Abstract
OBJECTIVES
This is an early feasibility clinical test of mitral loop cerclage annuloplasty to treat secondary mitral valve regurgitation.
BACKGROUND
Secondary mitral regurgitation is characterized by cardiomyopathy, mitral annular enlargement, and leaflet traction contributing to malcoaptation. Transcatheter mitral loop cerclage applies circumferential compression to the mitral annulus by creating a loop through the coronary sinus across the interventricular septum, protecting entrapped coronary arteries from compression, and interactive annular reduction under echocardiographic guidance. This is the first human test of mitral loop annuloplasty.
METHODS
Five subjects with severe symptomatic secondary mitral regurgitation underwent mitral loop cerclage, with echocardiographic and computed tomography follow-up over 6 months.
RESULTS
Mitral loop cerclage was successful in 4 of 5 subjects and aborted in 1 of the 5 because of unsuitable septal coronary vein anatomy. Immediately and over 6 months, measures of both mitral valve regurgitation (effective orifice area and regurgitation fraction) and chamber dimensions (left atrial and left ventricular volumes) were reduced progressively and ejection fractions increased. Two with persistent and permanent atrial fibrillation spontaneously reverted to sinus rhythm during follow-up. One subject experienced a small myocardial infarction from an unrecognized small branch coronary occlusion. Another, experiencing cardiogenic shock at baseline, died of intractable heart failure after 6 weeks.
CONCLUSIONS
In this first human test, mitral loop cerclage annuloplasty was successful in 4 of 5 attempts, caused reverse remodeling (reduction in secondary mitral regurgitation and heart chamber volumes), and suggested electrical remodeling (reversion of atrial fibrillation). Further evaluation is warranted.
Keywords: functional mitral regurgitation, mitral loop cerclage
Mitral valve regurgitation despite normal leaflets, also known as secondary or functional mitral valve regurgitation, is a common complication of left ventricular dysfunction that contributes to heart failure symptoms (1). It results from annular dilation and papillary traction causing leaflet malcoaptation. Percutaneous leaflet repair to relieve leaflet malcoaptation relieves symptoms (2,3) and produces favorable “reverse” left ventricle (LV) remodeling (4). Numerous alternative or adjunctive transcatheter strategies are under development (5).
Transcatheter mitral annuloplasty through the coronary sinus is attractive in its simplicity, but has been criticized because of the likelihood of compression of entrapped circumflex branch coronary arteries, because of the discordant rotational orientation of the mitral valve commissures in relation to the coronary sinus, and because of nonoverlapping planes of the mitral annulus and coronary sinus (6–8). To address these limitations, we developed transcatheter mitral cerclage annuloplasty, which incorporates an arch-like device to protect entrapped coronary arteries against compression, which delivers circumferential annular tension that enhances leaflet coaptation irrespective of rotational orientation of the mitral commissure in relation to the coronary sinus, and which accomplishes septal-lateral apposition irrespective of high atrial variants of the coronary sinus (9). Thereafter, we reported preclinical results of a new “mitral loop cerclage” catheter system (10). Herein we report the results of the first human test of mitral loop cerclage.
METHODS
PATIENTS AND STUDY DESIGN
This open-label, single-arm, prospective, first-in-human feasibility study was approved by the Korea Food and Drug Administration and by the institutional ethics board of Pusan National University Yangsan Hospital (NCT02471664).
All subjects for this feasibility study consented in writing and were selected by the institutional multi-disciplinary heart team. They were eligible to participate if they had New York Heart Association functional class III or IV heart failure with severe secondary mitral valve regurgitation despite ≥3 months of guideline-directed optimal medical therapy (1). Severe secondary mitral valve regurgitation was defined as effective regurgitant orifice area of ≥0.20 cm2, regurgitant volume of ≥30 ml, or regurgitant fraction of ≥50% (1). Key exclusion criteria were primary or degenerative mitral valve regurgitation, left ventricular ejection fraction of <25%, concurrent aortic valve disease, eligibility for surgical revascularization and concurrent mitral valve surgery, concurrent pacemakers, cardiac resynchronization or defibrillator devices, advanced atrioventricular node conduction block, or severe primary tricuspid valve disease.
This single-center study was sponsored and funded by Tau PNU Medical Company, Ltd., Pusan, Korea, and was designed by the first and senior authors (Y.H. Park, M.K. Chon, and J.H. Kim).
MITRAL LOOP CERCLAGE SYSTEM
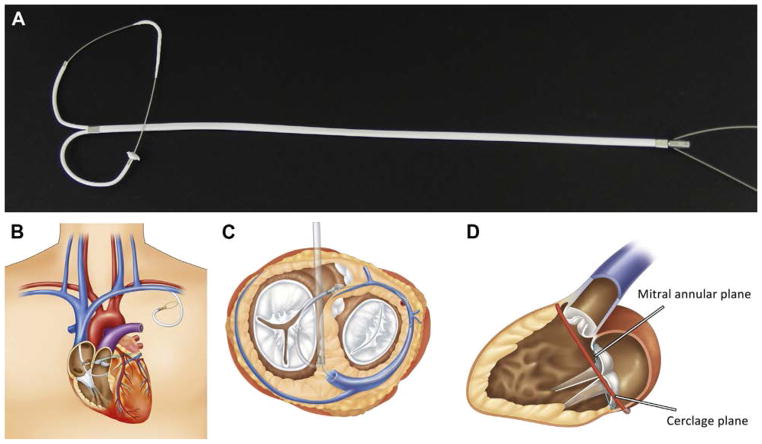
The purpose-built mitral loop cerclage catheter system (Tau-PNU Medical Co, Ltd, Pusan, Korea) has been described previously and has 4 implanted components (Figure 1) (10). One is a cerclage tension element made of stainless steel (previously described as a “cerclage rope”). The tension element contains an arch-like coronary artery protection element. The 2 free ends of the tension element are connected through a bifid coronary sinus tricuspid bridge device (previously described as the “CSTV”) that straddles and protects the septal tricuspid leaflet and coronary conduction system. The bridge extends to the left subclavian vein where it maintains tension. An adjustable extravascular lock connects the tension element and the bridge subcutaneously in the left subclavicular fossa. The lock allows post-procedure readjustment of tension during echocardiography, if desired.
FIGURE 1. Mitral Loop Cerclage Design and Devices.
(A) All 4 elements of the mitral loop cerclage implants assembled. (B) Device positioning. (C) The bridge device straddles the tricuspid valve between the coronary sinus and right ventricular septum. (D) Discordant planes of the mitral annulus and cerclage.
