Abstract
The Division of Lung Diseases of the NHLBI and the Cardiovascular Medical Education and Research Fund held a workshop to discuss how to leverage the anticipated scientific output from the recently launched “Redefining Pulmonary Hypertension through Pulmonary Vascular Disease Phenomics” (PVDOMICS) program to develop newer approaches to pulmonary vascular disease. PVDOMICS is a collaborative, protocol-driven network to analyze all patient populations with pulmonary hypertension to define novel pulmonary vascular disease (PVD) phenotypes. Stakeholders, including basic, translational, and clinical investigators; clinicians; patient advocacy organizations; regulatory agencies; and pharmaceutical industry experts, joined to discuss the application of precision medicine to PVD clinical trials. Recommendations were generated for discussion of research priorities in line with NHLBI Strategic Vision Goals that include: (1) A national effort, involving all the stakeholders, should seek to coordinate biosamples and biodata from all funded programs to a web-based repository so that information can be shared and correlated with other research projects. Example programs sponsored by NHLBI include PVDOMICS, Pulmonary Hypertension Breakthrough Initiative, the National Biological Sample and Data Repository for PAH, and the National Precision Medicine Initiative. (2) A task force to develop a master clinical trials protocol for PVD to apply precision medicine principles to future clinical trials. Specific features include: (a) adoption of smaller clinical trials that incorporate biomarker-guided enrichment strategies, using adaptive and innovative statistical designs; and (b) development of newer endpoints that reflect well-defined and clinically meaningful changes. (3) Development of updated and systematic variables in imaging, hemodynamic, cellular, genomic, and metabolic tests that will help precisely identify individual and shared features of PVD and serve as the basis of novel phenotypes for therapeutic interventions.
Keywords: pulmonary vascular disease, pulmonary hypertension, precision medicine, master protocol, Pulmonary Vascular Disease Phenomics
For centuries, doctors, scientists, and patients have known that the manifestations of a disease and its response to any particular therapy varied widely among individuals. Yet, medical practice, including treatment of pulmonary vascular disease (PVD) and pulmonary hypertension (PH), has been based on inclusive disease definitions, with a one-size-fits-all treatment approach, despite the likelihood that multiple mechanisms of disease lead to PH (1). To improve specificity of treatments, several programs funded by the Division of Lung Diseases of the NHLBI have sought to subtype certain diseases on the basis of clinical, biochemical, and molecular biomarkers to lay the groundwork for therapies targeted to an individual’s specific characteristics (2–5). The Pulmonary Vascular Disease Phenomics (PVDOMICS) project will study multiple etiologies that can lead to PH, PAH, or other manifestations of pulmonary vascular disease (7). This goal is the promise of personalized medicine using a precision medicine approach (6).
Inadequacies of Therapy of PH Using the Traditional Approach
From 1995 until the present, there have been 12 medicines approved for treatment of pulmonary arterial hypertension (PAH) (8). The relatively small impact of these treatments on the 6-minute-walk distance (6MWD) and functional class underlies the need for continued investigations into therapeutic options in PVD (9). The treatment effects on symptoms and exercise capacity are limited, with only one drug showing an improvement in survival (10). There are many reasons for the limited responses that need to be addressed if we are to make future clinical trials for PH more successful. First, patient phenotypes have not been mechanistically defined and delineated. The clinical classification of pulmonary hypertension was established with the World Health Organization in 1998 to incorporate all the associated conditions that may affect the development or progression of pulmonary hypertension (11). There is considerable heterogeneity and ambiguity among and within groups that makes it difficult to identify a single group of patients who may have a common underlying pathophysiology (12). The most common form of PH today is associated with heart failure with preserved ejection fraction (HFpEF), yet there appears to be more uncertainty on how to define this group of patients than any other (13).
A second problem is the lack of satisfactory clinical trial endpoints. Although the 6MWD has been the primary endpoint in most of the PAH registration trials, no minimum change has been established as a requirement (14) (Table 1). Because walking can be affected by many physiologic variables independent of the pulmonary circulation, it becomes difficult to attribute small changes solely to a drug effect on PAH. Also, because mortality rates in recent PH trials have been low, survival as an endpoint may require longer studies and/or larger enrollment. Survival may significantly add to cost in clinical trials as an endpoint but is clearly a major measure of efficacy. The more recently tested “time to clinical worsening” endpoint may be useful to determine durability of a treatment effect but does not inform a physician about the efficacy of a specific therapy in a given patient (15). In addition, there have never been endpoints that reveal the manner in which a drug is working in the pulmonary circulation in these patients. Despite more than 25 years of therapies, there are no clear data that answer whether any of the existing medications reverse the disease, halt progression of the disease, or even affect the rate of progression of disease beyond symptoms and functional limitation.
Table 1.
Reported Change in 6-Minute-Walk Distance in Patients Randomized to Active Therapy in the Pulmonary Arterial Hypertension Registration Trials
| Drug | Change in 6-Minute-Walk Distance (m) |
|---|---|
| Epoprostenol (i.v.) | 31 |
| Bosentan | 36 |
| Ambrisentan | 45 |
| Macitentan | 12 |
| Sildenafil | 45 |
| Tadalafil | 33 |
| Riociguat | 30 |
| Iloprost (inhaled) | 18 |
| Treprostinil (s.c.) | 10 |
| Treprostinil (i.v.) | Not measured |
| Treprostinil (inhaled) | 14 |
| Treprostinil (oral) | 13 |
| Selexipag | 4 |
Definition of abbreviations: i.v. = intravenous; s.c. = subcutaneous.
