Abstract
Rationale: Newly characterized type 2 innate lymphoid cells (ILC2s) display potent type 2 effector functionality; however, their contribution to allergic airways inflammation and asthma is poorly understood. Mucosal biopsy used to characterize the airway mucosa is invasive, poorly tolerated, and does not allow for sequential sampling.
Objectives: To assess the role of ILC2s during nasal allergen challenge in subjects with allergic rhinitis using novel noninvasive methodology.
Methods: We used a human experimental allergen challenge model, with flow cytometric analysis of nasal curettage samples, to assess the recruitment of ILC2s and granulocytes to the upper airways of subjects with atopy and healthy subjects after allergen provocation. Soluble mediators in the nasal lining fluid were measured using nasosorption.
Measurements and Main Results: After an allergen challenge, subjects with atopy displayed rapid induction of upper airway symptoms, an enrichment of ILC2s, eosinophils, and neutrophils, along with increased production of IL-5, prostaglandin D2, and eosinophil and T-helper type 2 cell chemokines compared with healthy subjects. The most pronounced ILC2 recruitment was observed in subjects with elevated serum IgE and airway eosinophilia.
Conclusions: The rapid recruitment of ILC2s to the upper airways of allergic patients with rhinitis, and their association with key type 2 mediators, highlights their likely important role in the early allergic response to aeroallergens in the airways. The novel methodology described herein enables the analysis of rare cell populations from noninvasive serial tissue sampling.
Keywords: ILC2, Th2, allergic airway inflammation, asthma
At a Glance Commentary
Scientific Knowledge on the Subject
Elevated numbers of type 2 innate lymphoid cells (ILC2s) have been identified at mucosal barrier surfaces in several atopic diseases, including the sputum of patients with atopy and severe asthma. However, the contribution of ILC2s to allergic airway inflammation and asthma, and their kinetics after allergen exposure, are poorly understood, partly due to current upper airway sampling techniques that do not allow serial sampling over the course of an allergic response.
What This Study Adds to the Field
Here we demonstrate, for the first time, that ILC2s are recruited to the upper airways of subjects with atopy and asthma in response to nasal allergen challenge, which is associated with increased symptom severity and markers of type 2 inflammation, and suggests a central role for ILC2s in orchestrating type 2 responses. The novel methodology of nasal curettage, followed by flow cytometric analysis, enables the detailed identification of mucosal cell infiltrates during the allergic response and presents wide applications in drug development and clinical diagnosis.
Type 2 immune responses are characterized by the secretion of the cytokines IL-4, IL-5, and IL-13 in response to the epithelial-derived cytokines IL-25, IL-33, and thymic stromal lymphopoeitin (TSLP), which are released by airway epithelial cells after exposure to allergen or virus (1–5). Exaggerated type 2 responses drive the pathogenesis of human allergic disease, including atopic asthma and allergic rhinitis (6–8).
In the context of allergen exposure, dendritic cells sense and subsequently present allergen to naive T cells and promote their differentiation toward T-helper type 2 (Th2) cells, which direct allergic inflammatory responses downstream and secrete type 2 cytokines. The release of these cytokines leads to IgE production, the recruitment and activation of eosinophils, in addition to directly affecting airways hyperresponsiveness and mucous production by goblet cells, which together promote the pathological features seen in allergic disease.
Type 2 innate lymphoid cells (ILC2s) are a recently characterized group of effector cells of the innate immune response that also have the capacity to produce large quantities of the type 2 cytokines, especially IL-5 and IL-13 (9–12). An increasing number of animal studies suggest that ILC2s may play an important early role in the initiation of Th2 responses to aeroallergens during allergic lung inflammation, bridging the innate and adaptive immune responses (13, 14). ILC2s have been identified in humans (6, 15), in whom they express the prostaglandin D2 (PGD2) chemoattractant receptor also found on Th2 cells (CRTh2) (15). PGD2 has recently been shown to be important in both ILC2 recruitment and activation, in addition to cysteinyl leukotrienes, and innate cytokines IL-25, IL-33, and TSLP (15–17).
Elevated numbers of ILC2s have been identified across a number of mucosal tissues, in a variety of human type 2–mediated diseases (18–21), notably in nasal polyps from patients with chronic rhinosinusitis, which suggests a specific role for ILC2s in type 2–mediated disease (15, 22). However, the exact contribution of ILC2s during the orchestration of the allergic response in humans is unclear. One challenge in achieving this has been a lack of repeatable, minimally invasive tissue sampling strategies that allow the analysis of inflammatory cell kinetics. We investigated the localized cellular responses of the nasal mucosa to a topical nasal allergen challenge (NAC) in a group of subjects with allergic rhinitis, using the novel method of applying flow cytometric analysis to nasal curettage samples.
Nasal curettage samples are minimally invasive, well tolerated, and collected without necessity for analgesia, which lends these samples to routine and sequential sampling. Using this novel technique, we identified the presence of a population of human ILC2s in the localized response to allergen provocation, enhancing our knowledge of the role of these recently characterized cells in human type 2 disease. Some of the results of these studies were previously reported in the form of an abstract (23).
Methods
Ethics and Consent
This study was approved by the London Bridge Research Ethics Committee (reference 12/LO/1278) and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Informed consent was obtained from all subjects before their participation.
Study Participants
We recruited nonsmoking volunteers with moderately severe asthma with allergic rhinitis. Study volunteers had an Asthma Control Questionnaire score greater than 0.75 (24), were on treatment that included inhaled corticosteroids (ICSs) or a combination inhaler (long-acting β agonist [LABA] + ICS). After a histamine challenge, subjects with asthma had objective airway hyperresponsiveness with a concentration of histamine required to reduce FEV1 by 20% <8 µg/ml, and evidence of atopy on skin prick testing (at least one positive skin prick test on a panel of 10 aeroallergens, including grass). Histamine challenge and skin prick tests were performed following standard protocols, as previous described (6). Nonsmoking, healthy volunteers without atopy were also recruited. Full inclusion and exclusion criteria are listed in Table 1, which included the use of antihistamines and oral or nasal steroids as a factor for exclusion. Baseline characteristics of study volunteers are shown in Table 2.
