Abstract
Objectives
To determine the prevalence of pre-frailty among HIV-infected persons and associations with pre-frailty and frailty in this population.
Design, Setting and Participants
From a contemporary, prospective observational cohort of HIV-infected persons (SUN Study), we determined, using a cross-sectional analytic study design, the proportions of non-frail, pre-frail, and frail persons by the respective presence of 0, 1-2, and ≥ 3 of 5 established frailty criteria: unintentional weight loss, exhaustion, physical-inactivity, weak-grip and slow-walk. We evaluated associations with pre-frailty/frailty using multivariate analysis.
Results
Of 322 participants assessed (79% men, 58% white non-Hispanic, median age 47 years, 95% on combination antiretroviral therapy [cART], median CD4 + cell count 641 cells/mm3 and 93% HIV RNA < 400 copies/mL), 57% were non-frail, 38% pre-frail, and 5% frail. Age increased from non-frailty through frailty. Notably, however, half of pre-frail and frail participants were < 50 years, and of those, 42% and 100%, respectively, were long-term unemployed (versus 16% of non-frail counterparts). In multivariate analysis, pre-frail/frail participants were more likely to have Hepatitis C seropositivity (adjusted odds ratio [aOR] 3.24, 95% CI: 1.35-7.78), a history of AIDS-defining-illness (aOR 3.51, 95% CI: 1.82-6.76), greater depressive symptoms (aOR 1.16, 95% CI:1.09-1.23), higher D-dimer levels (aOR 2.94, 95% CI:1.10-7.87), and were less likely to be white non-Hispanic (aOR 0.35, 95% CI: 0.20-0.61).
Conclusions
Pre-frailty and frailty are prevalent in the cART era and are associated with unemployment even among persons < 50 years. Pre-frailty appears to be an intermediate state in the spectrum from non-frailty through frailty and our characterization of pre-frailty/frailty suggests complex multifactorial associations.
Keywords: Pre-frailty, frailty, HIV, human immunodeficiency virus
Introduction
Frailty is a state of diminished reserve and increased vulnerability due to an accumulation of deficits in multiple physiological domains (1). It is characterized by weight loss, exhaustion, physical inactivity, reduced muscle strength, and slowed motor function but medical and psychosocial comorbidity are also evident (1-6).
Immunodeficiency is associated with HIV-related frailty (6-8), and to higher levels of markers of monocyte/macrophage and T-cell activation (9). The additional contributions of coagulopathy and endocrine dysfunction, as seen in age-related frailty (10, 11), remain to be elucidated.
Lastly, frailty among older HIV-uninfected persons is typically preceded by a subclinical pre-frail state that transitions to frailty once a threshold of physiological dysregulation is reached (3). However, the prevalence and significance of pre-frailty among HIV-infected populations is not well-described. We evaluated the prevalence of non-frailty, pre-frailty, and frailty among HIV-infected participants in the SUN Study (Study to Understand the Natural History of HIV and AIDS in the Era of Effective Therapy) and examined associations of pre-frailty and frailty with multiple behavioral and clinical factors and biomarkers of inflammation.
Methods
Study Population
The SUN Study is a closed, prospective, observational cohort study funded by the Centers for Disease Control and Prevention (CDC) that monitored the clinical course of persons in the combination antiretroviral therapy (cART) era at seven HIV-specialty clinics in four US cities: St. Louis, MO; Providence, RI; Minneapolis, MN; and Denver, CO. Seven hundred HIV-infected participants were enrolled between March 1, 2004 and June 30, 2006. The study's design and methods have been described previously (12). Participants were generally healthy adults receiving routine outpatient care and were either treatment naïve, or had only received combination antiretroviral therapy (cART). Participant data were abstracted from medical charts and entered into an electronic database (Clinical Practice Analyst; Cerner Corporation, Vienna, VA) by trained staff.
Additional data were collected through biannual study visits and included comprehensive testing for sexually transmitted diseases (STD), and an audio computer-assisted interview about health-related information; tobacco, alcohol, and recreational drug use, assessment of depression (PRIME MD depression screener) and general function (Short Form 12 [SF-12]) (12)). The study protocol was approved and annually reviewed by the CDC and participating sites′ institutional review boards.
Frailty assessment
Frailty assessments, using a well-validated and applied frailty appraisal as devised by Fried et al. (1), were incorporated into the SUN Study in June 2010 and prospectively conducted bi-annually thereafter. Only initial frailty assessment data from June 2010 to April 2011 were used for this analysis. The frailty assessment took a few minutes to perform by trained study coordinators. Non-frailty, pre-frailty, and frailty were defined by the presence of 0, 1-2, and ≥ 3 of 5 frailty criteria, respectively: unintentional weight loss, physical inactivity, exhaustion, weak grip strength, and slow walk, with modification to accommodate physical inactivity (Table 2).