To ease targeting and snaring of the guide wire traversing the interventricular septum from the anterior interventricular vein into the right ventricle, a homemade transfemoral target capture device was prepared using a Wallstent system (18 ± 60 mm, Boston Scientific, Marlborough, Massachusetts). The distal tip of the Wallstent was sutured to its delivery catheter, and the Wallstent delivery catheter was placed in the right ventricular outflow tract (RVOT) in tandem with a loop snare (Amplatzer Gooseneck 6-F, Medtronic, Minneapolis, Minnesota) prepositioned around its shaft. Configured in this way, the Wallstent serves as an over-the-wire RVOT snare (Figure 2B, Online Video 1).
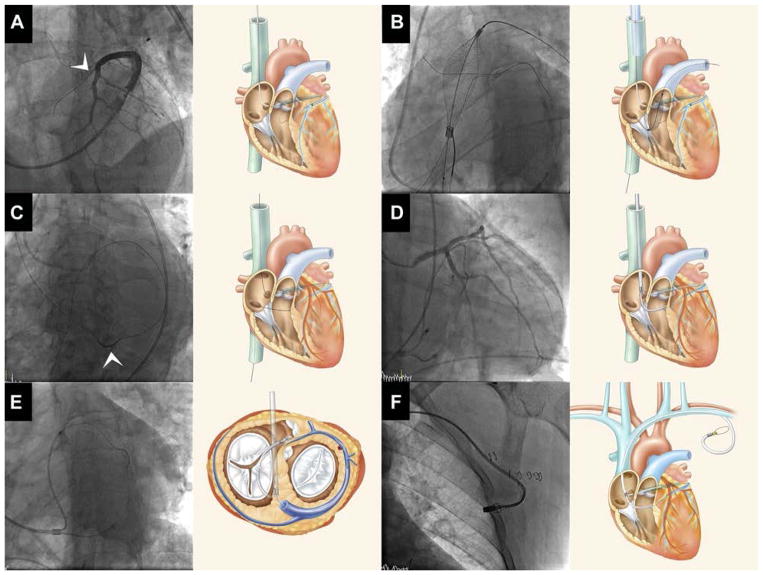
FIGURE 2. Representative Mitral Loop Cerclage Procedure.
(A) Coronary venography through a balloon wedge catheter identifies a basal septal perforator coronary vein (arrow head) through which a guidewire is advanced (Online Video 1). (B) A target-capture device is positioned into the right ventricular outflow tract to ensnare the guidewire that crossed from the coronary vein. (C) The guidewire is exchanged for tension device containing an integrated coronary artery protection element (arrow head). (D) During application of tension, the protection element prevents compression of entrapped circumflex coronary artery branches. (E) In this caudal left anterior oblique projection, the mitral loop cerclage system is shown to surround the mitral annular plane. (F) The tension locking device was embedded in the left subclavicular pocket.
TECHNIQUE OF MITRAL LOOP CERCLAGE ANNULOPLASTY
Under moderate sedation (n = 3) or general anesthesia (n = 2), 19-F × 15-cm introducer sheaths (Oscor, Palm Harbor, Florida) were placed both in the left subclavian vein (via a pacemaker-like pocket) and a femoral vein. This allowed the operator to stand at the right groin. Transesophageal echocardiography (TEE) or transthoracic echocardiography was used under anesthesia or sedation, respectively, to adjust tension interactively to reduce MR. Heparin was administered to achieve an activated clotting time of >300 s. A balloon-tip coronary sinus guiding catheter (Cello, Medtronic) was placed from the left subclavian vein into the great cardiac vein and a pressurized contrast venogram performed. Through the coronary sinus guide, a dual-lumen 0.014-inch microcatheter (Crusade, Kaneka Medix, Osaka, Japan) was positioned in the basal septal perforator coronary vein over a soft 0.014-inch guidewire (Whisper, Abbott, Chicago, Illinois) and then used to direct a stiff-tip 0.014-inch peripheral guidewire (Astato XS 20, Asahi, Japan) to traverse the interventricular septum into the RVOT (Figure 2A). A transfemoral balloon wedge end hole catheter was advanced across the major tricuspid orifice into the pulmonary artery and then used to exchange for the Wallstent/snare combination in the RVOT to serve as a target and capture system (Figure 2B). Once inside the RVOT, the traversing guidewire was snared and externalized through the femoral vein. The tension element was connected using heat-shrink tubing (polyether block amide, Cobalt Polymers, Cloverdale, California) to the guidewire and then pulled through the coronary sinus and interventricular septum into position (Figure 2C). The distal tip of this guidewire was transferred from the femoral to the subclavian sheath using a loop snare. Next the bifid coronary sinus tricuspid valve bridge device was advanced over the 2 free ends of the tension element. Diagnostic coronary arteriog-raphy allowed fine positioning of the incorporated coronary artery protection element (Figure 2D). Tension was applied interactively during transthoracic echocardiography or TEE to achieve the intended reduction in mitral annular dimension and mitral valve regurgitation (Figure 2E). Finally, the tension was locked in the left subclavicular pocket and the skin incision closed (Figure 2F).
IMAGING
Imaging was performed at baseline, 1, 3, and 6 months after mitral loop cerclage. Contrast-enhanced gated cardiac computed tomography (CT) used a 128-detector dual-source system (Somatom Definition Flash, Siemens Healthineers, Erlangen, Germany). Chamber volumes measured using semiautomated contours on a dedicated CT workstation (Aquarius iNtuition, TeraRecon Inc., Foster City, California).
Echocardiographic parameters were recorded according to guidelines (11) by experienced sonographers with a transthoracic echocardiography system using an iE33 ultrasound system equipped with S5-1 transducer (Philips Medical Systems, Andover, Massachusetts). Tricuspid regurgitation was graded integrating multiple parameters according to American Heart Association/American College of Cardiology guidelines (1).
Mitral loop cerclage used transthoracic echocardiography or TEE and concurrent fluoroscopic coronary arteriography to guide positioning of the coronary artery protection element and measure annular dimensions and mitral regurgitation during interactive application of tension.
DATA ANALYSIS
The primary efficacy endpoint was change in mitral regurgitation severity after 1 month follow up. The primary safety endpoint was freedom from major adverse cardiac events after 1 month, defined as death, myocardial infarction, cardiac tamponade, device-related cardiac surgery, stroke, and new conduction disorder requiring permanent pacing. Other adverse events are classified according to the Second Valve Academic Research Consortium guidelines (12).