Comparisons between drugs should not be made from these results, as the trials varied with respect to the severity of the pulmonary arterial hypertension and the use of background therapies. The minimal improvement for a patient to acknowledge a real benefit has been reported to between 54 and 80 m (47). Exercise training in patients who are stable on optimal therapy can increase their 6-minute-walk distance by 96 m (48).
Third, there is limited understanding of the underlying mechanism of action of any treatment in human PH. Future trials need to incorporate endpoints that inform not only if a drug is effective but also where it is working and how it is working and its effect on modifying the underlying disease.
Why a Precision Medicine Approach Is Appropriate for PVD
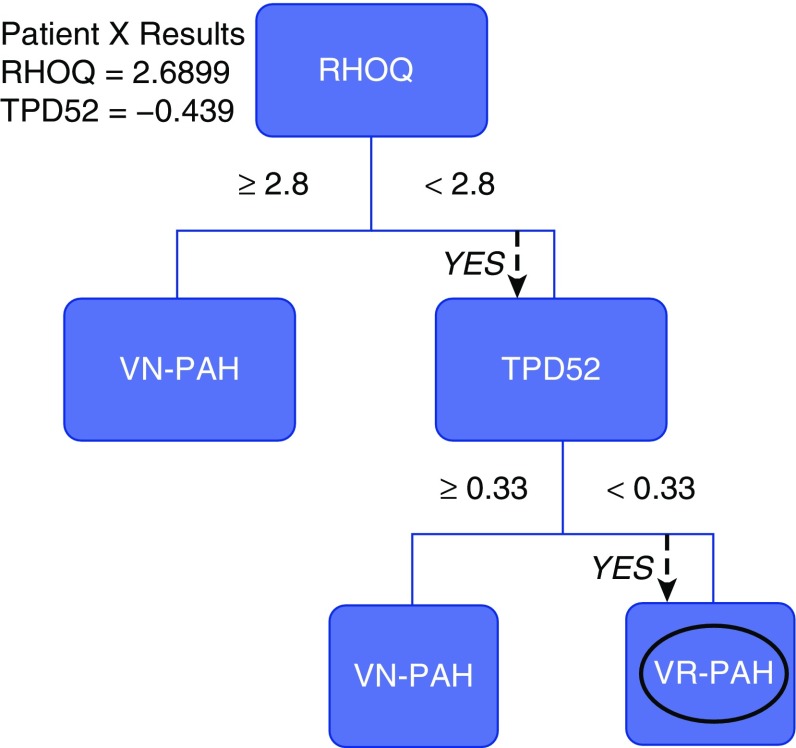
The recent launch of the Precision Medicines Initiative has generated interest in exploring this approach for several diseases (6). To be successful, it will require a clear understanding of the fundamental processes underlying PVD and the identification of biological measures that will identify a specific phenotype that will predict the efficacy of a specified treatment. In actuality, a precision medicine approach is currently part of the assessment of patients with PAH. At the initial right heart catheterization of a suspected patient, vasodilator challenge is a recommended practice to determine if the patient has pulmonary vasoreactivity (16). Those who are vasoreactive can be treated with calcium channel blocker drugs, with an expectation of markedly improved clinical symptoms and up to decades of survival (17, 18). Although this revelation was made years before the Precision Medicines Initiative was established, it supports the notion that identifying a highly predictive biomarker will enable effective treatment of PAH. Proof that genetic phenotyping is possible and potentially of use in treating PH also comes from the case of vasoreactive PAH. Recent work has shown that this endophenotype can be identified in the peripheral blood using RNA expression patterns (19, 20) (Figure 1) and potentially through the identification of a pattern of genetic variants (21).
Figure 1.
Genomic decision tree to differentiate vasodilator-responsive pulmonary arterial hypertension (VR-PAH) from vasodilator-nonresponsive pulmonary arterial hypertension (VN-PAH). The figure shows an example of a decision tree based on the primary gene RHOQ with a secondary gene, TPD52. RHOQ encodes a cytoskeletal protein involved in insulin-mediated signaling, TPD52 encodes a protein in vesicle-mediated transfer, and DSG2 is a desmosomal cadherin involved in Wnt/B-catenin signaling. Numbers shown within the tree are for the performance of the tree in the test cohort, and in the upper right is the performance in the validation cohort. This genomic decision tree correctly identified five of five vasodilator-responsive patients in the validation cohort. Adapted by permission from Reference 19.
PVDOMICS, Linking Phenotypes with Biological Mechanisms: Seeking Precision Insights
The 2010 NHLBI Pulmonary Vascular Strategic Plan identified the development of comprehensive cohorts to define phenotypes, integrating “omics” technologies and systems approaches as a top priority in PVD (2). The overall goal of the PVDOMICS network is to perform clinical phenotyping (demographic, physiologic, clinical chemistries, and imaging) and endophenotyping (genomic, proteomic, metabolomic, coagulomic, cell, and/or tissue based) across all PH groups to deconstruct the traditional classification and define new meaningful subclassifications of patients with PVD (22). The long-term goal is use of endophenotypes/biomarkers for early diagnosis, at-risk screening, and personalized approaches for interventions and/or prevention of PVD. Perhaps the most innovative aspect of the analysis will be to compare all omics data without regard to PH group designation to generate a new, more accurate classification of PVD leading to PH (22) (Figure 2).
Figure 2.
Schematic approach to acquire basic and phenotypic information on multiple individuals and groups of patients with pulmonary vascular disease (PVD). This process should lead to insights and therapies that are more directed at specific mechanisms of disease than is now possible. The omic plan in Pulmonary Vascular Disease Phenomics includes genomic DNA, mRNA expression, proteomics, metabolic variation (especially with exercise and with right ventricular energetics), coagulation profiles, endothelial cell function, and lung and cellular imaging. Data will be linked agnostically to cardiopulmonary function data and to patient clinical features and medical history. Comparators will include normal control subjects and disease control subjects, such as patients with emphysema or pulmonary fibrosis (22).
