Summary
Background
Nivolumab has shown improved survival in the treatment of advanced non-small-cell lung cancer (NSCLC) previously treated with chemotherapy. We assessed the safety and activity of combination nivolumab plus ipilimumab as first-line therapy for NSCLC.
Methods
The open-label, phase 1, multicohort study (CheckMate 012) cohorts reported here were enrolled at eight US academic centres. Eligible patients were aged 18 years or older with histologically or cytologically confirmed recurrent stage IIIb or stage IV, chemotherapy-naive NSCLC. Patients were randomly assigned (1:1:1) by an interactive voice response system to receive nivolumab 1 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks, nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks, or nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks until disease progression, unacceptable toxicities, or withdrawal of consent. Data from the latter two cohorts, which were considered potentially suitable for further clinical development, are presented in this report; data from the other cohort (as well as several earlier cohorts) are described in the appendix. The primary outcome was safety and tolerability, assessed in all treated patients. This ongoing study is registered with ClinicalTrials.gov, number NCT01454102.
Findings
Between May 15, 2014, and March 25, 2015, 78 patients were randomly assigned to receive nivolumab every 2 weeks plus ipilimumab every 12 weeks (n=38) or nivolumab every 2 weeks plus ipilimumab every 6 weeks (n=40). One patient in the ipilimumab every-6-weeks cohort was excluded before treatment; therefore 77 patients actually received treatment (38 in the ipilimumab every-12-weeks cohort; 39 in the ipilimumab every-6-weeks cohort). At data cut-off on Jan 7, 2016, 29 (76%) patients in the ipilimumab every-12-weeks cohort and 32 (82%) in the ipilimumab every-6-weeks cohort had discontinued treatment. Grade 3–4 treatment-related adverse events occurred in 14 (37%) patients in the ipilimumab every-12-weeks cohort and 13 (33%) patients in the every-6-weeks cohort; the most commonly reported grade 3 or 4 treatment-related adverse events were increased lipase (three [8%] and no patients), pneumonitis (two [5%] and one [3%] patients), adrenal insufficiency (one [3%] and two [5%] patients), and colitis (one [3%] and two [5%] patients). Treatment-related serious adverse events were reported in 12 (32%) patients in the ipilimumab every-12-weeks cohort and 11 (28%) patients in the every-6-weeks cohort. Treatment-related adverse events (any grade) prompted treatment discontinuation in four (11%) patients in the every-12-weeks cohort and five (13%) patients in the every-6-weeks cohort. No treatment-related deaths occurred. Confirmed objective responses were achieved in 18 (47% [95% CI 31–64]) patients in the ipilimumab every-12-weeks cohort and 15 (38% [95% CI 23–55]) patients in the ipilimumab every-6-weeks cohort; median duration of response was not reached in either cohort, with median follow-up times of 12·8 months (IQR 9·3–15·5) in the ipilimumab every-12-weeks cohort and 11·8 months (6·7–15·9) in the ipilimumab every-6-weeks cohort. In patients with PD-L1 of 1% or greater, confirmed objective responses were achieved in 12 (57%) of 21 patients in the ipilimumab every-12-weeks cohort and 13 (57%) of 23 patients in the ipilimumab every-6-weeks cohort.
Interpretation
In NSCLC, first-line nivolumab plus ipilimumab had a tolerable safety profile and showed encouraging clinical activity characterised by a high response rate and durable response. To our knowledge, the results of this study are the first suggestion of improved benefit compared with anti-PD-1 monotherapy in patients with NSCLC, supporting further assessment of this combination in a phase 3 study.
Funding
Bristol-Myers Squibb.
Introduction
In patients who have advanced non-small-cell lung cancer (NSCLC) without targetable mutations, first-line treatment is platinum-based combination chemotherapy. This approach has been the standard of care for the past three decades with few improvements in outcomes,1,2 and is characterised by moderate-to-severe toxicities, including haematological adverse events and non-haematological toxicities, such as fatigue, nausea, vomiting, and alopecia. The proportion of patients who achieved a response to chemotherapy remains in the 30% range;1,3,4 responses are rarely durable, and nearly half of patients die within 1 year.3–5 A profound need exists for treatment strategies to improve long-term survival in patients with newly diagnosed advanced NSCLC.
Research in context.
Evidence before this study
We searched PubMed for reports published between Jan 1, 2010, and Aug 24, 2016, without language restrictions, using the search terms “immunotherapy”, “nivolumab”, “pembrolizumab”, “durvalumab”, “atezolizumab”, “ipilimumab”, “tremelimumab”, “anti-PD-1”, “anti-PD-L1”, or “anti-CTLA-4” with “combination” and “lung”. Anti-PD-1 monotherapies have shown improved survival compared with docetaxel in patients with advanced non-small-cell lung cancer (NSCLC) previously treated with chemotherapy. Recently, a response rate of 23% was reported with the combination of durvalumab plus tremelimumab in patients with advanced NSCLC, most of whom had been previously treated with chemotherapy. In patients with melanoma, the combination of nivolumab plus ipilimumab showed improved response and survival compared with either agent alone. The present study was designed to examine the safety and efficacy of nivolumab plus ipilimumab in patients with chemotherapy-naive, advanced NSCLC.
Added value of this study
The results of this trial show that the combination of nivolumab and ipilimumab is well tolerated and is associated with promising, durable, clinical activity. Response rates were at least comparable with those achieved with first-line platinum-based chemotherapy and seemed to exceed the activity expected with nivolumab monotherapy based on previously reported phase 1 data, especially in patients with PD-L1-positive NSCLC.
Implications of all the available evidence
To our knowledge, the results of this study represent the first suggestion of improved benefit relative to anti-PD-1 monotherapy in patients with NSCLC and highlight the potential role for immunotherapy combinations as first-line treatment for NSCLC. Based on these findings, first-line combination treatment with nivolumab and ipilimumab is being prospectively assessed in patients with advanced NSCLC in an ongoing phase 3 study.
Antibodies that inhibit immune checkpoints such as CTLA-4 and PD-1 have improved outcomes for patients with several different types of cancers.6–12 In NSCLC, nivolumab (a fully human IgG4 antibody against PD-1) improves overall survival compared with docetaxel in patients with previously treated advanced NSCLC.10,11 The proportion of patients responding to nivolumab ranges from 15% to 20% in unselected patients,10,11,13 and responses have tended to be durable, persisting for months or years even after discontinuation of therapy.13
Since PD-1 and CTLA-4 modulate effector T-cell activation, proliferation, and function through distinct, complementary mechanisms,14 the combination of nivolumab plus ipilimumab (a fully human IgG1 antibody against CTLA-4) represents a rational approach to improve antitumour immunity. In patients with metastatic melanoma, the combination of nivolumab and ipilimumab has enhanced activity relative to either monotherapy,15,16 and median overall survival was not reached after a minimum of 2 years follow-up.17 The combination is approved in the USA and Europe for patients with melanoma.