Additional outcome measures at 1 and 6 months include: 1) change of mitral regurgitation severity by regurgitant volume and effective regurgitant orifice; 2) change in mitral annulus geometry (septal lateral dimension); 3) change in mitral valve regurgitation; 4) change in left ventricle volumes; 5) change in New York Heart Association heart failure symptom classification; 6) procedural technical success rate; 7) serious adverse events; and 8) major adverse cardiac events.
RESULTS
PATIENTS AND PROCEDURES
Eight patients were screened. Three were excluded because of unwillingness to participate in one, clinical deterioration requiring heart transplantation in another, and spontaneous improvement after medical treatment in the third.
Five subjects underwent attempted mitral loop cerclage. Baseline characteristics, procedure success, and complications are detailed in Table 1. All patients had marked LV enlargement (Figure 3), but 2 had overall preserved LV systolic function. The procedure was successful in 4 of the 5 attempts. It was aborted in 1 subject who had atypical coronary venous anatomy. In retrospect, baseline CT angiography identified a suitable septal perforator vein—not attempted—that arose from an anterolateral branch (Figure 4). Dual antiplatelet agents (aspirin 100 mg and clopidogrel 75 mg) were used for ≥3 months in subjects after successful mitral loop cerclage (Table 2).
TABLE 1.
Baseline Characteristics (n = 5)
| Case
|
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sex/age (yrs) | F/76 | M/71 | F/74 | M/62 | M/68 |
|
| |||||
| Mechanism of FMR | Ischemic MR | Annular dilation | Annular dilation | Nonischemic cardiomyopathy and leaflet tethering | Nonischemic cardiomyopathy and leaflet tethering |
|
| |||||
| Regurgitant volume (ml) | 47.8 | 71.9 | 62.6 | 38.6 | 70.3 |
|
| |||||
| EROA (cm2) | 0.24 | 0.42 | 0.34 | 0.27 | 0.78 |
|
| |||||
| LVEDD (mm) | 75 | 63 | 65 | 77 | 84 |
|
| |||||
| LVEDV (ml) | 191 | 229 | 212 | 262 | 342 |
|
| |||||
| LVEF (%) | 65 | 61 | 58 | 34 | 37 |
|
| |||||
| EuroSCORE II (%) | 4.57 | 7.42 | 14.78 | 12.99 | 20.85 |
|
| |||||
| STS score (%) | 2.86 | 4.42 | 5.67 | 7.35 | 9.60 |
|
| |||||
| NYHA functional class/ACC FMR stage | III/D | IV/D | IV/D | III/D | IV/D |
|
| |||||
| Medical history | |||||
| Hypertension | + | − | + | + | − |
| Diabetes | − | − | − | + | − |
| Previous PCI | − | − | − | − | − |
| Previous CABG | − | − | − | − | − |
| Chronic obstructive pulmonary disease | − | − | − | − | − |
| HF hospitalization in the last 12 months | − | 2 | 2 | 5 | 3 |
|
| |||||
| Blood pressure (systolic/diastolic), mm Hg | 110/70 | 80/54 | 136/93 | 146/71 | 100/66 |
|
| |||||
| Imaging guidance | AX + TTE | AX + TTE | AX + TEE | AX + TEE | AX + TTE |
|
| |||||
| Procedure success | Yes | No* | Yes | Yes | Yes |
|
| |||||
| Medical therapy | |||||
| ACE inhibitor/ARB | + | + | + | + | − |
| Beta-blocker | + | + | + | + | + |
| Diuretic | + | + | + | + | + |
| Statin | + | + | + | − | − |
| Digoxin | − | + | − | + | + |
Dimension and volume data were from cardiac computed tomography.
Procedure was aborted due to unsuitable anatomy of proximal septal vein. The subject was discharged next day without any complication.
ACC = American College of Cardiology; ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; AX = fluoroscopy angiography; CABG = coronary artery bypass graft; EROA = effective regurgitant orifice area; FMR = functional mitral regurgitation; HF = heart failure; LVEDD = left ventricular end-diastolic dimension; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; MR = mitral regurgitation; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; STS = Society of Thoracic Surgeons; TTE = transthoracic echocardiography.
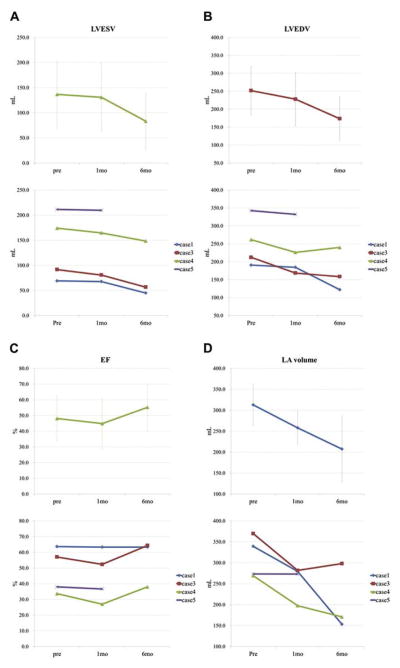
FIGURE 3. Effect of Mitral Loop Cerclage on Cardiac Chamber Dimensions (Reverse Remodeling).
Left ventricular end-systolic (LVESV) (A), end-diastolic (LVEDV) (B), ejection fraction (EF) (C), and left atrial (LA) volumes (D) all improve over time, suggesting reverse remodeling. Data are presented as in Figure 5.
FIGURE 4. Comparison of Basal Septal Coronary Veins.
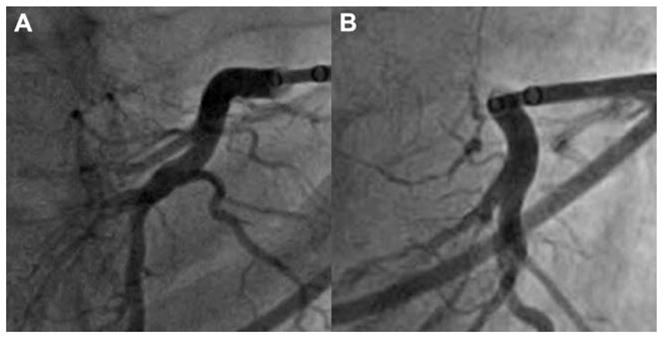
(A) A good septal vein in Patient #1. (B) A less suitable septal vein in Patient #2.
TABLE 2.