Although it seems obvious that knowledge of the molecular mechanisms of drug effects is necessary to move the field forward, no registration clinical trial to date has included biological samples for this type of analysis.
A genomic approach to precision medicine in PVD will be especially critical. Genetic variants are particularly well suited to apply to precision medicine because they have high specificity and thus can be measured only once. In addition, DNA is routinely extracted and can be performed on samples drawn at any time in the therapeutic intervention. RNA expression patterns, although less stable than DNA, have been validated in PAH and can be drawn in peripheral blood. Genetic variants and gene expression patterns can be used to characterize the likelihood of a patient responding to a given drug, as illustrated by their association with clinical outcomes in patients treated with endothelin receptor antagonists (21).
The Role of Large Data Analytics
Reanalysis of Prior Clinical Trials
One need not wait for newly defined endophenotypes to apply precision medicine strategies in PH. Clinical data from registration trials are now available to allow deeper analysis and the investigation of secondary evaluations and associations that may not have been evident on first analysis. Randomized clinical trials have enrolled thousands of patients with PAH with detailed assessments at baseline and randomization to active therapy and placebo. Much of the data from these clinical trials are not used in the primary and secondary analyses of drug effects, and it may be difficult to study comparative effectiveness in individual studies. Yet, when harmonized in individual participant data metaanalyses, these data may be used to answer important questions in PAH, such as the response to PAH therapy by sex and race (23) and the comparative effectiveness of treatment and risk of adverse events in distinct types of PAH, such as connective tissue disease (24, 25). A precision medicine approach, where treatments are studied in those most likely to respond, may be available by metaanalyzing multiple studies, achieving the large sample sizes often needed for such analyses.
To achieve the full potential of these completed studies and to optimize the design of future clinical trials in PAH, the creation of common data elements for PAH would facilitate both harmonization of legacy studies and start-up of future studies. Sponsors and investigators involved in the planning phase of studies should adhere to new guidelines for data sharing from the Academy of Medicine and the International Committee of Medical Journal Editors (26, 27).
Application of Machine Learning
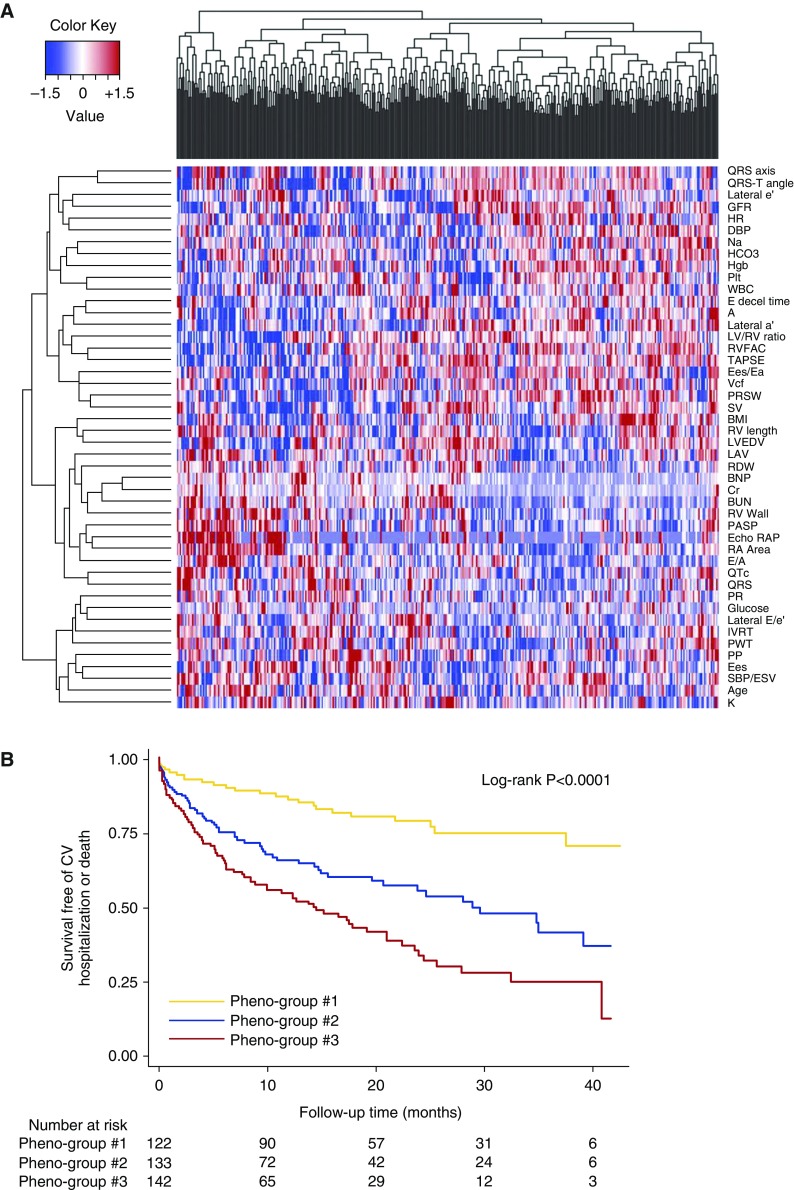
Machine learning uses algorithms to iteratively learn from data. Unsupervised machine learning is a form of statistical learning that seeks to find patterns (clusters) within datasets without prior knowledge of any specific outcome and is useful for classifying heterogeneous clinical syndromes. Supervised machine learning, in which the computer learns from labeled data to make predictions, can also be used to advance precision medicine, such as in predicting treatment responders in a post hoc analysis of a clinical trial. Deep learning is a branch of machine learning that is increasing in popularity due to its ability to process highly nonlinear data that require modeling of increasingly higher levels of abstractions across multiple processing layers (28, 29). Examples of uses of deep learning in medicine include automated processing and diagnosis of medical images for making clinical predictions from large amounts of data from the electronic health record, a process that has been termed “deep patient” (30). Although the current focus on precision medicine is on -omics type data, the data that are used for machine learning can be of any type (e.g., -omics of any kind; environmental data; lifestyle data, such as accelerometry; electronic health record data). Indeed, for many clinical syndromes, such as PH, the genomic-centric approach may not be sufficient. PH is a complex clinical syndrome with multiple etiologies and complex pathophysiology. In addition, because accessing the diseased tissue is not readily available, large-scale gene expression analyses and tissue characterization of the pulmonary vasculature are not possible. Thus, for clinical syndromes such as PH, we must leverage nongenetic data coupled with machine learning to resolve the heterogeneity of the PH syndrome, which will allow for more targeted therapeutics. With improvements in phenotyping techniques, including imaging, proteomics, and metabolomics, we can take advantage of modern “big data” analytics. For example, in patients with HFpEF, a common cause of PH, the combination of deep echocardiographic phenotyping with unsupervised model-based clustering-based machine learning (i.e., “phenomapping”; Figure 3) resulted in the detection of three mutually exclusive subcategories of HFpEF that differed greatly in their clinical characteristics, pathophysiology, and outcomes (31). All of these machine learning techniques could ultimately be used in PH to identify specific subtypes of PVD for future clinical studies.
