Abstract
Rationale: Leukocyte recruitment to sites of allergic inflammation depends on the local production of priming cytokines, chemokines, and potentially other mediators. Previously, we showed that eosinophils (Eos) express numerous orphan G-protein–coupled receptors, including Epstein-Barr virus–induced gene 2 (EBI2). Despite its contribution to inflammatory diseases, the role of EBI2 in pulmonary eosinophilia is unknown.
Objectives: To determine whether oxysterol ligands for EBI2 are increased in asthma exacerbation, and if or how they promote Eos pulmonary migration.
Methods: EBI2 ligands and pulmonary eosinophilia were measured in the bronchoalveolar lavage fluid from patients with mild asthma 48 hours after acute allergen challenge. In vitro, the ability of EBI2 ligands alone or in combination with IL-5 priming to induce the migration of human blood Eos was assessed.
Measurements and Main Results: EBI2 was constitutively and stably expressed in peripheral blood Eos. Eos treated with the EBI2 ligands showed significantly increased transwell migration that was enhanced by priming with physiologic doses of IL-5. Migration was suppressed by inhibitors of the prolyl isomerase Pin1 or extracellular signal–regulated kinases (ERK) 1/2 or by pertussis toxin. EBI2 signaling activated Pin1 isomerase activity through a cascade that was sensitive to ERK inhibitors and pertussis toxin. The concentration of EBI2 ligands was significantly increased in the bronchoalveolar lavage fluid 48 hours after segmental allergen challenge and strongly correlated with the increased numbers of Eos, lymphocytes, and neutrophils in the airways.
Conclusions: Oxysterols are increased in inflamed airways after allergen challenge and, through G-protein subunit α, ERK, and Pin1 signaling, likely participate in the regulation of Eos migration into the lung in people with asthma.
Keywords: asthma, eosinophils, migration, EBI2, Pin1
At a Glance Commentary
Scientific Knowledge on the Subject
Inhibition or ablation of chemokine or IL-5 function/expression has not eliminated eosinophilic inflammation in asthma. These data suggest the existence of additional, biologically relevant proinflammatory mediators that contribute to allergic inflammation in humans. These mediators could be targeted to further reduce inflammation and pulmonary pathology.
What This Study Adds to the Field
In this study, we found that segmental allergen challenge increases the level of airway oxysterol Epstein-Barr virus–induced gene 2 (EBI2) ligands, which was strongly correlated with leukocyte infiltration. In vitro, EBI2 ligands induced eosinophil migration through a Gα(i)-, extracellular signal–regulated kinase–, and Pin1-dependent process. Thus, the oxysterol–EBI2 signaling is likely another mechanism for leukocyte and eosinophil recruitment to allergic airways, and EBI2 antagonists combined with Pin1 inhibitors may have therapeutic potential.
In patients with asthma, inhaled allergen triggers eosinophil (Eos) infiltration into the bronchial mucosa and airway lumen from the peripheral blood (1). This recruitment occurs in direct response to the pulmonary release of a variety of lipid mediators, chemoattractants, and cytokines. Eotaxins 1–3; regulated upon activation, normal T-cell expressed and secreted (RANTES); macrophage inflammatory protein (MIP)-1α; and monocyte-chemotactic-protein-3 are among the best-studied and most potent Eos attractants and are produced by pulmonary epithelial and endothelial cells soon after contact with allergen. Once secreted, they interact with CCR3 on the surface of Eos, triggering migration and the release of reactive oxygen species and cationic proteins (degranulation), escalating epithelial cell damage, acute airway inflammation, and pathology.
Although clearly important in rodent models, interference with chemokine function has yielded conflicting results in humans with asthma. For example, whereas genomic knockout of CCR3 abrogated experimental asthma in rodents (2), treatment of patients with CCR3 inhibitors only reduced pulmonary Eos accumulation by approximately 40% (3, 4). This may reflect the loss of surface CCR3 expression by airway Eos, possibly after exposure to IL-3 and RANTES (5–8). Similarly, polymorphisms in CCR3 were not associated with asthma susceptibility or eosinophilia (9). Moreover, although anti-IL-5 treatment significantly reduced the number of blood and sputum Eos, those effects were only observed in a subgroup of patients with steroid-refractory asthma (10, 11). These observations suggest that other mediators, in addition to the known chemokine–cytokine/receptor pairs, contribute to allergic inflammation in humans with asthma.
We have previously shown that peripheral blood Eos express numerous orphan G-protein–coupled receptors (GPRs), including Epstein-Barr virus–induced gene 2 (EBI2; also known as GPR183) (12). EBI2, a seven-transmembrane GPR, was initially implicated as a guidance receptor to position B cells within lymph nodes and spleen (13). Polymorphisms or knock-out of EBI2 impairs the positioning of activated B cells and was associated with inflammatory autoimmune diseases (14, 15). Subsequent studies demonstrated that the receptor was also expressed on dendritic cells, neutrophils, T cells, and astrocytes after immune challenge (16–18).
EBI2 binds to a family of 7-, 25-, and/or 27-hydroxycholesterols (HCs) (oxysterols) that are produced and released by inflamed tissue, including the lung (19). The endogenous ligands for EBI2 are oxidized derivatives of cholesterol and linked to multiple physiologic processes, such as fat metabolism, bile acid synthesis, and lipid transport. On ligand engagement, EBI2 typically signals via inhibitory G-protein subunit α (Gα[i]) to induce intracellular calcium mobilization, mitogen-activated protein kinase (MAPK) activation, and cell proliferation (20, 21), but can also signal via G-protein–independent pathways, such as β-arrestin (17, 22). Although oxysterol-dependent activation of the liver X receptor has been linked to the acquired immune response (23), the aggregate effect of EBI2 signaling also contributes to cell migration.
Pin1, a cis-trans peptidyl prolyl-isomerase, plays a key role in the regulation of innate and adaptive immunity and the expression and signaling of cytokines (24). Pin1 was originally implicated in cell proliferation in part through control of cyclin D1 phosphorylation and stability (25). Pin1 specifically binds to and isomerizes pSer/pThr-Pro peptide bonds, altering target protein conformation, location, levels, and/or function. Previously, we have shown that pharmacologic blockade or genomic deletion of Pin1 prevents the development of experimental asthma in rodents (26, 27).
In the present study, we tested the hypothesis that EBI2 directs Eos migration through a signaling cascade that requires Pin1. We show that segmental allergen challenge increases the level of EBI2 ligands (EBI2-Ls) in the airway that was strongly correlated with leukocyte infiltration into lung. In vitro, EBI2-Ls induced Eos migration through a Gα(i)-, extracellular signal–regulated kinase (ERK)-, and Pin1-dependent process. Therefore, the oxysterol–EBI2 signaling is likely another mechanism for leukocyte and Eos recruitment to allergic airways, and EBI2 antagonists combined with Pin1 inhibitors may have therapeutic potential.
