Abstract
Transcaval access has been used successfully for over 200 transcatheter aortic valve replacements, large-bore percutaneous left ventricular assist devices, and thoracic endovascular aortic aneurysm repairs. This review teaches how to plan transcaval access and closure based on computed tomography. The main planning goals are to: 1) identify calcium-free crossing targets in the abdominal aorta along with optimal fluoroscopic projection angles and level with respect to lumbar vertebrae; 2) identify obstacles such as interposed bowel or pedunculated aortic atheroma; 3) plan covered stent bailout; and 4) identify jeopardized vascular branches such as renal arteries that might be obstructed by bailout covered stents if employed. The aorta and inferior vena cava are segmented (sculpted) using an image reconstruction workstation and crossing targets are highlighted. Important measurements such as aortic lumen diameter and target distance from renal arteries, aortoiliac bifurcation, and right femoral vein puncture site are reported to assist the operator. The proposed classification for transcaval feasibility has been revised, making some previously unfavorable candidates now feasible or favorable based on procedural success to date. Transcaval access allows percutaneous introduction of large devices into the aorta despite small or diseased iliofemoral arteries. By following these simplified procedures, both operators and imaging specialists can easily prepare comprehensive treatment plans.
Keywords: aorto-caval, caval-aortic, computed tomography, structural heart disease, transcatheter aortic valve replacement, transcatheter electrosurgery, transcaval
More than 200 transcaval transcatheter aortic valve replacement (TAVR) procedures have been performed to date, still with no directly attributable death or open surgical bailout. Transcaval access has been applied to other applications, including large-bore percutaneous left ventricular assist devices for cardiogenic shock as well as thoracic endovascular aortic aneurysm repairs (1). Enrollment in the 100-subject National Heart, Lung, and Blood Institute–sponsored transcaval TAVR investigational device exemption protocol is now complete and results will be reported soon after follow-up is completed (NCT02280824).
There is sufficient experience with the procedure that it can be accomplished with a modicum of planning and of training. Even though commercially available cardiac occluder devices are permeable and therefore not immediately hemostatic, preliminary experience with these devices suggest the procedure may be reasonably safe and effective for patients who are not eligible for conventional trans-femoral access (2).
In 2014 we published a brief pictorial guide on how to plan transcaval access and closure based on computed tomography (CT), including identification of unfavorable candidates ineligible for the investigational device exemption protocol (3). Many such unfavorable patients nevertheless underwent successful transcaval access and closure by experienced operators, outside of the protocol. As a result, our classification of suitability for transcaval access has evolved.
The purpose of this tutorial review is to provide updated guidance on how to plan the procedure (Central Illustration). We recommend both operators and imaging specialists master these considerations.
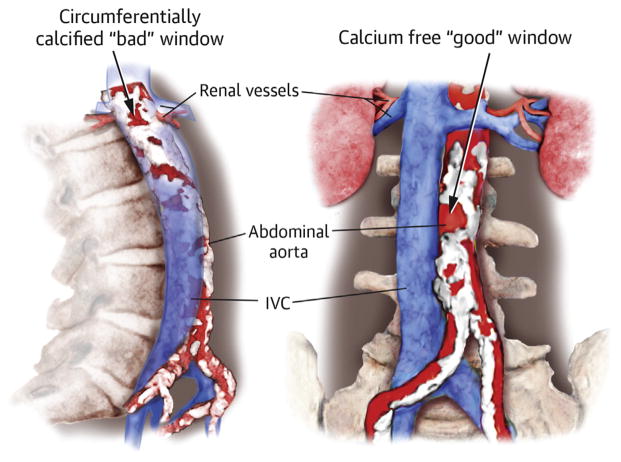
CENTRAL ILLUSTRATION.
Locating Calcium-Free Crossing Targets
THE GOALS OF COMPUTED TOMOGRAPHY PLANNING
Computed Tomography planning for transcaval access and closure amounts to anatomic patient selection, and covers several key objectives.
OBJECTIVE 1: FIND A CALCIUM-FREE CROSSING TARGET
Calcium is impenetrable for transcaval crossing systems and sheaths. The main goal is to find a calcium-free window (Figure 1) that will allow a sheath and heart valve to traverse but not further injure the aorta. We recommend the target not be surrounded circumferentially by a calcific rim (Figure 1A, lower arrow) and that the calcium-free window be larger than the sheath outer diameter in at least 1 dimension (≥7 mm for CoreValve Evolut R [Medtronic, Minneapolis, Minnesota] with a separate 18-F introducer sheath; ≥7.6 to 8.6 mm for Sapien 3 [Edwards Lifesciences, Irvine, California] and expandable sheaths). This is because it may be more difficult to advance a sheath through narrow and circumferentially calcified windows in the aortic wall (Figure 1A, lower arrow).