Given the established safety and activity of nivolumab monotherapy in previously treated advanced NSCLC and the long-term survival reported with combination immunotherapy in melanoma, this portion of the phase 1 multicohort CheckMate 012 study was designed to assess nivolumab plus ipilimumab as first-line therapy for patients with advanced NSCLC. Separate cohorts of this study in which nivolumab was given as monotherapy or in combination with other therapies have been reported previously.18,19
Methods
Study design and participants
This was an open-label, phase 1, multicohort study done at nine academic centres in the USA and three in Canada. Patients in the cohorts reported here were enrolled at eight of the US centres (appendix p 2). Eligible patients were aged 18 years or older with histologically or cytologically confirmed recurrent stage IIIb or stage IV NSCLC with radiographic evidence of measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.20 Patients were required to be chemotherapy-naive for advanced NSCLC; previous neoadjuvant and adjuvant chemotherapies for locally advanced disease were allowed (with no washout periods specified) as long as all other eligibility criteria were met. Previous oral tyrosine kinase inhibitors were also allowed in the adjuvant or metastatic disease setting if administration had been completed at least 2 weeks before randomisation. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; adequate haematological, hepatic, and renal function; and life expectancy of at least 3 months. Patients with stable, treated brain metastases were eligible if they had completed radiotherapy, surgery, or radiosurgery at least 2 weeks before enrolment and did not have active cerebral oedema requiring steroid treatment. Collection of pretreatment tumour tissue (excisional, incisional, or core needle) was required, but biomarker analyses (eg, PD-L1 expression) were not used to select patients. Baseline laboratory tests required to assess eligibility included white blood cell counts, neutrophils, platelets, haemoglobin, serum creatinine, alanine amino-transferase, aspartate aminotransferase, and total bilirubin. Patients with a history of autoimmune disease were not eligible, nor were those with previous malignancies (unless a complete remission was achieved at least 2 years prior to study entry). Another exclusion criterion was a need for immunosuppressive doses of systemic corticosteroids, although use of local cortico-steroids (with minimal absorption), physiological replacement doses of systemic corticosteroids, or a brief course of corticosteroids for treatment of non-autoimmune conditions or delayed nausea were allowed.
The study protocol was approved by the institutional review board at each participating centre. The study was done in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, as defined by the International Conference on Harmonisation. All patients provided written informed consent before enrolment.
Randomisation and masking
Eligible patients were randomly assigned to receive nivolumab 1 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks, nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks, or nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks intravenously (appendix p 3). An interactive voice response system was used to assign patients (1:1:1) to these three cohorts. Randomisation was stratified by histology (squamous vs non-squamous vs not otherwise specified). Patients and investigators were not masked to treatment.
Procedures
The main text of this paper focuses on results obtained in the two nivolumab 3 mg/kg cohorts, which were considered potentially suitable for further clinical development. Results obtained in the nivolumab 1 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks cohort are described in the appendix (pp 12–16). Treatment was to be continued until disease progression, unacceptable toxicities, or consent withdrawal (appendix pp 3, 8). Patients could be treated beyond initial progression as long as they were considered by the investigator to be deriving clinical benefit and tolerating study treatment. No nivolumab or ipilimumab dose reductions were allowed, but administration of either drug could be delayed for adverse events (appendix p 6). Ipilimumab could be discontinued and nivolumab continued as monotherapy if the investigator was able to attribute adverse events to ipilimumab only. Treatment beyond initial progression was to be discontinued if an additional 10% increase in tumour burden beyond initial progression was measured on subsequent imaging.
Safety was assessed throughout the study (appendix p 7) by the investigators. Two follow-up visits for safety assessments were required after discontinuing study treatment: one at 30 days and one at 100 days after the last dose or date of discontinuation. Categories of select adverse events (those with a potential immunological cause) were based on a prespecified list of terms from the Medical Dictionary for Regulatory Activities version 18.1. The severity of adverse events was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, and the causal association with study drugs was determined by the investigators. Tumour response was monitored using spiral CT with contrast or MRI at weeks 11, 17, and 23, and then every 12 weeks until disease progression (investigator-assessed per RECIST version 1.1). For patients treated beyond initial progression, following a scan done 6 weeks after documentation of progression, tumour assessments continued on the original schedule if the investigator felt that the patient continued to derive clinical benefit without a further increase in tumour burden of more than 10%. Patients who discontinued for reasons other than progressive disease or consent withdrawal continued tumour assessments on the protocol-defined schedule until documented progression. Patients were followed for survival every 12 weeks after discontinuing study treatment.
PD-L1 was assessed retrospectively in fresh or archival pretreatment tumour samples collected before the first dosing date with the use of a validated immunohistochemistry assay (Dako, North America, Carpinteria, CA, USA)21 that used a rabbit antihuman PD-L1 antibody (clone 28–8; Epitomics, Burlingame, CA, USA). Tumour PD-L1 expression was measured as percentage of tumour cells with membrane staining (any intensity) in a section containing at least 100 evaluable tumour cells.
Outcomes
The primary outcome of the study was the frequency of adverse events and serious adverse events occurring up to 100 days after the last dose of study drug. Secondary outcomes comprised the proportion of patients achieving a confirmed objective response, median duration of response, and progression-free survival at 24 weeks using tumour assessments reported by investigators and defined according to RECIST version 1.1 (excluding clinical progression). Objective response was defined as the proportion of all treated patients with either a confirmed complete response or partial response. Duration of response was defined from the first documented objective response and progression-free survival was defined from the date of first dose of study medication; both were defined as the time to the date of first disease progression or death, if death occurred within 100 days of the final dose of nivolumab or ipilimumab, whichever was administered last. Among patients without previous RECIST-defined progression, patients who died beyond 100 days and those who remained alive were censored at the last tumour assessment date (before subsequent therapy). Progression-free survival at 24 weeks was defined as the proportion of all treated patients who remained progression-free at 24 weeks and survived through 24 weeks. Median progression-free survival, overall survival, overall survival at 1 year, and associations between clinical activity and tumour PD-L1 expression, smoking status, and EGFR mutation status were assessed as prespecified exploratory endpoints.
Statistical analysis
Recruitment of about 30 patients per cohort was planned with the goal of identifying safe regimens for future clinical development, with “safe” defined as less than 25% of treated patients (seven or fewer of 30) discontinuing study drug due to treatment-related adverse events by week 17. At 30 treated patients per cohort, the probability of observing seven or fewer such events was 76% or 28% if the true toxicity rate was 20% or 30%, respectively, providing false-negative rates of 24% and false-positive rates of 28% deemed acceptable.
Analyses were based on a Feb 18, 2016, database lock. All patients who received at least one dose of study drug were eligible for safety and efficacy assessments. Patient demographics and frequency of adverse events were summarised with descriptive statistics. For determination of the total number of patients with an adverse event, patients were counted only once at the preferred term, once at the system organ class, and once at the patient level. Two-sided 95% exact CIs were calculated for the proportion of patients achieving an objective response using the Clopper-Pearson method. Estimated time-to-event endpoints were calculated with the Kaplan-Meier method, with two-sided 95% CIs for medians and rates calculated using standard methods. All statistical analyses were done with SAS version 9.04.
This ongoing study is registered with ClinicalTrials.gov, number NCT01454102.
Role of the funding source
The funder provided the study drug and worked with the investigators to design the study and to collect, analyse, and interpret the data. The report was prepared by the corresponding author with input from all coauthors and with editorial assistance from professional medical writers, funded by the sponsor. The authors and professional medical writers had full access to the raw data, and the decision to submit the report for publication was made by all authors.