Figure 3.
Phenomapping for novel classification of heart failure with preserved ejection fraction. (A) Phenotype heat map (phenomap) of heart failure with preserved ejection fraction. Columns represent individual study participants; rows represent individual phenotypes. Red indicates increased value of a phenotype; blue indicates decreased value of a phenotype. (B) Survival free of cardiovascular (CV) hospitalization or death stratified by phenogroup. Reprinted by permission from Reference 31.
Considerations in the Conduct of Clinical Trials for a Precision Medicine Approach
Primary Endpoints
Early clinical trials in PAH were of short duration, with the most common primary endpoint being the 6MWD. Metaanalysis had demonstrated that change in 6MWD did not correlate with other important endpoints, including all-cause death, hospitalization, or initiation of rescue therapy (14). Recently, time to clinical worsening has been used as a primary endpoint in PAH trials (15), with the definition to include time to (1) all-cause mortality, (2) hospitalization for PAH, and (3) disease progression, defined as worsening functional class and a reduction in 6MWD. Although applicable to group data, this endpoint will not be helpful for a personalized approach.
A clinical endpoint that incorporates how an individual patient feels and functions during a drug intervention is necessary for successful personalized treatments. Patient-reported outcomes have not been included as primary endpoints in any of these trials. Recently, the Pulmonary Hypertension Association queried patients regarding measures of drug efficacy using a vignette describing a patient with a newly diagnosed chronic, progressive disease being prescribed a therapeutic regimen specifically for patients linked with their genetic makeup. The responses revealed that patients mostly want improvement in how they feel. Symptom reduction, increased exercise capacity, and improved quality of life dominated their perceived goals, whereas improved survival, cure, and reduced disease progression were about half as important. We suggest that endpoints in future clinical trials include patient preferences as important measurements of efficacy (32).
Secondary Endpoints
The clinical trials in PH have largely ignored informative endpoints essential for understanding how a drug is working in patients and its effect on the underlying disease process. Yet, it is possible with current tools to acquire these data. These include:
-
•
Mechanism of action. When a drug may affect vascular receptors in different circulatory beds, it would be important to ascertain on which vessels and tissues the clinical effects are manifest.
-
•
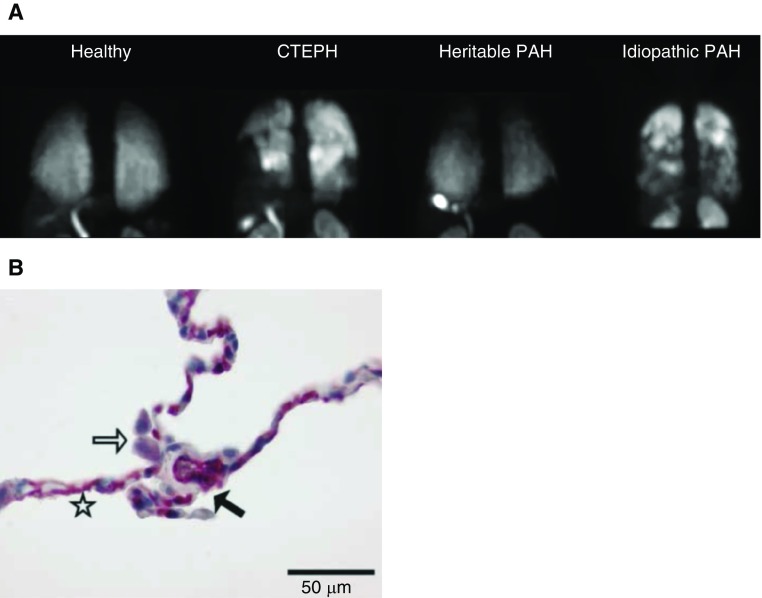
Disease modification. Decades ago, the severity of PVD was estimated in patients with PAH and congenital heart disease with a simple pulmonary wedge angiogram (33). In the future, higher-resolution microvascular imaging may provide an overall picture of disease progression or reversal. Computed tomography technology has now been used in a more comprehensive way in PVD (34). Studies have also shown that molecular imaging of the human pulmonary vascular endothelium is possible using an adrenomedullin receptor ligand (35) (Figure 4). Positron emission tomography scans can demonstrate increased lung 18F-fluorodeoxyglucose uptake in animal models and patients and reveal changes with effective treatments (36), (Figure 5).