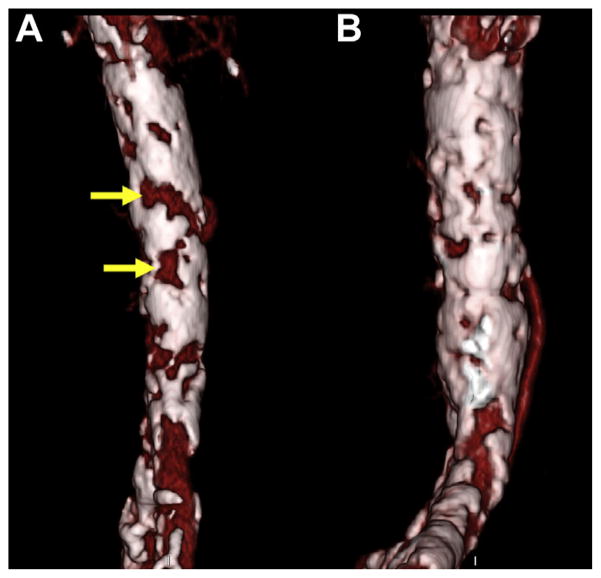
FIGURE 1. En Face View of the Right Abdominal Aorta as Seen From the Aorta.
This view is helpful to identify calcium-free targets. (A) Non-circumferentially calcified window measuring 5 × 10 mm (upper arrow) and circumferentially calcified window measuring 7 × 4 mm (lower arrow). (B) Porcelain abdominal aorta with no suitable calcium-free regions.
OBJECTIVE 2: AVOID IMPORTANT OBSTACLES
Assure the introducer sheath will not traverse bowel interposed between inferior vena cava (IVC) and aorta (Figure 2A). This seems more common among elderly. Bowel position may change dynamically, and operators can consciously choose to exit the IVC posteriorly away from bowel, and intravascular volume expansion can change the position of IVC, but we recommend excluding crossing targets if there is the possibility of bowel injury.
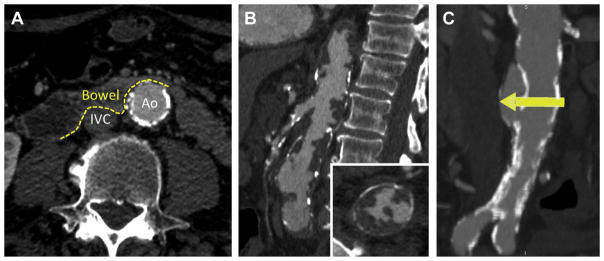
FIGURE 2. Anatomical Hazards to Avoid.
(A) Interposed bowel (dashed line, inferior margin) between the inferior vena cava (IVC) and aorta (Ao). (B) Pedunculated aortic atheroma on sagittal and axial image (inset). (C) Calcified aortic dissection at crossing target (yellow arrow).
Pedunculated aortic atheroma (Figure 2B) might lead to atheroembolization during crossing, closing, or manipulating accessory devices such as snares or pigtail catheters. Similarly, aortic dissection (Figure 2C) at the crossing target may be disrupted or extended by advancing the introducer sheath, and dissection above or below the target may be disrupted by the closure device and accessories. Worse, chronic dissections may interfere with closure device apposition to the aortic wall.
By contrast, aortic ectasia and aneurysm are not contraindications to transcaval access, and indeed focal outpouchings serve as desirable zones to seat closure devices.
Another challenge is the leftward aorta, which refers to aortas that from the perspective of the transcaval crossing catheter move leftward away from the IVC as the catheter is advanced. The result is tangential crossing of the aortic wall, which is difficult. We therefore avoid transcaval access when targets are more than approximately 20° leftward.
More esoteric obstacles include IVC filters, which should be removed before transcaval access is attempted, and polyester aortic grafts, which have been successfully traversed (4).
OBJECTIVE 3: IDENTIFY JEOPARDIZED VASCULAR BRANCHES AND INTRAVASCULAR STRUCTURES
Crossing targets should be safely away from important vascular branches or intravascular structures. Intravascular disc components of closure devices should not obstruct flow to the right renal arteries or main left renal veins. Closure devices should be far enough away from aorto-iliac stents that they do not interfere with each other. Covered stents placed for emergency bailout should not occlude renal arteries, solitary patent mesenteric branches, or iliac arteries. Accessory renal arteries should be identified clearly so that the operator is cognizant should these be jeopardized, to measure risk and benefit of access and bailout techniques.