Results
Initial cohorts specified in an early amendment to the CheckMate 012 protocol assessed the safety and efficacy of the combination of nivolumab and ipilimumab given in the following schedules: nivolumab 1 mg/kg plus ipilimumab 3 mg/kg or nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses, followed by nivolumab 3 mg/kg every 2 weeks until disease progression, unacceptable toxicity, or withdrawal of consent (appendix pp 12–16).22 These schedules were characterised by poor tolerability, with 25 (51%) of 49 patients having grade 3–4 treatment-related adverse events, including three treatment-related deaths, and a discontinuation rate of up to 33% due to grade 3–4 treatment-related adverse events. Four additional cohorts specified in subsequent protocol amendments evaluated lower doses of both agents or less frequent dosing of ipilimumab, or both. These four regimens were nivolumab 1 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four cycles, nivolumab 1 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks, and nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks or 6 weeks (appendix p 3). These regimens were better tolerated, although clinical activity was suboptimal when nivolumab was given at the lower dose (1 mg/kg).23 Overall, of the six nivolumab plus ipilimumab regimens assessed, the results obtained with the first four are presented in the appendix (pp 12–16). Here, we focus on results obtained with the last two regimens, which were considered potentially suitable for further clinical development: nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg given either every 12 weeks or every 6 weeks.
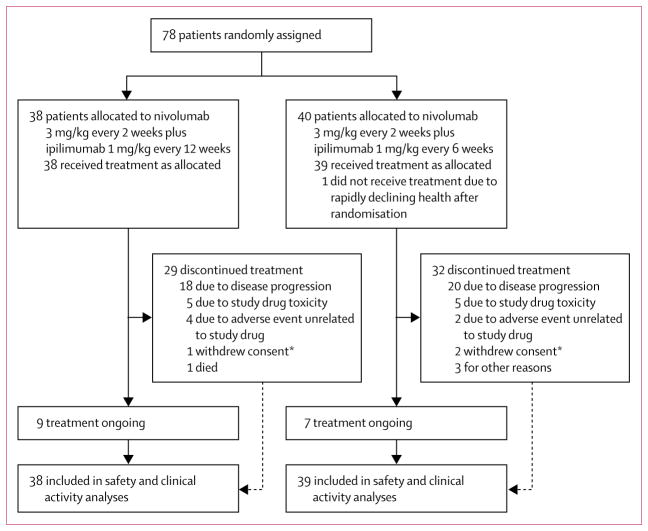
Between May 15, 2014, and March 25, 2015, 78 patients with chemotherapy-naive advanced NSCLC were randomly assigned to treatment with nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks (n=38, all were treated) or every 6 weeks (n=40, of whom 39 were treated; figure 1). The one patient in the ipilimumab every-6-week dosing schedule group who did not receive treatment had a rapid decline in health after randomisation to this cohort and was therefore never treated. All 77 treated patients were assessed for safety and clinical activity. The two cohorts were balanced for ECOG performance status, disease stage, histology, and EGFR mutations (table 1). The cohort that received ipilimumab every 12 weeks had fewer never-smokers and fewer male patients than the every-6-weeks cohort (table 1). 61 (79%) of 77 patients had tumour samples in which PD-L1 could be assessed (31 [82%] in the every-12-weeks cohort and 30 [77%] in the every-6-weeks cohort); PD-L1 expression was 1% or greater in 44 (72%) samples overall (21 [68%] in the ipilimumab every-12-weeks cohort and 23 [77%] in the ipilimumab every-6-weeks cohort). Tumour PD-L1 expression was not quantifiable at the time of analysis in 16 (21%) of 77 patients (seven [18%] of those in the ipilimumab every-12-weeks cohort and nine [23%] in the ipilimumab every-6-weeks cohort), either because of inadequate tissue amount or quality (n=4 in both cohorts) or because no tumour tissue specimen was available for assessment (n=3 in the ipilimumab every-6-weeks cohort; n=5 in the ipilimumab every-12-weeks cohort). The lack of tumour tissue for these latter eight patients represented a protocol violation. Tissue for one of these patients in the every-6-weeks cohort was provided after the database lock, and that patient’s tumour PD-L1 expression level was subsequently determined to be less than 1%.
Figure 1. Trial profile.
*Includes patient requests to discontinue study drug.
Table 1.
Baseline characteristics
| Nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks (n=38) | Nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks (n=39) | |
|---|---|---|
| Age (years) | 68 (58–73) | 62 (57–73) |
|
| ||
| Sex | ||
| Men | 17 (45%) | 24 (62%) |
| Women | 21 (55%) | 15 (38%) |
|
| ||
| ECOG performance status | ||
| 0 | 12 (32%) | 16 (41%) |
| 1 | 26 (68%) | 21 (54%) |
| Not reported | 0 | 2 (5%) |
|
| ||
| Disease stage | ||
| Stage IIIb | 4 (11%) | 1 (3%) |
| Stage IV | 34 (89%) | 38 (97%) |
|
| ||
| Histology | ||
| Non-squamous | 31 (82%) | 33 (85%) |
| Squamous | 7 (18%) | 6 (15%) |
|
| ||
| EGFR mutation status | ||
| Mutant | 4 (11%) | 4 (10%) |
| Wild type | 28 (74%) | 26 (67%) |
| Unknown | 6 (16%) | 9 (23%) |
|
| ||
| PD-L1 expression quantifiable* | 31 (82%) | 30 (77%) |
| ≥1% | 21/31 (68%) | 23/30 (77%) |
| ≥5% | 16/31 (52%) | 19/30 (63%) |
| ≥10% | 13/31 (42%) | 15/30 (50%) |
| ≥25% | 10/31 (32%) | 8/30 (27%) |
| ≥50% | 6/31 (19%) | 7/30 (23%) |
|
| ||
| Smoking status | ||
| Current | 7 (18%) | 4 (10%) |
| Former | 29 (76%) | 25 (64%) |
| Never | 2 (5%) | 9 (23%) |
| Unknown | 0 | 1 (3%) |
|
| ||
| Previous surgery | 29 (76%) | 26 (67%) |
|
| ||
| Previous radiotherapy | 20 (53%) | 16 (41%) |
|
| ||
| Previous systemic therapy | 10 (26%) | 9 (23%) |
| Regimen setting† | ||
| Adjuvant therapyठ| 8 (21%) | 6 (15%) |
| Neoadjuvant therapy‡ | 2 (5%) | 2 (5%) |
| Metastatic disease | 1 (3%) | 4 (10%) |
| Systemic therapy in the metastatic setting | ||
| Erlotinib | 1 (3%) | 4 (10%) |
| Other targeted therapy¶ | 1 (3%) | 3 (8%) |
Data are n (%), n/N (%), or median (IQR), unless otherwise stated. Data for KRAS status and history of CNS metastases were not routinely collected and are unavailable. ECOG=Eastern Cooperative Oncology Group.
Tumour PD-L1 expression was not quantifiable in 16 (21%) of 77 patients in the two cohorts, because of either inadequate tissue amount or quality (n=8) or because no tumour tissue specimen was available for assessment (n=8); tissue for one of these patients was provided after database lock.
More than one setting per patient might be reflected in the frequency.