Methods
Research Volunteers for the Bronchoalveolar Lavage Study
Bronchoscopy with bronchoalveolar lavage (BAL) was approved by the University of Wisconsin-Madison Center for Health Sciences Human Subjects Committee and each participant provided written informed consent. Subjects had a history of mild asthma, positive skin prick test, a reversibility to albuterol (180 μg) of greater than 12%, and/or airway hyperresponsiveness to methacholine (the provocative concentration of methacholine producing a 20% fall in FEV1 was <8 mg/ml). Subjects were nonsmokers, were not receiving asthma controller medications, and did not have evidence of a respiratory infection or asthma exacerbation within the previous 4 weeks. Segmental bronchial provocation with allergen (SBP-Ag) and preparation of BAL fluid was performed as described previously (28) and with a brief summary in the online supplement.
Detection of Oxysterols in BAL Fluid by Mass Spectrometry
Detailed methods have previously been described (29). Crude sterols in BAL fluids were extracted with dichloromethane/methanol (1:1) and 10N KOH before isolation using solid-phase extraction columns. Quantitative analysis of oxysterols was performed using a tertiary Shimadzu LC-20XR HPLC system (Shimadzu Corporation, Kyoto, Japan) equipped with an ultrasonic degasser, column oven, and autosampler. Oxysterols were resolved with a binary solvent gradient after injection of 30 μl extract. Integration of areas under elution curves was done using ChemStation software (CSS Analytical Co Inc., Shawnee, KS). Quantitative values were calculated using isotope dilution and single-point calibration through a relative response factor calculation. The relative response factor standard was run at the beginning, middle, and end of each sample set; typically, 54 samples per set.
Cell Migration Assay
CytoSelect 24-Well Cell Migration Assay kit (Cell Biolabs, Inc., San Diego, CA) was used. Cells were seeded in the top of the insert in a medium with or without 10% fetal bovine serum, while chemoattractants were placed in the well below. Inhibitors and IL-5 (for priming) were added directly to the cell suspension before stimulation with chemoattractants. Cells migrated through the pores (5 μm) toward the chemoattractants and were lysed and stained with CyQuant GR dye (from the assay kit) before fluorescence measurement in a plate reader at 480 nm/520 nm.
Pin1 Activity Assay
Activity was measured as described previously (30) with slight modifications.
Statistics
All P values were calculated using GraphPad Prism 7.01 (GraphPad Software, La Jolla, CA). For comparing multiple groups, we performed nonparametric one-way analysis of variance followed by post hoc Tukey test. Data are represented as mean ± SD; P less than 0.05 was considered statistically significant.
Results
EBI2 Ligands Are Increased in Human Asthma
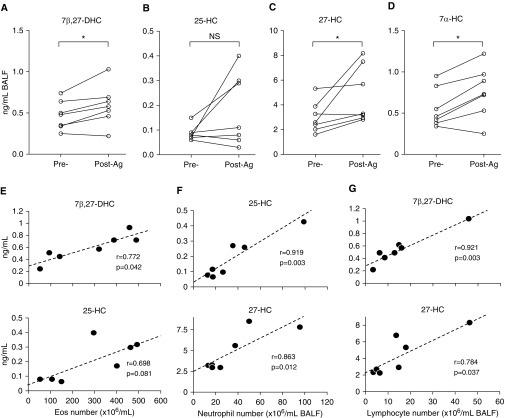
EBI2 oxysterol ligands are up-regulated in a variety of human inflammatory diseases, including atherosclerosis, autoimmunity, diabetes, and cancer (20, 31). Thus, we hypothesized that oxysterol production would be elevated in the BAL fluid of patients with allergic pulmonary inflammation. Therefore, seven patients with mild asthma (see Table E1 in the online supplement) were subjected to SBP-Ag (see online supplement). After 48 hours, BAL fluid was collected, and cellular infiltrate before and after provocation was analyzed. Consistent with previous observations (32), total BAL cell counts increased by approximately fourfold from a baseline of 126 ± 59 × 104 to 516 ± 200 × 104 per milliliter of BAL fluid (see Table E2). Absolute Eos numbers in BAL showed the greatest change and were increased by approximately 40-fold. We then analyzed BAL fluid from these patients for EBI2-Ls (17, 21) by mass spectrometry (29, 33). Of the eight known EBI2-Ls, 25-HC showed a trend to increase after SBP, whereas 7β,27-dihydroxycholesterol (7β,27-DHC), 27-HC, and 7α-HC were significantly increased (Figures 1A–1D). Interestingly, the BAL concentration of 7β,27-DHC, 25-HC, and 27-HC was strongly positively correlated with inflammatory cell counts (Eos, neutrophils, and lymphocytes) with 7β,27-DHC correlating best with Eos and lymphocyte counts and 25-HC correlating best with neutrophilia (Table 1, Figures 1E–1G). These data show that multiple EBI2-Ls are increased in the BAL after acute allergic stimulation and likely participate in Eos, neutrophil, and lymphocyte recruitment/retention in allergic airway disease.
Figure 1.
Epstein-Barr virus–induced gene 2 ligands are increased in bronchoalveolar lavage fluid (BALF) after segmental allergen challenge. (A–D) BALF was collected before and 48 hours after segmental allergen challenge of patients (n = 7) with mild asthma. After removal of cells, the supernatants were subjected to mass spectrometry. (E–G) Pearson correlation coefficients (r) were measured to evaluate the linear relationship between ligand concentration and cell numbers in BAL from after segmental allergen challenge. *P < 0.05 by paired Student's t test. Ag = allergen challenge; DHC = dihydroxycholesterol; Eos = eosinophil; HC = hydroxycholesterol; NS = nonsignificant.
Table 1.
Pearson Correlation Coefficients and P Values for Comparisons of BAL Cells and EBI2 Oxysterol Ligands (n = 7)
| Ligands | Total BAL Cells |
Eosinophils |
Neutrophils |
Lymphocytes |
Macrophages |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | |
| 7β,27-DHC | 0.689 | 0.087 | 0.772 | 0.042 | 0.225 | 0.628 | 0.921 | 0.003 | −0.133 | 0.777 |
| 25-HC | 0.649 | 0.115 | 0.698 | 0.081 | 0.919 | 0.003 | 0.648 | 0.116 | −0.333 | 0.466 |
| 27-HC | 0.667 | 0.102 | 0.681 | 0.092 | 0.863 | 0.012 | 0.784 | 0.037 | −0.257 | 0.578 |
| 7α-HC | 0.342 | 0.453 | 0.430 | 0.335 | −0.250 | 0.589 | 0.523 | 0.229 | −0.081 | 0.863 |
Definition of abbreviations: BAL = bronchoalveolar lavage; DHC = dihydroxycholesterol; EBI2 = Epstein-Barr virus–induced gene 2; HC = hydroxycholesterol.
r, Pearson correlation coefficient; cells are per milliliter of BAL fluid recovered; neutrophils and lymphocytes (but not eosinophils) are logarithmically transformed.