Table 1.
Study Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Subjects with allergic rhinitis and asthma | Age 18–55 yr | Current use of antihistamines, nasal steroids, LTRA, or tiotropium |
| Clinical diagnosis of asthma | Current symptoms of rhinitis | |
| Positive skin prick test to timothy grass pollen (in a panel of 10 aeroallergens) | Smoking history in the past 6 mo | |
| Histamine PC20 < 8 μg/ml (or <12 μg/ml and bronchodilator response ≥12%) | History of clinically relevant systemic disease or respiratory disease (other than asthma) | |
| Daily ICS (daily dose ≥400 μg fluticasone or equivalent) or ICS and LABA | OCS treatment in the previous 3 mo | |
| ACQ score > 0.75 | Pregnant or breastfeeding women | |
| Healthy subjects | Age 18–55 yr | Smoking history in the past 6 mo |
| PC20 > 8 μg/ml and bronchodilator response <12% | Positive skin prick test | |
| History or current symptoms of atopic disease such as allergic rhinitis, asthma, or eczema | ||
| Shortness of breath at screening | ||
| History of respiratory or significant systemic disease | ||
| Use of ICS or OCS in the previous 3 mo | ||
| Current use of LABA, nasal spray, antihistamine, LTRA, or tiotropium | ||
| Pregnant or breastfeeding women |
Definition of abbreviations: ACQ = Asthma Control Questionnaire, ICS = inhaled corticosteroid; LABA = long-acting β2 agonist; LTRA = leukotriene receptor antagonist; OCS = oral corticosteroid; PC20 = concentration of histamine required to reduce FEV1 by 20%.
Table 2.
Baseline Characteristics of Study Subjects
| Allergic Rhinitis (n = 9) | Healthy (n = 8) | P Value | |
|---|---|---|---|
| Age, yr | 28 (25, 37.5) | 35 (25.3, 40) | NS |
| Sex, F/M | 4/5 | 5/3 | NS |
| Baseline FEV1% predicted | 87 (77.5, 94.5) | 106.5 (92, 115) | 0.06 |
| PC20, mg/ml | 0.48 (0.08, 1.5) | >8 | N/A |
| ICS daily dose beclomethasone/equivalent, µg | 400 (250, 650) | 0 | N/A |
| Number of positive skin pricks | 4 (2.5, 5) | 0 | N/A |
| IgE, IU/ml | 196 (76.05, 906) | 23.2* (11.4, 41.3) | 0.005 |
| ACQ | 1.67 (1.3, 2.75) | N/A | N/A |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; ICS = inhaled corticosteroid; N/A = not applicable; NS = nonsignificant; PC20 = concentration of histamine required to reduce FEV1 by 20%.
All atopic patients were skin prick positive to timothy grass, Phleum pratense. Data are displayed as median (25th, 75th percentiles) values, and statistical analysis was carried out using Mann-Whitney U tests.
n = 7 healthy subjects.
Study Design
Study volunteers meeting the inclusion criteria underwent a single NAC, between October and March outside the UK grass pollen season, using timothy grass pollen (Aquagen SQ [ALK 225], Phleum pratense, Number 4, freeze dried extract (ALK Abello, Reading, UK). A working concentration of 1 μg of allergen in 100 μl of solution was administered by nasal spray into each nostril. Nasosorption of nasal mucosal lining fluid was performed, as previously described (6), prechallenge and at 2-hour intervals up to 8 hours after challenge. Nasal curettage sampling was performed after nasosorption at baseline and at 6 hours after the challenge. Total nasal symptom scores (TNSS) and clinical observations were assessed at serial intervals before and after challenge in all study subjects (see Figure E1 in the online supplement). TNSS represents the cumulative score of patient-assessed runny nose, nasal blockage, itching and sneezing, each scored from 0 to 3 using a questionnaire. Additional details on clinical methodology are provided in the online supplement.
Nasal Curettage Sampling
Nasal curettage samples were collected using a plastic Rhino-probe curette (Arlington Scientific, Springville, UT). Two samples were taken from the surface of the nasal inferior turbinate using a gentle scraping motion and collected into fluorescence-activated cell sorter buffer before processing into a single-cell suspension, using a 21-gauge needle. Nasal curettage samples are smaller than nasal biopsies, up to 60 µm in depth (determined by electron microscopy, unpublished data).
Flow cytometry and fluorescence-activated cell sorting analysis of nasal mucosa tissue
Nasal cells were incubated in normal human serum (Sigma-Aldrich, Shaftesbury, UK) to prevent nonspecific binding before surface staining for the following populations: eosinophils (HLA-DR−, CD9+ CD16−), neutrophils (HLA-DR−, CD16+ CD9), and ILC2s (lineage− [CD2, CD3, CD14, CD16, CD19, CD56, CD235a] CD123− FcεRIα−, CD127+ CRTh2+). CD45 positive selection was used to identify ILC2s in a subgroup of study volunteers, which did not affect ILC2 enumeration. Numbers of ILC2s, eosinophils, and neutrophils were calculated for each subject by multiplying their percentage of total live cells, after flow cytometric analysis, by the total number of nasal cells stained. ILC2 cells were sorted and analyzed using a Becton Dickinson AriaIIIU Cell Sorter. Granulocytes were analyzed using a Becton Dickinson Fortessa LSR-SORP (Oxford, UK). Further details on the cytometer settings, and antibodies and buffers used are provided in the online supplement (see Tables E1 and E2).
Immunoassays
IL-2, IL-4, IL-5, IL-6, CXCL8/IL-8, IL-12p40, IL-13, IL-17, IFN-γ, CCL11/eotaxin-1, CCL17/TARC, CCL22/MDC, and CCL26/eotaxin-3 levels were analyzed using the Meso-Scale Discovery (MSD) (Rockville, MD) V-Plex array platform and a Sector Imager 2400 (MSD). PGD2 levels were analyzed using a PGD2-MOX enzyme immunoassay (Cayman Chemicals, Ann Arbor, MI) and run on a Spectra Max Plus 384 plate reader (Molecular Devices, Wokingham, UK) with SoftMax Pro 5.4.5 software (Molecular Devices).