Includes pemetrexed, gemcitabine, cisplatin, bevacizumab, carboplatin, paclitaxel, docetaxel, etoposide, and vinorelbine.
One patient in each cohort received erlotinib as part of adjuvant therapy.
Includes afatinib, alectinib, crizotinib, and the MET inhibitor LYM2875388.
At the time of this analysis, median follow-up was 12·8 months (IQR 9·3–15·5) in the ipilimumab every-12-weeks cohort and 11·8 months (6·7–15·9) in the ipilimumab every-6-weeks cohort. At data cut-off on Jan 7, 2016, across cohorts, 61 (79%) patients had discontinued study treatment (29 [76%] in the every-12-weeks cohort and 32 [82%] in the every-6-weeks cohort), most commonly because of disease progression (figure 1, appendix p 8). 11 (29%) patients in the ipilimumab every-12-weeks cohort and 15 (38%) patients in the ipilimumab every-6-weeks cohort had died, mostly from disease progression (nine [82%] of 11 in the ipilimumab every-12-weeks cohort and 13 [87%] of 15 in the ipilimumab every-6-weeks cohort). One patient in the ipilimumab every-12-weeks cohort died from acute respiratory failure and one from an unknown cause; one patient in the ipilimumab every-6-weeks cohort died from a pulmonary embolism and one from a lung infection; none of these events were judged to be treatment-related. Nine (24%) patients in the ipilimumab every-12-weeks cohort and seven (18%) patients in the ipilimumab every-6-weeks cohort remained on treatment at the time of database lock.
The median duration of nivolumab treatment was 9.4 months (IQR 3.6–12.8) in the ipilimumab every-12-weeks cohort and 4.1 months (1.9–10.1) in the ipilimumab every-6-weeks cohort (appendix p 8). The median duration of ipilimumab treatment was 9.7 months (IQR 5.5–13.8) in the ipilimumab every-12-weeks cohort and 3·4 months (1.4–9.4) in the ipilimumab every-6-weeks cohort. Relative dose intensities for both agents were well balanced across the two cohorts (appendix p 8). Relative dose intensities of 90% or greater were achieved in 25 (66%) and 26 (67%) patients for nivolumab and 33 (87%) and 33 (85%) patients for ipilimumab in the ipilimumab every-12-weeks and the ipilimumab every-6-weeks cohorts, respectively.
Any-grade treatment-related adverse events were reported in 31 (82%) of 38 patients in the ipilimumab every-12-weeks cohort and 28 (72%) of 39 in the ipilimumab every-6-weeks cohort; grade 3–4 treatment-related adverse events occurred in 14 (37%) patients in the ipilimumab every-12-weeks cohort and 13 (33%) in the ipilimumab every-6-weeks cohort (table 2). The most frequently observed categories of treatment-related select adverse events were skin (15 [39%] in the ipilimumab every-12-weeks cohort and 14 [36%] in the ipilimumab every-6-weeks cohort), gastrointestinal (nine [24%] and nine [23%]), and endocrine (four [11%] and eight [21%]; table 3). The most frequent grade 3–4 treatment-related adverse events overall were increased lipase (three [8%] in the ipilimumab every-12-weeks cohort and none in the every-6-weeks cohort), pneumonitis (two [5%] and one [3%]), adrenal insufficiency (one [3%] and two [5%]), and colitis (one [3%] and two [5%]; table 2). Treatment-related serious adverse events were reported in 12 (32%) patients in the ipilimumab every-12-weeks cohort and 11 (28%) patients in the every-6-weeks cohort (appendix p 9). A similar proportion of patients in both cohorts discontinued treatment because of treatment-related adverse events (table 3). These events occurred early (within the first 3 months of treatment) for one patient in the every-12-weeks cohort and three patients in the every-6-weeks cohort (two of whom had not yet received a second dose of ipilimumab). Late discontinuations (after 3 months) were similar across both cohorts (three in the every-12-weeks cohort and two in the every-6-weeks cohort). The most common treatment-related adverse event leading to discontinuation was pneumonitis (any grade, two [5%] in both cohorts; table 3). There were no treatment-related deaths at the time of analysis (table 2).
Table 2.
Treatment-related adverse events
| Nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks (n=38) | Nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks (n=39) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |
| Any event | 17 (45%) | 13 (34%) | 1 (3%) | 15 (38%) | 11 (28%) | 2 (5%) |
|
| ||||||
| Pruritus | 9 (24%) | 0 | 0 | 5 (13%) | 0 | 0 |
|
| ||||||
| Diarrhoea | 7 (18%) | 1 (3%) | 0 | 8 (21%) | 0 | 0 |
|
| ||||||
| Nausea | 6 (16%) | 0 | 0 | 5 (13%) | 1 (3%) | 0 |
|
| ||||||
| Fatigue | 5 (13%) | 1 (3%) | 0 | 8 (21%) | 1 (3%) | 0 |
|
| ||||||
| Increased amylase | 5 (13%) | 1 (3%) | 0 | 0 | 0 | 0 |
|
| ||||||
| Maculopapular rash | 5 (13%) | 0 | 0 | 3 (8%) | 1 (3%) | 0 |
|
| ||||||
| Pyrexia | 5 (13%) | 0 | 0 | 2 (5%) | 0 | 0 |
|
| ||||||
| Rash | 5 (13%) | 1 (3%) | 0 | 3 (8%) | 1 (3%) | 0 |
|
| ||||||
| Arthralgia | 4 (11%) | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Decreased appetite | 4 (11%) | 0 | 0 | 5 (13%) | 0 | 0 |
|
| ||||||
| Anaemia | 3 (8%) | 0 | 0 | 2 (5%) | 1 (3%) | 0 |
|
| ||||||
| Increased lipase | 3 (8%) | 2 (5%) | 1 (3%) | 0 | 0 | 0 |
|
| ||||||
| Dyspnoea | 2 (5%) | 1 (3%) | 0 | 0 | 0 | 0 |
|
| ||||||
| Pneumonitis | 2 (5%) | 2 (5%) | 0 | 1 (3%) | 1 (3%) | 0 |
|
| ||||||
| Vomiting | 2 (5%) | 0 | 0 | 2 (5%) | 1 (3%) | 0 |
|
| ||||||
| Acute kidney injury | 1 (3%) | 1 (3%) | 0 | 0 | 0 | 0 |
|
| ||||||
| Increased alanine aminotransferase | 1 (3%) | 0 | 0 | 0 | 1 (3%) | 0 |
|
| ||||||
| Increased aspartate aminotransferase | 1 (3%) | 0 | 0 | 0 | 1 (3%) | 0 |
|
| ||||||
| Increased blood creatinine | 1 (3%) | 1 (3%) | 0 | 3 (8%) | 0 | 0 |
|
| ||||||
| Pancreatitis | 1 (3%) | 1 (3%) | 0 | 0 | 0 | 0 |
|
| ||||||
| Reduced lymphocyte count | 1 (3%) | 1 (3%) | 0 | 1 (3%) | 0 | 0 |
|
| ||||||
| Adrenal insufficiency | 0 | 1 (3%) | 0 | 3 (8%) | 2 (5%) | 0 |
|
| ||||||
| Cataract | 0 | 0 | 0 | 0 | 1 (3%) | 0 |
|
| ||||||
| Colitis | 0 | 1 (3%) | 0 | 1 (3%) | 2 (5%) | 0 |
|
| ||||||
| Dehydration | 0 | 0 | 0 | 0 | 1 (3%) | 0 |
|
| ||||||
| Diabetes | 0 | 0 | 0 | 0 | 0 | 1 (3%) |
|
| ||||||
| Encephalopathy | 0 | 0 | 0 | 0 | 1 (3%) | 0 |
|
| ||||||
| Generalised rash | 0 | 0 | 0 | 4 (10%) | 0 | 0 |
|
| ||||||
| Hypokalaemia | 0 | 1 (3%) | 0 | 1 (3%) | 0 | 0 |
|
| ||||||
| Hyponatraemia | 0 | 1 (3%) | 0 | 1 (3%) | 0 | 0 |
|
| ||||||
| Hyperthyroidism | 0 | 0 | 0 | 4 (10%) | 0 | 0 |
|
| ||||||
| Increased transaminases | 0 | 0 | 0 | 0 | 0 | 1 (3%) |
|
| ||||||
| Oesophagitis | 0 | 0 | 0 | 0 | 1 (3%) | 0 |
|
| ||||||
| Pneumonia | 0 | 0 | 0 | 0 | 1 (3%) | 0 |
|
| ||||||
| Pulmonary embolism | 0 | 1 (3%) | 0 | 0 | 0 | 0 |
|
| ||||||
| Radiation necrosis | 0 | 1 (3%) | 0 | 0 | 0 | 0 |
|
| ||||||
| Respiratory distress | 0 | 0 | 0 | 0 | 1 (3%) | 0 |
Data are n (%). Grade 1–2 treatment-related events in 10% or more of patients in either treatment cohort and all grade 3 and 4 events are reported. There were no treatment-related deaths at the time of analysis. Events were reported between the first dose date and 100 days after the last dose of nivolumab or ipilimumab (whichever was the latest). The causal relation (related or not related) between study drugs and adverse events was determined by the investigators. Some patients had more than one adverse event.