Table 2. Prevalence of individual frailty criteria among SUN study participants with complete data available.
| Frailty criteria | Pre-frail* (n=121) | Frail (n=15) |
|---|---|---|
| Unintentional weight loss | 13 (11%) | 1 (7%) |
| More than 10 pounds or ≥5% of weight loss occurred during the last year. | ||
| Physical inactivity | 35 (29%) | 15 (100%) |
| Participants answering “3” when asked whether their health limits vigorous activities (eg running, lifting heavy objects, participating in strenuous sports (1 = not at all, 2 = a little, or 3 = a lot). | ||
| Exhaustion | 76 (63%) | 11 (73%) |
| Participants answering 2 or 3 to either one of two statements – How often in the last week have you felt that: (a) everything you did was an effort or (b) I could not ‘get going’. 0 = rarely (< 1 day), 1 = some of the time (1-2 days), 2 = occasionally (3-4 days) or 3 = most of the time (5-7 days) | ||
| Weak grip strength | 9 (7%) | 11 (73%) |
| Strength, kg, median (IQR) | 36 (29-44) | 29 (23-39) |
| Cut-points according to BMI: | ||
| M: ≤24 kg/m2 ≤29 kg; 24.1-26.0 kg/m2 ≤30 kg; 26.1-28.0 kg/m2 ≤30 kg; >28 kg/m2 ≤32 kg. | ||
| W: <23 kg/m2<17 kg; 23.1-26.0 kg/m2 <17.3 kg; 26.1-29.0 kg/m2 <18 kg; >29 kg/m2<21 kg. | ||
| Slow walking speed (n=121) | 20 (17%) | 7 (54%) |
| Time, seconds, median (IQR) | 4 (3-5) | 8 (4-9) |
| Cut-points according to height: | ||
| M: ≤173 cm ≥7 sec; >173 cm ≥6 sec. | ||
| W: ≤159 cm ≥7 sec; >159 cm ≥6 sec. | ||
Grip strength was determined from the average of three measurements of the dominant hand using a dynamometer (1). Walking was measured at usual pace over a 15-foot track (average of 2 trials) (1).
Excluding 10 persons who had 1-2 frailty criteria but could not be definitely assigned pre-frailty or frailty status due to missing values at the defining variables. M, men; W, women
Data and definitions
Data collected at the time of initial frailty assessment and retrospectively through study enrollment were used. Data at initial frailty assessment included, race/ethnicity (white non-Hispanic versus [vs.] all others), employment status (fulltime/part-time employed vs. unemployed), tobacco use (ever/current vs. never), and recent (past 30 days) alcohol use, as well as the number of days per month in which alcohol was consumed. Long-term unemplyment and long-term alcohol use were defined as reports of unemployment and recent alcohol use at ≥ 75% of previous visits, respectively.
The variable for ‘past depression score’ was calculated as the median of all scores from PRIME MD at previous study visits. ‘Current depression score’ was the score from PRIME MD at initial frailty assessment. Similar assessments were made for statements of perceived general health and past health limitations to moderate activities using the SF-12 (12). ‘Past physical inactivity’ was present if the median response to past health limitations to moderate activities was “limited a lot“.
HIV-related data included history of AIDS-defining opportunistic illnesses (ADI), and cART, CD4+ cell count and HIV viral load history. A plasma HIV RNA concentration of < 400 copies/mL defined viral suppression. Hepatitis C seropositivity defined hepatitis C infection, and detectable serum surface antigen defined active hepatitis B infection. A body mass index (BMI) ≥ 30 kg/m2 defined obesity and a BMI < 18.5 kg/m2 defined underweight. Chronic kidney disease was defined as a glomerular filtration rate (eGFR) < 60 ml/min/1.73m2 calculated using Modification of Diet in Renal Disease (MDRD) formula. The Diabetes Research and Training Center Radioimmunoassay Core Laboratory (Washington University School of Medicine) used stored serum and plasma collected at study entry from all participants to assay select biomarker levels retrospectively. Serum and plasma used for these assays were iced, centrifuged, and stored at –70°C within 24 hours of collection until time of batched assay. We assayed for the following biomarkers: high sensitivity C-reactive protein (hs-CRP), interleukin-1 (IL-1), IL-6, tumor necrosis factor-α (TNF-α), 25-hydroxyvitamin D, D-dimer, soluble CD-14, and lipoprotein A.
Statistical analysis
Univariate analyses
Descriptive statistics were used to compare characteristics between non-frail, pre-frail and frail groups. Associations between non-frailty, pre-frailty, and frailty were assessed using the chi-square test for categorical variables; a p-value of < 0.001 was considered significant using the Bonferroni correction. For normally distributed and non-normally distributed continuous variables, one-way analysis of variance and the Kruskal-Wallis tests were used, respectively; a p-value of < 0.05 was considered significant for these tests.