Statistical Analysis
Statistical analysis was performed using Prism 6 (GraphPad Software, La Jolla, CA). Data are presented as median (25th, 75th percentiles) with statistical differences within groups determined using Wilcoxon’s signed rank test and between groups using Mann-Whitney U tests. Kruskal-Wallis tests were used to assess differences between groups where appropriate. Correlations were investigated using Spearman’s correlation coefficient. Differences were considered statistically significant at P values <0.05. All P values are two-sided.
Results
Seventeen volunteers (nine subjects with atopy and asthma and eight healthy subjects) underwent a single NAC with timothy grass pollen. No subjects withdrew. One subject with asthma required a single dose of a short-acting bronchodilator after the challenge.
Subjects with Atopy Experience Rapid Onset of Nasal Symptoms after NAC
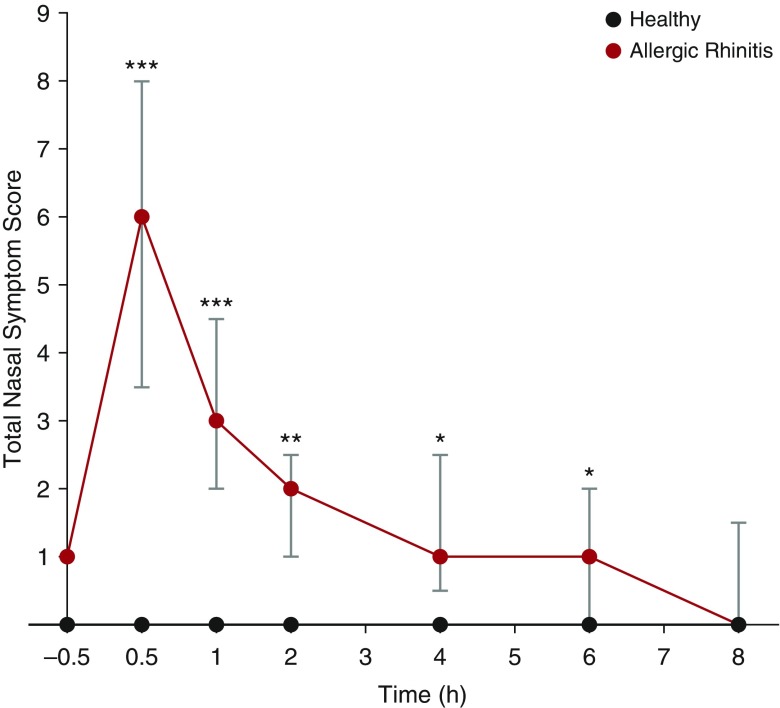
After NAC, subjects with atopy displayed a rapid and significant increase in their TNSS, peaking within the first 30 minutes. This increase in symptoms persisted for up to 6 hours before returning to baseline (Figure 1). Healthy subjects experienced minimal symptoms throughout the course of the study duration.
Figure 1.
Increased nasal symptom scores in subjects with atopy after nasal allergen challenge (NAC). Nasal symptoms were assessed at baseline (30 min before NAC) and at the time points shown after NAC (0 h) in subjects with atopy (n = 9) and healthy controls (n = 8) using a nasal symptom questionnaire. Subjects scored the severity of their nasal running, blockage, itching, and sneezing from 0 to 3; the total nasal symptom score represents the cumulative score. Statistical analysis was performed using a Kruskal-Wallis nonparametric analysis of variance and Mann-Whitney U tests; data are displayed as median (25th, 75th percentiles). *P < 0.05; **P < 0.01; ***P < 0.001.
Allergen-induced Granulocyte Recruitment to the Nasal Mucosa Is Increased in Subjects with Atopy
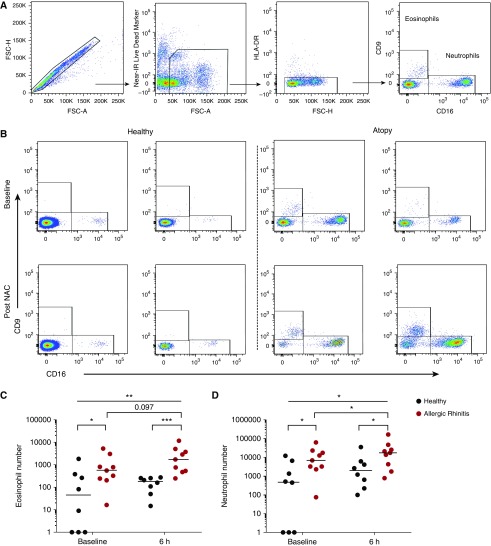
We characterized the granulocytic response in the nasal submucosa by flow cytometric analysis. Single cells were gated on the basis of size and granularity, live cells selected, and neutrophils and eosinophils were identified based on their expression of the markers HLA-DR, CD9, and CD16 (Figure 2A). Subjects with atopy had significantly increased numbers of eosinophils present in the nasal mucosa at baseline compared with healthy subjects; this was increased further after the allergen challenge, with a trend toward increased eosinophil recruitment to the upper airways after NAC in atopy, which was not present in healthy subjects (Figures 2B and 2C). We also observed significantly higher neutrophil numbers in subjects with allergic rhinitis compared with control subjects at baseline, along with significant recruitment after NAC (Figures 2B and 2D).
Figure 2.
Increased recruitment of granulocytes to the upper airways of subjects with atopy after an allergen challenge. Subjects with atopy (n = 9) and healthy control subjects (n = 8) were challenged intranasally with timothy grass pollen. Nasal curettage samples were collected from the inferior turbinate, and granulocytes were enumerated via flow cytometric analysis. (A) Eosinophils (CD9+ CD16−) and neutrophils (CD9− CD16+) were determined within the single, live, HLA-DR− gate. (B) Representative flow cytometric plots display eosinophils and neutrophils from two healthy subjects and two subjects with atopy at baseline and 6 hours after nasal allergen challenge (NAC). (C) Eosinophil and (D) neutrophil numbers for healthy subjects and subjects with atopy at baseline at 6 hours after NAC are shown. Bars represent median values. Kruskal-Wallis tests were used to assess differences between groups; further statistical analysis for paired data was calculated using Wilcoxon matched-pairs test, and unpaired data were calculated using the Mann-Whitney U test. *P < 0.05; **P < 0.01; ***P < 0.001; P < 0.1 displayed. FSC-A/H = forward scatter area/height; IR = infrared.
