Abstract
Object
Endoscopic third ventriculostomy (ETV) with choroid plexus cauterization (CPC) represents a viable treatment option for congenital hydrocephalus in infants less than 2 years of age. Imaging studies complement clinical data in the evaluation of treatment success or failure. The objectives of this study were to investigate novel radiographic markers—CSF turbulence and choroid plexus (CP) visualization—and their ability to reflect or predict clinical outcomes following ETV/CPC.
Methods
Hydrocephalic patients under 2 years of age initially treated by ETV/CPC at the senior authors’ institution from March 2013 to February 2014 were retrospectively reviewed. Clinical data as well as visualization of CSF turbulence and CP on pre- and post-operative fast-sequence magnetic resonance imaging (FS-MRI) were recorded. Radiographic images were reviewed by a blinded observer based on specific criteria for visualization of CSF turbulence and CP. Data were collected and analyzed using descriptive statistics, including Fisher’s exact test for comparisons. The research team obtained appropriate Institutional Review Board (IRB) approval for this study, without the necessity for informed consent.
Results
Among 32 patients (53% male, 47% female) studied, 18/32 (56%) responded favorably to initial or repeat ETV/CPC, with 13/32 (41%) requiring one surgery. Of the 19 (59%) patients failing initial ETV/CPC, 8/19 (42%) underwent repeat ETV/CPC with 5/8 (63%) responding favorably. Radiographic CSF turbulence appeared more frequently following ETV/CPC failure than after ETV/CPC success (55% vs. 18%, p = 0.02). The sensitivity and specificity of CSF turbulence as a radiographic marker for ETV/CPC failure were 80% and 58%, respectively. Radiographic depiction of CP disappearance following ETV/CPC from pre- to post-operative imaging occurred in 20/30 patients (67%). Among patients responding unsuccessfully to ETV/CPC and ultimately requiring secondary shunt insertion, 71% (10/14) demonstrated CP persistence on post-operative imaging. In contrast, 6% (1/18) of patients treated successfully by ETV/CPC demonstrated presence of CP on follow-up imaging. This difference reached statistical significance (p = 0.0001). Visualization of CP persistence despite ETV/CPC reflected treatment failure with 91% sensitivity and 81% specificity. The sensitivity of either or both radiographic markers suggesting ETV/CPC failure was 77%, while their specificity (both markers absent indicating ETV/CPC success) was 81%.
Conclusions
Radiographic markers correlate with clinical outcomes following treatment of infantile hydrocephalus with ETV/CPC. Specifically, CSF turbulence may indicate ongoing pathologic CSF flow dynamics while CP absence following ETV/CPC may predict shunt independence.. Future studies incorporating prospective review and formal intra-/inter-observer reliability estimates may help corroborate the utility of these radiographic markers.
Keywords: CSF turbulence, choroid plexus, endoscopic third ventriculostomy (ETV), choroid plexus cauterization (CPC), radiographic markers
With the addition of choroid plexus cauterization (CPC), endoscopic third ventriculostomy (ETV) has increased in popularity in recent years as an acceptable and potentially favorable treatment modality for hydrocephalus in young children. The application of ETV combined with CPC (ETV/CPC) to neonates or infants with new onset hydrocephalus due to various etiologies represents a recent development nationally and globally. Despite potentially lower success rates in this patient population, ETV/CPC provides an alternative treatment modality for CSF diversion that avoids the risks inherent to permanent indwelling shunt hardware.
Radiographic examinations contribute significantly to the evaluation of patients with previously treated hydrocephalus. The combination of imaging and clinical data may reveal inadequate or unsuccessful treatment and the need for surgical revision. Failure of initial ETV/CPC can be managed with repeat endoscopic exploration and ETV, along with more extensive CPC as necessary. Alternatively, secondary shunt insertion may be performed, with or without preceding endoscopic re-exploration.
Various radiographic markers that correlate with ETV success or failure have been used clinically and explored in recent literature. These radiographic findings include the ventriculostomy site ‘flow void’ and third ventricular parameters, such as floor contour, width, and mid-sagittal cross-sectional area.6 These markers corroborate clinical findings to help determine if a patient has responded favorably to ETV/CPC or requires further treatment, either with repeat endoscopy or shunt implantation. This study investigates two additional imaging findings in the setting of ETV/CPC: cerebrospinal fluid (CSF) turbulence and choroid plexus (CP) visualization. The authors explored whether radiographic evidence of CSF turbulence and CP visualization correlated with clinical outcomes following ETV/CPC..