Bold indicates P < 0.05.
EBI2 Is Expressed by Eos and Directs Migration
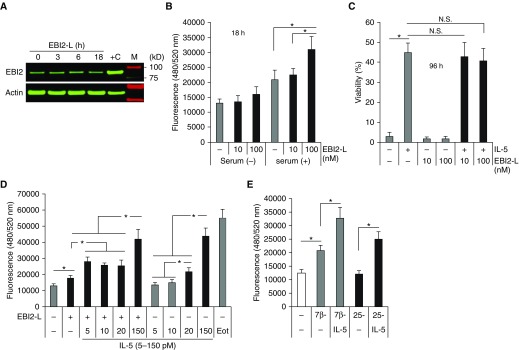
These data suggested that EBI2 and its ligands play a role in allergic airway inflammation. EBI2 (GPR183) is highly expressed in lung (34) and most immune cells after activation (16, 17), although we are unaware of any reports regarding its expression by Eos. RNA Seq revealed 24 GPRs, including EBI2, were expressed above baseline by human peripheral blood Eos (see Table E3) (12). Western blotting confirmed that EBI2 was present in resting Eos or those treated with the prosurvival cytokine IL-5 or EBI2-L (Figure 2A and data not shown). Therefore, EBI2–oxysterol signaling apparatus is present in Eos and based on our in vivo data above, likely functional and physiologically relevant for the migration of human Eos into allergen-challenged lung.
Figure 2.
Epstein-Barr virus–induced gene 2 (EBI2) is expressed by eosinophil (Eos) and directs migration. (A) Freshly purified blood Eos were treated with EBI2 ligand (EBI2-L) (7α, 25-dihydroxycholesterol [DHC]) (100 nM = 40.3 ng/ml) for the indicated times. Whole-cell lysates were subjected to immunoblots with anti-EBI2 and β-actin as shown. +C: Ramos cell lysate served as positive control. (B) Cell migration assay was performed (see Methods) in the presence (+) or absence (−) of 10% fetal bovine serum and EBI2-L. Migrated cells were lysed and stained with DNA dye. (C) Cells were cultured for 96 hours in the presence or absence of EBI2-L (10–100 nM) and IL-5 (150 pM) in the medium containing 10% fetal bovine serum before viability assay. (D) Cells were primed in the presence of different doses of IL-5 (5–150 pM) for 10 minutes before induction of migration with EBI2-L (100 nM) for 3 hours. Eotaxin (50 ng/ml) used as a positive control. (E) Cells were primed with IL-5 (10 pM) before induction of migration with other EBI2-L (6 μM of 7β,27-DHC, or 152 μM of 25-DHC) for 3 hours. Data are expressed as mean ± SD. *P < 0.05. The data are from at least four independent experiments. Eot = eotaxin; N.S. = nonsignificant.
To verify this, we performed cell migration assays using transwell chambers. Peripheral blood Eos was added to the upper chamber and EBI2-L was added to the lower chamber. After incubation for 18 hours, migrated cells were lysed and quantitated (see Methods). In the presence of 10% fetal bovine serum, the EBI2-L 7α,25-DHC (100 nM = 40.3 ng/ml) induced significant migration into the lower chamber (Figure 2B). We observed similar results after migration for 3 hours, indicating that most of cell migration occurs in first 2–3 hours. The difference in lower chamber cell counts was not caused by variation in cell viability because at 96 hours of culture, daily supplementation with 7α,25-DHC (10–100 nM) had no effect on Eos survival either alone or in combination with IL-5 (5–150 pM) (Figure 2C and data not shown). Because BAL IL-5 increases 1.7-fold (5 pM = 13 pg/ml) in patients with asthma (35) and also acts as an independent Eos chemoattractant at high concentration (>150 pM) (36, 37), we primed the cells with different doses of IL-5 for 10 minutes before the induction of migration for 3 hours. High-dose IL-5 (20–100 pM) had similar chemoattractant activity as the EBI2-L alone (100 nM) (EC50 = 2 nM for the receptor activation) or eotaxin (50 ng/ml) (Figure 2D). Interestingly, the EBI2-induced migration was augmented when cells were primed with pathophysiologically relevant doses of IL-5 (5–10 pM).
We have obtained similar data with other EBI2-Ls, including 7β,27-DHC (6 μM) and 25-HC (152 μM) (Figures 1 and 2E) but at higher doses than their EC50 (17, 21). These results are consistent with postchallenge in vivo events in which Eos would be expected to encounter both elevated IL-5 and EBI2-L in the blood and airway after pulmonary allergen exposure. To understand the specificity of the ligands, we isolated human peripheral blood mononuclear cells and neutrophils, and tested their response to 7α,25-DHC, 7β,27-DHC, and 27-HC (Figure 1). All freshly isolated, resting cells had poor response to the ligands. However, prior activation of T cells with anti-CD3/CD28 (see Figure E1) or phorbol myristate acetate/ionomycin (data not shown) increased cell migration to 7α,25-DHC and 7β,27-DHC, as did neutrophils primed with granulocyte-macrophage colony–stimulating factor (GM-CSF) (see Figure E1), suggesting EBI2 also regulates the trafficking of other inflammatory cell types (Figures 1F and 1G; see Table E2) in addition to Eos.
Gα(i), cAMP, and ERK Signaling Are Required for EBI2-directed Eos Migration
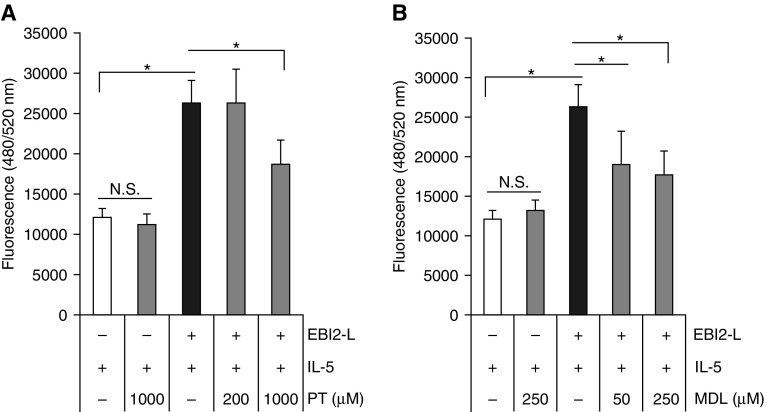
EBI2 signaling can drive MAPKs activation through Gα(i)-dependent or –independent mechanisms (18, 20). Gα(i) blocks G-protein α stimulatory (Gα[s]) function, decreasing the activity of adenylyl cyclases (ACs) 1, 5, and 6 (38). Gβ/Gγ subunits have the opposite effect and can directly stimulate ACs 2, 4, and 7. Thus, the overall intracellular cAMP levels are an aggregate reflection of the levels and activity of Gα(i), Gα(s), and Gβ/γ. To identify which GPR pathway EBI2 signaling used (39), we used purified Eos, which were preincubated with either pertussis toxin (PT) (inhibitor of Gα[i]) (40) or the pan-AC inhibitor MDL-12330A (MDL) (41, 42). Interestingly, both inhibitors had no effect on Eos only treated with IL-5 but suppressed cell migration induced by IL-5-priming and EBI2-L (Figures 3A and 3B). These data suggest that EBI2 signaling is dually regulated by Gα(i) and AC, presumably with AC downstream of Gα(i) independent of IL-5 signaling.