Multivariate analyses
Pre-frail and frail categories were collapsed into one outcome to evaluate factors independently associated with pre-frailty/frailty. Categorical variables were analyzed using chi square. For normally distributed and non-normally distributed continuous variables, student t-test and Mann-Whitney test were used, respectively. In order to generate odds ratios (OR) for each continuous variable, all were analyzed individually using logistic regression. Using clinically relevant cut-points, where possible, D-dimer was categorized by a cut-point of > 0.34 µg/mL (13), hsCRP by a cut-point of > 3.00 mg/L (14) and 25 hydroxyvitamin D levels by a cut-point of < 30 ng/mL (15). The upper quartile levels of TNF-α, sCD14 and lipoprotein A were calculated for all persons and the respective values used as cut-points to calculate the OR categorized by upper quartile vs. the lower quartiles. Cut-points of 200 cells/mm3 and 350 cells/mm3 were used to categorize nadir and current CD4+ counts, respectively. All variables associated with pre-frailty/frailty at p < 0.10 in univariate analyses were initially included in a multivariate logistic regression model. Variables which were non-significant in the multivariable model (p > 0.05) were then eliminated and the model re-fit. The Hosmer-Lemeshow test was used to assess the model's calibration (goodness of fit). All analyses were performed using SPSS version 20.0 (Chicago, IL).
Results
Overall, 346 SUN participants underwent a frailty assessment, however, 24 (7%) participants were excluded from analysis due to missing components of the assessment; all had no positive frailty criteria and exact frailty status could not be ascertained. Ten additional participants also had missing components of the assessment but had 1-2 frailty criteria present. This latter group was included for analysis of the pre-frail/frail group vs. the non-frail group and their characteristics are also presented below.
Therefore of 322 (93%) participants with sufficient data for study analysis; 79% were men, 58% were white non-Hispanic with median age 47 years, 24% had a history of ADI, current median CD4+ cell count was 641 cells/mm3, 95% were taking cART, and 93% were virologically suppressed (Table 1).
Table 1. Characteristics of SUN study participants by frailty status.
| Characteristic | Non-frail (n =176) | Pre-frail* (n =121) | Frail (n =15) | p-value |
|---|---|---|---|---|
| Demographics and behaviors | ||||
| Age, years† | 46 (40-52) | 48 (43-54) | 53 (45-54) | 0.03 |
| Male sex | 149 (85%) | 86 (71%) | 13 (87%) | 0.01 |
| Race | <0.001 | |||
| White, non-Hispanic | 119 (68%) | 57 (47%) | 6 (40%) | |
| Black, non-Hispanic | 38 (22%) | 47 (39%) | 6 (40%) | |
| Hispanic | 15 (9%) | 13 (11%) | 3 (20%) | |
| Other/unknown | 4 (2%) | 4 (3%) | 0 | |
| Current unemployment, n =311 | 48 (29%) | 63 (52%) | 13 (87%) | <0.001 |
| Long-term unemployment, n =265 | 29 (19%) | 44 (46%) | 9 (82%) | <0.001 |
| Current tobacco use | 50 (29%) | 53 (44%) | 7 (47%) | 0.02 |
| Ever tobacco use | 98 (57%) | 78 (65%) | 13 (87%) | 0.03 |
| Alcohol use last 30 days, n =311 | 123 (74%) | 73 (60%) | 5 (33%) | 0.001 |
| No. of days alcohol used per month†, n =311 | 3 (0-8) | 2 (0-6) | 0 (0-1) | 0.006 |
| Long-term alcohol use | 137 (78%) | 73 (60%) | 5 (38%) | 0.001 |
| Past physical inactivity | 2 (1%) | 9 (7%) | 7 (47%) | <0.001 |
| Perception of general health | ||||
| Current response on study†, n =301 | very good (excellent-good) | good (very good-good) | fair (good-fair) | <0.001 |
| Excellent | 42 (24%) | 9 (7%) | 0 | |
| Good | 29 (17%) | 41 (34%) | 5 (33%) | |
| Fair | 8 (5%) | 22 (20%) | 5 (33%) | |
| Poor | 1 (1%) | 6 (6%) | 3 (20%) | |
| Past responses on study †, n =309 | very good (very good-good) | good (very good-good) | fair (good-fair) | <0.001 |
| Excellent | 37 (21%) | 11 (9%) | 0 | |
| Very good | 93 (53%) | 33 (28%) | 1 (7%) | |