Table 3.
Treatment-related select adverse events and treatment-related adverse events leading to discontinuation of both study drugs
| Nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks (n=38) | Nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks (n=39) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |
| Select adverse events | ||||||
|
| ||||||
| Endocrine | 3 (8%) | 1 (3%) | 0 | 6 (15%) | 2 (5%) | 0 |
| Adrenal insufficiency | 0 | 1 (3%) | 0 | 3 (8%) | 2 (5%) | 0 |
| Hyperthyroidism | 0 | 0 | 0 | 4 (10%) | 0 | 0 |
| Gastrointestinal | 7 (18%) | 2 (5%) | 0 | 7 (18%) | 2 (5%) | 0 |
| Diarrhoea | 7 (18%) | 1 (3%) | 0 | 8 (21%) | 0 | 0 |
| Colitis | 0 | 1 (3%) | 0 | 1 (3%) | 2 (5%) | 0 |
| Hepatic | 1 (3%) | 0 | 0 | 0 | 1 (3%) | 1 (3%) |
| Increased alanine aminotransferase | 1 (3%) | 0 | 0 | 0 | 1 (3%) | 0 |
| Increased aspartate aminotransferase | 1 (3%) | 0 | 0 | 0 | 1 (3%) | 0 |
| Increased transaminases | 0 | 0 | 0 | 0 | 0 | 1 (3%) |
| Pulmonary | 2 (5%) | 2 (5%) | 0 | 1 (3%) | 1 (3%) | 0 |
| Pneumonitis | 2 (5%) | 2 (5%) | 0 | 1 (3%) | 1 (3%) | 0 |
| Renal | 1 (3%) | 2 (5%) | 0 | 3 (8%) | 0 | 0 |
| Acute kidney injury | 1 (3%) | 1 (3%) | 0 | 0 | 0 | 0 |
| Increased blood creatinine | 1 (3%) | 1 (3%) | 0 | 3 (8%) | 0 | 0 |
| Skin | 14 (37%) | 1 (3%) | 0 | 12 (31%) | 2 (5%) | 0 |
| Pruritus | 9 (24%) | 0 | 0 | 5 (13%) | 0 | 0 |
| Maculopapular rash | 5 (13%) | 0 | 0 | 3 (8%) | 1 (3%) | 0 |
| Rash | 5 (13%) | 1 (3%) | 0 | 3 (8%) | 1 (3%) | 0 |
| Generalised rash | 0 | 0 | 0 | 4 (10%) | 0 | 0 |
|
| ||||||
| Adverse events leading to discontinuation of both study drugs | ||||||
|
| ||||||
| Any event | 2 (5%) | 2 (5%) | 0 | 2 (5%) | 2 (5%) | 1 (3%) |
| Pneumonitis | 1 (3%) | 1 (3%) | 0 | 1 (3%) | 1 (3%) | 0 |
| Colitis | 0 | 1 (3%) | 0 | 0 | 0 | 0 |
| Infusion-related reaction | 1 (3%) | 0 | 0 | 0 | 0 | 0 |
| Facial nerve disorder | 0 | 0 | 0 | 1 (3%) | 0 | 0 |
| Oesophagitis | 0 | 0 | 0 | 0 | 1 (3%) | 0 |
| Increased transaminases | 0 | 0 | 0 | 0 | 0 | 1 (3%) |
Data are n (%). Select adverse events are those with a potential immunological cause. Grade 1–2 treatment-related select adverse events in 10% or more of patients in either treatment cohort and all grade 3–4 events are reported; all treatment-related adverse events leading to discontinuation are reported. There were no treatment-related deaths at the time of analysis. Events were reported between the first dose date and 100 days after the last dose of nivolumab or ipilimumab (whichever was the latest). The causal relation (related or not related) between study drugs and adverse events was determined by the investigators. Some patients had more than one adverse event.
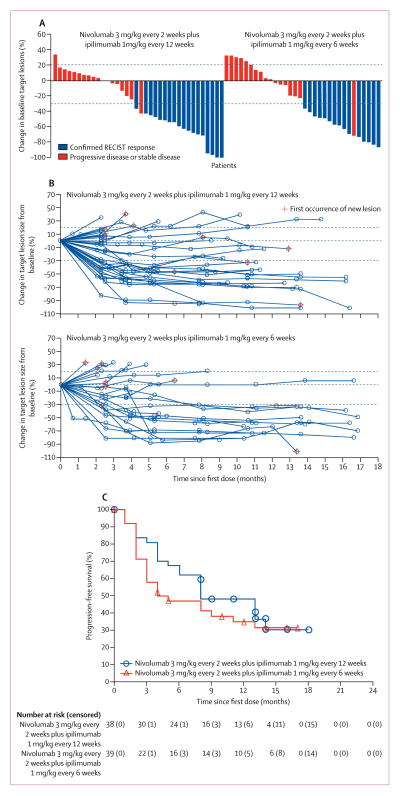
Confirmed objective responses were achieved in 18 (47%) patients in the every-12-weeks cohort and 15 (38%) in the every-6-weeks cohort, all of which were partial responses (table 4, figure 2). Two partial responses (one in each cohort) were subsequently found to be complete pathological responses upon excisional biopsy of residual lesions after study treatment. 14 (78%) of 18 responders in the ipilimumab every-12-weeks cohort and 12 (80%) of 15 responders in the ipilimumab every-6-weeks cohort had a response by the first scan (week 11; figures 2A, B). 13 (72%) responses in the ipilimumab every-12-weeks cohort and 12 (80%) responses in the ipilimumab every-6-weeks cohort were ongoing as of the last tumour assessment before censoring (appendix p 4). Median duration of response had not been reached in either cohort (table 4).