-
•
Right ventricular (RV) function and pulmonary vascular compliance (37). Advancing knowledge about the molecular, cellular, and functional characteristics of the RV and pulmonary arteries will accelerate progress in the treatment of PH (38, 39). It is a valid strategy to develop effective therapies that will be directed toward the RV, as the morbidity and mortality in PAH have been correlated best with hemodynamics and RV ejection fraction. Knowledge of whether new medications work solely on the RV, or on both the RV and pulmonary circulation, is of critical importance.
-
•
Prognostic and predictive biomarkers. The profiling of metabolites, including lipids, sugars, nucleotides, amino acids, and amines, is particularly relevant to the understanding of RV–pulmonary vascular dysfunction (39). The goal is to identify biomarker signatures that provide a more rational approach to clinical phenotypes and that will predict favorable responses to each of multiple therapies (or their combination) and subsequently test resulting hypotheses in future, larger studies. Ideally, it would be very useful to establish biomarkers as surrogate endpoints in clinical trials, which could then be incorporated into adaptive trial designs(40).
Figure 4.
Selective adrenomedullin receptor ligand as an imaging modality of the pulmonary vascular endothelium. (A) Molecular single-photon emission computed tomography imaging of the pulmonary circulation with 99mTc-PulmoBind, a selective adrenomedullin receptor ligand, in a healthy human and in subjects with pulmonary hypertension. (B) Intense staining (red) of the adrenomedullin receptor in human lung capillaries. The star indicates the alveolar wall with intensively stained capillaries, the open arrow indicates slightly positive alveolar macrophages, and the solid arrow indicates the septal wall. CTEPH = chronic thromboembolic pulmonary hypertension; PAH = pulmonary arterial hypertension. Adapted by permission from References 34 and 50.
Figure 5.
Lung fluorodeoxyglucose (FDG) uptake in patients with pulmonary arterial hypertension (PAH) are shown in comparison to normal control patients. (A) 18FDG uptake in the idiopathic PAH (IPAH) patient group was increased compared with control subjects. (B) Two-tissue compartment model analysis demonstrated a significantly higher k3 in lungs of patients with IPAH than in control subjects, consistent with increased intracellular glucose metabolism. (C, a) Computed tomographic thorax image (transverse view). (b) Computed tomographic thresholding to define lungs. (c) Defined region of interest in computed tomographic view of lung parenchyma. (d) Coregistration of region of interest with positron emission tomography image. (e) Representative map of lung parametric FDG score in region of interest. (f) Distribution of voxels with top 25% FDG scores in region of interest. (D) Representative three-dimensional parametric map generated from computed per-voxel FDG scores from a patient with IPAH showing uneven FDG uptake within the lung. CTD = connective tissue disease. Adapted by permission from Reference 36.
Considerations in the Design of Clinical Trials
Advances in regulatory science need to be exploited in PVD, given its orphan disease status. Several strategies have been identified that can be applied to phase II and phase III precision medicine trials (40–44) (Table 2). Predictive enrichment strategies are used to select subjects for study who have the greatest chance of benefit on the basis of a validated biomarker. Adaptive clinical trials evaluate a treatment by measuring appropriate outcomes on a prescribed schedule and then modify the trial protocol in a prospective strategy on the basis of the observed effects. Modifications can be made in an adaptive manner to the dose and schedule of drug, patient selection to include enrichment with responsive patients, and avoidance of nonresponders. A factorial study design allows investigators to test multiple hypotheses at once. A crossover study design has greater power than a parallel trial design for the same number of participants. A randomized discontinuation trial is optimal for studying long-term, noncurative therapies, especially when the use of placebo is considered unethical. An N-of-1 trial design involves multiple crossover experiments performed over predefined time periods to compare the effects of different treatments on outcome measures within an individual patient. A patient enrolled in an N-of-1 trial undergoes baseline measurement of a specific outcome measure followed by an intervention for a prespecified time period, after which performance on the outcome measures is reassessed. After a drug washout period, the same experimental design is repeated to measure the effect of a second therapy on the same outcome measures.
Table 2.
Characteristics of Proposed and Established Study Designs in Pulmonary Arterial Hypertension
| Trial Type | Design | Advantage | Limitation |
|---|---|---|---|
| Randomized controlled trial | Patients randomized to study agent or placebo and outcomes assessed at follow-up | Placebo control demonstrates efficacy | Expense |
| Ethics of placebo use | |||
| Powered adequately to determine effect | Subpopulations not well studied | ||
| Factorial design | ≥2 factors, each with ≥2 levels: 2 × 2 factorial design; drug A + placebo B; placebo A + placebo B; placebo A + active B; active A + active B | Test multiple hypotheses at once | Potential interaction between agents |
| Test combination of agents | |||
| Crossover study | Each subject is administered a particular therapy at different time points | Within-subject analysis possible | Rapid clinical deterioration may affect results and limit eligibility of patients |
| Smaller sample size necessary | |||
| Randomized discontinuation trial | Responders to drug therapy are randomly assigned to placebo or continued treatment | Removal of patients who are therapy nonresponders is an element of study design | Adverse events may occur on withdrawal of drug |
| N-of-1 clinical trial | Multiple crossover experiments over a predefined time period | Individualized therapeutic response identified | Limited statistical power, generalizability of findings to other patients unknown |
Adapted by permission from Reference 49.