| Good | 35 (20%) | 52 (43%) | 3 (21%) | |
| Fair | 9 (6%) | 21 (18%) | 10 (72%) | |
| Poor | 1 (1%) | 3 (3%) | 0 | |
| Co-morbidities | ||||
| Body mass index, kg/m2 † | 26.9 (24.2-30.6) | 26.1 (22.9-29.6) | 26.1 (23.0-29.8) | 0.32 |
| Obese BMI | 26% | 22% | 27% | 0.91 |
| Underweight BMI | 1% | 3% | 4% | 0.09 |
| Hepatitis C seropositivity | 10 (6%) | 24 (20%) | 5 (33%) | 0.001 |
| Active hepatitis B nfection | 6 (1%) | 3 (3%) | 2 (13%) | 0.08 |
| Current depression score†, n=306 | 3 (0-5) | 6 (2-11) | 8 (4-10) | <0.001 |
| Past depression score†, n=255 | 3 (1-7) | 5 (3-10) | 7 (3-9) | <0.001 |
| Hemoglobin, g/L† | 15.1 (14.4-15.9) | 14.3 (13.0-15.3) | 14.6 (13.1-15.6) | <0.001 |
| Creatinine, mg/dL† | 1.0 (0.9-1.1) | 0.9 (0.8-1.1) | 1.0 (0.9-1.4) | <0.001 |
| eGFR <60mL/min/1.73m2, n =258 | 3% | 4% | 30% | <0.001 |
| Current STD, n=312 | 14 (8%) | 4 (3%) | 0 (0%) | 0.13 |
| Ever STD, n =266 | 56 (32%) | 28 (29%) | 0 (0%) | 0.02 |
| HIV-related factors | ||||
| Time since HIV diagnosis, years† | 9.4 (6.7-12.8) | 11.8 (8.1-14.4) | 10.4 (7.4-12.1) | 0.03 |
| History of ADI | 26 (15%) | 38 (31%) | 8 (53%) | < 0.001 |
| Current cART | 166 (94%) | 112 (96%) | 15 (100%) | 0.58 |
| Total time on cART, years† | 7.5 (5.6-10.2) | 8.2 (5.9-10.8) | 6.8 (5.9-9.7) | 0.7 |
| CD4+ cell count, cells/mm3† | ||||
| Nadir | 209 (100-295) | 149 (45-257) | 154 (70-175) | 0.04 |
| Current | 649 (470-813) | 619 (418-795) | 641 (460-943) | 0.87 |
| Viral suppression, n =203 | 121 (94%) | 66 (99%) | 8 (89%) | 0.13 |
| Biomarkers, median (IQR) | ||||
| hs-CRP, mg/L | 1.6 (0.8-3.3) | 2.0 (0.9-4.2) | 3.5 (0.9-7.6) | 0.91 |
| TNF-α, pg/mL, n=285 | 13.0 (10.3-18.9) | 13.7 (11.2-24.4) | 15.1 (10.1-339.0) | < 0.001 |
| IL-6, pg/mL, n=288 | 2.9 (2.0-5.0) | 3.0 (2.0-6.5) | 9.2 (2.1-4641.5) | < 0.001 |
| D-dimer, µg/L | 0.11 (0.04-0.23) | 0.14 (0.06-0.27) | 0.20 (0.10-0.45) | 0.001 |
| sCD14, ng/mL‡ | 1136.5 ± 24.0 | 1233.6 ± 33.8 | 1326.7 ± 91.5 | 0.06 |
| Lipoprotein A, mg/mL | 10.0 (4.0-30.0) | 17.0 (6.0-45.0) | 13.0 (6.0-33.0) | 0.1 |
| 25-hydroxy vitamin D, ng/mL‡ | 25.4 ± 0.9 | 21.4 ± 1.1 | 21.8 ± 3.5 | 0.07 |
Comparison of all three groups.
Excluding 10 persons who had 1-2 frailty criteria but could not be definitely assigned pre-frailty or frailty status due to missing values at the defining variables.
median, (interquartile range, IQR),
mean ± standard error. ADI, AIDS-defining illness; cART, combination antiretroviral therapy; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; sCD14, soluble CD14; STD, sexually transmitted diseases; TNF-α, tumor necrosis factor-α.
In total, 176 (57%) participants were non-frail and 146 (43%) had ≥ 1 frailty criteria present. Excluding the ten participants mentioned above; 121 (38%) participants were definitively defined as pre-frail, and 15 (5%) as frail. Most participants were categorized as pre-frail by exhaustion (63%), then physical inactivity (29%). Most persons were categorized as frail by physical inactivity (100%), exhaustion (73%), and weak grip strength (73%) (Table 2). Of note, current depression scores were greater among participants who reported physical inactivity, median 8 (4-13) vs. no physical inactivity 3 (1-7), or exhaustion, median 7 (3-11) vs. no exhaustion 3 (1-6); both p < 0.001. Age increased from non-frailty through frailty (Table 1), although half of pre-frail (67/121) and frail (7/15) participants were < 50 years of age (Figure 1). Notably, 42% of pre-frail and 100% of frail participants < 50 years of age were long-term unemployed compared with 16% of non-frail ones. Findings were similar for current unemployment. Lastly, 53% of frail participants perceived their current general health to be either fair or poor compared with 26% of pre-frail and 6% of non-frail participants (p < 0.001). Furthermore, 47% of frail participants reported past physical inactivity compared with 7% of pre-frail and 1% of non-frail ones, (p < 0.001).