Methods
A retrospective review of magnetic resonance imaging (MRI) scans obtained previously for clinical purposes in hydrocephalic patients under 2 years of age was performed. The imaging modality mainly consisted of Shunt Function or Fast Sequence MRI (FS-MRI) incorporating single-shot T2 turbo spin echo (TSE) sequences (axial, coronal, and sagittal). Study patients underwent initial treatment for hydrocephalus via ETV/CPC at the authors’ institution from March 2013 to February 2014. Clinical records of these patients were reviewed to determine initial operative dates, clinical outcomes, and dates of repeat surgery for hydrocephalus (repeat ETV or secondary shunt insertion), when applicable. Patients with pre-operative standard MRI or FS-MRI, post-operative FS-MRI, and documented post-operative clinical follow-up evaluations were selected for this study. Because this study investigated both success and failure following neuro-endoscopic treatment, a minimum duration of clinical follow-up was not incorporated as an exclusion criteria. The research team obtained appropriate Institutional Review Board (IRB) approval for this study, without the necessity for informed consent.
Imaging sequences were selected for review based on specific criteria regarding ETV/CPC outcome and timing. FS-MRI scans were designated as reflecting ETV/CPC failure if repeat surgery was performed within 2 weeks from the imaging date. For the investigation of CSF turbulence, the latest FS-MRI scan available for review (in cases of ETV/CPC success) and the last FS-MRI examination prior to repeat surgical intervention (when applicable, and while fulfilling the above criterion) were selected. In this fashion, a study patient responding favorably to repeat neuro-endoscopic treatment may have up to 2 imaging examinations included (1 ‘failure’ FS-MRI scan preceding repeat surgery, 1 ‘success’ FS-MRI scan occurring at the latest follow-up). Similarly, a study patient undergoing repeat ETV/CPC followed by shunt insertion may offer 2 FS-MRI scans for review (1 ‘failure’ scan preceding repeat neuro-endoscopy, 1 ‘failure’ scan preceding shunt insertion). Patients managed with secondary shunt insertion following initial ETV/CPC failure would contribute 1 imaging examination for review (1 ‘failure’ scan preceding shunt insertion), as would patients responding favorably to initial ETV/CPC (1 ‘success’ scan at the latest follow-up). This mode of image selection led to data analysis for CSF turbulence recognition on a per-patient or per-scan basis, depending on ETV/CPC outcome.
For evaluation of CP visualization, the latest follow-up FS-MRI scan available for review was selected, with a minimum requirement of image acquisition at least 4 weeks from the initial ETV/CPC date. In contrast to image selection for CSF turbulence, this selection process provided 1 FS-MRI scan per study patient for review, stratified by clinical ETV/CPC outcome (ETV/CPC ‘success’ reflecting shunt independence, ETV/CPC ‘failure’ indicating eventual shunt insertion). The minimum 4-week requirement was incorporated to allow sufficient time for the disappearance or involution of CP glomus following CPC. Without this constraint, imaging in the setting of early ETV/CPC failure might create a false correlation between treatment failure and residual CP that has not yet become radiographically occult. The latest follow-up FS-MRI scan seemed the most reasonable selection for review since it provided the greatest time interval to observe the effects of CPC, it provided a consistent method of image selection, and because CP visualization should not be expected to change from ‘absent’ to ‘present’ over serial scans preceding the latest imaging study. The serial FS-MRI scans occurring prior to the last follow-up examination were reviewed to check for internal consistency with respect to CP visualization (CP ‘presence’ on the last follow-up scan should be matched by CP ‘presence’ on prior scans, while CP ‘absence’ should not be followed by CP ‘presence’ on subsequent scans).
The surgical approach remained consistent among pediatric neurosurgeons on the study team. During initial treatment of hydrocephalus, standard ETV was performed via a coronal entry site (typically right-sided) in the mid-pupillary line. This site often coincided with the lateral apex of the anterior fontanelle, obviating bone removal; however, removal of adjacent or surrounding bone was performed with rongeurs or high-speed drill as necessary. Following linear dural opening (when possible), the flexible endoscope was advanced into the lateral ventricle and through the Foramen of Monro after identifying intra-ventricular anatomy. The ventriculostomy was performed through the floor of the third ventricle, anterior to the impression of the mammillary bodies and posterior to the infundibular recess and impression of the dorsum sella. Following ETV and exploration of the pre-pontine cistern, with lysis of the membrane of Lilequist and any arachnoid adhesions, extensive CPC was performed in the ipsilateral and contralateral bodies, atria, and temporal horns of both lateral ventricles. Additional time and focus was devoted to thorough coagulation of the CP glomus bilaterally. Following thorough CPC, the ventriculostomy site was re-explored for patency. The durotomy was closed primarily (when possible), and the wound was closed in standard two-layer fashion.
For recurrent or progressive hydrocephalus, attending surgeons would evaluate the necessity for repeat treatment based on clinical (symptoms of elevated intracranial pressure [ICP], accelerated head growth, fullness or bulging of the anterior fontanel [AF], suture diastasis) and imaging information. Similarly, attending surgeons decided to perform repeat endoscopic treatment versus secondary shunting based on their discretion. Typically, the patient would be positioned to allow for a repeat endoscopic exploration first and ventricular shunt insertion as necessary. The attending surgeon additionally assessed the need for repeat or expanded CPC (typically not required) following repeat ETV.