Type 2 Cytokines and Chemokines Are Induced after Allergen Challenge in Atopy
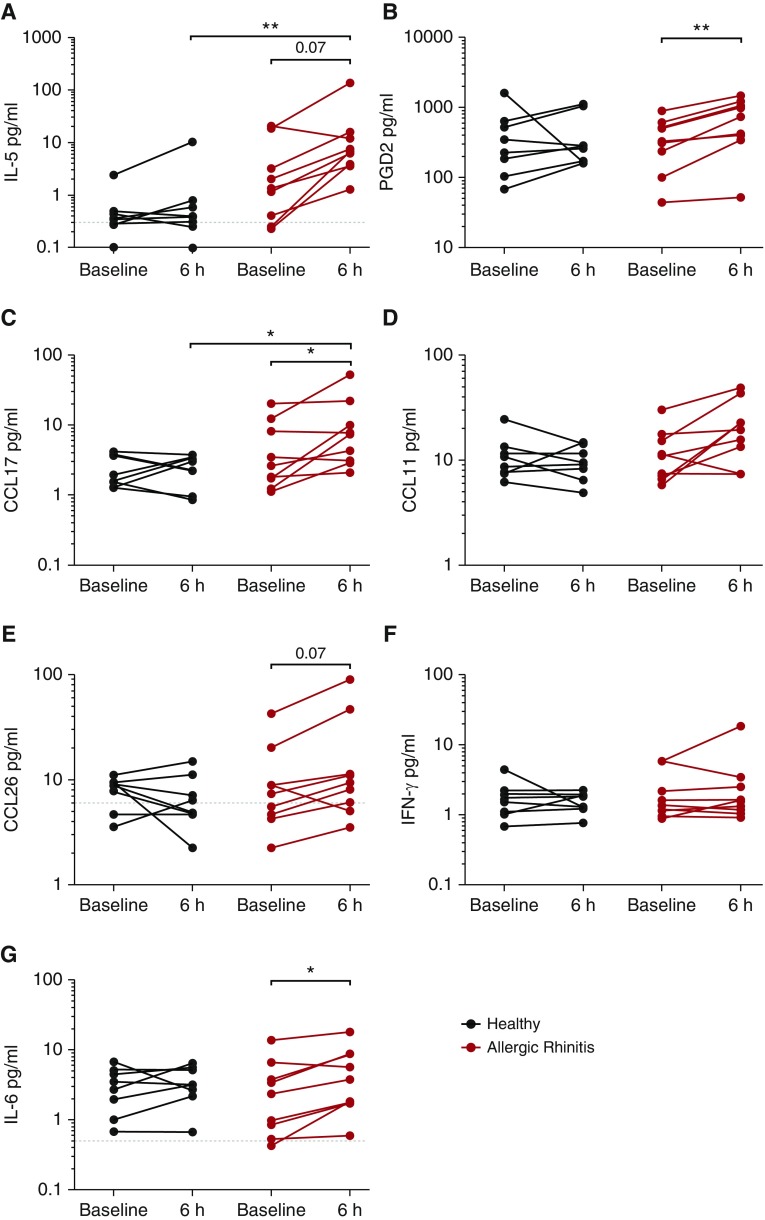
We next sought to determine the nasal levels of mediators associated with the type 2 response. Levels of each mediator are shown at 6 hours after NAC, with full time courses displayed in the online supplement (see Figure E2). NAC evoked a trend toward induction of IL-5 in the upper airways of subjects with atopy, with levels significantly elevated in subjects with allergies after an allergen challenge compared with healthy control subjects (Figure 3A). Similar trends were observed when we measured PGD2, which is associated broadly with allergic inflammatory responses, but has recently been indicated as being the major chemotactic factor for ILC2s (25). Subjects with atopy had a highly significant increase in PGD2 after NAC, which was not observed in healthy subjects (Figure 3B). The Th2 cell chemokine CCL17/TARC was also significantly induced in the airways of patients with atopy after an allergen challenge, with levels significantly increased in the upper airways compared with healthy subjects 6 hours after NAC (Figure 3C). There was a trend toward the induction of the eosinophil chemokine CCL26/eotaxin-3 (Figure 3E) at 6 hours after NAC in subjects with atopy, which was consistent with the increase in eosinophil number observed in this group after NAC (Figure 2C). This pattern of induction was not seen in healthy subjects. In contrast, there was no induction of type 1 cytokine IFN-γ after the challenge in either group (Figure 3F). The proinflammatory mediator IL-6 was elevated 6 hours after NAC in subjects with atopy (Figure 3G). Median levels of IL-13, CCL22/MDC, CXCL8/IL-8, and IL-12p40 are displayed in Table E3. No differences were detected in these mediators between the healthy subjects and subjects with atopy, at baseline or 6 hours after NAC, nor were levels altered after the challenge. IL-2, IL-4, and IL-17 were below the assay limit of detection.
Figure 3.
Induction of type 2 mediators in the nasal lining fluid of subjects with atopy after nasal allergen challenge (NAC). Nasal lining fluid samples were collected using nasosorption, at baseline (30 min before NAC) and at 2-hour intervals up to 8 hours after NAC. Levels of (A) IL-5, (B) prostaglandin D2 (PGD2), (C) CCL17/TARC, (D) CCL11/eotaxin-1, (E) CCL26/eotaxin-3, (F) IFN-γ, and (G) IL-6 were determined in subjects with atopy and healthy control using Meso-Scale Discovery and a PGD2-methoxime enzyme immunoassay. Levels 6 hours after NAC are displayed. Bars represent median values, and dashed lines represent the limit of detection. Statistical analysis for paired data was calculated using Wilcoxon matched-pairs test, and unpaired data were calculated using the Mann-Whitney U test. *P < 0.05; **P < 0.01; P < 0.1 displayed. CCL = chemokine ligand.