Figure 1.
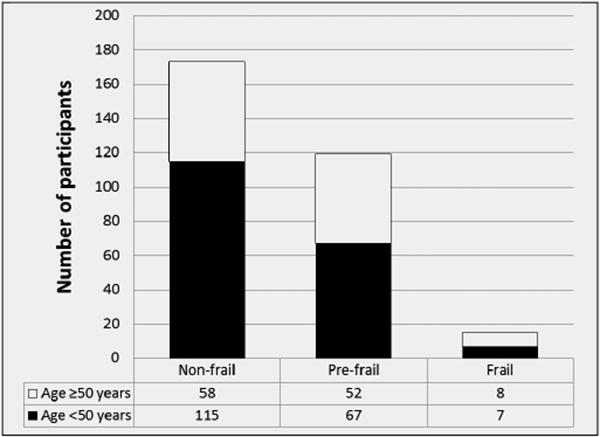
Non-frail, pre-frail* and frail SUN study participants by age
*excluding 10 participants with 1-2 frailty criteria but missing additional data such that they could not be assigned pre-frailty or frailty status with certainty.
Compared with non-frail participants, pre-frail/frail participants had lower odds of being white non-Hispanic (aOR 0.35, 95%CI: 0.20-0.61), higher odds of hepatitis C seropositivity (aOR 3.24, 95% CI: 1.35-7.78), and higher odds of history of ADI (aOR 3.51, 95% CI: 1.82-6.76) (Table 3). Current depression scores were greater (aOR 1.16, 95% CI: 1.09-1.23) and D-dimer levels were higher (aOR 2.94, 95% CI: 1.10-7.87).
Table 3. Factors independently associated with pre-frail/frail versus non-frail status among SUN study participants.
| Characteristics | Non-frail (n=176) | Pre-frail/frail* (n=146) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-frail/frail versus non-frail | Pre-frail/frail versus non-frail | |||||||
| OR | 95%CI | p-value | OR | 95%CI | p-value | |||
| Demographics and behaviors | ||||||||
| Age, years† | 46 (40-52) | 48 (44-54) | 1.04 | 1.01-1.06 | 0.01 | |||
| Male sex | 149 (85%) | 104 (71%) | 0.45 | 0.26-0.77 | 0.003 | |||
| White, non-Hispanic race | 119 (68%) | 66 (45%) | 0.4 | 0.25-0.62 | < 0.001 | 0.35 | 0.20-0.61 | <0.001 |
| Current unemployment, n=311 | 48 (29%) | 85 (59%) | 3.48 | 2.18-5.58 | < 0.001 | |||
| Long-term unemployment, n=265 | 29 (19%) | 58 (52%) | 4.59 | 2.65-7.95 | < 0.001 | |||
| Current tobacco use, n=313 | 50 (28%) | 67 (48%) | 2.26 | 1.41-3.60 | 0.001 | |||
| Ever tobacco use, n=313 | 98 (52%) | 98 (70%) | 1.79 | 1.12-2.86 | 0.02 | |||
| Alcohol use last 30 days, n=297 | 123 (74%) | 80 (55%) | 0.43 | 0.27-0.69 | < 0.001 | |||
| Alcohol use per month, days† | 3 (0-8) | 1 (0-1) | 0.95 | 0.92-0.99 | 0.001 | |||
| Long-term alcohol use, n=314 | 137 (78%) | 83 (60%) | 0.41 | 0.25-0.67 | < 0.001 | |||
| Past inactivity | 1 (1%) | 17 (12%) | 23.2 | 3.04-175.9 | < 0.001 | |||
| Perception of general health | ||||||||
| Current response on study†, n=311 | very good (excellent-good) | Good | 2.71 | 2.04-3.60 | < 0.001 | |||
| (very good-fair) | ||||||||
| Excellent | 42 (24%) | 10 (7%) | ||||||
| Very good | 86 (49%) | 44 (31%) | ||||||
| Good | 29 (17%) | 51 (35%) | ||||||
| Fair | 8 (5%) | 29 (20%) | ||||||
| Poor | 1 (1%) | 11 (8%) | ||||||
| Past responses on study †, n =318 | very good (very good-good) | Good (very good-fair) | 2.6 | 1.95-3.46 | < 0.001 | |||
| Excellent | 37 (21%) | 11 (8%) | ||||||
| Very good | 93 (53%) | 37 (25%) | ||||||
| Good | 35 (20%) | 58 (40%) | ||||||
| Fair | 9 (6%) | 34 (26%) | ||||||
| Poor | 1 (1%) | 4 (3%) | ||||||
| Co-morbidities | ||||||||
| Body mass index, kg/m2 † | 26.9 (24.2-30.6) | 26.2 (23.0-29.9) | 0.99 | 0.96-1.02 | 0.18 | |||
| Obese BMI | 26% | 25% | 0.94 | 0.56-1.56 | 0.81 | |||
| Underweight BMI | 1% | 4% | 6.35 | 0.73-55.0 | 0.09 | |||