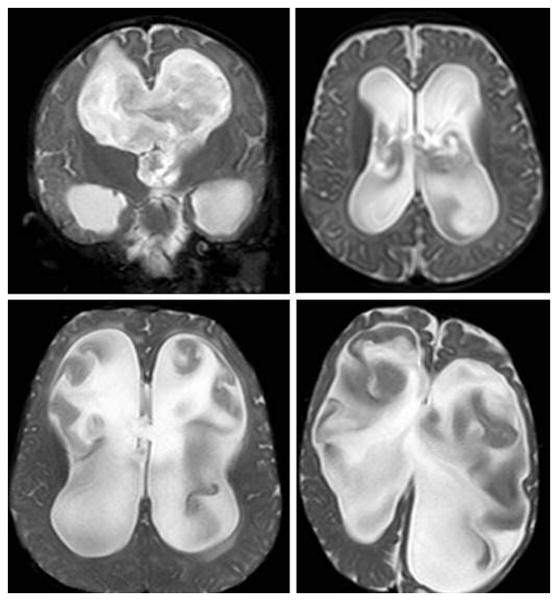
FS-MRI scans were reviewed by one blinded observer for the presence or absence of two markers: CSF turbulence and CP visualization. For this pilot study, the single observer specialized in pediatric neurosurgery at the fellowship level and represented a contributing author. Visualization of CSF turbulence entailed three requirements (Fig. 1):
Figure 1.
Examples of Radiographic Cerebrospinal Fluid (CSF) Turbulence.
These FS-MRI coronal and axial T2 slices demonstrate the presence of radiographic CSF turbulence in at least two of three supra-tentorial ventricles.
The presence of irregular, heterogenous, or hypointense signal intensity in a pattern suggesting non-linear CSF flow
The presence of this signal intensity (i) in at least two of three supra-tentorial ventricles
The distinction of this signal intensity (i) from imaging artifact due to patient movement
Similarly, radiographic evaluation of CP entailed specific designations (Fig. 2):
Figure 2.

Example of Radiographic Choroid Plexus (CP) Presence.
This FS-MRI axial T2 slice demonstrates the typical appearance of CP glomus within at least one ventricular atrium, consistent with the study’s definition of ‘CP presence.’
Clear visualization of the glomus in at least one ventricular atrium denoting ‘CP presence’
Lack of or diminutive size of the glomus in both ventricular atria denoting ‘CP absence’
These radiographic criteria were established prior to imaging review.
Qualitative determinants of CSF turbulence and CP visualization were used based on the capabilities and limitations of FS-MRI, the preferred imaging study for neonates and infants requiring radiographic evaluation following treatment for CSF diversion at the senior author’s (C.J.R.) institution. Subjective interpretation of the lack of clearly visualized CP glomus, or only scant CP glomus remaining within the ventricular atria, served as surrogate markers for CP absence. The observer was blinded to both patient outcome and chronologic order of the MRI scans (ie at which point in the patient’s clinical course the radiographic examination occurred). Primary outcome variables included ETV/CPC clinical outcome and radiographic visualization of CSF turbulence and CP.
Data were collected and analyzed using descriptive statistics, including Fisher’s exact test for comparisons. The sensitivity and specificity of CSF turbulence and CP visualization as radiographic markers for ETV/CPC failure were calculated as well. . Serial imaging examinations evaluated for CP visualization were reviewed for internal consistency and as a surrogate method for testing intra-observer reliability. Specifically, the number of imaging examinations following any post-operative FS-MRI scan with CP ‘absence’ was tabulated for all patients. Out of these scans, the number of imaging studies demonstrating an inconsistent result (CP ‘presence’ following a previous designation of CP ‘absence’) was identified. The number of inconsistent results were compared to the total number of scans from above, to estimate a crude measure of internal consistency.