Figure 3.
G-protein α and cAMP mediate Epstein-Barr virus–induced gene 2 (EBI2) signaling. (A and B) Eosinophils were pretreated for 10 minutes with pertussis toxin or adenylyl cyclase inhibitor (MDL-12330A) before priming with IL-5 (10 pM, 10 min) and subsequent induction of migration with EBI2 ligand (100 nM) for 3 hours. *P < 0.05. The data are from at least four independent experiments. EBI2-L = Epstein-Barr-virus-induced gene 2 ligand; MDL = MDL-12330A; N.S. = nonsignificant; PT = pertussis toxin.
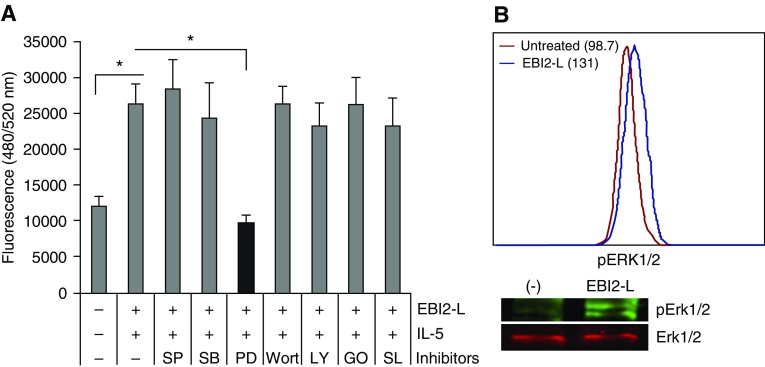
EBI2 engagement is known to activate MAPK pathways in diverse cell types, including transformed cells and glia (20). Therefore, before transwell migration assays, Eos were preincubated with a variety of kinase inhibitors. Although inhibitors for c-Jun N-terminal kinases, p38, phosphatidylinositol-3 kinases (PI3K), protein kinase C-α/β2, and ribosomal S6 kinase had no effect, the MAPK/ERK kinase (MEK) 2 inhibitor (PD98059) reproducibly blocked cell migration induced by IL-5 and EBI2-L (Figure 4A). Western blots and flow cytometry analysis (Figure 4B) revealed the activation of ERK1/2 after EBI2 signaling. Therefore, EBI2 triggers a cascade involving Gα(i), AC, and ERK, all of which are required for Eos migration.
Figure 4.
Extracellular signal–regulated kinase (ERK) mitogen-activated protein kinase (MAPK) mediates Epstein-Barr virus–induced gene 2 (EBI2) signaling. (A) Eosinophils were pretreated with kinase inhibitors (5 μM SP600125 [SP] for c-Jun N-terminal kinases, 5 μM SB203580 [SB] for p38 MAPK, 50 μM PD98059 [PD] for ERK MAPK, 200 nM wortmannin [Wort] for phosphatidylinositol-3 kinases, 10 μM LY294002 [LY] for phosphatidylinositol-3 kinases, 10 nM Gö6976 [GO] for protein kinase C-α/β, 100 nM SL0101-1 [SL] for p90 ribosomal S6 kinase) before priming with IL-5 (10 pM) and subsequent induction of migration with EBI2 ligand (EBI2-L) (100 nM) for 3 hours. (B) Cells were treated with EBI2-L alone for 10 minutes and subjected to flow cytometry and immunoblots for the activation of ERK1/2. *P < 0.05. The data are from at least four independent experiments.
Pin1 Is Required for EBI2-directed Eos Migration
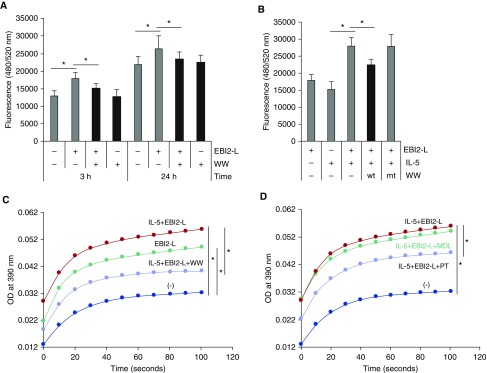
We have shown that pharmacologic blockade of or genetic deletion of Pin1 significantly reduced Eos inflammation and airway fibrosis in experimental rodent models of asthma (26, 27). Although some of the reductions reflected increased apoptosis of pulmonary Eos, these changes did not account for all of the observed differences. Thus, we hypothesized that Pin1 may also play a role in human Eos migration induced by EBI2-L. To test this possibility, we incubated Eos with a specific Pin1 inhibitor before measuring transwell cell migration. As shown (Figure 5A), dominant negative WW peptide of Pin1 alone had no effect on random cell migration; however, it significantly suppressed EBI2-L–induced and IL-5–enhanced cell migration at multiple time points (3 h and 24 h). Control peptides (W34A nonfunctional mutant peptide) unable to compete with endogenous Pin1 also had no effect on migration (Figure 5B).
Figure 5.
Pin1 is required for Epstein-Barr virus–induced gene 2 (EBI2)-directed eosinophil migration. (A) Eosinophils were pretreated with dominant negative TAT-WW of Pin1 peptide (WW, 10 nM) for 10 minutes before induction of migration with EBI2 ligand (EBI2-L) (100 nM) alone for the indicated times. (B) Cells were pretreated with TAT-WW (wt) or with its nonfunctional mutant (mt) before priming with IL-5 (10 pM) and subsequent induction of migration with EBI2 ligand for 3 hours. (C and D) Cells were treated as in B for 10 minutes. Cell lysates were subjected to Pin1 isomerase activity assay as described in Methods. In D, 1,000 μM of pertussis toxin and 250 μM of MDL were used. *P < 0.05. The data are from at least four independent experiments. MDL = MDL-12330A; OD = optical density; PT = pertussis toxin.
These data suggested that Pin1 isomerase activity might be regulated by EBI2 signaling. Thus, Eos were primed and/or treated with EBI2-L before cell lysis and Pin1 activity assay. As shown (Figure 5C), 10-minute exposure to the ligand markedly increased Pin1 cis-trans isomerase activity that was further enhanced when cells were primed with low dose IL-5. The specificity of the assay for Pin1 was confirmed by the inhibitory effect of dominant negative WW peptide (Figure 5C). Collectively, these data suggest that Pin1 isomerase activity is required for and regulates cell migration directed by the ligands.