| Hepatitis C seropositivity | 10 (6%) | 33 (23%) | 4.85 | 2.30-10.23 | <0.001 | 3.24 | 1.35-7.78 | 0.008 |
| Active hepatitis B infection | 6 (3%) | 5 (3%) | 1.01 | 0.30-3.37 | 0.99 | 1.16 | 1.09-1.23 | <0.001 |
| Current depression score†, n=306 | 3 (0-5) | 7 (3-11) | 1.17 | 1.11-1.23 | < 0.001 | |||
| Past depression score†, n=255 | 3 (1-7) | 6 (3-10) | 1.13 | 1.06-1.19 | < 0.001 | |||
| Hemoglobin, g/L † | 15.1 (14.4-15.9) | 14.3 (13.0-15.3) | 0.72 | 0.62-0.84 | < 0.001 | |||
| Creatinine, mg/dL† | 0.98 (0.86-1.07) | 0.92 (0.80-1.11) | 0.75 | 0.30-1.92 | 0.65 | |||
| eGFR < 60mL/min/1.73m2, n=258 | 3% | 8% | 3.07 | 0.90-10.5 | 0.06 | |||
| Current STD, n=312 | 14 (8%) | 4 (3%) | 0.31 | 0.10-0.95 | 0.03 | |||
| Ever STD, n=266 | 56 (37%) | 29 (25%) | 0.56 | 0.33-0.96 | 0.04 | |||
| HIV-related factors | ||||||||
| Time since HIV diagnosis, years† | 9.4 (6.7-12.8) | 11.4 (8.2-14.4) | 1.06 | 1.02-1.11 | 0.001 | |||
| History of ADI | 26 (15%) | 51 (35%) | 3.1 | 1.81-5.30 | < 0.001 | 3.51 | 1.82-6.76 | <0.001 |
| Current cART | 166 (94%) | 140 (96%) | 1.41 | 0.50-3.96 | 0.52 | |||
| Total time on cART, years† | 7.5 (5.6-10.2) | 8.1 (5.9-10.5) | 1.03 | 0.96-1.10 | 0.49 | |||
| Nadir CD4+ cell count, cells/mm3 †,§ | 209 (100-295) | 149 (46-250) | 1.2 | 1.10-1.40 | 0.002 | |||
| <200 cells/mm3 | 85 (49%) | 96 (66%) | 2.06 | 1.31-3.23 | 0.002 | |||
| Current CD4+ cell count, cells/mm3 † | 649 (470-813) | 619 (418-794) | 1 | 0.99-1.00 | 0.25 | |||
| ≥350 cells/mm3 | 152 (88%) | 114 (84%) | 0.71 | 0.37-1.39 | 0.32 | |||
| Viral suppression, n=215 | 122 (91%) | 78 (96%) | 2.56 | 0.70-9.35 | 0.14 | |||
| Biomarkers | ||||||||
| D-dimer, µg/L† | 0.11 (0.04-0.23) | 0.14 (0.07-0.28) | 3.21 | 1.40-7.37 | 0.02 | 2.94 | 1.10-7.87 | 0.03 |
| D-dimer >0.34 µg/L | 19 (11%) | 33 (23%) | 2.46 | 1.33-4.56 | 0.003 | |||
| hs-CRP, mg/L† | 1.6 (0.8-3.3) | 1.9 (0.9-4.4) | 1.01 | 0.98-1.04 | 0.08 | |||
| hs-CRP >3.00 mg/L | 48 (27%) | 55 (38%) | 1.62 | 1.01-2,59 | 0.05 | |||
| TNF-α, pg/mL†,‖, n=298 | 13.0 (10.3-18.9) | 13.9 (11.2-25.1) | 1.02 | 1.00-1.04 | 0.02 | |||
| TNF-α >20.5 pg/mL | 31 (19%) | 42 (30%) | 1.79 | 1.05-3.05 | 0.03 | |||
| sCD14, ng/mL‡,‖ | 1136.5 ± 24.0 | 1249.7 ± 30.0 | 1.01 | 1.00-1.02 | 0.003 | |||
| sCD14 >1361 ng/mL | 30 (17%) | 50 (34%) | 2.54 | 1.51-4.27 | <0.001 | |||
| Lipoprotein A†, ‖ | 10.0 (4.0-30.0) | 19.0 (6.3-45.8) | 1.1 | 1.01-1.18 | 0.01 | |||
| Lipoprotein A >38.8 mg/mL | 34 (20%) | 45 (31%) | 1.85 | 1.10-3.09 | 0.02 | |||
| 25-hydroxyvitamin D, ng/mL‡ | 25.4 ± 0.9 | 22.7 ± 1.0 | 0.98 | 0.96-1.00 | 0.04 | |||
| 25-hydroxyvitamin D <30 ng/mL | 121 (70%) | 112(77%) | 1.49 | 0.90-2.46 | 0.12 | |||
including all participants with at least 1 frailty criterion;
median (IQR);
mean ± standard error;
pet 100 cells/mm3 decrease in CD4+ cells;
per 10 unit increase. ADI, AIDS-defining illness; cART, combination antiretroviral therapy; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; sCD14, soluble CD14; STD, sexually transmitted diseases; TNF-α, tumor necrosis factor-α.