Moreover, there should be sufficient distance away from branch vessels to allow covered stents to completely cover the closure device disk and achieve hemostasis when necessary.
OBJECTIVE 4: PLAN CLOSURE
We now recognize that the distance between aorta and IVC does not appear to be important to procedural success or adequacy of closure.
We close the transcaval access port with a first-generation Amplatzer Duct Occluder (ADO1-1, St. Jude Medical, St. Paul, Minnesota), specifically the 8 mm/6 mm device for 18-F sheaths or smaller used for CoreValve Evolut R (Medtronic, Minneapolis, Minnesota) or Symetis Acurate neo (Symetis, Eclubens, Switzerland), and with the 10 mm/8 mm device for expandable sheaths used for Sapien 3 (Edwards Lifesciences, Irvine, California), which achieve a transient outer diameter of 7.5 mm or larger. The former has a 12 mm aortic disc, and the latter has a 16 mm aortic disc.
OBJECTIVE 5: PLAN COVERED-STENT BAILOUT
The transcaval treatment plan should allow the operator to select a covered stent for standby (usually a self-expanding limb extender for an endovascular aortic aneurysm occluder system), and which femoral artery access site should be used to deliver it (and therefore should be selected for snare and pigtail catheter access).
OBJECTIVE 6: REPORT KEY FINDINGS
We recommend a tabular report to guide the operator during the procedure (Table 1).
TABLE 1.
Recommended Report Content for Transcaval Planning, With an Example
| Feature | Example Value and Detail |
|---|---|
| Recommendation | Favorable (no high-risk features) Feasible (having features that increase difficulty or risk) Unfavorable (having features that contraindicate or significantly increase risk and difficulty) |
| Basis for recommendation | Detail such as “having no high-risk features” or “planned on noncontrast Computed Tomography” |
| Target entry site lumbar vertebra | L3.0 = middle of lumbar vertebra; L3.5 = midway between L3 and L4; see text for a further description of this nomenclature |
| Projection angles | 30° RAO and 60° LAO; these are the angles with regard to horizontal or vertical with the centers of the IVC and aorta at the crossing target |
| Interposed and nearby structures | Example: bowel anterior |
| Aortic lumen diameter (+3/0/−3 cm) | Above 16 mm/at target 15 mm/below 14 mm to help select bailout covered stent device |
| Target distance below renal artery | 25 mm (to aid in planning closure and covered stent) |
| Target distance above aortoiliac bifurcation | 65 mm (to aid in planning closure and covered stent) |
| Working sheath distance to target | 25 cm from RFV puncture site to aortic entry along theoretical vascular centerline |
| Covered-stent bailout limb access and size | L 4.5 mm/R 4.0 mm minimum lumen dimension of L and R iliofemoral arteries to guide arterial access and covered stent bailout |
| Mesenteric arteries | Celiac and SMA patent; to determine risk of mesenteric ischemia if inferior mesenteric artery becomes occluded |
| Other caveats | Calcium is mostly anterior at the target; compression fractures cause L3 to L4 to correspond to iliac crests; leftward aorta 15°; SMA 80% ostial stenosis; accessory L renal artery 10 mm superior to target; patient rotated 10° leftward on Computed Tomography table. These are examples of miscellaneous comments important to the operator. |
IVC = inferior vena cava; L = left; LAO = left anterior oblique; R = right; RAO = right anterior oblique; RFV = right femoral vein; SMA = superior mesenteric artery.
First we identify the target with respect to lumbar spines and the iliac crests, to allow the operator to coregister the findings from this analysis with the radiographic projections during the procedure.
Second, we identify key measurements, such as recommended working angles for the fluoroscopy system, to assure proper orientation of the transcaval crossing catheter with regard to the aortic snare that serves as a bullseye target. Other key measurements include distance from the femoral vein puncture site to assure adequate sheath working length, distance from key renal and iliac branches, and measurements as described previously to allow the operator to select a suitable bailout covered stent. As mentioned already, aortocaval distance does not seem important.
Perhaps most important, the analysis should include a summary recommendation of anatomic eligibility. Our criteria are summarized in (Table 2), having 3 categories of progressively higher perceived operator difficulty or risk.