Table 4.
Clinical activity
| Nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks (n=38) | Nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks (n=39) | |
|---|---|---|
| Confirmed objective response* | 18 (47% [31–64]) | 15 (38% [23–55]) |
|
| ||
| Confirmed disease control† | 30 (79% [63–90]) | 22 (56% [40–72]) |
| Best overall response‡ | ||
| Complete response | 0 | 0 |
| Partial response | 18 (47%) | 15 (38%) |
| Stable disease | 12 (32%) | 7 (18%) |
| Stable disease for ≥6 months | 6 (16%) | 3 (8%) |
| Progressive disease | 5 (13%) | 11 (28%) |
| Unable to determine | 3 (8%) | 6 (15%) |
|
| ||
| Ongoing responses§ | 13 (72%) | 12 (80%) |
|
| ||
| Median duration of response (months)¶ | NR (11·3–NR) | NR (8·4–NR) |
|
| ||
| Median progression-free survival (months) | 8·1 (5·6–13·6) | 3·9 (2·6–13·2) |
|
| ||
| Progression-free survival at 24 weeks | 68% (50–80) | 47% (31–62) |
|
| ||
| 1 year overall survival | NC | 69% (52–81) |
Data are n (% [95% CI]), % (95% CI), median (95% CI), or n (%), unless otherwise stated. NR=not reached (due to insufficient number of events, insufficient follow-up, or both). NC=not calculated (when more than 25% of patients were censored).
Includes patients with initial observations of complete response and partial response that were subsequently confirmed by repeat scans done no earlier than 4 weeks after the original observation.
Includes patients with initial observations of complete response and partial response that were subsequently confirmed by repeat scans done no earlier than 4 weeks after the original observation, and patients with best overall response of stable disease.
Tumour assessments up to initial disease progression or initiation of subsequent anticancer therapy, whichever occurred first, were considered for best overall response assessment.
Includes patients with confirmed complete response or partial response who neither progressed nor died within 100 days of the final dose of nivolumab or ipilimumab, whichever was latest.
Time from first response to documented progression or death within 100 days of final nivolumab or ipilimumab dose (whichever was latest), or last tumour assessment before subsequent therapy. Estimated median duration of response values were determined from Kaplan-Meier curves.
Figure 2. Characteristics of response and progression-free survival.
(A) Best percentage change in target lesion tumour burden from baseline. Maximum percentage reduction in target lesion tumour burden until disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 progression. Positive change in tumour burden indicates tumour growth; negative change in tumour burden indicates tumour reduction. Horizontal lines denote 30% decrease and 20% increase indicating objective response and progressive disease, respectively, as per RECIST version 1.1. Not all reductions of 30% or greater from baseline were partial responses. (B) Percentage change in target lesion tumour burden from baseline over time. Horizontal lines denote 30% decrease, 20% increase, and no change. For both (A) and (B), only patients with baseline target lesion assessment and one or more post-baseline target lesion assessments were included (nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks, n=36; nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks, n=33). (C) Kaplan–Meier curves of progression-free survival. Symbols denote censored observations. All data in this figure were based on a Feb 18, 2016, database lock.
Progression-free survival at 24 weeks was 68% (95% CI 50–80) in patients receiving ipilimumab every 12 weeks and 47% (31–62) in those receiving ipilimumab every 6 weeks (table 4). Median progression-free survival was longer in the ipilimumab every-12-weeks cohort than in the every-6-weeks cohort (table 4, figure 2C). 23 (61%) patients in the ipilimumab every-12-weeks cohort and 25 (64%) patients in the every-6-weeks cohort had progressed or died. Overall survival at 1 year was 69% (95% CI 52–81) in the every-6-weeks cohort (table 4). Overall survival at 1 year in the ipilimumab every-12-weeks cohort and median overall survival in both cohorts have not been reported due to a large proportion of patients (27 [71%] in the every-12-weeks cohort and 24 [62%] in the every-6-weeks cohort) having been censored at the time of analysis.
In patients with 1% or greater tumour PD-L1 expression (21 [68%] of 31 patients in the every-6-weeks cohort and 23 [77%] of 30 in the every-12-weeks cohort), objective responses were achieved in 12 patients in the ipilimumab every-12-weeks cohort and 13 patients in the ipilimumab every-6-weeks cohort (57% in both cohorts; appendix p 10). Median progression-free survival in these patients was 8·1 months (95% CI 5·6-not reached) in the ipilimumab every-12-weeks cohort (n=21) and 10·6 months (3·6-not reached) in the ipilimumab every-6-weeks cohort (n=23), and 24-week progression-free survival was 80% (55–92) and 65% (42–81), respectively. The magnitude of clinical benefit achieved with the combination treatment was enhanced with higher PD-L1 expression. Pooling the two cohorts, of the 13 patients with 50% or greater PD-L1 expression (who comprised 21% of the 61 PD-L1-evaluable patients), 12 had a confirmed objective response and one had an unconfirmed response.
Three (30%) of ten patients with less than 1% PD-L1 expression in the ipilimumab every-12-weeks cohort and none of seven with less than 1% PD-L1 expression in the every-6-weeks cohort had an objective response (appendix p 10). Notably, one patient in the ipilimumab every-6-weeks cohort who had a confirmed partial response and was ultimately found to have a pathological complete response was determined after database lock to have tumour PD-L1 expression less than 1% (otherwise noted in this analysis to be PD-L1 unknown).
Confirmed responses were recorded in both smokers and never-smokers across both cohorts, although the overall proportion of patients achieving an objective response was higher in current and former smokers than in never-smokers (46% [30 of 65] vs 27% [three of 11]). In patients with EGFR-mutant NSCLC, four (50%) of eight had an objective response; appendix p 5). Of these eight patients with EGFR-mutant NSCLC, tumour PD-L1 expression levels were 1% or more in seven and 50% or more in three; clinical activity by PD-L1 expression is shown on appendix p 11.
Discussion
Results from this phase 1 trial show that first-line nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks or every 6 weeks is tolerable, with a notable improvement in safety compared with previous schedules.22 This treatment schedule also showed encouraging clinical activity, which was particularly enhanced in patients with tumour PD-L1 expression of 1% or higher.
With median follow-up of 12·8 and 11·8 months, the frequency of grade 3–4 treatment-related adverse events was similar in the two cohorts, and the rate of discontinuation because of treatment-related adverse events was similar to that reported with nivolumab monotherapy (with median follow-up of 14·3 months).18 No treatment-related deaths had occurred at the time of analysis. Although select adverse events occurred at higher rates compared with monotherapy (eg, for skin, 39% in the ipilimumab every-12-weeks cohort and 36% in the ipilimumab every-6-weeks cohort vs 25% with monotherapy), adverse events were consistent with expected events for either nivolumab or ipilimumab monotherapy, suggesting that no novel toxicities occur when therapies are combined. No significant increases in pulmonary, gastrointestinal, or renal toxicities or discontinuation due to treatment-related adverse events were noted when ipilimumab was given every 6 weeks compared with every 12 weeks.
