephalosporin-induced neurotoxicity: clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann Pharmacother. 2008;42:1843–1850. doi: 10.1345/aph.1L307. [DOI] [PubMed] [Google Scholar]
- 36.Sonck J, Laureys G, Verbeelen D. The neurotoxicity and safety of treatment with cefepime in patients with renal failure. Nephrol Dial Transplant. 2008;23:966–970. doi: 10.1093/ndt/gfm713. [DOI] [PubMed] [Google Scholar]
- 37.Shea YF, Mok MY, Cheng KC, Hon FK, Chu LW. Delayed recovery from ertapenem induced encephalopathy: case-report and a possible mechanism. Int J Clin Pharm. 2013;35:535–537. doi: 10.1007/s11096-013-9812-x. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharyya S, Darby RR, Raibagkar P, Gonzalez Castro LN, Berkowitz AL. Antibiotic-associated encephalopathy. Neurology. 2016;86:963–971. doi: 10.1212/WNL.0000000000002455. [DOI] [PubMed] [Google Scholar]
- 39.Leite TT, Macedo E, Martins IdaS, Neves FM, Libório AB. Renal outcomes in critically ill patients receiving propofol or midazolam. Clin J Am Soc Nephrol. 2015;10:1937–1945. doi: 10.2215/CJN.02330315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strøm T, Johansen RR, Prahl JO, Toft P. Sedation and renal impairment in critically ill patients: a post hoc analysis of a randomized trial. Crit Care. 2011;15:R119. doi: 10.1186/cc10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Zhong D, Lei L, Jia Y, Zhou H, Yang B. Propofol prevents renal ischemia-reperfusion injury via inhibiting the oxidative stress pathways. Cell Physiol Biochem. 2015;37:14–26. doi: 10.1159/000430329. [DOI] [PubMed] [Google Scholar]
- 42.Luo C, Yuan D, Li X, Yao W, Luo G, Chi X, Li H, Irwin MG, Xia Z, Hei Z. Propofol attenuated acute kidney injury after orthotopic liver transplantation via inhibiting gap junction composed of connexin 32. Anesthesiology. 2015;122:72–86. doi: 10.1097/ALN.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 43.Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27:928–937. doi: 10.1038/ki.1985.101. [DOI] [PubMed] [Google Scholar]
- 44.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189:658–665. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]