Results
This study incorporated 32 patients (53% male, 47% female) with mean age at first ETV/CPC of 17.1 weeks (standard deviation [SD] = 20.0 weeks; range = [0.4 weeks, 96.9 weeks]) (representing actual age since birth). Etiologies of hydrocephalus included prematurity with intra-ventricular hemorrhage (IVH) (47%), myelomeningocele (MMC) (19%), congenital aqueductal stenosis (19%), and other causes (compressive arachnoid cyst, obstructive hydrocephalus due to tumor, meningitis, and congenital communicating hydrocephalus) (16%) (Table 1). With a mean follow-up of 10.6 months (SD = 4.3 months; range = [1.0 months, 21.0 months]), approximately 56% (18/32) of patients responded favorably to ETV/CPC (initial or repeat surgery), remaining shunt independent (Fig. 3). Patients with MMC (5/6, 83%) and congenital aqueductal stenosis (5/6, 83%) exhibited the highest ETV/CPC success rates, while premature neonates with IVH (6/15, 40%) demonstrated the lowest ETV/CPC success rate (Fig. 3. Initial ETV/CPC carried a 41% (13/32) success rate, while 63% (5/8) of patients undergoing repeat ETV/CPC remained shunt independent (Fig. 3). Following initial ETV/CPC failure, 58% (11/19) of patients underwent secondary shunt insertion (Fig. 3). Repeat surgical intervention occurred at a mean duration of 7.8 weeks (range: [1.1 weeks, 26.6 weeks]) following initial ETV/CPC failure and 2.2 weeks (range: [2.0 weeks, 2.4 weeks]) following unsuccessful repeat neuro-endoscopy. CSF turbulence appeared in 55% (12/22) of imaging examinations performed in study patients experiencing ETV/CPC failure, while appearing in 18% (3/17) of FS-MRI scans from study patients responding favorably to ETV/CPC (Table 2, Fig. 4–5). This difference reached statistical significance (p = 0.02, Fisher’s exact test). The sensitivity of CSF turbulence as a radiographic marker for ETV/CPC failure was 80%, while the specificity was 58% (Table 2). Radiographic suggestion of CSF turbulence appeared in 1 study patient’s pre-treatment MRI (1/32, 3%). The mean duration of time between initial ETV/CPC and imaging prior to repeat surgery was 7.5 weeks (range: [0.6 weeks, 26.4 weeks]), while the average time interval between repeat neuro-endoscopy and subsequent imaging prior to VP shunt insertion (only occurring in 3 patients) was 1.9 weeks (range: [1.9 weeks, 2.0 weeks]). Latest follow-up imaging in cases of ETV/CPC success (with initial or repeat surgery) occurred on average 30.0 weeks (range: [4.9 weeks, 62.7 weeks]) following the most recent neuro-endoscopic procedure.
Table 1.
Study Population Gender and Etiology of Hydrocephalus
| Gender or Hydrocephalus Etiology | Proportion | Percentage |
|---|---|---|
| Male | 17/32 | 53% |
| Female | 15/32 | 47% |
| Prematurity, Intra-Ventricular Hemorrhage (IVH) | 15/32 | 47% |
| Myelomeningocele (MMC) | 6/32 | 19% |
| Congenital Aqueductal Stenosis | 6/32 | 19% |
| Other (Arachnoid Cyst, Tumor, Meningitis) | 5/32 | 16% |
Abbreviations: CPC = choroid plexus cauterization; ETV = endoscopic third ventriculostomy.
Figure 3.
Flow Diagram for Study Patient Cohort.
This flow diagram demonstrates the total study patient cohort stratified by endoscopic third ventriculostomy/choroid plexus cauterization (ETV/CPC) success or failure (on initial or repeat attempt) and performance of secondary shunt insertion (when necessary).
Table 2.
CSF Turbulence as a Radiographic Marker for Clinical Outcomes following ETV/CPC
| Study Cohort | ETV/CPC Success | ETV/CPC Failure | p-value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| CSF Turbulence Present | 3/17 (18%) | 12/22 (55%) | 0.02 | 12/15 (80%) | -- |
| CSF Turbulence Absent | 14/17 (82%) | 10/22 (45%) | -- | -- | 14/24 (58%) |
Abbreviations: CPC = choroid plexus cauterization; CSF = cerebrospinal fluid; ETV = endoscopic third ventriculostomy
Figure 4.
Presence of Radiographic Cerebrospinal Fluid (CSF) Turbulence in the Setting of Treatment Failure.
This FS-MRI axial T2 slice (A) acquired during a period of initial success following endoscopic third ventriculostomy/choroid plexus cauterization (ETV/CPC) lacks any radiographic signs of CSF turbulence. However, follow-up imaging over 5 months later (same study patient) in the setting of delayed ETV/CPC failure (B) demonstrates findings consistent with CSF turbulence.
Figure 5.
Radiographic Investigation of Cerebrospinal Fluid (CSF) Turbulence in the Setting of Treatment Failure and Success.
These FS-MRI axial T2 slices demonstrate the presence of CSF turbulence in the setting of ETV/CPC failure (A), with the resolution of CSF turbulence following clinically successful treatment by ventriculoperitoneal shunting (B) in the same study patient.
Radiographic depiction of adequate CPC (CP ‘presence’ changing to CP ‘absence’) occurred after 67% (20/30) of neuro-endoscopic procedures (incorporating those with the appropriate pre- and post-operative imaging studies) (Fig. 6). Residual CP appeared in 71% (10/14) of latest follow-up FS-MRI scans in patients failing ETV/CPC and ultimately requiring shunt insertion (Table 3). In contrast, CP was present in 6% (1/18) of latest follow-up FS-MRI scans in patients responding favorably to ETV/CPC and remaining shunt independent. This difference reached statistical significance (p = 0.0001, Fisher’s exact test) (Table 3). The sensitivity of CP visualization as a radiographic marker for ETV/CPC failure reached 91%, while the specificity was 81% (Table 3). The mean duration of time between initial ETV/CPC and latest follow-up imaging for CP visualization evaluation was 23.0 weeks (range: [4.0 weeks, 62.7 weeks]).