TABLE 2.
Revised Classification of Transcaval Feasibility Features
| Classification | Features |
|---|---|
| Favorable | All of the following: A calcium-free window >10 mm in diameter No important interposed structures (hemiazygos or lumbar plexus veins OK; lumbar artery not OK; renal vein not OK) Centerline distance from femoral vein at lower femoral head to aortic entry ≥7 cm less than working length of intended introducer sheath Target >15 mm below lowest renal artery and >15 mm above aortoiliac bifurcation |
| Feasible (having features that may increase difficulty or risk) | Any of the following: Circumferentially calcified window (as long as diameter is ≥2 mm than expanded outer diameter of intended valve and sheath) or noncircumferential calcified window (if its smallest dimension is ≤2 mm less than expanded outer diameter of intended valve and sheath) Centerline distance from femoral vein at lower femoral head to aortic entry 5–7 cm less than working length of intended sheath Aortic fabric graft Planned on noncontrast Computed Tomography, masking intraluminal pathology |
| Unfavorable (having features that contraindicate or significantly increase risk and difficulty) | Any of the following: Porcelain abdominal aorta (confluent calcification) without a calcium-free target window larger than the intended introducer sheath Centerline distance from femoral vein at lower femoral head to aortic entry that will allow transcaval purchase of <5 cm of working length of intended sheath Proximity to renal arteries ≤15 mm Pedunculated abdominal aortic atheroma that might be embolized by the transcaval procedure or devices Bilateral iliofemoral artery occlusion that impede transfemoral snare maneuvers and covered stent bailout Celiac and superior mesenteric artery obstruction such that covered stent occlusion of the inferior mesenteric arteries would induce bowel ischemia Leftward aortic angle with regard to vertical approximately >20° that would impede catheter and sheath advancement Other high risk features (e.g., permanent IVC filter, aortic stent, stent graft at target) |
| Not important | Aortocaval distance Aortic aneurysm or ectasia, even with lamellated thrombus Interposed lumbar venous structures |
Abbreviations as in Table 1.
DETAILS OF COMPUTED TOMOGRAPHY PLANNING
STEP 1: ACQUIRE A DIAGNOSTIC COMPUTED TOMOGRAPHY
Transcaval procedures can be planned using the same Computed Tomography examination and contrast exposure used to plan the primary procedures (e.g., TAVR or thoracic endovascular aortic aneurysm repair). Computed Tomography planning requires abdomen and pelvis Computed Tomography with thin-slice reconstructions (≤1.5 mm), otherwise calcium is exaggerated. Contrast enhancement is necessary to identify intravascular hazards such as dissection and pedunculated atheroma, although because these are infrequent, we plan and classify transcaval procedures as intermediate risk when using noncontrast Computed Tomography.
These are best performed using Computed Tomography systems having 64 or more detector rows. Lower peak tube voltage (80 or 100 kV) not only reduces radiation exposure but also increases vascular contrast attenuation and may allow lower contrast doses.
Computed Tomography planning requires an image reconstruction workstation capable of carving (segmenting) the aortic wall images from surrounding structures. Preferably the workstation also can segment the IVC and can apply color to highlight structures (e.g., calcium) and partial transparency to allow the operator to visualize spatial relationships. Planners have used Vitrea (Vital Images, Minnetonka, Minnesota), iNtuition (TeraRecon, Foster City, California), 3mensio (Pie Medical, Maastricht, the Netherlands), and the low-cost Osirix (Pixmeo, Bernex, Switzerland).
STEP 2: SELECT AND VIEW THIN-SLICE ABDOMEN AND PELVIS COMPUTED TOMOGRAPHY SERIES
Select a Computed Tomography series that is contrast enhanced and having thin-slice (≤1.5 mm) reconstructions. Typically, this will have at least 300 slices. Confirm that the selected images represent the correct patient to be included in the report.
Set the image window and level so that calcium appears bright white, aortic contrast appears bright gray, and other structures appear dim gray (Figures 3A and 3B).
FIGURE 3. How to Set Image Window and Level Settings for Transcaval Analysis.