Allergen Challenge Causes Enhanced ILC2 Recruitment to the Nasal Mucosa in Subjects with Atopy
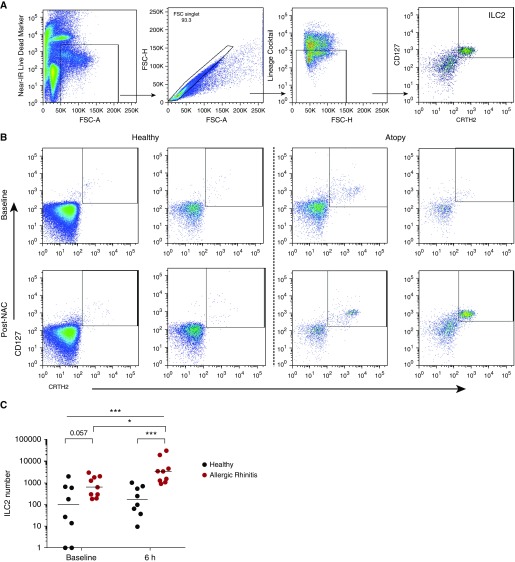
We quantified ILC2s within the nasal mucosa, by flow cytometry, identifying them as live, single cells that were negative for lineage markers (CD2, CD3, CD14, CD16, CD19, CD56, and CD235a), CD123, and FcεR1α, and were CD127+ CRTh2+ cells (Figure 4A). At baseline, subjects with atopy had enriched ILC2 numbers compared with healthy subjects (Figures 4B and 4C). In support of a role for ILC2s in the allergic response, subjects with atopy demonstrated a significant induction of ILC2s 6 hours after the challenge, whereas no change was observed in the healthy subjects. After an allergen challenge, ILC2 numbers were significantly higher in subjects with atopy compared with healthy subjects (Figures 4B and 4C).
Figure 4.
Individuals with atopy have increased recruitment of type 2 innate lymphoid cells (ILC2s) to the upper airways after an allergen challenge. Subjects with atopy (n = 9) and healthy control subjects (n = 8) were challenged intranasally with timothy grass pollen. Nasal curettage samples were collected from the inferior turbinate. (A) ILC2s were enumerated via flow cytometry analysis using the following gating strategy: live, single, lineage− (CD2, CD3, CD14, CD16, CD19, CD56, CD235a) CD123− FcεR1α−, and CD127+ CRTh2+ cells. (B) Representative flow cytometric plots of CD127+ CRTh2+ ILC2s from two healthy subjects and two subjects with atopy are shown at baseline and 6 hours after nasal allergen challenge (NAC). (C) ILC2 numbers from healthy subjects and subjects with atopy before and after NAC are displayed; bars represent median values. Kruskal-Wallis tests were used to assess differences between groups, and further statistical analysis for paired data was calculated using Wilcoxon matched-pairs test, and unpaired data were calculated using the Mann-Whitney U test. *P < 0.05; ***P < 0.001; P < 0.1 displayed. FSC-A/H = forward scatter area/height; IR = infrared.
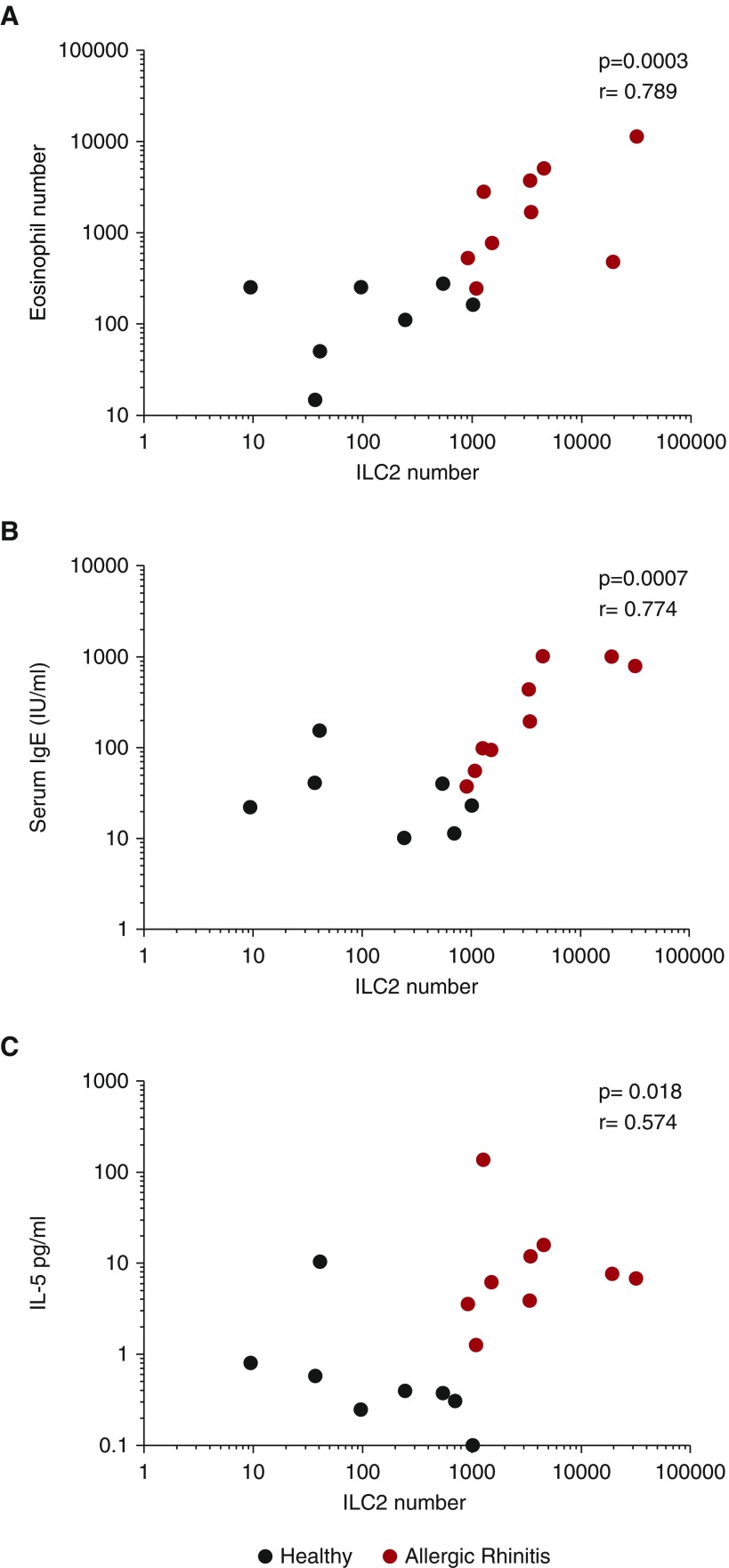
ILC2 Recruitment Correlates with Markers of Type 2 Responses
Having established that ILC2s were recruited to the upper airway during NAC, we then assessed their association with other parameters of type 2 responses. Nasal ILC2 numbers 6 hours after allergen challenge were positively correlated with nasal eosinophil numbers 6 hours after NAC, and with baseline serum IgE and IL-5 levels 6 hours after NAC (Figures 5A–5C), which suggested that ILC2 infiltration was increased after the allergen challenge in the subjects with atopy with Th2-high, eosinophil-mediated disease. Table 3 displays the statistical analysis for all the parameters correlated with ILC2s, for the total study population, and the subjects with atopy alone.