Figure 6.
Radiographic Depiction of Choroid Plexus (CP) Before and After Endoscopic Third Ventriculostomy and Choroid Plexus Cauterization (ETV/CPC).
These FS-MRI axial T2 slices reflect CP ‘presence’ before (A, C) and CP ‘absence’ (based on study definitions) after (B, D) clinically successful ETV/CPC in two respective study patients. The duration of time between serial imaging scans was approximately 12 weeks (A, B) and 9 weeks (C, D), in the same study patients, respectively.
Table 3.
CP Visualization as a Radiographic Marker for Clinical Outcomes following ETV/CPC
| Study Cohort | ETV/CPC Success | ETV/CPC Failure | p-value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| CP Present | 1/18 (6%) | 10/14 (71%) | .0001 | 10/11 (91%) | -- |
| CP Absent | 17/18 (94%) | 4/14 (29%) | -- | -- | 17/21 (81%) |
Abbreviations: CP = choroid plexus; CPC = choroid plexus cauterization; CSF = cerebrospinal fluid; ETV = endoscopic third ventriculostomy
Seven study patients (22%) underwent repeat surgical intervention prior to the 4-week minimum time constraint imposed on radiographic CP evaluation. Within this subset of patients, average duration of time between initial and repeat surgery was 2.1 weeks (range: [1.1 weeks, 3.7 weeks]). Following study protocol, the latest follow-up FS-MRI scan was still used for CP evaluation in these patients, and the preceding imaging examinations were checked for internal consistency. One patient demonstrated CP ‘absence’ at 1.4 weeks following surgery and at the latest follow-up scan (4.4 weeks). Five study patients exhibited CP persistence on all post-operative scans consistently, including the early and latest follow-up imaging examinations. Within the subset of 7 patients, one demonstrated an inconsistent finding of CP ‘absence’ on the early post-operative scan (3.6 weeks from surgery) while exhibiting CP ‘presence’ on all subsequent imaging studies (including the latest follow-up scan at 9.6 weeks from initial ETV/CPC). One additional study patient (outside of this subset) demonstrated a similar inconsistent finding among their post-operative FS-MRI scans. Based on the method described above, this resulted in an internal inconsistency rate of approximately 9% (2 inconsistent scans/22 total post-operative scans following CP disappearance). This measure of 91% internal consistency may be considered as a crude but surrogate marker for intra-observer reliability with the specific type of FS-MRI evaluation described here.
Regarding visualization of the radiographic markers in combination, the presence of both CSF turbulence and CP (following the same criteria for evaluation of each marker individually) always reflected ETV/CPC failure (4/4 post-operative scans), but this combination occurred in only 4/20 (20%) post-operative ‘failure’ FS-MRI scans. The presence of either or both markers occurred in 17/20 (85%) of imaging studies from patients demonstrating ETV/CPC failure, while occurring in 5/18 (28%) of scans from patients responding favorably to ETV/CPC (Table 4). This difference reached statistical significance (p = 0.0007, Fisher’s exact test). The sensitivity and specificity of using either or both radiographic markers as an indication of ETV/CPC failure were 77% (17/22) and 81% (13/16), respectively (Table 4).
Table 4.
Combination of CSF Turbulence and CP Visualization as Radiographic Markers for Clinical Outcomes following ETV/CPC
| Study Cohort | ETV/CPC Success | ETV/CPC Failure | p-value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Either or Both Markers Present | 5/18 (28%) | 17/20 (85%) | .0007 | 17/22 (77%) | -- |
| Both Markers Absent | 13/18 (72%) | 3/20 (15%) | -- | -- | 13/16 (81%) |
Abbreviations: CP = choroid plexus; CPC = choroid plexus cauterization; CSF = cerebrospinal fluid; ETV = endoscopic third ventriculostomy
Discussion
Radiographic markers can be used to help reflect or predict the clinical outcomes of pediatric patients with hydrocephalus following ETV or combined ETV/CPC. These represent important adjuncts to evaluation and management in the neonatal population due to the occasional paucity of clinical markers of success or failure. Previously studied radiographic characteristics of ETV site patency or ventricular size include a CSF flow-void traversing the third-ventriculostomy site, computerized ventricular volume analysis, frontal-occipital horn ratio (FOR), third ventricular width, and third ventricular mid-sagittal cross-sectional area.1,2,5,6 Despite functional diversion of CSF flow when successful, ETV produces more subtle ventricular size changes than traditional shunting.6,11 This study presents two additional radiographic markers—CSF turbulence and CP visualization—that may be used to reflect clinical outcomes in hydrocephalic neonates and infants following ETV/CPC. These radiographic markers have not been reported or studied previously in children treated for hydrocephalus.