The proportion of patients achieving a response with combination nivolumab plus ipilimumab in unselected advanced NSCLC is at least similar to, if not improved, relative to that achieved with standard platinum-based chemotherapy (about 30%), with an improved safety profile and durable responses. Furthermore, nivolumab plus ipilimumab had increased clinical activity in patients whose tumours expressed PD-L1, with the proportion of patients achieving a response rising to more than 90% in patients with 50% or more tumour PD-L1 expression. One patient in each cohort (including one who was negative for PD-L1 expression on the tumour cell membrane) had radiographic partial responses that were subsequently documented as pathological complete responses.
A limitation of this study was that it was not designed or powered to directly compare safety and efficacy between the treatment schedules examined, resulting in small sample sizes and lack of stratification by relevant baseline characteristics. Additionally, some baseline characteristics (eg, KRAS mutation and history of CNS metastasis) were not routinely collected and therefore could not be examined as subsets. There were moderate differences in clinical activity between the ipilimumab every-6-weeks and every-12-weeks cohorts, which might be related in part to imbalances in baseline characteristics or might change with longer follow-up time or both. There were fewer current or former smokers in the ipilimumab every-6-weeks cohort than in the every-12-weeks cohort, and smoking has been linked to high mutational load and higher response to immunotherapy.13,24,25 However, even with these imbalances, the proportion of patients achieving an objective response in the two cohorts was similar at tumour PD-L1 expression 1% or greater and median progression-free survival was not compromised in either cohort. Additionally, disease progression in the ipilimumab every-6-weeks cohort occurred early, with 44% of patients with disease progression experiencing progression or dying before their first imaging assessment (compared with 18% in the ipilimumab every-12-weeks cohort). Since there was no signal of increased toxicity in the every-6-weeks cohort, the rapid progression in nearly half of the patients with progression in this cohort (and the resulting shorter median duration of response and median progression-free survival compared with the every-12-weeks cohort) is likely to be indicative of incidental imbalances in the baseline populations in the context of small sample sizes, rather than suggesting intrinsic differences in clinical activity between ipilimumab given every 6 weeks or every 12 weeks. Based on the tolerability and promising clinical activity observed in both cohorts, an initial hypothesis was that more frequent dosing of ipilimumab might be important for maintaining long-term response. After integrating observations from other tumour types in which greater ipilimumab exposure was associated with improved activity,26,27 the nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks regimen was chosen for further development in NSCLC.
Different doses and schedules of nivolumab plus ipilimumab have been chosen for further development in other tumour types. For patients with melanoma, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four cycles followed by nivolumab 240 mg every 2 weeks is the approved regimen, based on the foundational single-agent activity of ipilimumab given at 3 mg/kg for four doses and the results of phase 1–3 studies.15,16,26 This regimen has been chosen for development in extensive-stage small-cell lung cancer, based on the results of a phase 1/2 study;27 however, as noted in the appendix, this regimen was not tolerable and had suboptimal activity in NSCLC. Meanwhile, for patients with renal cell carcinoma, the regimen of nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four cycles followed by nivolumab 3 mg/kg every 2 weeks seemed to be optimal.28 The explanations for tumour type-specific differences in tolerability and activity based on the dose and schedule of nivolumab and ipilimumab are uncertain and require further examination. Nevertheless, the approach for determining the optimal regimen for patients with each tumour type has been to use data obtained with each type of cancer, along with a synthesis of the experience across tumour types.
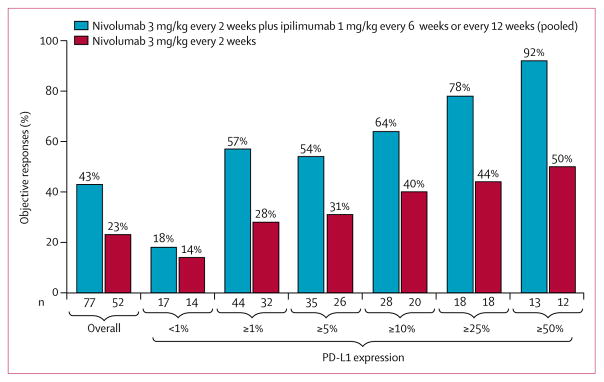
Another limitation of this study was that only indirect comparisons were possible between the nivolumab plus ipilimumab cohorts reported here and the nivolumab monotherapy or nivolumab plus chemotherapy combination cohorts of this study that have been reported previously, since these treatments were not randomised.18,19 However, clinical activity with nivolumab plus ipilimumab might be enhanced relative to what has been previously achieved with nivolumab monotherapy, especially in patients with tumour PD-L1 expression of at least 1%. In patients with increasing levels of PD-L1 expression, more of these patients achieved an objective response with nivolumab plus ipilimumab than in the cohort of CheckMate 012 assessing first-line nivolumab monotherapy (PD-L1 expression ≥1%: 57% vs 28%; PD-L1 expression ≥50%: 92% vs 50%;18 figure 3). Similarly, in patients with PD-L1 levels of 1% or higher, median progression-free survival was prolonged with the combination compared with nivolumab monotherapy (ipilimumab every-12-weeks cohort: 8·1 months [95% CI 5·6–not reached]; ipilimumab every-6-weeks cohort: 10·6 months [3·6–not reached]; monotherapy cohort, 3·5 months [<0·1+ to 28·0+]18). In light of recently reported positive results from KEYNOTE-024,29 a phase 3 study of pembrolizumab versus chemotherapy in patients with tumour PD-L1 expressed on 50% or more tumour cells, randomised studies would be needed to examine whether the addition of ipilimumab to nivolumab indeed expands benefit compared with anti-PD-1 monotherapy in this subgroup, as well as across the range of levels of PD-L1 expression.
Figure 3. Objective responses across tumour PD-L1 expression levels.
Combination data based on a Feb 18, 2016, database lock; monotherapy data based on a March 17, 2015, database lock. This trial was not randomised across combination and monotherapy cohorts.
An urgent clinical challenge in NSCLC is the identification of optimal combination strategies in the first-line setting to expand the breadth of patients who can benefit. Although nivolumab plus ipilimumab seems to improve responses in several specific subgroups in the current study, activity in patients negative for PD-L1 remains unclear. The proportion of patients with less than 1% tumour PD-L1 expression responding to nivolumab plus ipilumumab was similar to that seen with nivolumab monotherapy (about 18%, including one pathological complete response; figure 3) but another study combining the anti-CTLA-4 antibody tremelimumab with the anti-PD-L1 antibody durvalumab reported a proportion of response of 40% (four of ten patients)30—although notably the sample sizes in both studies were small. Immunotherapy plus chemotherapy might provide an additional approach to improving the proportions of patients achieving responses, although the potential for achieving durable responses remains uncertain in initial studies.19,31,32
In conclusion, the combination of nivolumab plus ipilimumab is tolerable with promising clinical activity, including high response rates in patients with PD-L1-positive tumours and the potential for deep and durable responses. These findings represent the first evidence, to our knowledge, of improved benefit through immunotherapy combinations in the first-line treatment of NSCLC. Several phase 3 studies are ongoing to assess dual checkpoint inhibitor blockade or immunotherapy plus chemotherapy (eg, NCT02453282, NCT02367781, NCT02578680, and NCT02477826 [CheckMate 227—a prospective phase 3 study of nivolumab, nivolumab plus ipilimumab, or nivolumab plus chemotherapy versus chemotherapy for the first-line treatment of patients with advanced NSCLC]). These efforts collectively aim for an improved first-line strategy (or strategies) for patients with advanced NSCLC.