Figure 5.
Markers of type 2 responses correlate with type 2 innate lymphoid cell (ILC2) recruitment. Subjects with atopy (n = 9) and healthy control subjects (n = 8) were challenged intranasally with timothy grass pollen. The number of ILC2s in the upper airways 6 hours after nasal allergen challenge (NAC) correlated with (A) eosinophil number 6 hours after NAC, (B) baseline serum IgE levels (n = 7 healthy control subjects), and (C) IL-5 levels 6 hours after NAC, using Spearman’s correlation coefficient.
Table 3.
Spearman’s Correlation Coefficient Analysis of Upper Airway Type 2 Innate Lymphoid Cell Numbers at 6 Hours after Nasal Allergen Challenge with Study Parameters
| Allergic Rhinitis and Healthy (P Value) | Allergic Rhinitis Alone (P Value) | |
|---|---|---|
| Eosinophils at 6 h | 0.0003 | 0.1475 |
| Neutrophils at 6 h | 0.0104 | 0.0503 |
| IL-5 at 6 h | 0.0179 | 0.2125 |
| PGD2 at 6 h | 0.2942 | 0.3125 |
| CCL17 at 6 h | 0.0854 | 0.9484 |
| CCL11 at 6 h | 0.1589 | 0.9816 |
| CCL26 at 6 h | 0.4609 | 0.8432 |
| IFN-γ at 6 h | 0.6458 | 0.6436 |
| IL-6 at 8 h | 0.744 | 0.81 |
| Number of positive skin pricks | N/A | 0.2307 |
| SPT area | N/A | 0.6357 |
| Baseline serum IgE | 0.0007 | 0.002 |
| AUC TNSS | 0.0025 | 0.7847 |
Definition of abbreviations: AUC = area under the curve; CCL = chemokine ligand; N/A = not applicable; PGD2 = prostaglandin D2; SPT = skin prick test; TNSS = total nasal symptom score.
Correlative analysis was carried out with type 2 innate lymphoid cell number in the upper airway at 6 hours after challenge with the above parameters, analyzing both subjects with allergic rhinitis and healthy subjects and subjects with allergic rhinitis alone using Spearman’s correlation coefficient.
Discussion
Increased understanding of the pathophysiology underlying allergic responses in the airway has led to greater characterization of disease subsets and ultimately the development of new therapeutic approaches. The recent characterization of a novel immune cell family, the innate lymphoid cells, and the subsequent identification of a role for ILC2s in executing type 2 effector functionality places them as a focal point for investigation in atopic disease (10, 21). We used a clinical model of an allergen challenge and novel sampling methodology to identify ILC2s as major responders during the acute phase allergic response in the upper airways.
To our knowledge, our study was the first to demonstrate that subjects with atopy and asthma had a strong trend toward enriched accumulation of ILC2s in the nasal mucosa outside of allergy season compared with healthy control subjects at baseline. Furthermore, after an allergen provocation, only subjects with atopy had a significant increase in the number of ILC2s in the upper airways, which resulted in a significantly greater number being present compared with healthy control subjects. This enhanced recruitment occurred rapidly in the first 6 hours after allergen exposure.
We identified increased numbers of eosinophils in the nasal mucosa of patients with allergic rhinitis, both before and after allergen challenge, which were historically associated with the allergic immune response in asthma. We also identified enriched neutrophils in the upper airways in subjects with atopy and asthma. Although neutrophil influx to the lower airways in asthma was demonstrated previously, and believed to reflect the response to the proinflammatory milieu induced after allergen provocation (26, 27), we demonstrated influx to the upper airways after an allergen challenge. These observations were consistent with previous grass pollen studies that demonstrated the presence of a nasal neutrophil RNA signature after an allergen provocation (28). We also observed increased levels of the proinflammatory mediator IL-6 in our subjects with atopy, which was shown to play a crucial role in neutrophil trafficking (29). Interestingly, ILC2s were demonstrated to be a possible source of IL-6 production (17), although in this study, nasal levels did not correlate with ILC2 numbers and would require further interrogation.
Smith and colleagues previously identified elevated ILC2 numbers in the sputum of patients with eosinophilic asthma (21), and numbers of IL-13+ ILC2s in the peripheral blood of patients with asthma were shown to be associated with level of asthma control (30). We demonstrated that the recruitment of ILC2s was correlated with known features of type 2 inflammation, such as baseline total IgE and IL-5 secretion after challenge, in addition to eosinophil numbers. This suggested that ILC2s are a major component of the localized mucosal cellular immune response to allergen provocation in individuals with allergic rhinitis and asthma.