CSF Turbulence
Radiographic appearance of turbulent CSF flow represents an imaging artifact, typically best appreciated on T2-weighted MRI sequences (including the FS-MRI turbo spin echo sequences used in this study) given the relative hyperintensity of CSF (Fig. 1, 4, 5).9,10 Signal loss due to elevated CSF flow velocity or turbulence has been observed clinically and reported in both humans and dogs.7,8 For instance, Sherman and Citrin reported the frequent occurrence of CSF flow-voids or signal dropout within the cerebal aqueduct of Sylvius and 4th ventricle among MRI studies of adult patients without intra-ventricular abnormalities.8 The cerebral aqueduct represents a common location for CSF flow voids due to increased velocity of flow from a larger volume (third ventricle) through a region with comparatively smaller cross-sectional area (cerebral aqueduct).9,10 Pulsatile flow-related parameters of high velocity and turbulence produce alterations in CSF signal intensity by various mechanisms. Radiographic appearance of CSF turbulence reflects motion-induced phase changes during MR image acquisition, with explanations extending beyond the scope of this article. In simplistic terms, turbulence involves multiple randomly oriented velocity vectors of CSF flow with different signal phases, resulting in a heterogenous pattern of signal loss.7,9,10 Consequently, turbulent non-laminar flow produces CSF flow voids and signal heterogeneity.4,9
As demonstrated by the findings in this article, the radiographic appearance of CSF turbulence may reflect underlying pathologic CSF flow dynamics and/or reduced intracranial compliance, signaling potential failure of ETV/CPC in certain patients.7,10 Prior studies have reported higher incidence of CSF flow void signs and signal heterogeneity in patients with ventriculomegaly as compared to individuals with normal sized ventricles.9,10 In this study, radiographic evidence of CSF turbulence appeared in 55% of patients following ETV/CPC failure as compared to 18% of patients following successful endoscopic treatment, reaching statistical significance (p = 0.02; Table 2). A CSF flow void through an ETV site typically suggests patency and provides reassurance.9,10 In contrast, the distinct radiographic appearance of CSF turbulence as described in this study may reflect pathologic CSF flow dynamics and signal treatment failure, with modest sensitivity of 80% (Table 2).
Radiographic depiction of CSF turbulence might reflect the elevated amplitudes of intra-ventricular CSF pulsations thought to be instrumental in progressive ventriculomegaly of infants with hydrocephalus.12,13 While ETV may function as a pulsation absorber, progressive stenosis or scarring at the ventriculostomy site reduces its pulsation-absorption capacity.13 Scarring across an opened but previously undisturbed anatomic surface (third ventricular floor) might influence ventricular compliance sufficiently to produce CSF pulsation artifacts such as turbulent flow. The new appearance of CSF turbulence in a hydrocephalic infant’s FS-MRI following ETV/CPC could represent progression of inadequately treated hydrocephalus. Cauterization of the CP might also function by reducing the amplitude of intraventricular CSF pulsations, as previously described.12,13 Based on the hydrodynamic CSF pulsation model proposed previously in the literature, it follows that CSF turbulence—reflecting pathologic CSF flow dynamics—and CP persistence despite ETV/CPC might represent useful radiographic markers of treatment failure.
Choroid Plexus (CP) Visualization
Visualization of CP extends the utility of radiographic markers following ETV/CPC or upon consideration of secondary shunt insertion (Fig. 2, 6). . Among patients exhibiting clinical ETV/CPC failure, 71% (10/14) demonstrated persistence of CP on post-operative imaging, while only 6% (1/18) of patients responding favorably to ETV/CPC demonstrated CP persistence, representing a statistically significant difference (p = 0.0001, Table 3). In this study, CP presence on post-operative FS-MRI reflected clinical ETV/CPC failure and predicted ultimate secondary shunt insertion with reasonably high sensitivity (91%). Additionally, the absence of CP following ETV/CPC—suggesting radiographically adequate CPC—predicted ETV/CPC success and shunt independence with moderate specificity (81%). While demonstrating the potential utility of CP visualization as a radiographic marker, this finding also emphasizes the importance of CPC in the treatment of hydrocephalic neonates, as suggested by prior works.13,14
Of those individuals (10/30, 33%) with persistent CP following ETV/CPC, 70% (7/10) had developed hydrocephalus due to prematurity with IVH. Six (86%) of these 7 patients with prematurity, post-hemorrhagic hydrocephalus, and CP persistence despite ETV/CPC ultimately required shunt insertion. The high rate of CP persistence in the cohort of IVH patients may reflect the difficulty of achieving extensive CPC due to scarring of the CP in this population. Furthermore, the relatively low success rate (40%) of ETV/CPC and high shunting rate of neonates with prematurity/IVH in this small sample reflects the clinical experience of the study authors.