Clinical and Laboratory Follow-Up Data
| Case#
|
||||
|---|---|---|---|---|
| 1 | 3 | 4 | 5 | |
| Periprocedural outcomes | ||||
|
| ||||
| Bleeding complications (class)* | IIIa | IIIa | I | I |
| Complications | Focal myocardial infarction | Loss of tension, minor; acute kidney injury score = 2. | New-onset RBBB | |
| Blood pressure (systolic/diastolic), mm Hg | ||||
| Baseline | 110/70 | 136/93 | 146/71 | 100/66 |
| Post-procedure | 98/57 | 121/84 | 130/51 | 94/62 |
| NYHA functional class/ACC FMR stage | ||||
| Baseline | III/D | IV/D | IV/D | IV/D |
| Post-procedure | II | I–II | I | III |
| 1 month | I–II | I–II | I | III |
| 3 months | I | I–II | I–II | |
| 6 months | I | I–II | I–II | |
| Radiation dose (total, mGy) | 2,964 | 2,521 | 1,475 | 5,878 |
| Contrast media volume (ml) | 400 | 350 | 200 | 350 |
| Left circumflex size (mm) | 3.7 | 4.2 | 3.3 | 3.8 |
| Left circumflex length (mm) | 105 | 130 | 116 | 121 |
| Marginal/posterolateral branches | ||||
| n | 2 | 3 | 1 | 1 |
| Length to first branch (mm) | 31.5 | 32.4 | 15.9 | 13.8 |
| Diameter of first branch (mm) | 1.87 | 3.0 | 1.1 | 1.9 |
|
| ||||
| Laboratory data | ||||
|
| ||||
| Troponin I (ng/ml) | ||||
| Baseline | <0.01 | 0.03 | 0.08 | 0.33 |
| 1 month | 0.14 | 0.04 | 0.08 | 0.53 |
| 3 months | 0.01 | 0.06 | 0.06 | — |
| 6 months | 0.01 | 0.04 | 0.03 | — |
| CK-MB (ng/ml) | ||||
| Baseline | 1.3 | 1.4 | 2.1 | 2.1 |
| 1 month | 1.0 | 2.6 | 1.4 | 35.2 |
| 3 months | 1.5 | 1.9 | 2.0 | — |
| 6 months | 3.6 | 2.1 | 1.3 | — |
| BNP (pg/ml) | ||||
| Baseline | 228 | 3,269 | 489 | 2,404 |
| 1 month | 277 | 56 | 681 | 1,235 |
| 3 months | 132 | 79 | 131 | — |
| 6 months | 70 | 150 | 250 | — |
| Creatinine (mg/dl) | ||||
| Baseline | 1.00 | 0.92 | 1.36 | 1.72 |
| 1 month | 1.87 | 1.10 | 1.33 | 0.92 |
| 3 months | 1.59 | 1.65 | 1.59 | — |
| 6 months | 1.54 | 1.72 | 1.28 | — |
| eGFR (MDRD) (ml/min/1.73 m2) | ||||
| Baseline | 53.9 | 59.6 | 53.0 | 39.7 |
| 1 month | 26.1 | 48.5 | 54.4 | >60.0 |
| 3 months | 31.5 | 30.4 | 44.3 | — |
| 6 months | 32.6 | 28.9 | 56.9 | — |
According to Bleeding Academic Research Consortium definition for bleeding criteria.
BNP = brain natriuretic peptide; CK-MB = creatinine kinase myocardial band; eGFR = estimated glomerular filtration rate; MDRD = Modification of Diet in Renal Disease; RBBB = right bundle branch block; other abbreviations as in Table 1.
Procedures were performed using moderate sedation and transthoracic echocardiographic guidance in 3 and general anesthesia and TEE in 2. The total fluoroscopy time was 79 ± 20 min, time required to cross the interventricular septum with a guidewire was 43 ± 35 min, and the total procedure time was 169 ± 40 min.
IMPACT OF MITRAL LOOP CERCLAGE OF CARDIAC CHAMBERS AND MITRAL REGURGITATION
Mitral loop cerclage tension was applied interactively during echocardiography. Progressive tension application consistently reduced mitral regurgitation to a minimum; further tension exacerbated mitral regurgitation in the 2 subjects. The lock device was applied to fix the tension associated with minimum mitral regurgitation.
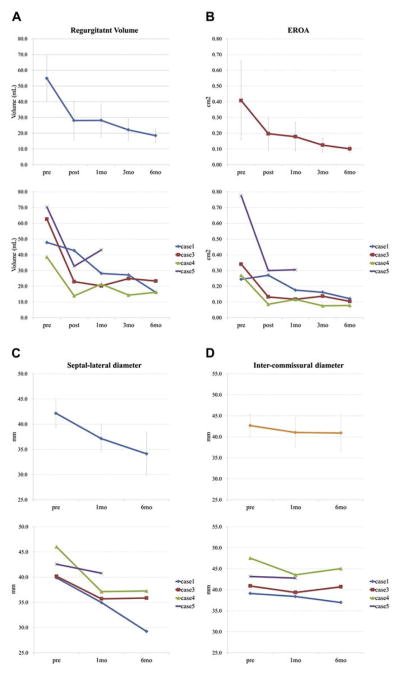
Tables 3 and 4 and Figures 3, 5, 6, and 7 show echocardiographic and CT findings during the 6-month follow-up period. Mitral loop cerclage significantly reduced annular dimension. The post-procedure septal lateral dimension was reduced from 44.2 ± 5.2 mm to 37.9 ± 4.1 mm (average 14.2%) by echocardiography.
TABLE 3.