If Pin1 was required for directed Eos migration, we reasoned that other inhibitors of EBI2 signaling, namely PT and MDL, would also negatively impact Pin1 activity. Thus, cells were pretreated with PT or MDL before IL-5 and EBI2-L. As shown (Figure 5D), although PT and PD98059 (MEK/ERK inhibitor) (data not shown) significantly attenuated Pin1 activity, MDL had no effect. This suggests that AC signaling does not impact Pin1 and that ERK activation needed for both migration and Pin1 activation must be triggered by Gα(i) independently of AC.
Discussion
In this study, we demonstrated that human blood Eos constitutively express EBI2 and its ligands strongly induce cell migration in concert with IL-5 priming. The chemotactic effects of EBI2-Ls were also observed in activated human T cells and neutrophils, although their responses were relatively poor. Relevant to allergic diseases, the endogenous EBI2 oxysterol ligands were significantly increased in the lung after segmental allergen challenge, an accepted experimental surrogate for acute asthma. At the molecular level, EBI2 receptor signaling was mediated via Gα(i), cAMP, and ERK1/2 MAPK. Pin1 was also required for Eos migration, functioning downstream of Gα(i) and ERK, although the molecular targets regulated by the isomerase that are involved in migration remain to be elucidated. Thus, along with established chemokines, EBI2-Ls may facilitate Eos and other immune cell migration and airway inflammation (see Table E2).
Much evidence implicates C-C chemokines in allergic inflammation. RANTES, eotaxins, MIP-1, and monocyte-chemotactic protein 3/4 are dramatically increased in rodent models and humans with asthma (1, 2). Chemoattractants are released by activated Th2 lymphocytes, macrophages, epithelial cells, and mast cells (1, 2), and show variable specificity and potency on Eos. These molecules bind to and signal through a common receptor, CCR3, and function as powerful chemoattractants, in vitro. Despite this, the role of these molecules in the induction of eosinophilic inflammation in patients with asthma remains unclear. For example, BAL Eos showed significantly reduced CCR3 levels after exposure to airway IL-3/RANTES (5–7), attenuating CCR3-mediated signaling. Although the CCR3 agonist MIP-1 is a powerful Eos chemoattractant in mice (43), another agonist (eotaxin-3) shows limited activity in humans (8). Prosurvival cytokines IL-5 and GM-CSF show modest chemotactic activity but very potently prime Eos for enhanced chemotactic responsiveness to suboptimal concentrations of chemokines (44) and oxysterols (Figure 2D). In aggregate these data suggest Eos respond to a complex combination of signals that are only partially predictable based on the cell-surface receptor they engage.
The bioactive oxysterols that were increased after allergen challenge (Figure 1) are potentially a new class of Eos chemoattractants that also show biologic activity toward other inflammatory cells that express EBI2, including neutrophils and lymphocytes (see Table E2 and Figure E1). The most potent physiologic EBI2-L is 7α,25-DHC (17, 21). The short half-life of these compounds quenches their signaling in normal tissues (36). However, during pathology involving tissue damage, such as atherosclerosis, cataracts, and Alzheimer disease (45), EBI2-Ls dramatically increase as we have seen after segmental allergen challenge (Figure 1). Blockade or knock-out (13) of EBI2-L–mediated signaling greatly reduced innate and adaptive immune responses (45), processes essential for responses to infectious, allergic, or inflammatory diseases and cancer. These observations suggest a diverse array of chemotactic signals contribute to immune cell trafficking and activation.
We propose that oxysterols and chemokines are nonredundant signaling molecules because of the differences in their production and metabolism. Oxysterols likely are more short-acting and thus locally relevant because of their rapid production by many cell types and their relatively short half-life. In contrast, chemokines secretion is slower and restricted to fewer cell types but capable of communicating over greater distances (e.g., between lung and bone marrow). Thus, both types of chemoattractants likely function in a spatial and temporal cooperative manner. It remains to be determined if oxysterol production and/or levels can be used as a biomarker for asthma severity or Eos inflammation during disease exacerbations.
In addition to EBI2, Eos expressed a large number of orphan GPRs (see Table E3) (12). Although some ligand-receptor pairs have been identified, their functional roles remain poorly characterized. We have recently shown that many orphan GPRs, including EBI2, are markedly increased in total BAL cells 48 hours after allergen challenge (46), although the cellular source was not definitively determined. These included GPR35, GPR65, and GPR77 (see Table E3), all of which are highly expressed by untreated blood Eos and have been associated with asthma and allergic disease (47–49). Eos lacking GPR65 failed to increase cAMP production or prolong survival after acid treatment (47). GPR77/C5L2 (coded by C5AR2 gene in human) knockout mice were protected from eosinophilic inflammation and airway hyperresponsiveness (49). The latter may reflect the function of GPRs on airway smooth muscle cells that interact with integrins (50). Whether GPR183-deficient mice are similarly protected from challenge-induced eosinophilic pulmonary inflammation is unknown. Taken together, these data suggest that orphan GPR signaling plays a variety of roles in Eos function and asthma pathogenesis.
The signaling that regulates Eos migration in response to various chemoattractants is complex and includes kinase cascades involving MAPKs (51–53) and PI3Ks (54–56). Other activated kinases during Eos migration include protein kinase C (57), Src (58), and Janus kinase 2 (59). Eotaxin, an extremely potent and Eos-selective chemokine rapidly induced tyrosine phosphorylation of multiple intracellular proteins (including ERK1/2), calcium mobilization, actin reorganization, and changes in cell shape (51). In addition to recruitment, eotaxin induces Eos degranulation (60). Coimmunoprecipitation studies revealed that eotaxin binding triggered internalization of the GPR CCR3 and intracellular association with Src family kinases (Hck and c-Fgr) (61). Kinetically similar, oxysterol–GPR EBI2 interactions induced the rapid (1–5 min) recruitment of Gα(i), with subsequent cAMP activation, calcium mobilization (19, 20), and ERK1/2 MAPK phosphorylation (Figures 3B and 4). These data suggest that oxysterol-induced signaling uses a distinct, initial mechanism from CCR3-bound chemokines, but one that eventually integrates into the common pathways required for cell migration.