Radiographic demonstration of CP persistence or disappearance following ETV/CPC has not been reported or studied previously in the literature for comparison. However, near-complete CPC has been shown to correlate with higher rates of treatment success in hydrocephalic infants. Specifically, greater than or equal to 90% CPC (by surgeon’s intra-operative estimation) correlated with 82% success rate of ETV/CPC in infants with hydrocephalus from various etiologies in a Hydrocephalus Clinical Research Network (HCRN) retrospective study.3 Additional studies have shown that the extent of CPC (along with other independent variables including age and hydrocephalus etiology) impacts ETV/CPC success rates in Ugandan children with hydrocephalus.14,15 Therefore, radiographic markers that reflect the extent or completeness of CPC may help predict the success of ETV/CPC in hydrocephalic infants. The time-course of CP disappearance on FS-MRI following CPC could not be evaluated in this study due to the retrospective nature of data analysis and the variable timing of imaging performed after surgery. Of note, 3 study patients underwent early follow-up imaging (11 days, 13 days, and 17 days after ETV/CPC) demonstrating CP persistence with subsequent imaging at later time points (12.1 weeks, 57.0 weeks, and 50.3 weeks, respectively) showing CP disappearance without intervening surgery. Future prospective studies with imaging performed at specific time intervals may allow assessment of the time-dependent nature of CP disappearance following CPC.
CSF Turbulence and CP Visualization in Combination
Either or both radiographic markers appeared in 85% of ‘failure’ FS-MRI scans from patients undergoing repeat treatment for hydrocephalus. This represented a statistically significant difference from the presence of either or both markers in only 28% of post-operative scans in patients responding favorably to ETV/CPC (p = 0.0007, Table 4). Furthermore, the absence of both radiographic markers on post-operative imaging predicted clinical ETV/CPC success with moderate specificity (81%, Table 4). With a higher sensitivity rating (91%), CP persistence seemed to represent a stronger individual predictor of ETV/CPC failure than CSF turbulence visualization (sensitivity 80%) in this retrospective study. However, the investigation of CSF turbulence in the context of treatment failure should not require a minimum time interval (occurring as early as 1.4 weeks post-operatively for one patient within this study) as may be required for CP evaluation following CPC. Because it can be appreciated on post-operative imaging without the necessity of a minimum time interval for evaluation, radiographic CSF turbulence may reflect neuro-endoscopic treatment failure on a time-independent, per-scan basis. In contrast, CP persistence (despite CPC) may reflect ETV/CPC failure over time and ultimate necessity for shunt insertion.
Study Limitations
Despite the potential applicability of these two radiographic markers, weaknesses of this study should be recognized. The limited sample size (32) and incorporation of one blinded observer represent quantitative shortcomings. This research effort represented a pilot study, explaining the incorporation of a single, blinded observer. Future studies consisting of more study subjects and multiple observers, with inter-observer reliability estimates, could corroborate and strengthen the current findings. The second stage of this research endeavor involves the analysis of intra- and inter-observer reliability of these radiographic markers, currently underway at the senior author’s (C.J.R) institution. Nevertheless, the method described in this study provided for estimation of an internal consistency rate exceeding 90%, as described above. . Additionally, the incorporation of a consistent imaging schedule following surgery in future prospective studies would provide more uniform data and may suggest the time course of CP disappearance following ETV/CPC. A minimum duration of clinical follow-up was not used here since this retrospective study highlighted the identification of radiographic markers of ETV/CPC failure rather than prolonged success.
Uniform distinction of CSF turbulence and CP visualization represent qualitative challenges that can be mitigated by strict adherence to the requirements listed within the Methods section (Fig. 1, 2, 4–6). The limited capabilities of FS-MRI necessitated utilization of qualitative markers for CSF turbulence and CP visualization. Subjective interpretation of the absence of CP glomus or the presence of only scant residual glomus within the ventricular atria qualified as surrogate markers for CP absence. While FS-MRI represents a useful imaging modality in multiple age groups for patients requiring radiographic evaluation following CSF diversion, these imaging sequences lack adequate resolution and anatomic definition to consistently depict choroid plexus within the lateral ventricular temporal horns or bodies. Therefore, the study authors selected radiographic visualization of the CP glomus as the most reliable and consistent marker for determining adequate or inadequate CPC. This additionally reflects the intra-operative focus on thorough CP glomus cauterization during CPC at the senior author’s (C.J.R) institution. The use of CP glomus as a surrogate marker for CP to determine the adequacy of CPC represents a study limitation imposed by the imaging modality (FS-MRI) most frequently used for clinical purposes in the infantile hydrocephalic population at the senior author’s institution. However, head computed tomography would likely result in similar imaging limitations while standard MRI carries the non-trivial risks and requirements for sedation and/or anesthesia in this young population.
Importantly, the modest sensitivity and specificity levels for visualization of CSF turbulence and CP prohibit excessive or isolated reliance on these radiographic markers. These radiographic markers could be used in conjunction with other imaging modalities including cardiac-gated MRI or CSF-flow studies to help assess hydrocephalic infants following treatment, as adjuncts to clinical evaluation.