Echocardiographic Data
| Baseline (n = 4) | Post-Procedure (n = 4) | 1 Month (n = 4) | 3 Months (n = 3) | 6 Months (n = 3) | |
|---|---|---|---|---|---|
| Regurgitant volume (ml) | 54.8 ± 14.3 | 28.0± 12.4 | 28.2± 10.6 | 22.1± 6.8 | 18.5 ± 4.1 |
| EROA (cm2) | 0.41± 0.25 | 0.20± 0.10 | 0.18± 0.09 | 0.12± 0.04 | 0.10 ± 0.02 |
| MV septal lateral dimension (mm) | 41.5± 2.6 | 35.7± 3.4 | 35.3± 3.2 | 34.3± 3.2 | 34.2 ± 3.3 |
| Mitral orifice area (cm2) | 4.9± 0.5 | 4.8± 0.8 | 4.7± 0.9 | 3.9± 0.9 | 4.2 ± 0.7 |
| Trans-MV mean pressure gradients (mm Hg) | 1.0± 0.5 | 0.6± ± 0.1 | 0.9± 0.4 | 0.5± 0.1 | 0.9 ± 0.4 |
| SPAP (mm Hg) | 51.2± 8.9 | 42.1± 10.7 | 49.1± 21.0 | 31.3± 5.8 | 39.6 ± 0.6 |
| LVEDD (mm) | 66.4± 8.1 | 67.6± 7.8 | 64.5± 10.2 | 61.9± 8.0 | 58.4 ± 5.1 |
| LVESD (mm) | 55.7± 11.0 | 55.1± 13.6 | 52.3± 13.3 | 46.8± 9.8 | 41.4 ± 10.9 |
| Vena contracta (mm) | 6.4± 1.0 | 4.7± 1.0 | 4.6± 1.2 | 4.2± 1.6 | 4.1 ± 1.4 |
| LVEDV (ml) | 140.5± 62.5 | 146.7± 50.0 | 144.4± 52.1 | 115.4± 30.7 | 102.6 ± 35.7 |
| LVESV (ml) | 77.8± 46.7 | 82.4± 44.7 | 82± 48.9 | 62.7± 37.7 | 55.1 ± 32.1 |
| Ejection fraction (%) | 47.7± 12.5 | 46.8± 13.9 | 47.2± 17.5 | 48.4± 17.1 | 48.9 ± 11.4 |
| MR grade (0–4) | 3.8± 0.5 | 2.8± 0.5 | 2.5± 0.6 | 2.0± 1.0 | 1.7 ± 0.6 |
| MR Vmax (cm/s) | 526± 91 | 547± 57 | 520± 27 | 535± 37 | 554 ± 57 |
| MR VTI (cm) | 153± 48 | 151± 29 | 164± 18 | 180± 11 | 188 ± 48 |
| PISA radius (cm2) | 0.9± 0.2 | 0.6± 0.2 | 0.6± 0.1 | 0.5± 0.1 | 0.5 ± 0.0 |
| TR grade (0–4) | 1.8± 1.0 | 1.3± 0.5 | 1.7± 1.2 | 0.7± 0.6 | 0.7 ± 0.6 |
| TR Vmax (m/s) | 3.2± 0.3 | 2.8± 0.5 | 3.2± 0.9 | 2.3± 0.3 | 2.7 ± 0.0 |
Values are mean ± SD.
EROA = effective regurgitant orifice area; MR = Mitral regurgitant; MV = mitral valve; PISA = proximal isovelocity surface area; SPAP = systolic pulmonary artery pressure; TR = tricuspid regurgitation; VTI = velocity time integral; other abbreviations as in Table 1.
TABLE 4.
Computed Tomography Measurement Data
| Baseline (n = 4) | 1 Month (n = 4) | 6 Months (n = 3) | |
|---|---|---|---|
| MV septal lateral dimension (mm) | 42.2 ± 2.9 | 37.1 ± 2.6 | 34.1 ± 4.3 |
|
| |||
| Intercommissural dimension (mm) | 42.7 ± 3.6 | 41.0 ± 2.5 | 40.9 ± 4.0 |
|
| |||
| Left ventricular outflow tract (mm) | |||
| Systole | 19.8 ± 2.7 | 20.6 ± 3.5 | 20.4 ± 4.5 |
| Diastole | 21.5 ± 2.2 | 22.1 ± 2.3 | 21.4 ± 3.7 |
|
| |||
| LV base (3 chamber, mm) | |||
| Systole | 58.6 ± 5.5 | 54.7 ± 5.0 | 51.9 ± 8.4 |
| Diastole | 61.2 ± 4.3 | 57.6 ± 3.1 | 55.6 ± 5.0 |
|
| |||
| LVEDD (mm) | 75.2 ± 7.8 | 72.5 ± 8.8 | 66.5 ± 7.7 |
|
| |||
| LVESD (mm) | 63.7 ± 13.1 | 59.3 ± 15.8 | 49.7 ± 12.4 |
|
| |||
| LA volume (ml) | 312.8 ± 49.7 | 258.0 ± 40.4 | 207.3 ± 79.0 |
|
| |||
| LVEDV (ml) | 251.8 ± 67.4 | 227.9 ± 73.6 | 173.5 ± 60.4 |
|
| |||
| LVESV (ml) | 136.5 ± 67.2 | 130.7 ± 68.0 | 83.3 ± 56.7 |
|
| |||
| Ejection fraction (%) | 48.1 ± 14.5 | 44.8 ± 16.2 | 55.2 ± 14.9 |
Values are mean ± SD.
Abbreviations as in Table 1.
FIGURE 5. Effect of Mitral Loop Cerclage on Mitral Regurgitation and Geometry.
Immediate and follow-up measurements are shown with mean ±SD on the top row and individual subject data on the bottom row. (A) Regurgitant volume. (B) Effective regurgitant orifice area (EROA). (C) Septal-lateral annular dimension. (D) Intercommissural annular dimension.
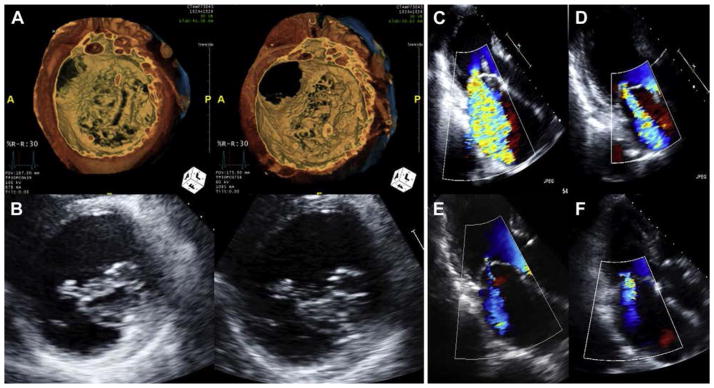
FIGURE 6. Improved Mitral Regurgitation and Leaflet Coaptation.
(A) Volume-rendered computed tomography before (left) and after (right) mitral loop cerclage in Patient #4. (B) Echocardiography before (left) and after (right) mitral loop cerclage in Patient #5. (C to F) Serial echocardiography in Patient #3 showing improvement, from baseline, 30 days, 3 months, and 6 months, respectively, in mitral regurgitation severity.