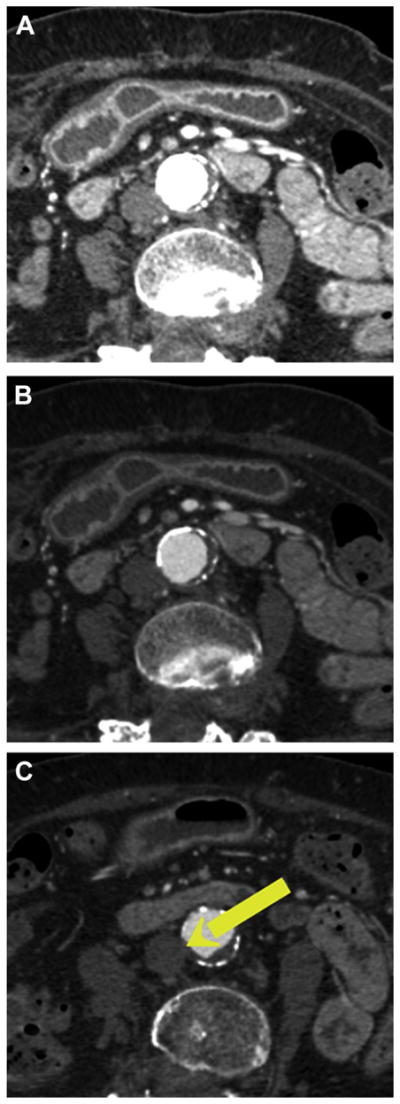
Image window sets the dynamic range and specifies the Hounsfield unit value beyond which objects are displayed white. Level is the centerofthe scale. These values changethecontrastand brightness, respectively. (A) Typical default Computed Tomography soft tissue settings (window 400, level 40) make aortic calcification appear indistinguishable from intravenous contrast. (B) Vascular settings (window 800, level 250) clearly distinguish calcification (bright white) from intra-aortic contrast (gray) and noncalcified atheroma (dark gray). (C) An electronic arrow marks the proposed transcaval crossing target.
Start by scrolling through all abdominal slices in cross-sections (axials) from the celiac artery to the aortoiliac bifurcation to identify potential crossing targets and hazards. Mark these candidate targets with electronic arrows (Figure 3C).
STEP 3: SCULPT (SEGMENT) THE AORTA AND (OPTIONALLY) IVC
Use the workstation features to segment the Computed Tomography volume scans. This means create or modify regions of interest for the aorta and the IVC, usually by drawing vessel borders along different axial slices and then interpolating between them. Many workstations automatically can segment the contrast-filled arteries but not usually the IVC. Combine these regions of interest to create 3-dimensional depictions of the aorta and IVC. These steps are called sculpting in Vitrea, and volume-rendering in Osirix (Figures 4A and 4B).
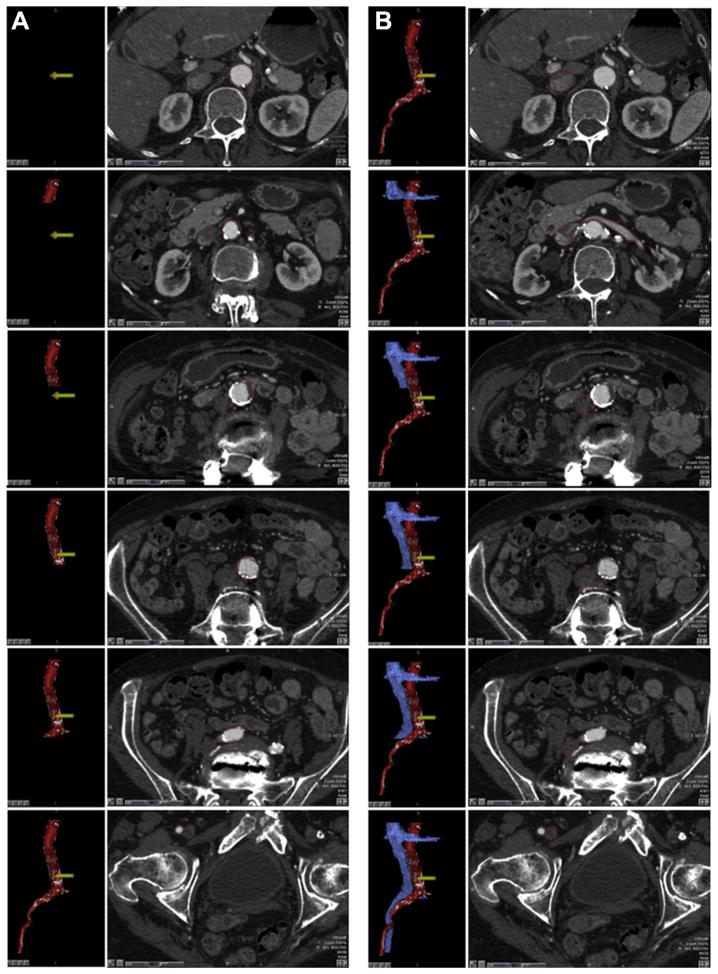
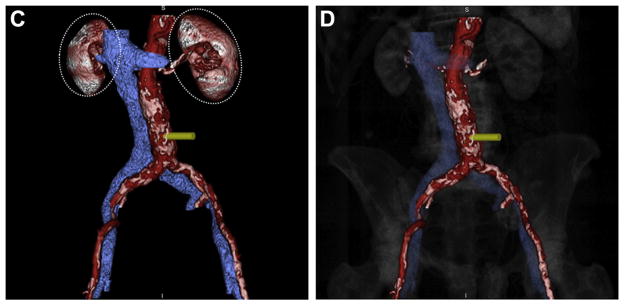
FIGURE 4. Segmentation and Visualization of the Aorta and Cava.