Acknowledgments
We thank the patients and their families, as well as the investigators and study teams, for making this trial possible; ONO Pharmaceutical Company Limited, Osaka, Japan; the staff at Dako, North America for collaborative development of the PD-L1 IHC 28-8 pharmDx assay; Suresh Alaparthy, George Klinger, and Kyle Brown for serving as protocol managers; Allen Chen and Faith Nathan for serving as medical monitors; and Tina Chatterjee for statistical programming. This study was funded by Bristol-Myers Squibb. Medical writing and editorial assistance were provided by Zenab Amin, Wendy Sacks, and Anne Cooper of StemScientific, funded by Bristol-Myers Squibb.
Footnotes
See Online for appendix
Contributors
MDH, NAR, WJG, SA, XL, and SJA designed the study. MDH, NAR, JWG, SNG, HB, JRB, NER, DEG, LQC, WJG, SA, and SJA collected the data. MDH and TCY designed the figures. All authors analysed and interpreted the data, contributed to drafting the report, and provided final approval to submit for publication.
Declaration of interests
MDH has received grants from Bristol-Myers Squibb during the conduct of the study, and personal fees from Cowen, Bristol-Myers Squibb, Genentech, AstraZeneca, Iniovo, Merck, and Neon outside the submitted work. NAR has served in a leadership role and owned stock in Gritstone Oncology; has received personal fees from Merck Sharp & Dohme, Amgen, Roche, AstraZeneca, Bristol-Myers Squibb, and Novartis; and has received research funding from AstraZeneca outside the submitted work. JWG has received research funds from Bristol-Myers Squibb during the conduct of study. SNG has received grants from Bristol-Myers Squibb during the conduct of the study; personal fees from Janssen Pharmaceuticals, Bristol-Myers Squibb, and ARIAD; and research funds from Genentech, ARIAD, AstraZeneca, Boehringer Ingelheim, Incyte, and Pfizer outside the submitted work. HB has received honoraria from Bristol-Myers Squibb and Celgene; personal fees from Bristol-Myers Squibb, Lilly, Clovis, Genentech, and Pfizer; and non-financial support from Bristol-Myers Squibb, Lilly, Genentech, and Clovis outside the submitted work. JRB has served as a consultant for Celgene, Lilly, Merck, and Bristol-Myers Squibb (uncompensated), and received research funds from Bristol-Myers Squibb, MedImmune/AstraZeneca, and Merck outside the submitted work. NER has received honoraria from Bristol-Myers Squibb as an adviser and speaker, Celgene, and Heat Biologics, and personal fees from Celgene outside the submitted work. DEG has owned stock in Gilead Sciences; received research funds from ImmunoGen, ArQule, Synta Pharmaceuticals, Genentech, Celgene, ImClone Systems, and BerGenBio; received personal fees from Oxford University and Clinical Decision Support–Oncology; and received travel accommodations from Eli Lilly and ArQule outside the submitted work. LQC received an institutional study grant and personal consulting fees from Bristol-Myers Squibb during the course of the study; and personal consulting and advisory fees from Amgen, Emergent BioSolutions, Sanofi Genzyme, and Seattle Genetics; institutional study grants and personal consulting and advisory fees from Novartis, Bristol-Myers Squibb, Merck, and Pfizer; and institutional grants from Eli Lilly/Imclone, OSI Pharma, Genentech, VentiRx, Incyte, AstraZeneca/MedImmune, and NCCN/GlaxoSmithKline outside the submitted work. RAJ has received honoraria from Bristol-Myers Squibb, Boehringer Ingelheim, AstraZeneca, Roche Canada, and Merck Sharp & Dohme; served as a consultant for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, and Roche Canada; and received research funds from AstraZeneca/MedImmune, Bristol-Myers Squibb, Merck Sharp & Dohme, and Novartis outside the submitted work. FAS served as a consultant with Bristol-Myers Squibb during the conduct of the study; owned stock in Eli Lilly and AstraZeneca; received honoraria from Eli Lilly, AstraZeneca, Bristol-Myers Squibb, Merck Serono, Genentech, Merck/Schering Plough, and Boehringer Ingelheim; served as a consultant for Eli Lilly, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Recombio, Synta Pharmaceuticals, and Bristol-Myers Squibb; and received research funds from Boehringer Ingelheim outside the submitted work. SAL has received honoraria from Pfizer, Bayer AG, Novartis, and Boehringer Ingelheim outside the submitted work. WJG, SA, TCY, and XL were employed by and owned stock in Bristol-Myers Squibb during the conduct of study. SJA has owned stock in Cellular Biomedicine Group; received honoraria from AstraZeneca, Bristol-Myers Squibb, Merck, and Boehringer Ingelheim; served as a consultant for Bristol-Myers Squibb, AstraZeneca, Merck, and Boehringer Ingelheim; and received non-financial support from Bristol-Myers Squibb, AstraZeneca, Merck, and Boehringer Ingelheim outside the submitted work.
References
- 1.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 2.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 3.Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:4349–57. doi: 10.1200/JCO.2012.47.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–34. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 6.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Reckamp K, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–19. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–12. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–18. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 15.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–68. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gettinger SN, Rizvi N, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small cell lung cancer. J Clin Oncol. 2016;34:2980–87. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34:2969–79. doi: 10.1200/JCO.2016.66.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Phillips T, Simmons P, Inzunza HD, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015;23:541–49. doi: 10.1097/PAI.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonia SJ, Gettinger S, Goldman JW, et al. Safety and efficacy of first-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 2014;90(suppl):S32–3. [Google Scholar]
- 23.Rizvi NA, Gettinger SN, Goldman JW, et al. Safety and efficacy of first-line nivolumab (NIVO; anti-programmed death-1 [PD-1]) and ipilimumab in non-small cell lung cancer (NSCLC) J Thorac Oncol. 2015;10:S176. [Google Scholar]
- 24.Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–34. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in nonsmall cell lung cancer. Science. 2015;348:124–28. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–95. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 28.Hammers H, Plimack ER, Infante JR, et al. Expanded cohort results from CheckMate 016: a phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2015;33(suppl) doi: 10.1200/JCO.2016.72.1985. abstr 4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016 doi: 10.1056/NEJMoa1606774. published online Oct 8 http://www.nejm.org/doi/full/10.1056/NEJMoa1606774. [DOI] [PubMed]
- 30.Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gadgeel S, Stevenson J, Langer C, et al. Pembrolizumab plus chemotherapy as front-line therapy for advanced NSCLC: KEYNOTE-021 cohorts A-C. J Clin Oncol. 2016;34(suppl) abstr 9016. [Google Scholar]
- 32.Liu SV, Powderly JD, Camidge DR, et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with platinum-based doublet chemotherapy in patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2015;33(suppl) abstr 8030. [Google Scholar]