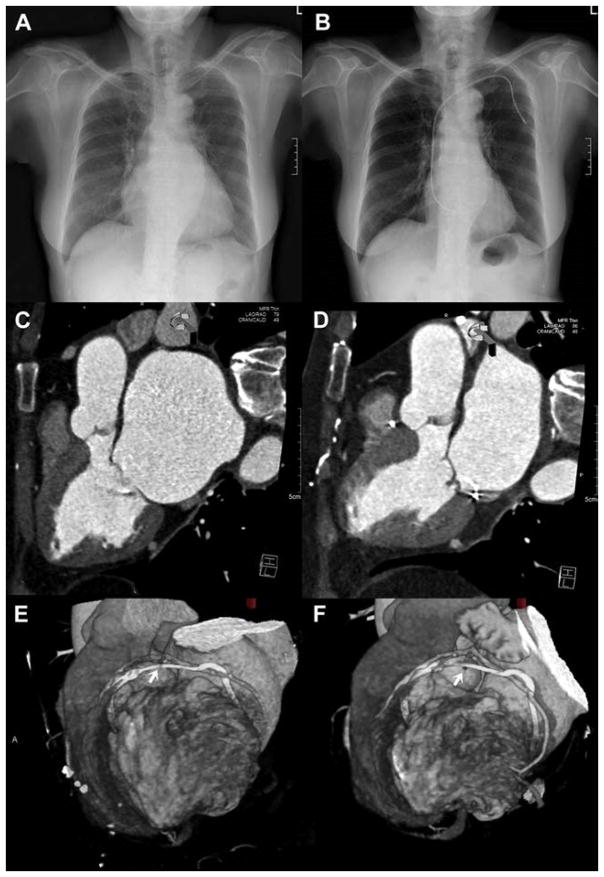
FIGURE 7. Evidence of Reduced Chamber Dimensions (Reverse Remodeling) in Subject 1.
(A and B) Baseline and 6 months posteroanterior radiograph shows reduced cardiothoracic ratio. (C and D) Multiplanar reformatted computed tomography (CT) shows left atrium in diastole before and after mitral loop cerclage. (E and F) Surface rendered CT shows positioning of arch-like coronary artery protection element at baseline and 6 months (arrows = compromised small branch).
Mitral loop cerclage imparted a sustained reduction in mitral valve regurgitation (Figure 5). Both regurgitant volume and effective regurgitant orifice area decreased immediately and continued to decrease during the 6-month follow-up. After 6 months, regurgitant volume and effective regurgitant orifice area reduced about 66% and 76%, respectively (Figure 6). Mitral loop cerclage imparted a significant reduction in left atrial and both systolic and diastolic left ventricular chamber size (Figures 3 and 7), decreasing an average 34% (left atrium), 31% (LV end-diastolic), and 39% (LV end-systolic) after 6 months. Accordingly, the LV ejection fraction did not deteriorate (48.1 ±14.5% to 55.2 ± 14.9%) (Figure 3). In addition, mitral loop cerclage was associated with a significant and sustained reduction in tricuspid regurgitation (Table 3).
REVERSION OF ATRIAL FIBRILLATION
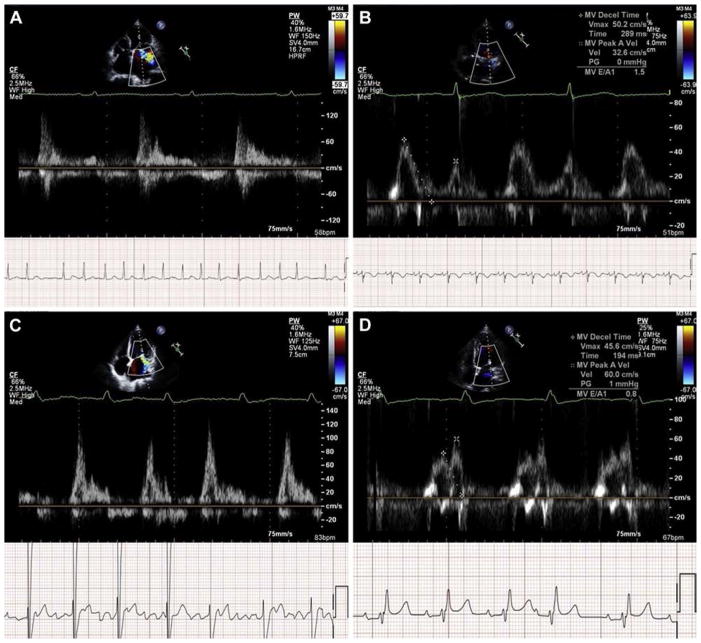
Mitral loop cerclage temporally corresponded with restoration of sinus rhythm in this early feasibility study. Two subjects with persistent and permanent atrial fibrillation at baseline spontaneously reverted to sinus rhythm immediately and 3 months, respectively, after mitral loop cerclage. Figure 8 shows the return of atrial mechanical contraction.
FIGURE 8. Reversion of Atrial Fibrillation to Sinus Rhythm.
Baseline (A and C) and 3 and 6 months (B and D) pulse-wave Doppler shows atrial fibrillation followed by restoration of sinus rhythm and atrial contraction after mitral loop cerclage in Patient #3 (A and B) and Patient #4 (C and D).
One additional subject had reported irregular tachypalpitation at baseline. She had brief paroxysmal atrial fibrillation and identical tachypalpitation 1 week after the procedure. Neither atrial fibrillation nor tachypalpitation recurred during the 6-month follow-up.
SAFETY AND COMPLICATIONS
The primary safety endpoint of freedom from major adverse cardiac events was met in 4 of the 5 subjects (80%). One subject experienced a focal myocardial infarction (akinetic area was 1.6% of LV mass assessed by CT) related to unrecognized entrapment of a small obtuse marginal branch (Figures 7E and 7F).
No subject experienced atrioventricular block. One subject developed new right bundle branch block attributed to traction against the interventricular septum from the coronary sinus tricuspid valve bridge device. This subject reverted from atrial fibrillation to sinus rhythm at the 3-month follow-up. In this subject, CT showed the right ventricular limb of the coronary sinus tricuspid valve bridge device to be too short, and as a result the tension element was tightly apposed to the interventricular septum.
In 1 subject, the coronary sinus tricuspid valve bridge device spontaneously migrated on post-procedure day 2 and caused recurrent mitral valve regurgitation. The subclavicular pocket was opened and tension reapplied with mitigation of mitral valve regurgitation.
One subject, who was in cardiogenic shock at baseline requiring mechanical circulatory support, and who had a severely dilated left ventricle (left ventricular end-diastolic dimension of 84 mm) died of refractory heart failure at 6 weeks after the procedure, and he did not undergo autopsy.