A role for Pin1 in EBI2 signaling was not unexpected given prior data (24, 26, 27). Pin1 is required for enhanced cytokine gene expression by immune cells and fibroblasts in response to tissue injury or damage in asthma, fibrosis, and infection. Pin1 blockade suppressed GM-CSF or IL-5 production and their prosurvival signaling in activated human Eos as well as in rodent model of asthma (26, 27). At a molecular level, ERK MAPK activation was critical for in vitro survival cytokine expression and in vivo prosurvival signaling in Eos (62–64). The increased level of pulmonary oxysterols (Figure 1) and their activation of ERK1/2 (Figure 4B) via EBI2 provide an additional dimension for defining and understanding of eosinophilic accumulation in the airway of subjects with asthma. Pin1 is required for the EBI2 signaling but not for eotaxin signaling (Chu and colleagues, unpublished data). The molecular mechanism by which Pin1 selectively modulates oxysterol-EBI2 signaling remains elusive. Potential targets include β-arrestin-1/2, protein kinase A, ERK1/2, regulator of G-protein signaling 14, p21-activated kinases, MAPK/ERK kinase kinase kinase 2, and PI3K, all of which have been implicated in Gα(i) signaling and cytoskeleton reorganization (17, 21, 65–67). Nevertheless, we were unable to coimmunoprecipitate these molecules with Pin1 (not shown) despite Eos activation with high-dose EBI2-L. Future studies using mass spectrometry–based proteomics approach should help to delineate the molecule mechanisms.
In summary, we have shown that oxysterol–EBI2 signaling is relevant to human asthma through a complex signaling cascade involving Gα(i), cAMP, ERK, and Pin1. Oxysterols can be pharmacologically manipulated through drugs directed at rate-limiting enzymes in their synthetic pathway that could provide additional approaches to reduce inflammation in allergic diseases.
Acknowledgments
Acknowledgment
The authors thank the participants in the bronchoalveolar lavage study for their time and commitment to this research. The authors thank Elizabeth Schwantes, M.S., and Lei Shi, Ph.D., for processing bronchoalveolar lavage samples; research coordinators Mary Jo Jackson, B.S.N., Holly Eversoll, B.S.N., Michele Wolff, M.S.N., and Evelyn Falibene, B.S., for patient recruitment and screening; Paul Fichtinger, B.S., and Elizabeth Schwantes for eosinophil purification; Richard Cornwell, M.D., and Keith Meyer, M.D., for assistance with bronchoscopies; and David Russell, Ph.D., for the mass spectrometry analysis (UT-Southwestern Medical Center).
Footnotes
Supported by National Institutes of Health grant P01HL088594 (J.S.M. and N.N.J.), General Clinical Research Center grant M01 RR03186, and University of Wisconsin Institute for Clinical and Translational Research grant NCRR/NIH 1UL1 TR000427.
Author Contributions: Conceived and designed the experiments, Z.-J.S., N.N.J., L.C.D., and J.S.M. Performed the experiments, Z.-J.S., J.H., V.P.K., K.L., and E.A.K. Data analysis, Z.-J.S., J.H., J.G.M., V.P.K., E.A.K., and J.S.M. Wrote the paper, Z.-J.S. and J.S.M.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201608-1580OC on January 26, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 2.Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, Martin TR, Gerard NP, Gerard C. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci USA. 2002;99:1479–1484. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauvreau GM, Boulet LP, Cockcroft DW, Baatjes A, Cote J, Deschesnes F, Davis B, Strinich T, Howie K, Duong M, et al. Antisense therapy against CCR3 and the common beta chain attenuates allergen-induced eosinophilic responses. Am J Respir Crit Care Med. 2008;177:952–958. doi: 10.1164/rccm.200708-1251OC. [DOI] [PubMed] [Google Scholar]
- 4.Neighbour H, Boulet LP, Lemiere C, Sehmi R, Leigh R, Sousa AR, Martin J, Dallow N, Gilbert J, Allen A, et al. Safety and efficacy of an oral CCR3 antagonist in patients with asthma and eosinophilic bronchitis: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2014;44:508–516. doi: 10.1111/cea.12244. [DOI] [PubMed] [Google Scholar]
- 5.Nagase H, Kudo K, Izumi S, Ohta K, Kobayashi N, Yamaguchi M, Matsushima K, Morita Y, Yamamoto K, Hirai K. Chemokine receptor expression profile of eosinophils at inflamed tissue sites: decreased CCR3 and increased CXCR4 expression by lung eosinophils. J Allergy Clin Immunol. 2001;108:563–569. doi: 10.1067/mai.2001.118292. [DOI] [PubMed] [Google Scholar]
- 6.Dulkys Y, Kluthe C, Buschermöhle T, Barg I, Knöss S, Kapp A, Proudfoot AE, Elsner J. IL-3 induces down-regulation of CCR3 protein and mRNA in human eosinophils. J Immunol. 2001;167:3443–3453. doi: 10.4049/jimmunol.167.6.3443. [DOI] [PubMed] [Google Scholar]
- 7.Elsner J, Dulkys Y, Kimmig D, Wells TN, Proudfoot AE, Kapp A. Aminooxypentane-RANTES induces CCR3 activation and internalization of CCR3 from the surface of human eosinophils. Int Arch Allergy Immunol. 2001;124:227–229. doi: 10.1159/000053719. [DOI] [PubMed] [Google Scholar]
- 8.Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, Molkentin JD, Rothenberg ME. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005;280:13952–13961. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Chang HS, Kim JH, Park SM, Lee YM, Uh ST, Rhim T, Chung IY, Kim YH, Park BL, et al. Genetic effect of CCR3 and IL5RA gene polymorphisms on eosinophilia in asthmatic patients. J Allergy Clin Immunol. 2007;120:1110–1117. doi: 10.1016/j.jaci.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 11.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 12.Shen ZJ, Hu J, Esnault S, Dozmorov I, Malter JS. RNA Seq profiling reveals a novel expression pattern of TGF-β target genes in human blood eosinophils. Immunol Lett. 2015;167:1–10. doi: 10.1016/j.imlet.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatto D, Brink R. B cell localization: regulation by EBI2 and its oxysterol ligand. Trends Immunol. 2013;34:336–341. doi: 10.1016/j.it.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460:1122–1126. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinig M, Petretto E, Wallace C, Bottolo L, Rotival M, Lu H, Li Y, Sarwar R, Langley SR, Bauerfeind A, et al. Cardiogenics Consortium. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature. 2010;467:460–464. doi: 10.1038/nature09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatto D, Wood K, Caminschi I, Murphy-Durland D, Schofield P, Christ D, Karupiah G, Brink R. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat Immunol. 2013;14:446–453. doi: 10.1038/ni.2555. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Yang XV, Wu J, Kuei C, Mani NS, Zhang L, Yu J, Sutton SW, Qin N, Banie H, et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011;475:519–523. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 18.Rutkowska A, Preuss I, Gessier F, Sailer AW, Dev KK. EBI2 regulates intracellular signaling and migration in human astrocyte. Glia. 2015;63:341–351. doi: 10.1002/glia.22757. [DOI] [PubMed] [Google Scholar]