Regions of interest depicting the (A) aorta and (B) vena cava are drawn manually or automatically using image reconstruction workstations. As these structures are defined, an accompanying surfaced-rendered 3-dimensional image is generated (red-colored aorta with white calcium, blue vena cava). (C) Distracting contrast-enhanced structures such as the kidneys (dashed ovals) can be removed from the 3-dimensional surface rendering. (D) Highly transparent (90%) depiction of the bony structures provides anatomic landmarks for the operator.
Assure the aortic segmentation includes all calcium within the aortic wall. Assure the ostia of the main renal artery and vein branches are segmented because they are important structures for the operator to recognize.
Cut away distracting contrast-filled structures including kidneys and mesenteric vessels beyond their ostia (Figure 4C). Add highly transparent (>90%) depictions of the spine to allow the operator visually to register the planned target with fluoroscopic lumbar vertebral landmarks (Figure 4D).
STEP 4: DEPICT CALCIUM AND REGIONS OF INTEREST FOR DISPLAY
We emphasize the critical importance of depicting calcium and its distribution near the intended crossing target. Calcium is unfavorable whether superficial, deep, or thin (eggshell). The selection of window, level, and color can exaggerate or underrate the severity of aortic wall calcium (Figure 5).
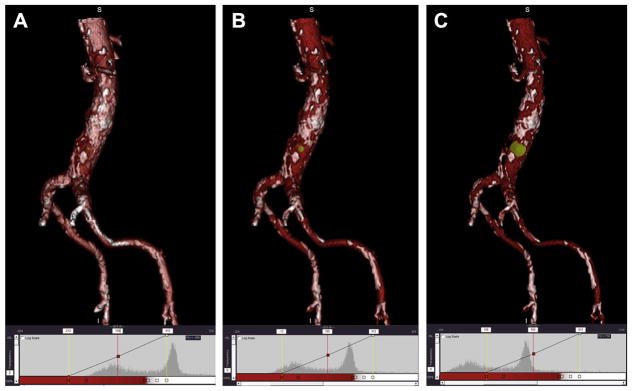
FIGURE 5. Proper Adjustment of 3-Dimensional Surface Rendering Display Level to Visualize Aortic Calcification as Seen From the Cava at Proposed Target.
(A) Setting an image level inappropriately low at 100 confuses exaggerates the extent of calcification. The image intensity histogram depicts that a majority of the pixels are above the 200 level (red line). (B) Appropriate image level of 300 discriminates vascular contrast (red) from aortic calcification (white) on the 3-dimensional surface rendering and on the histogram by evenly dividing the 2 populations of pixels. (C) An excessive image level of 500 results in artificially suppressing the appearance of calcium and will misleadingly depict a larger calcium-free target window. The corresponding histogram shows that most of the pixels are below the threshold level of 500 (red line). The proposed target appears as a yellow arrowhead or dot.
Optionally recolor the aortic segmentation so that voxels having approximately >350 Hounsfield units are depicted in white, and voxels having lower attenuation are depicted in bright red. Then apply transparency (also known as opacity) to at least 30%, so that the operator can view calcium through the right aortic wall. Far-wall calcium is usually and serendipitously volume-averaged away. Also, optionally apply color to the IVC segmentation (e.g., blue) with high transparency (at least 50%).
STEP 5: FINALIZE CROSSING TARGET(S)
A circular calcium-free target is best entered at its most caudal portion because catheters and sheaths exert upward force that may force the crossing cephalad.
When there are multiple possible targets, lower targets are generally preferred over higher targets because they allow deeper purchase of the introducer sheath into the aorta.