The Challenges
The Challenge from the Regulatory Perspective
The development of biomarker-based approaches to personalized medicine in cardiovascular disease has been challenging, in part, because most cardiovascular therapies treat acquired syndromes that develop over many years and represent the end result of several pathophysiological mechanisms. Success in designing clinical trials for personalized medicine will require the selection of patient populations with attributes that can be targeted or that predict outcome and the use of appropriate enrichment strategies once such attributes are identified. In oncology, the ability to identify specific molecular targets has resulted in therapies that work in small populations but with a magnitude of benefit that is amplified. Cardiovascular studies approach hypertension and heart failure as population-based diseases and test which disease responds to specific drugs through trial and error. Although there has been a long-recognized need for the importance of understanding different pathways in PH, the current therapies work primarily via non–pulmonary-specific vasodilation effects.
Considering the relatively modest benefits that PAH drugs possess, regulatory agencies have tolerated considerable uncertainties in the safety profile resulting from much smaller safety databases than are typically expected of chronic therapies. This uncertainty will be aggravated by further reduction in the size of development programs targeting progressively more constrained populations. This means that the benefits with such targeted therapies will need to be fundamentally larger than those of current drugs (e.g., mortality, avoidance of hospitalization, or functional or symptomatic improvements unequivocally large enough for individual subjects to perceive as meaningful). More reliance on post-marketing surveillance of efficacy and safety will be inevitable.
The Food and Drug Administration (FDA) has permitted the use of drugs that have a proven benefit on some clinical endpoint, even if that endpoint is not wholly satisfactory to the field. In PAH, the 6MWD is an example. If a drug has a satisfactory safety profile and gives a statistically significant increase in the 6MWD, the drug is approvable. If post-marketing use of the drug identifies a significant toxicity, the FDA has the responsibility and right to either demand a boxed warning or to decertify a drug from the marketplace. The FDA is poised to be a partner in the evolution of precision medicine on the basis of better understanding of shared mechanisms of disease in PH cohorts and will work with trialists and the pharmaceutical industry to develop satisfactory trial designs and to aid in innovative approaches toward PH (Figure 2).
Pharmaceutical Industry Challenges with Precision Medicine Clinical Trials
The advances in genomics and understanding of individual responses to efficacy and safety of therapeutics have challenged the traditional “one size fits all” drug-development paradigm. As the cost of drug development has accelerated, with the average cost for one new approved drug a staggering $2.6 billion (2000–2010), the industry is looking for more efficient drug development pathways. Although the overall likelihood of regulatory approval from phase I for all drug candidates is approximately 10%, the success rate increases with use of selective biomarkers and in rare diseases (45, 46). New drugs for PVD will require new models of collaboration among academia, industry, and the FDA. These consortia must address the organizational complexity to gain the potential benefits. These include the sharing and/or combination of biomarker databases, agreement on intellectual property rights, standardization of operating procedures in a clinical consortium, decisions on funding and influence within a consortium, and how prioritization and other decisions are made. Industry needs to be open-minded as to what constitutes a true commercial advantage that must be protected while maintaining the spirit of open collaboration and, most important, the interests of the patients.
Clinical trials in PAH have many challenges. The sample size required to establish the utility of novel therapeutics is increasing for reasons that include the increasing prevalence of combination therapy, the ethical problems of placebo treatment in naive patients, and the small incremental improvements anticipated in traditional endpoints. These changes necessitate high enrollment numbers for adequate powering. Heterogeneity of treatment effects is a problematic issue. Specifically, those who benefit most from novel treatments are usually the most severely affected by PAH, but small in number. Conversely, the least severely affected who are available to study are those who receive little benefit from the treatments. However, these less ill patients are exposed to the same risks for side effects as the most severely affected people. In addition, the least severely affected patients with PAH also have the least room for improvement in response to effective novel treatment, leading to increased clinical trial sample sizes.
The cost of new medicines will continue to draw attention, as niche drugs usually come with high price tags. Political tensions will probably increase when patients are denied access to precision medicines for financial reasons. The FDA is not in a position to intercede in this area, but societal demands will require a dialogue between the payers and the drug sponsors.
Summary of Recommendations to the NHLBI
-
1.
A national effort, involving all the stakeholders to coordinate biosamples and biodata from all funded programs to a web-based repository so that information can be shared and correlated with other and all research projects. This could be considered as an element of future multicenter trials.
-
2.
Coordination of genomic data with the National Precision Medicine Initiative so that large genetic databases can be used to detect genotype–phenotype relationships.
-
3.
Creation of a task force, inclusive of the principal stakeholders, to develop a Master Clinical Trials Protocol for PVD that will apply precision medicine principles to future interventional clinical trials. With the input and approval of regulatory agencies, the Master Protocol would provide a reasonable path for drug development (phase II) and registration (phase III) while it addresses the needs of academia, clinicians, and patients. Specifically:
-
•
Patient-centered outcome measures that incorporate patient needs and preferences should be identified in PVD and tested and validated in future clinical trials along with traditional medical outcomes measures.
-
•
As the development of precision medicine initiatives alter the size and composition of clinical trials, statistical expertise and FDA insights will be needed to allow for flexible and innovative statistical design.
-
•
There is a need for testing of newer endpoints, both primary and secondary, that represent well-defined and clinically meaningful changes that accurately reflect whether a drug is working in a given patient.
-
•
Continued development of static and dynamic imaging, hemodynamic, cellular, genomic, and metabolic variables that will identify patients for their personal features. Such development should be hypothesis based where possible to reveal differences that can lead to improved trials development whose effects can be objectively measured.
-
•
Footnotes
Supported by the NHLBI, National Institutes of Health, and the Cardiovascular Medical Research and Education Fund.
This report should not be construed to represent the Food and Drug Administration’s views or policies.