Other procedure-related adverse events include sustained Valve Academic Research Consortium-2 acute kidney injury grade 2 in a subject who required multiple iodine contrast exposures, and a small catheter-induced retrograde coronary sinus dissection that had no clinical sequelae. There were no infections.
DISCUSSION
This was the first human application of transcatheter mitral loop cerclage annuloplasty. The procedure was successful in 4 of 5 subjects and aborted in 1 of 5 because of unsuitable coronary vein anatomy. Mitral loop cerclage reduced immediate and progressive 6-month reduction of mitral valve regurgitation, and favorable (“reverse”) remodeling of the left atrial and left ventricular chamber volumes. Remarkably, mitral loop cerclage seemed also to induce electrical remodeling of the atria manifest as spontaneous reversion so sinus rhythm among the 3 subjects with paroxysmal, persistent, and permanent atrial fibrillation, respectively.
Complications were few, and included otherwise clinically inconsequential failure to cross the inter-ventricular septum in 1 subject with seemingly unsuitable coronary vein anatomy. Another experienced a small procedural myocardial infarction from an unrecognized small branch coronary occlusion. Another subject, who experienced cardiogenic shock at baseline, died of intractable heart failure after 6 weeks, despite a technically successful mitral loop cerclage procedure.
Mitral loop cerclage accomplished the immediate goal of reducing mitral annular dimensions, for example, the septal-lateral diameter, by 14%. Later reverse remodeling may contribute to further MR reduction. This compares favorably with 6% achieved using Mitralign transcatheter annular plication (Mitralign, Tewksbury, Massachusetts) (13), and Coapsys (Myocor, Inc., Maple Grove, Minnesota), 16% (14). These geometric changes are less pronounced than after surgical ring annuloplasty. Moreover, our subjects had greater baseline LV dimensions (7.5 ± 0.8 cm) compared with 6.3 ± 0.9 cm (Mitralign) and 6.1 ± 0.6 cm (Cardioband, Valtech, Or Yehuda, Israel); greater chamber dilation predicts poorer response to surgical annuloplasty (15).
Most interesting was the mechanical remodeling detected even in this small cohort. Mitral loop cerclage, by virtue of circumferential application of tension and of discordant annular and cerclage planes, seems to reduce cardiac dimensions even early, as it did in animals (9). In experimental animals with ischemic mitral regurgitation, undersized Paneth mitral annuloplasty sufficient to abolish mitral regurgitation led to reduced global and regional LV dimensions and modestly increased end-systolic elastance (16). In patients undergoing undersized surgical mitral annuloplasty for secondary mitral regurgitation, both pre-operative and post-operative global end-systolic myocardial wall stress predicted clinical improvement, suggesting that reduced end-systolic stress achieved by annuloplasty may contribute to reverse remodeling (17), and may interrupt the vicious cycle of mitral regurgitation and progressive ventricular dysfunction. Remarkably, there was also a suggestion in our experience of electrical remodeling (reversion of atrial fibrillation to sinus rhythm in 2 subjects), as a possible functional correlate of the geometric remodeling (18).
Mitral loop cerclage addresses shortcomings of other coronary sinus annuloplasty approaches, also known as “indirect” annuloplasty. First, our subjects may not have been eligible for the only marketed coronary sinus annuloplasty device (Carillon Mitral Contour, Cardiac Dimensions, Inc., Kirkland, Washington) because of likely compression of major coronary artery branches underneath the coronary sinus. In the largest published experience using that device, 17 of 53 subjects were excluded because of unsuitable anatomy (19). Second, mitral loop cerclage delivers annular tension circumferentially along a plane slightly discordant from the mitral annulus (Figure 1D) in a way that may compensate for coronary sinus anatomy running high along the left atrial free wall (9). By comparison, the planar relations of the compressive vectors seem to be different between the ARTO device (MVRX, Inc., Belmont, California) (20) and cerclage because cerclage pulls against the interventricular septum, whereas ARTO pulls against the interatrial septum. Accordingly, and similar to mitral loop cerclage, the investigational Coapsys device accomplished mid or basal extrinsic compression of the left ventricle, and reduced or abolished mitral regurgitation with an average septal-lateral reduction magnitude of 16% (14). We observed that cerclage enhanced leaflet coaptation in our small study (Figures 6A and 6B ). Nonsurgical tension readjustment, as in subject 3, seemed to be a useful and desirable feature.
Annuloplasty including cerclage may have value as an adjunct to mitral leaflet repair. Standalone surgical edge-to-edge leaflet repair has shown unsatisfactory long-term results (21). Both Carrilon (22) and Cardio-band (23) transcatheter annuloplasty devices been used to treat recurrent mitral regurgitation after Mitraclip (Abbott) procedures. Moreover, annuloplasty such as cerclage may provide a useful “landing zone” to implant transcatheter valves in native mitral valve disease. These concepts warrant further investigation.
STUDY LIMITATIONS
Limitations of this study include the small sample size of an early feasibility investigation, absence of a matching control cohort, and only a 6-month follow-up. Limitations of stand-alone annuloplasty in general includes high recurrence rates of secondary mitral regurgitation (24) and no direct therapy of concurrent subvalvular pathology, such as leaflet tethering. That said, we witnessed reverse remodeling even in this small study that effectively reduced leaflet tethering in follow-up.
CONCLUSIONS
In this small feasibility study, mitral loop cerclage annuloplasty was successful in 4 of 5 attempts, reduced mitral regurgitation severity, and caused reverse geometric and possible electrical remodeling. Further clinical evaluation is warranted.
PERSPECTIVES.
WHAT IS KNOWN?
Secondary mitral regurgitation is caused by annular dilation and leaflet traction causing malcoaptation. Mitral cerclage annuloplasty is a transcatheter technique that applies circumferential tension around the mitral annulus.
WHAT IS NEW?
In this first-in-human application of mitral loop cerclage annuloplasty, mitral annular geometry was reduced, mitralregurgitation reduced, and during a 6-month follow-up there was evidence of reverse remodeling and restoration of sinus rhythm.
WHAT IS NEXT?
Additional clinical experience will provide further insight into the safety, value, and mechanism of benefit of mitral loop cerclage annuloplasty.
Acknowledgments
The authors are grateful to Jun-Oh Kim for procedure assistance and Su-Jin Jung for data analysis.