We use a simple nomenclature to describe the crossing target with respect to the lumbar vertebra. For example, L3.0 represents the middle of the L3 vertebral body, and L3.5 represents the middle of the L3 to L4 interspace, which is halfway between the middle of L3 and L4 bodies.
In most patients, the top of the superior iliac crest corresponds to the L4 to L5 interspace in the anteroposterior projection. When this is not the case, as in lumbar compression fractures common in this population, it can be helpful to convey this in the report.
STEP 6: PREPARE AND REPORT REPRESENTATIVE IMAGES AND MEASUREMENTS
Measure the crossing projections. This is the angle, with respect to horizontal, of the line connecting the center of the IVC and the aorta at the crossing target. It can be helpful to report pairs of orthogonal angles (e.g., 20° right anterior oblique and 70° left anterior oblique), the former for crossing and the latter for viewing the aortic snare as a bullseye target.
Measure the average diameter of the aorta at the target and ±3 cm above and below to allow planning the bailout covered stent size.
The diameter of the nearby IVC, in the left-right dimension, is optional and can help the operator select a guiding catheter shape.
A sagittal view of the vertebral spine from L1-S1 should be provided with the target indicated. A corresponding coronal view should allow the operator visually to register the lumbar vertebrae with the superior iliac crests.
We prefer to provide progressively thick oblique coronal slices of the aorta at the crossing target, thinnest to depict the calcium-free target in cephalad-caudal dimension and thickest to depict the target and aorta in the context of the spine (Figure 6). Technically this last view should be a sum of voxels to resemble the fluoroscopy projection.
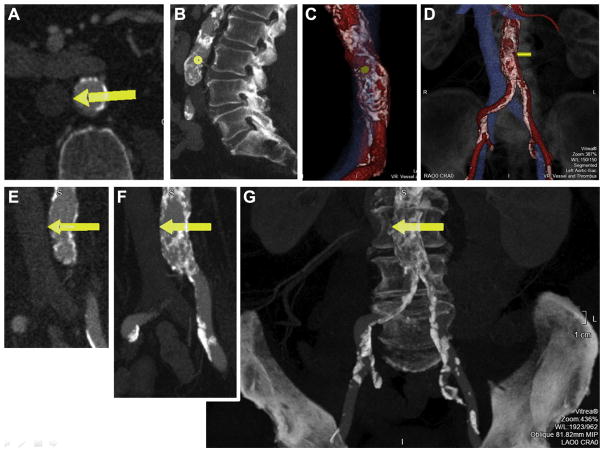
FIGURE 6. Sample Roadmap Images Included in a Report.
We find it helpful to post these images in the procedure room. (A) Axial thin slice at the crossing target (yellow arrow). (B) Sagittal thick slice reconstruction along the vertebral column to indicate the crossing target. (C) En face view of the calcium-free target window on the aorta as viewed from the cava. (D) Three-dimensional surface rendering with translucent bony landmarks. Coronal oblique views (rotated to match fluoroscopy projection angle) depicting the crossing target with (E) thin-slice depiction, (F) thicker slice depiction (slice thickness = aortic thickness) to convey aortic calcification burden, and (G) thickest slice to demonstrate calcification in relation to the spine and pelvis to mimic the expected fluoroscopy pattern during the procedure.
TRANSCAVAL ACCESS WITHOUT COMPUTED TOMOGRAPHY PLANNING
In urgent or emergency procedures, transcaval access can be obtained without formal Computed Tomography planning. The typical indications are conversion to transcaval after failed transfemoral access, or emergency percutaneous left ventricular assist device implantation for cardiogenic shock. Especially the latter can justify the theoretical possibility of traversing interposed bowel, which would be expected to be lethal.
If available, an old abdominal Computed Tomography should be found to survey for calcium. Similarly, if available, cone-beam (C-arm fluoroscopy based) Computed Tomography can be performed to assess for calcium if not interposed structures. Calcium in the aortic wall can sometimes be identified by cinefluoroscopy while the patient or x-ray system moves, either during respiration or during left-right gantry rotation.