The positive association of post-NAC ILC2 numbers with both post-NAC eosinophil numbers and IL-5 levels, as well as baseline IgE, suggested that ILC2 responses were associated with a Th2-high clinical endotype. In particular, the correlation with baseline IgE highlighted a novel potential use of serum IgE as a surrogate marker for ILC2 responses in this subset of subjects with chronic lung disease. This is of specific interest in the context of clinical trials because it would provide a rapid and easily performed test to help stratify individuals with asthma into groups based on the likelihood of their ILC responsiveness. A number of CRTh2 antagonists underwent clinical investigation (31–35). Because of the importance of CRTh2 in identifying human ILC2s, combined with recent evidence that PGD2 engagement of this receptor on ILC2s induces their migration and activation (25), would make CRTh2 antagonists an attractive therapeutic approach against ILC2-mediated allergic inflammation.
Although the mechanisms by which ILC2s are recruited to sites of inflammation remain unclear, after NAC in this study, we reported significantly increased PGD2 levels in the nasal lining fluid of subjects with allergic rhinitis, which could act to recruit ILC2s to the upper airways. Mast cell degranulation during the allergic response is believed to be the major source of PGD2, with eosinophils also contributing to the allergic response (36, 37).
In addition, ILC2s are also known to express CCR4, the receptor for the chemokines CCL22 (MDC) and CCL17 (TARC), which are necessary for T-cell trafficking (20). We observed a significant increase in nasal levels of CCL17 in subjects with atopy after NAC, which suggested this chemokine could also be involved in chemotaxis of ILC2s from the periphery.
One of the key features of ILC2s is their capacity to produce large quantities of the type 2 cytokines, particularly IL-5 and IL-13. We observed increased levels of IL-5 in the upper airways of subjects with atopy after NAC compared with healthy subjects without atopy. Because of the short duration of the sampling period, it was unlikely that this would be due to CD4+ T-helper cells alone; however, mast cells were another likely source (38). Further investigation would be required to determine the absolute relative contribution for each cell population in this setting.
It was previously shown that ILC2s could represent as many as 50% of the total IL-5–producing cells after allergen provocation in mouse models (39), and together this would suggest that ILC2s play a key role in orchestrating the recruitment of the effector cells of the allergic response, via the early production of the type 2 mediators. The strong correlation observed between ILC2s and eosinophil recruitment after NAC strengthened this observation.
Although the significant recruitment of ILC2s to the nasal mucosa in subjects with allergies undoubtedly represented a pathological mechanism of their allergic rhinitis, it remained difficult to ascertain whether the observed trend for increased ILC2 numbers present in the upper airways at baseline were directly related to the allergic rhinitis in the subjects or their underlying asthma. Recent work by Bartemes and colleagues (40), who studied the capability of ILC2s isolated from peripheral blood to generate type 2 responses, demonstrated that this process was enhanced in asthma alone and not allergic rhinitis. Furthermore, ILC2s were increased in the sputum of patients with severe asthma, compared with those with mild disease (21). Although these studies suggested that the ILC2 responses observed were related to asthma phenotype and that ILC2s were involved in the pathogenesis of asthma, it is clear that further work is required to distinguish differences in ILC2 responses in relation to specific disease states. These could include a direct comparison of upper airway responses between individuals with atopy and asthma and individuals with atopy but without asthma after allergen provocation, which would directly address the relative contribution of atopic and asthmatic status to ILC2 biology. We believe it likely that substantial shared mechanisms of cellular inflammation exist between atopy and asthma.
Our subjects with atopy and asthma were all on ICS, which might have suppressed the degree of ILC2 responses observed, with some reports suggesting that systemic steroid use could result in an approximately 50% reduction in ILC2 numbers (41). It would therefore be interesting to investigate whether greater ILC2 responses are seen in steroid-naive individuals with atopy and asthma.
In this study, we established a novel technique for the analysis of the local nasal mucosal immune response, using flow cytometric analysis of nasal curettage samples. This allowed for direct and accurate quantification of discrete cell populations, alongside their in-depth phenotypic analysis within the nasal mucosa. In the context of a NAC model, this allowed for the careful and complex assessment of the underlying mechanisms of allergic inflammation in a safe, reproducible, and noninvasive fashion that permitted serial sampling. It could also provide a useful clinical tool for stratifying individuals with active symptoms of rhinitis and monitoring responses to both local and/or systemic treatment.
In summary, this study provided evidence for a population of ILC2s in the nasal mucosa of subjects with atopy and asthma, which was significantly increased after allergen provocation and was associated with cardinal features of upper airways allergic inflammation. These changes suggested that ILC2s might play an important role in the pathogenesis of allergen-mediated type 2 disease in the upper airways, highlighting a need for greater understanding of their role in the lower airways in asthma, and further identifies them as a target for future therapeutic interventions.
Acknowledgments
Acknowledgment
The authors gratefully acknowledge the St. Mary’s Flow Cytometry Core Facility, National Heart and Lung Institute, Imperial College London, for support and access to equipment.
Footnotes
Supported by a Medical Research Council (MRC) and GlaxoSmithKline Strategic Alliance Program grant G1100238, the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme, and MRC and Asthma UK Centre grant G1000758. Financial support from the NIHR Biomedical Research Centre based at Guy's and St. Thomas' NHS Foundation Trust and King's College London and from the NIHR Leicester Respiratory Biomedical Research Unit (D.J.C.). S.L.J. is the Asthma UK Clinical Chair (grant CH11SJ) and is an NIHR Senior Investigator.