This work was supported by grants from Tau-PNU MEDICAL Co. and the R&D program of MOTIE/KIAT (No. N0000697), Pusan National University (to Dr. J.H. Kim), and NIH Z01-HL006040 (to Dr. Lederman). Drs. J.H. Kim and S.C. Sung have equity in Tau-PNU Medical Co., Ltd. Drs. J.H. Kim and R.J. Lederman are inventors on patents assigned to their employers Pusan National University (to Dr. J.H. Kim) and National Institutes of Health (to Drs. J.H. Kim and R.J. Lederman).
ABBREVIATIONS AND ACRONYMS
- CT
computed tomography
- LV
left ventricle
- RVOT
right ventricular outflow tract
- TEE
transesophageal echocardiography
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For a supplemental video and its legend, please see the online version of this article.
References
- 1.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ ACC guideline for the management of patients with valvular heart disease: are port of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:e521–643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 2.Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 3.Feldman T, Kar S, Elmariah S, et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation 5-Year Results of EVEREST II. J Am Coll Cardiol. 2015;66:2844–54. doi: 10.1016/j.jacc.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Foster E, Kwan D, Feldman T, et al. Percutaneous mitral valve repair in the initial EVEREST cohort: evidence of reverse left ventricular remodeling. Circ Cardiovasc Imaging. 2013;6:522–30. doi: 10.1161/CIRCIMAGING.112.000098. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann HC, Maisano F. Transcatheter therapy of mitral regurgitation. Circulation. 2014;130:1712–22. doi: 10.1161/CIRCULATIONAHA.114.009881. [DOI] [PubMed] [Google Scholar]
- 6.Choure AJ, Garcia MJ, Hesse B, et al. In vivo analysis of the anatomical relationship of coronary sinus to mitral annulus and left circumflex coronary artery using cardiac multidetector computed tomography. Implications for percutaneous coronary sinus mitral annuloplasty. J Am Coll Cardiol. 2006;48:1938–45. doi: 10.1016/j.jacc.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 7.Maselli D, Guarracino F, Chiaramonti F, Mangia F, Borelli G, Minzioni G. Percutaneous mitral annuloplasty: an anatomic study of human coronary sinus and its relation with mitral valve annulus and coronary arteries. Circulation. 2006;114:377–80. doi: 10.1161/CIRCULATIONAHA.105.609883. [DOI] [PubMed] [Google Scholar]
- 8.Tops LF, Van De Veire NR, Schuijf JD, et al. Noninvasive evaluation of coronary sinus anatomy and its relation to the mitral valve annulus: implications for percutaneous mitral annuloplasty. Circulation. 2007;115:1426–32. doi: 10.1161/CIRCULATIONAHA.106.677880. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kocaturk O, Ozturk C, et al. Mitral cerclage annuloplasty, a novel transcatheter treatment for secondary mitral valve regurgitation. Initial results in swine. J Am Coll Cardiol. 2009;54:638–51. doi: 10.1016/j.jacc.2009.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Sung SC, Chon MK, et al. Mitral loop cerclage as a variant form of mitral cerclage annuloplasty that adds a device (CSTV) for preventing potential complications : a preclinical proof of concept and feasibility study. Euro-Intervention. 2016;11:1669–79. doi: 10.4244/EIJV11I14A319. [DOI] [PubMed] [Google Scholar]
- 11.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 12.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;60:1438–54. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Nickenig G, Schueler R, Dager A, et al. Treatment of chronic functional mitral valve regurgitation with a percutaneous annuloplasty system. J Am Coll Cardiol. 2016;67:2927–36. doi: 10.1016/j.jacc.2016.03.591. [DOI] [PubMed] [Google Scholar]
- 14.Grossi EA, Woo YJ, Schwartz CF, et al. Comparison of Coapsys annuloplasty and internal reduction mitral annuloplasty in the randomized treatment of functional ischemic mitral regurgitation: impact on the left ventricle. J Thorac Cardiovasc Surg. 2006;131:1095–8. doi: 10.1016/j.jtcvs.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 15.Braun J, Bax JJ, Versteegh MIM, et al. Preoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. Eur J Cardiothorac Surg. 2005;27:847–53. doi: 10.1016/j.ejcts.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 16.Tibayan FA, Rodriguez F, Langer F, et al. Undersized mitral annuloplasty alters left ventricular shape during acute ischemic mitral regurgitation. Circulation. 2004;110(Suppl 11):98–102. doi: 10.1161/01.CIR.0000138395.45145.45. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Taniguchi K, Shudo Y, et al. Mechanism of beneficial effects of restrictive mitral annuloplasty in patients with dilated cardiomyopathy and functional mitral regurgitation. Circulation. 2010;122(Suppl 1):S3–9. doi: 10.1161/CIRCULATIONAHA.109.927855. [DOI] [PubMed] [Google Scholar]
- 18.Gertz ZM, Raina A, Saghy L, et al. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol. 2011;58:1474–81. doi: 10.1016/j.jacc.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Siminiak T, Wu JC, Haude M, et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur J Heart Fail. 2012;14:931–8. doi: 10.1093/eurjhf/hfs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers JH, Thomas M, Morice M-C, et al. Treatment of heart failure with associated functional mitral regurgitation using the ARTO system initial results of the first-in-human Mitral Valve Repair Clinical Trial (MAVERIC) J Am Coll Cardiol Intv. 2015;8:1095–104. doi: 10.1016/j.jcin.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 21.De Bonis M, Lapenna E, Maisano F. Long-term results (up to 18 years) of the edge-to-edge mitral valve repair without annuloplasty in degenerative mitral regurgitation: implications for the percutaneous approach. Circulation. 2014;130:S19–24. doi: 10.1161/CIRCULATIONAHA.113.007885. [DOI] [PubMed] [Google Scholar]
- 22.Abdelrahman N, Chowdhury MA, Nooryani AA, Elabbassi W. A case of dilated cardiomyopathy and severe mitral regurgitation treated using a combined percutaneous approach of MitraClip followed by CARILLON mitral contour system. Cardiovasc Revascularization Med. 2016;17:578–81. doi: 10.1016/j.carrev.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Latib A, Ancona MB, Ferri L, et al. Percutaneous direct annuloplasty with Cardioband to treat recurrent mitral regurgitation after MitraClip implantation. J Am Coll Cardiol Intv. 2016;9:e191–2. doi: 10.1016/j.jcin.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2016;374:344–53. doi: 10.1056/NEJMoa1512913. [DOI] [PMC free article] [PubMed] [Google Scholar]