Current CD4+ cell counts and the proportion of participants with viral suppression were comparable between groups. A sub-analysis of participants (n = 203) with full viral suppression on cART, demonstrated similar associations excluding hepatitis C seropositivity (results not shown).
Discussion
Among our contemporary cohort of predominantly middle-aged participants with well-controlled HIV infection and high median CD4+ cell counts, we demonstrated a prevalence of frailty of 5%. This was comparable to the lower prevalence reported among similarly aged HIV-infected cohorts (6-8) and among HIV-uninfected persons in their 7th decade of life (1, 3-5, 7, 16). Our pre-frailty prevalence of 38% was comparable to estimates reported among older HIV-uninfected persons (28-51%) (4, 7). Furthermore, half of pre-frail and frail participants in our study were < 50 years of age, a concerning finding given the many co-morbidities that affect HIV-infected persons as they age (17) and the higher mortality risk associated with pre-frailty/frailty as determined by the Veterans Aging Cohort (VACS) Index (18). Consistent with studies of the general population, our data also suggest that pre-frailty in the HIV population is an intermediary in the spectrum of progression from non-frailty to frailty (3, 5) and that it is a state more modestly associated with frailty-related factors such as medical and psychosocial comorbidity (6). In this cross-sectional analysis, several factors were associated with pre-frailty/frailty: high D-dimer, history of ADI, hepatitis C seropositivity, and greater current depression score. White non-Hispanic participants were less likely to be pre-frail/frail.
D-dimer, a marker of activation of the coagulation cascade has been associated with incident age-related frailty (19). A low-grade, chronic and systemic pro-inflammatory state negatively impacts multiple organ systems (11) and is a hallmark of aging, age-related frailty, HIV disease, and increasingly, HIV-related frailty (9). Longitudinal analyses of changes in biomarker levels during progression to frailty would be necessary to further elucidate the role of chronic inflammation and activation of the coagulation cascade in the development of frailty.
Our finding that history of ADI was associated with prevalent pre-frailty/frailty is consistent with other studies (6-8). A history of ADI (clinical immunodeficiency) may confer an increased risk of developing pre-frailty or frailty when, additional physiological insults ultimately accumulate due to aging, sociodemographic factors, lifestyle behaviors, comorbidities, and/or ongoing chronic inflammation (20). Furthermore, the additive effect of the number of past ADI may also increase the risk of becoming pre-frail/frail (6). Future longitudinal studies on prevention of ADI to ameliorate risk for pre-frailty/frailty might underscore the importance of earlier HIV diagnosis, treatment, and retention in care (20, 21).
Consistent with findings in the Women's Interagency Health Study, wherein, Hispanic HIV-infected women had significantly weaker grip strength than women from other racial/ethnic groups (8), we demonstrated a higher pre-frailty/frailty prevalence among persons of non-white race/ethnicity. Older HIV-uninfected black and Hispanic adults have a higher frailty prevalence (5, 22) and over classification of racial/ethnic minorities as pre-frail or frail could occur since the standardized height and BMI in frailty assessment criteria fail to account for normal differences in the range of these values among different racial/ethnic groups (16). However, differences in other factors associated with racial/ethnic disparities, such as poverty, unemployment, health-care utilization, comorbidity, HIV testing, retention in HIV-related care and antiretroviral adherence (21, 23, 24), may have contributed to our findings.