Author Contributions: J.D. performed the clinical aspects of the study. M.-B.T.-T. and A.d.R. assisted with screening volunteers and sampling. S.L.J., T.T.H., and D.J.J. supervised clinical aspects of the study. A.C. and J.D. performed clinical sample processing. T.T.H. and R.P.W. developed novel sample processing methods used in the study. M.P. performed fluorescence-activated cell sorting of ILC2s. A.C. and J.D. conducted flow cytometric analysis. R.P.W., D.J.C., and B.M.J.R. advised on flow cytometric analysis. A.C., J.D., and E.B. conducted MSD analysis. R.P.W. and M.R.E. supervised laboratory processing and analysis. R.P.W. conceived and designed the study.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201609-1846OC on January 13, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 3.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 4.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 5.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, Jerico Del-Rosario, Telcian AG, Nikonova A, Zhu J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beale J, Jayaraman A, Jackson DJ, Macintyre JD, Edwards MR, Walton RP, Zhu J, Ching YM, Shamji B, Edwards M, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6:256ra134. doi: 10.1126/scitranslmed.3009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Till S, Durham S, Dickason R, Huston D, Bungre J, Walker S, Robinson D, Kay AB, Corrigan C. IL-13 production by allergen-stimulated T cells is increased in allergic disease and associated with IL-5 but not IFN-gamma expression. Immunology. 1997;91:53–57. doi: 10.1046/j.1365-2567.1997.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 10.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halim TY, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, Salmond RJ, Liew FY. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 15.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 16.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mjösberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT, Jr., Rollins DR, Gorentla B, Liu W, Gorska MM, Chu H, Martin RJ, Alam R. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clinical Immunol. 2015;136:59–68.e14. doi: 10.1016/j.jaci.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O’Byrne PM, Gauvreau GM, Boulet LP, Lemiere C, Martin J, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, Liu YJ, Luong A. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–439. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhariwal J, Cameron A, Trujillo-Torralbo B, Del Rosario A, Paulsen M, Jackson DJ, Edwards MR, Cousins D, Hansel TT, Johnston SL, et al. Novel nasal sampling techniques identify ILC2s as important responders in asthma during nasal allergen challenge [abstract]. Am J Respir Crit Care Med 2016. 193:A7546 [Google Scholar]
- 24.Juniper EF, Bousquet J, Abetz L, Bateman ED GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Xue L, Salimi M, Panse I, Mjösberg JM, McKenzie AN, Spits H, Klenerman P, Ogg G. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoskins A, Reiss S, Wu P, Chen N, Han W, Do RH, Abdolrasulnia R, Dworski R. Asthmatic airway neutrophilia after allergen challenge is associated with the glutathione S-transferase M1 genotype. Am J Respir Crit Care Med. 2013;187:34–41. doi: 10.1164/rccm.201204-0786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nocker RE, Out TA, Weller FR, Mul EP, Jansen HM, van der Zee JS. Influx of neutrophils into the airway lumen at 4 h after segmental allergen challenge in asthma. Int Arch Allergy Immunol. 1999;119:45–53. doi: 10.1159/000024174. [DOI] [PubMed] [Google Scholar]
- 28.Leaker BR, Malkov VA, Mogg R, Ruddy MK, Nicholson GC, Tan AJ, Tribouley C, Chen G, De Lepeleire I, Calder NA, et al. The nasal mucosal late allergic reaction to grass pollen involves type 2 inflammation (IL-5 and IL-13), the inflammasome (IL-1β), and complement Mucosal Immunol[online ahead of print] 28 Sept 2016DOI: 10.1038/mi.2016.74 [DOI] [PubMed] [Google Scholar]
- 29.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181:2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 30.Jia Y, Fang X, Zhu X, Bai C, Zhu L, Jin M, Wang X, Hu M, Tang R, Chen Z. IL-13+ type 2 innate lymphoid cells correlate with asthma control status and treatment response. Am J Respir Cell Mol Biol. 2016;55:675–683. doi: 10.1165/rcmb.2016-0099OC. [DOI] [PubMed] [Google Scholar]
- 31.Hall IP, Fowler AV, Gupta A, Tetzlaff K, Nivens MC, Sarno M, Finnigan HA, Bateman ED, Rand Sutherland E. Efficacy of BI 671800, an oral CRTH2 antagonist, in poorly controlled asthma as sole controller and in the presence of inhaled corticosteroid treatment. Pulm Pharmacol Ther. 2015;32:37–44. doi: 10.1016/j.pupt.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Krug N, Gupta A, Badorrek P, Koenen R, Mueller M, Pivovarova A, Hilbert J, Wetzel K, Hohlfeld JM, Wood C. Efficacy of the oral chemoattractant receptor homologous molecule on TH2 cells antagonist BI 671800 in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2014;133:414–419. doi: 10.1016/j.jaci.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Collins LP, Hunter MG, Steiner J, Lewis T, Payton MA, Perkins CM, et al. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy. 2012;67:1572–1579. doi: 10.1111/all.12042. [DOI] [PubMed] [Google Scholar]
- 34.Singh D, Cadden P, Hunter M, Pearce Collins L, Perkins M, Pettipher R, Townsend E, Vinall S, O’Connor B. Inhibition of the asthmatic allergen challenge response by the CRTH2 antagonist OC000459. Eur Respir J. 2013;41:46–52. doi: 10.1183/09031936.00092111. [DOI] [PubMed] [Google Scholar]
- 35.Gonem S, Berair R, Singapuri A, Hartley R, Laurencin MF, Bacher G, Holzhauer B, Bourne M, Mistry V, Pavord ID, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4:699–707. doi: 10.1016/S2213-2600(16)30179-5. [DOI] [PubMed] [Google Scholar]
- 36.Luna-Gomes T, Magalhães KG, Mesquita-Santos FP, Bakker-Abreu I, Samico RF, Molinaro R, Calheiros AS, Diaz BL, Bozza PT, Weller PF, et al. Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. J Immunol. 2011;187:6518–6526. doi: 10.4049/jimmunol.1101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng X, Ramsden MK, Negri J, Baker MG, Payne SC, Borish L, Steinke JW.Eosinophil production of prostaglandin D2 in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2016. 138:1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, Hendriks RW. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–1116. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 40.Bartemes KR, Kephart GM, Fox SJ, Kita H.Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol 2014. 134:671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, Bethel KJ, Scott DR, Khorram N, Miller M, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155:126–135. doi: 10.1016/j.clim.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]