- 19.Baldan A, Gonen A, Choung C, Que X, Marquart TJ, Hernandez I, Bjorkhem I, Ford DA, Witztum JL, Tarling EJ. ABCG1 is required for pulmonary B-1 B cell and natural antibody homeostasis. J Immunol. 2014;193:5637–5648. doi: 10.4049/jimmunol.1400606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barroso R, Martínez Muñoz L, Barrondo S, Vega B, Holgado BL, Lucas P, Baíllo A, Sallés J, Rodríguez-Frade JM, Mellado M. EBI2 regulates CXCL13-mediated responses by heterodimerization with CXCR5. FASEB J. 2012;26:4841–4854. doi: 10.1096/fj.12-208876. [DOI] [PubMed] [Google Scholar]
- 21.Benned-Jensen T, Smethurst C, Holst PJ, Page KR, Sauls H, Sivertsen B, Schwartz TW, Blanchard A, Jepras R, Rosenkilde MM. Ligand modulation of the Epstein-Barr virus-induced seven-transmembrane receptor EBI2: identification of a potent and efficacious inverse agonist. J Biol Chem. 2011;286:29292–29302. doi: 10.1074/jbc.M110.196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011;475:524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 24.Esnault S, Shen ZJ, Malter JS. Pinning down signaling in the immune system: the role of the peptidyl-prolyl isomerase Pin1 in immune cell function. Crit Rev Immunol. 2008;28:45–60. doi: 10.1615/critrevimmunol.v28.i1.30. [DOI] [PubMed] [Google Scholar]
- 25.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 26.Esnault S, Rosenthal LA, Shen ZJ, Sedgwick JB, Szakaly RJ, Sorkness RL, Malter JS. A critical role for Pin1 in allergic pulmonary eosinophilia in rats. J Allergy Clin Immunol. 2007;120:1082–1088. doi: 10.1016/j.jaci.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Shen ZJ, Esnault S, Rosenthal LA, Szakaly RJ, Sorkness RL, Westmark PR, Sandor M, Malter JS. Pin1 regulates TGF-beta1 production by activated human and murine eosinophils and contributes to allergic lung fibrosis. J Clin Invest. 2008;118:479–490. doi: 10.1172/JCI32789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 29.McDonald JG, Smith DD, Stiles AR, Russell DW. A comprehensive method for extraction and quantitative analysis of sterols and secosteroids from human plasma. J Lipid Res. 2012;53:1399–1409. doi: 10.1194/jlr.D022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennig L, Christner C, Kipping M, Schelbert B, Rücknagel KP, Grabley S, Küllertz G, Fischer G. Selective inactivation of parvulin-like peptidyl-prolyl cis/trans isomerases by juglone. Biochemistry. 1998;37:5953–5960. doi: 10.1021/bi973162p. [DOI] [PubMed] [Google Scholar]
- 31.Daugvilaite V, Arfelt KN, Benned-Jensen T, Sailer AW, Rosenkilde MM. Oxysterol-EBI2 signaling in immune regulation and viral infection. Eur J Immunol. 2014;44:1904–1912. doi: 10.1002/eji.201444493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lilly CM, Nakamura H, Belostotsky OI, Haley KJ, Garcia-Zepeda EA, Luster AD, Israel E. Eotaxin expression after segmental allergen challenge in subjects with atopic asthma. Am J Respir Crit Care Med. 2001;163:1669–1675. doi: 10.1164/ajrccm.163.7.9812044. [DOI] [PubMed] [Google Scholar]
- 33.Stiles AR, Kozlitina J, Thompson BM, McDonald JG, King KS, Russell DW. Genetic, anatomic, and clinical determinants of human serum sterol and vitamin D levels. Proc Natl Acad Sci USA. 2014;111:E4006–E4014. doi: 10.1073/pnas.1413561111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenkilde MM, Benned-Jensen T, Andersen H, Holst PJ, Kledal TN, Lüttichau HR, Larsen JK, Christensen JP, Schwartz TW. Molecular pharmacological phenotyping of EBI2. An orphan seven-transmembrane receptor with constitutive activity. J Biol Chem. 2006;281:13199–13208. doi: 10.1074/jbc.M602245200. [DOI] [PubMed] [Google Scholar]
- 35.Hosoki K, Ying S, Corrigan C, Qi H, Kurosky A, Jennings K, Sun Q, Boldogh I, Sur S. Analysis of a panel of 48 cytokines in BAL fluids specifically identifies IL-8 levels as the only cytokine that distinguishes controlled asthma from uncontrolled asthma, and correlates inversely with FEV1. PLoS One. 2015;10:e0126035. doi: 10.1371/journal.pone.0126035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Effects of reactive oxygen and nitrogen metabolites on RANTES- and IL-5-induced eosinophil chemotactic activity in vitro. Am J Pathol. 1999;155:591–598. doi: 10.1016/S0002-9440(10)65154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sur S, Kita H, Gleich GJ, Chenier TC, Hunt LW. Eosinophil recruitment is associated with IL-5, but not with RANTES, twenty-four hours after allergen challenge. J Allergy Clin Immunol. 1996;97:1272–1278. doi: 10.1016/s0091-6749(96)70195-1. [DOI] [PubMed] [Google Scholar]
- 38.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol. 2000;279:F400–F416. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- 39.Benned-Jensen T, Madsen CM, Arfelt KN, Smethurts C, Blanchard A, Jepras R, Rosenkilde MM. Small molecule antagonism of oxysterol-induced Epstein-Barr virus induced gene 2 (EBI2) activation. FEBS Open Bio. 2013;3:156–160. doi: 10.1016/j.fob.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katada T. The inhibitory G protein G(i) identified as pertussis toxin-catalyzed ADP-ribosylation. Biol Pharm Bull. 2012;35:2103–2111. doi: 10.1248/bpb.b212024. [DOI] [PubMed] [Google Scholar]
- 41.Seifert R, Lushington GH, Mou TC, Gille A, Sprang SR. Inhibitors of membranous adenylyl cyclases. Trends Pharmacol Sci. 2012;33:64–78. doi: 10.1016/j.tips.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell JE, Ussher JR, Mulvihill EE, Kolic J, Baggio LL, Cao X, Liu Y, Lamont BJ, Morii T, Streutker CJ, et al. TCF1 links GIPR signaling to the control of beta cell function and survival. Nat Med. 2016;22:84–90. doi: 10.1038/nm.3997. [DOI] [PubMed] [Google Scholar]
- 43.Lukacs NW, Strieter RM, Shaklee CL, Chensue SW, Kunkel SL. Macrophage inflammatory protein-1 alpha influences eosinophil recruitment in antigen-specific airway inflammation. Eur J Immunol. 1995;25:245–251. doi: 10.1002/eji.1830250140. [DOI] [PubMed] [Google Scholar]