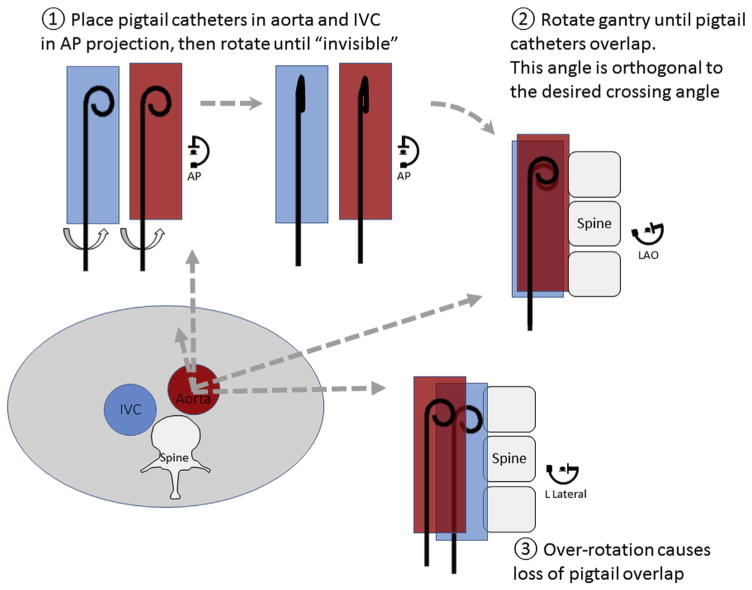
When crossing without Computed Tomography, the average target is the middle of the L3 lumbar vertebra, which is midway between the renal arteries and aortoiliac bifurcation. The following catheter trick can identify a satisfactory projection angle (Figure 7). Place a pigtail catheter in the aorta and another in the IVC, and rotate both so that they are best viewed in the lateral projection. Rotate the x-ray gantry obliquely until the catheters overlap. The best ad hoc crossing projection is orthogonal to this gantry position.
FIGURE 7. Finding the Optional Transcaval Projection Angle Without Using Computed Tomography.
The lower left figure depicts an abdominal cross-section at the level of the intended transcaval crossing, with a typical relationship of inferior vena cava (IVC), aorta, and spine. Pigtail catheters are placed in the IVC and the aorta and rotated in the anteroposterior projection until the pigtail curve is no longer visible. Then rotate the fluoroscopy gantry left-right until the 2 pigtail catheters overlap. A small-volume contrast injection can confirm appropriate positioning. Over-rotation also is depicted, at which point pigtail catheters no longer overlap. AP = anteroposterior projection angle; LAO = left anterior oblique.
SUMMARY AND CONCLUSIONS
We have outlined key principles in the Computed Tomography-based planning of transcaval access for introduction of large appliances into the aorta to avoid small or diseased iliofemoral access arteries. Targets are selected by identifying calcium-free targets on the right wall of the aorta, without interposed bystander structures, and away from important vascular branches. We describe measurements important for the transcaval operator and recipes for effective visual representation of the findings. Complete plans include contingencies for bailout covered stent implantation to avoid unintended morbidity.
With a firm understanding of these principals and semiautomated segmentation tools available on Computed Tomography image processing workstations, robust transcaval treatment plans can be generated in minutes.
Acknowledgments
The study was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Z01-HL006040.
The authors thank Jonathan Mazal and Bill Schenke of National Heart, Lung, and Blood Institute for image processing.
ABBREVIATIONS AND ACRONYMS
- IVC
inferior vena cava
- TAVR
transcatheter aortic valve implantation
Footnotes
Drs. Lederman, Rogers, and Greenbaum are listed as inventors on patent applications, assigned to their employers, related to transcaval access and closure. Dr. Greenbaum has served as a physician proctor for Edwards Lifesciences, Medtronic, and St. Jude Medical. Mr. Khan has reported that he has no relationships relevant to the contents of this paper to disclose. Dr. Fusari is a full-time employee of and surgeon proctor for Edwards Lifesciences. Dr. Chen is the National Heart, Lung, and Blood Institute core lab director for transcaval access.
References
- 1.Uflacker A, Lim S, Ragosta M, et al. Transcaval aortic access for percutaneous thoracic aortic aneurysm repair: initial human experience. J Vasc Interv Radiol. 2015;26:1437–41. doi: 10.1016/j.jvir.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol. 2014;63:2795–804. doi: 10.1016/j.jacc.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lederman RJ, Chen MY, Rogers T, et al. Planning transcaval access using CT for large transcatheter implants. J Am Coll Cardiol Img. 2014;7:1167–71. doi: 10.1016/j.jcmg.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lederman RJ, O’Neill WW, Greenbaum AB. Transcaval access for TAVR across a polyester aortic graft. Catheter Cardiovasc Interv. 2015;85:1270–3. doi: 10.1002/ccd.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]