Originally Published in Press as DOI: 10.1164/rccm.201701-0150WS on April 21, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D4–D12. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins IM, Moore TM, Blaisdell CJ, Abman SH. National Heart, Lung, and Blood Institute workshop: improving outcomes for pulmonary vascular disease. Circulation. 2012;125:2165–2170. doi: 10.1161/CIRCULATIONAHA.112.092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erzurum S, Rounds SI, Stevens T, Aldred M, Aliotta J, Archer SL, Asosingh K, Balaban R, Bauer N, Bhattacharya J, et al. Strategic plan for lung vascular research: an NHLBI-ORDR workshop report. Am J Respir Crit Care Med. 2010;182:1554–1562. doi: 10.1164/rccm.201006-0869WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dweik RA, Rounds S, Erzurum SC, Archer S, Fagan K, Hassoun PM, Hill NS, Humbert M, Kawut SM, Krowka M, et al. ATS Committee on Pulmonary Hypertension Phenotypes. An official American Thoracic Society statement: pulmonary hypertension phenotypes. Am J Respir Crit Care Med. 2014;189:345–355. doi: 10.1164/rccm.201311-1954ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin ED, West J, Loyd JE, Hemnes AR. Molecular medicine of pulmonary arterial hypertension from population genetics to precision medicine and gene editing. Am J Respir Crit Care Med. 2017;195:23–31. doi: 10.1164/rccm.201605-0905PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015;65:1976–1997. doi: 10.1016/j.jacc.2015.03.540. [DOI] [PubMed] [Google Scholar]
- 9.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, Weitzenblum E, Cordier JF, Chabot F, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 10.Macchia A, Marchioli R, Tognoni G, Scarano M, Marfisi R, Tavazzi L, Rich S. Systematic review of trials using vasodilators in pulmonary arterial hypertension: why a new approach is needed. Am Heart J. 2010;159:245–257. doi: 10.1016/j.ahj.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Simonneau G, Galiè N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 12.Ghofrani HA, Wilkins MW, Rich S. Uncertainties in the diagnosis and treatment of pulmonary arterial hypertension. Circulation. 2008;118:1195–1201. doi: 10.1161/CIRCULATIONAHA.106.674002. [DOI] [PubMed] [Google Scholar]
- 13.Borlaug BA. Invasive assessment of pulmonary hypertension: time for a more fluid approach? Circ Heart Fail. 2014;7:2–4. doi: 10.1161/CIRCHEARTFAILURE.113.000983. [DOI] [PubMed] [Google Scholar]
- 14.Savarese G, Paolillo S, Costanzo P, D’Amore C, Cecere M, Losco T, Musella F, Gargiulo P, Marciano C, Perrone-Filardi P. Do changes of 6-minute walk distance predict clinical events in patients with pulmonary arterial hypertension? A meta-analysis of 22 randomized trials. J Am Coll Cardiol. 2012;60:1192–1201. doi: 10.1016/j.jacc.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin VV, Badesch DB, Delcroix M, Fleming TR, Gaine SP, Galiè N, Gibbs JS, Kim NH, Oudiz RJ, Peacock A, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S97–S107. doi: 10.1016/j.jacc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Rich S, Brundage BH. High-dose calcium channel-blocking therapy for primary pulmonary hypertension: evidence for long-term reduction in pulmonary arterial pressure and regression of right ventricular hypertrophy. Circulation. 1987;76:135–141. doi: 10.1161/01.cir.76.1.135. [DOI] [PubMed] [Google Scholar]
- 18.Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 19.Hemnes AR, Trammell AW, Archer SL, Rich S, Yu C, Nian H, Penner N, Funke M, Wheeler L, Robbins IM, et al. Peripheral blood signature of vasodilator-responsive pulmonary arterial hypertension. Circulation. 2015;131:401–409. [Discussion, p. 409]. doi: 10.1161/CIRCULATIONAHA.114.013317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemnes AR, Zhao M, West J, Newman JH, Rich S, Archer SL, Robbins IM, Blackwell TS, Cogan J, Loyd JE, et al. Critical genomic networks and vasoreactive variants in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:464–475. doi: 10.1164/rccm.201508-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benza RL, Gomberg-Maitland M, Demarco T, Frost AE, Torbicki A, Langleben D, Pulido T, Correa-Jaque P, Passineau MJ, Wiener HW, et al. Endothelin-1 pathway polymorphisms and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:1345–1354. doi: 10.1164/rccm.201501-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.PVDOMICS protocol website. [accessed 2016 May 12]. Available from: https://pvdstudy.ccf.org/pvd/
- 23.Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, Kawut SM, Halpern SD. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest. 2012;141:20–26. doi: 10.1378/chest.11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee RL, Gabler NB, Praestgaard A, Merkel PA, Kawut SM. Adverse events in connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheumatol. 2015;67:2457–2465. doi: 10.1002/art.39220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee RL, Gabler NB, Sangani S, Praestgaard A, Merkel PA, Kawut SM. Comparison of treatment response in idiopathic and connective tissue disease-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:1111–1117. doi: 10.1164/rccm.201507-1456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United States Institute of Medicine. Committee on Strategies for Responsible Sharing of Clinical Trial Data, Institute of Medicine (US). Board on Health Sciences Policy. Sharing clinical trial data: maximizing benefits, minimizing risk. National Academy of Sciences; 2015 [accessed 2016 Jul 30]. Available from: http://nationalacademies.org/hmd/~/media/Files/Report%20Files/2015/SharingData/RAAG_ShareData_Web.pdf. [DOI] [PubMed]