- 44.Koenderman L, van der Bruggen T, Schweizer RC, Warringa RA, Coffer P, Caldenhoven E, Lammers JW, Raaijmakers JA. Eosinophil priming by cytokines: from cellular signal to in vivo modulation. Eur Respir J Suppl. 1996;22:119s–125s. [PubMed] [Google Scholar]
- 45.Olkkonen VM, Béaslas O, Nissilä E. Oxysterols and their cellular effectors. Biomolecules. 2012;2:76–103. doi: 10.3390/biom2010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esnault S, Kelly EA, Schwantes EA, Liu LY, DeLain LP, Hauer JA, Bochkov YA, Denlinger LC, Malter JS, Mathur SK, et al. Identification of genes expressed by human airway eosinophils after an in vivo allergen challenge. PLoS One. 2013;8:e67560. doi: 10.1371/journal.pone.0067560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kottyan LC, Collier AR, Cao KH, Niese KA, Hedgebeth M, Radu CG, Witte ON, Khurana Hershey GK, Rothenberg ME, Zimmermann N. Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood. 2009;114:2774–2782. doi: 10.1182/blood-2009-05-220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milligan G. Orthologue selectivity and ligand bias: translating the pharmacology of GPR35. Trends Pharmacol Sci. 2011;32:317–325. doi: 10.1016/j.tips.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, König P, Gerard NP, Gerard C, Wills-Karp M, Köhl J. A critical role for C5L2 in the pathogenesis of experimental allergic asthma. J Immunol. 2010;185:6741–6752. doi: 10.4049/jimmunol.1000892. [DOI] [PubMed] [Google Scholar]
- 50.Teoh CM, Tam JK, Tran T. Integrin and GPR crosstalk in the regulation of ASM contraction signaling in asthma. J Allergy (Cairo) 2012 doi: 10.1155/2012/341282. 2012:341282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P, Bacon KB. Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. J Immunol. 1999;163:1611–1618. [PubMed] [Google Scholar]
- 52.Adachi T, Stafford S, Kayaba H, Chihara J, Alam R. Myosin light chain kinase mediates eosinophil chemotaxis in a mitogen-activated protein kinase-dependent manner. J Allergy Clin Immunol. 2003;111:113–116. doi: 10.1067/mai.2003.27. [DOI] [PubMed] [Google Scholar]
- 53.Holub A, Byrnes J, Anderson S, Dzaidzio L, Hogg N, Huttenlocher A. Ligand density modulates eosinophil signaling and migration. J Leukoc Biol. 2003;73:657–664. doi: 10.1189/jlb.0502264. [DOI] [PubMed] [Google Scholar]
- 54.Kang BN, Ha SG, Ge XN, Reza Hosseinkhani M, Bahaie NS, Greenberg Y, Blumenthal MN, Puri KD, Rao SP, Sriramarao P. The p110δ subunit of PI3K regulates bone marrow-derived eosinophil trafficking and airway eosinophilia in allergen-challenged mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1179–L1191. doi: 10.1152/ajplung.00005.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lampinen M, Håkansson LD, Venge P. Albumin stimulation of eosinophil migration involves PI3-kinases and is associated with diminished eosinophil CD49d and CD49f expression. Int Arch Allergy Immunol. 2006;140:113–120. doi: 10.1159/000092412. [DOI] [PubMed] [Google Scholar]
- 56.Mishra RK, Scaife JE, Harb Z, Gray BC, Djukanovic R, Dent G. Differential dependence of eosinophil chemotactic responses on phosphoinositide 3-kinase (PI3K) Allergy. 2005;60:1204–1207. doi: 10.1111/j.1398-9995.2005.00845.x. [DOI] [PubMed] [Google Scholar]
- 57.Luschnig-Schratl P, Sturm EM, Konya V, Philipose S, Marsche G, Fröhlich E, Samberger C, Lang-Loidolt D, Gattenlöhner S, Lippe IT, et al. EP4 receptor stimulation down-regulates human eosinophil function. Cell Mol Life Sci. 2011;68:3573–3587. doi: 10.1007/s00018-011-0642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueki S, Kihara J, Kato H, Ito W, Takeda M, Kobayashi Y, Kayaba H, Chihara J. Soluble vascular cell adhesion molecule-1 induces human eosinophil migration. Allergy. 2009;64:718–724. doi: 10.1111/j.1398-9995.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 59.Li B, Zhang G, Li C, He D, Li X, Zhang C, Tang F, Deng X, Lu J, Tang Y, et al. Identification of JAK2 as a mediator of FIP1L1-PDGFRA-induced eosinophil growth and function in CEL. PLoS One. 2012;7:e34912. doi: 10.1371/journal.pone.0034912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tenscher K, Metzner B, Schöpf E, Norgauer J, Czech W. Recombinant human eotaxin induces oxygen radical production, Ca(2+)-mobilization, actin reorganization, and CD11b upregulation in human eosinophils via a pertussis toxin-sensitive heterotrimeric guanine nucleotide-binding protein. Blood. 1996;88:3195–3199. [PubMed] [Google Scholar]
- 61.El-Shazly A, Yamaguchi N, Masuyama K, Suda T, Ishikawa T. Novel association of the src family kinases, hck and c-fgr, with CCR3 receptor stimulation: A possible mechanism for eotaxin-induced human eosinophil chemotaxis. Biochem Biophys Res Commun. 1999;264:163–170. doi: 10.1006/bbrc.1999.1379. [DOI] [PubMed] [Google Scholar]
- 62.Shen ZJ, Esnault S, Malter JS. The peptidyl-prolyl isomerase Pin1 regulates the stability of granulocyte-macrophage colony-stimulating factor mRNA in activated eosinophils. Nat Immunol. 2005;6:1280–1287. doi: 10.1038/ni1266. [DOI] [PubMed] [Google Scholar]
- 63.Shen ZJ, Esnault S, Schinzel A, Borner C, Malter JS. The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax activation. Nat Immunol. 2009;10:257–265. doi: 10.1038/ni.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bates ME, Sedgwick JB, Zhu Y, Liu LY, Heuser RG, Jarjour NN, Kita H, Bertics PJ. Human airway eosinophils respond to chemoattractants with greater eosinophil-derived neurotoxin release, adherence to fibronectin, and activation of the Ras-ERK pathway when compared with blood eosinophils. J Immunol. 2010;184:7125–7133. doi: 10.4049/jimmunol.0900634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banquet S, Delannoy E, Agouni A, Dessy C, Lacomme S, Hubert F, Richard V, Muller B, Leblais V. Role of G(i/o)-Src kinase-PI3K/Akt pathway and caveolin-1 in β2-adrenoceptor coupling to endothelial NO synthase in mouse pulmonary artery. Cell Signal. 2011;23:1136–1143. doi: 10.1016/j.cellsig.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Shu FJ, Ramineni S, Hepler JR. RGS14 is a multifunctional scaffold that integrates G protein and Ras/Raf MAPkinase signalling pathways. Cell Signal. 2010;22:366–376. doi: 10.1016/j.cellsig.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]