Conclusions
This study presented two newly applied radiographic markers useful in the evaluation of hydrocephalic neonates and infants following ETV/CPC or under consideration for secondary shunting. The radiographic appearance of CSF turbulence may suggest the presence of pathologic CSF flow dynamics and signal ETV/CPC failure, while CP persistence may predict the ultimate necessity for shunt insertion despite neuro-endoscopic treatment. Radiographic evidence of adequate CPC, with the absence of CP on post-operative FS-MRI scans, suggested a higher likelihood of ETV/CPC success and shunt independence. The presence of either or both radiographic markers reflected ETV/CPC failure in statistically significant fashion, with only moderate sensitivity and specificity. Further prospective studies are warranted to confirm the predictive and clinical utility of these radiographic markers—CSF turbulence and CP visualization—in hydrocephalic neonates and infants undergoing ETV/CPC. Furthermore, future studies incorporating formal inter-/intra-observer reliability estimates may help corroborate the utility of these radiographic markers as adjuncts to clinical evaluation.
Footnotes
Note: Portions of this work were presented in oral format at the 43rd Annual Meeting of the AANS/CNS Section on Pediatric Neurological Surgery, Amelia Island, Florida, December 2014.
Disclosures: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. There were no sources of financial or material support for this work.
References
- 1.Di Rocco F, Grevent D, Drake JM, Boddaert N, Puget S, Roujeau T, et al. Changes in intracranial CSF distribution after ETV. Childs Nerv Syst. 2012;28:997–1002. doi: 10.1007/s00381-012-1752-6. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni AV, Drake JM, Armstrong DC, Dirks PB. Measurement of ventricular size: reliability of the frontal and occipital horn ratio compared to subjective assessment. Pediatr Neurosurg. 1999;31:65–70. doi: 10.1159/000028836. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni AV, Riva-Cambrin J, Browd SR, Drake JM, Holubkov R, Kestle JR, et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infants with hydrocephalus: a retrospective Hydrocephalus Clinical Research Network study. J Neurosurg Pediatr. 2014;14:224–229. doi: 10.3171/2014.6.PEDS13492. [DOI] [PubMed] [Google Scholar]
- 4.Malko JA, Hoffman JC, Jr, McClees EC, Davis PC, Braun IF. A phantom study of intracranial CSF signal loss due to pulsatile motion. AJNR Am J Neuroradiol. 1988;9:83–89. [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: A linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29:245–249. doi: 10.1159/000028730. [DOI] [PubMed] [Google Scholar]
- 6.Pindrik J, Jallo GI, Ahn ES. Changes in third ventricular size in pediatric patients undergoing endoscopic third ventriculostomy. Childs Nerv Syst. 2013;29:2027–2034. doi: 10.1007/s00381-013-2145-1. [DOI] [PubMed] [Google Scholar]
- 7.Scrivani PV, Freer SR, Dewey CW, Cerda-Gonzalez S. Cerebrospinal fluid signal-void sign in dogs. Vet Radiol Ultrasound. 2009;50:269–275. doi: 10.1111/j.1740-8261.2009.01532.x. [DOI] [PubMed] [Google Scholar]
- 8.Sherman JL, Citrin CM. Magnetic resonance demonstration of normal CSF flow. AJNR Am J Neuroradiol. 1986;7:3–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Sherman JL, Citrin CM, Bowen BJ, Gangarosa RE. MR demonstration of altered cerebrospinal fluid flow by obstructive lesions. AJNR Am J Neuroradiol. 1986;7:571–579. [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman JL, Citrin CM, Gangarosa RE, Bowen BJ. The MR appearance of CSF flow in patients with ventriculomegaly. AJR Am J Roentgenol. 1987;148:193–199. doi: 10.2214/ajr.148.1.193. [DOI] [PubMed] [Google Scholar]
- 11.St George E, Natarajan K, Sgouros S. Changes in ventricular volume in hydrocephalic children following successful endoscopic third ventriculostomy. Childs Nerv Syst. 2004;20:834–838. doi: 10.1007/s00381-004-0939-x. [DOI] [PubMed] [Google Scholar]
- 12.Stone SS, Warf BC. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infant hydrocephalus: a prospective North American series. J Neurosurg Pediatr. 2014;14:439–446. doi: 10.3171/2014.7.PEDS14152. [DOI] [PubMed] [Google Scholar]
- 13.Warf BC. Congenital idiopathic hydrocephalus of infancy: the results of treatment by endoscopic third ventriculostomy with or without choroid plexus cauterization and suggestions for how it works. Childs Nerv Syst. 2013;29:935–940. doi: 10.1007/s00381-013-2072-1. [DOI] [PubMed] [Google Scholar]
- 14.Warf BC. The impact of combined endoscopic third ventriculostomy and choroid plexus cauterization on the management of pediatric hydrocephalus in developing countries. World Neurosurg. 2013;79:S23 e13–25. doi: 10.1016/j.wneu.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Warf BC, Mugamba J, Kulkarni AV. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus in Uganda: report of a scoring system that predicts success. J Neurosurg Pediatr. 2010;5:143–148. doi: 10.3171/2009.9.PEDS09196. [DOI] [PubMed] [Google Scholar]