- 27.Taichman DB, Backus J, Baethge C, Bauchner H, de Leeuw PW, Drazen JM, Fletcher J, Frizelle FA, Groves T, Haileamlak A, et al. Sharing clinical trial data: a proposal from the International Committee of Medical Journal Editors. N Engl J Med. 2016;374:384–386. doi: 10.1056/NEJMe1515172. [DOI] [PubMed] [Google Scholar]
- 28.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 29.Chan SY, Loscalzo J. The emerging paradigm of network medicine in the study of human disease. Circ Res. 2012;111:359–374. doi: 10.1161/CIRCRESAHA.111.258541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miotto R, Li L, Kidd BA, Dudley JT. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci Rep. 2016;6:26094. doi: 10.1038/srep26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang C-C, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peacock AJ, Naeije R, Galiè N, Rubin L. End-points and clinical trial design in pulmonary arterial hypertension: have we made progress? Eur Respir J. 2009;34:231–242. doi: 10.1183/09031936.00107108. [DOI] [PubMed] [Google Scholar]
- 33.Rabinovitch M, Keane JF, Fellows KE, Castaneda AR, Reid L. Quantitative analysis of the pulmonary wedge angiogram in congenital heart defects: correlation with hemodynamic data and morphometric findings in lung biopsy tissue. Circulation. 1981;63:152–164. doi: 10.1161/01.cir.63.1.152. [DOI] [PubMed] [Google Scholar]
- 34.Lau EMT, Manes A, Celermajer DS, Galiè N. Early detection of pulmonary vascular disease in pulmonary arterial hypertension: time to move forward. Eur Heart J. 2011;32:2489–2498. doi: 10.1093/eurheartj/ehr160. [DOI] [PubMed] [Google Scholar]
- 35.Hagner S, Stahl U, Knoblauch B, McGregor GP, Lang RE. Calcitonin receptor-like receptor: identification and distribution in human peripheral tissues. Cell Tissue Res. 2002;310:41–50. doi: 10.1007/s00441-002-0616-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, Ashek A, Wang L, Fang W, Dabral S, Dubois O, Cupitt J, Pullamsetti SS, Cotroneo E, Jones H, et al. Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation. 2013;128:1214–1224. doi: 10.1161/CIRCULATIONAHA.113.004136. [DOI] [PubMed] [Google Scholar]
- 37.van de Veerdonk MC, Kind T, Marcus JT, Mauritz G-J, Heymans MW, Bogaard H-J, Boonstra A, Marques KMJ, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 38.Talati M, Hemnes A. Fatty acid metabolism in pulmonary arterial hypertension: role in right ventricular dysfunction and hypertrophy. Pulm Circ. 2015;5:269–278. doi: 10.1086/681227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 40.Grieve AP, Chow SC, Curram J, Dawe S, Harnisch LO, Henig NR, Hung HM, Ivy DD, Kawut SM, Rahbar MH, et al. Advancing clinical trial design in pulmonary hypertension. Pulm Circ. 2013;3:217–225. doi: 10.4103/2045-8932.109933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lythgoe MP, Rhodes CJ, Ghataorhe P, Attard M, Wharton J, Wilkins MR. Why drugs fail in clinical trials in pulmonary arterial hypertension, and strategies to succeed in the future. Pharmacol Ther. 2016;164:195–203. doi: 10.1016/j.pharmthera.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Wholley D. The biomarkers consortium. Nat Rev Drug Discov. 2014;13:791–792. doi: 10.1038/nrd4439. [DOI] [PubMed] [Google Scholar]
- 43.Printz C. I-SPY 2 may change how clinical trials are conducted: researchers aim to accelerate approvals of cancer drugs. Cancer. 2013;119:1925–1927. doi: 10.1002/cncr.28172. [DOI] [PubMed] [Google Scholar]
- 44. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for industry: adaptive design clinical trials for drugs and biologics. 2010 Feb [accessed 2016 Jul 30]. Available from: www.fda.gov/downloads/Drugs/.../Guidances/ucm201790.
- 45.Blumenschein GR, Jr, Saintigny P, Liu S, Kim ES, Tsao AS, Herbst RS, Alden C, Lee JJ, Tang X, Stewart DJ, et al. Comprehensive biomarker analysis and final efficacy results of sorafenib in the BATTLE trial. Clin Cancer Res. 2013;19:6967–6975. doi: 10.1158/1078-0432.CCR-12-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tufts Center for the Study of Drug Development. Briefing: cost of developing a new drug. 2014 Nov 18 [accessed 2016 Jul 30]. Available from: http://csdd.tufts.edu/files/uploads/Tufts_CSDD_briefing_on_RD_cost_study_-_Nov_18,_2014.
- 47.Rich S. The 6-minute walk test as a primary endpoint in clinical trials for pulmonary hypertension. J Am Coll Cardiol. 2012;60:1202–1203. doi: 10.1016/j.jacc.2012.03.080. [DOI] [PubMed] [Google Scholar]
- 48.Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, Halank M, Meyer FJ, Karger G, Buss J, Juenger J, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114:1482–1489. doi: 10.1161/CIRCULATIONAHA.106.618397. [DOI] [PubMed] [Google Scholar]
- 49.Ryan JJ, Rich JD, Maron BA. Building the case for novel clinical trials in pulmonary arterial hypertension. Circ Cardiovasc Qual Outcomes. 2015;8:114–123. doi: 10.1161/CIRCOUTCOMES.114.001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harel F, Langleben D, Provencher S, Fournier A, Finnerty V, Nguyen QT, Letourneau M, Levac X, Abikhzer G, Guimond J, et al. Molecular imaging of the human pulmonary vascular endothelium in pulmonary hypertension: a phase II safety and proof of principle trial. Eur J Nucl Med Mol Imaging. doi: 10.1007/s00259-017-3655-y. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]