Greater current depression scores have been consistently reported in numerous cross-sectional studies of age and HIV-related frailty (2, 4-6, 9). Depression may influence self-reporting of exhaustion and physical inactivity due to an overlap of symptoms with frailty. Furthermore, both depression and frailty are associated with racial/ethnic minorities, medical and psychosocial comorbidity, advanced HIV disease, hepatitis C co-infection and increased risk of death (24-27). However, depression can negatively impact motor function and contribute to disability (27) and depressive symptoms were strongly associated with incident frailty in older HIV-uninfected women in the Women's Health Initiative Study (5). Lastly, a study exploring the relationship between the constructs of depression (as defined by the Diagnostic Interview Schedule) and frailty showed that these are distinct but still overlapping syndromes (28). If one considers depression and frailty to be interrelated, then identification of depressive symptoms is imperative in the assessment of frailty state. Effective management of depression in the HIV-outpatient care setting is gaining prominence (29) and its impact on frailty states should be studied.
Hepatitis C seropositivity, a marker for a viral infection, that like HIV infection is associated with pro-inflammatory, psychiatric, psychosocial, and multisystem comorbidity (30-32), was also associated with frailty among HIV-infected men who have sex with men (33). Approximately a quarter of HIV-infected individuals in Europe and USA are Hepatitis C/HIV co-infected and at increased risk of cirrhosis, HIV-related disease, and overall mortality (32). Persons with co-infection may be at increased risk for pre-frailty and frailty as well and effective treatment of this co-infection to prevent pre-frailty or frailty warrants consideration.
Our analysis present some limitations. The low prevalence of frailty in this cohort likely reduced our capacity to robustly test for and detect associations. We also did not collect data on disability. We combined the categories of pre-frail and frail in an effort to increase our statistical power. Some nuances may have been lost on subsequent analyses of this combined group; for readers’ reference we presented the data in table 1 stratified by all three groups, with p-values calculated using alternative statistical analysis. Missing data may have further limited analysis. We obtained initial frailty assessment data up to six years after study enrollment and can only make inferences based on our retrospective examination of longitudinal data and biomarker results. Lastly, we analyzed data from a contemporary, predominantly male population and our findings may not be generalizable to other HIV-infected populations in the United States or elsewhere.
In conclusion, pre-frailty and frailty were prevalent in our cohort of HIV-infected adults receiving contemporary cART and was associated with unemployment, even among persons <50 years. Our analysis that included sociodemographic, clinical, and biomarker data corroborates the complex multifactorial associations with this syndrome. Furthermore, our data support the concept of pre-frailty as an intermediate state in the spectrum from non-frailty through frailty that may represent a population of persons most likely to benefit from prevention strategies as well as depression screening.
Acknowledgments
Funding: Centers for Disease Control and Prevention contract numbers 200-2002-00610, 200-2002-00611, 200-2002-00612, 200-2002-00613, 200-2007-23633, 200-2007-23634, 200-2007-23635, and 200-2007-23636.
Footnotes
Conflicts of interest: Turner Overton has served as a consultant or on an advisory board for the following companies: Gilead, Bristol Myers Squibb, Glaxo-Smith-Kline, Tibotec, Merck, and Monogram Sciences. Keith Henry has received research support from Gilead, and Glaxo-Smith-Kline/ViiV.
Disclaimer: The findings and conclusions from this review are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Prior presentation: These data were presented in part to the 19th Conference on Retroviruses and Opportunistic Infections (CROI), March 5-8, 2012, Seattle, Washington.
References
- 1.Fried L, Tangen CM, Waltson J, et al. Frailty in older persons: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56a:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57:453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 3.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 4.Avila-Funes JA, Helmer C, Amieva H, et al. Frailty among community-dwelling elderly people in France: the three-city study. J Gerontol A Biol Sci Med Sci. 2008;63:1089–1096. doi: 10.1093/gerona/63.10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 6.Önen NF, Agbebi A, Shacham E, Stamm KE, Önen AR, Overton ET. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect. 2009;59:346–352. doi: 10.1016/j.jinf.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. A Biol Sci Med Sci. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 8.Terzian AS, Holman S, Nathwani N. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health. 2009;18:1965–1974. doi: 10.1089/jwh.2008.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolick J, Jacobson L, Lopez J, et al. Frailty and circulating concentrations of proinflammatory cytokines and chemokines in HIV-infected and –uninfected men in the Multicenter AIDS Cohort Study (MACS). 3rd International Workshop on HIV and Aging; Baltimore. November 5-6, 2012; Abstract O_13. [Google Scholar]
- 10.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical morbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 11.Giunta S. Exploring the complex relations between inflammation and aging (inflamm-aging): anti-inflamm-aging remodeling of inflammaging, from robustness to frailty. Inflamm Res. 2008;57:558–563. doi: 10.1007/s00011-008-7243-2. [DOI] [PubMed] [Google Scholar]
- 12.Vellozzi C, Brooks JT, Bush TJ, et al. The study to understand the natural history of HIV and AIDS in the era of effective therapy (SUN Study) Am J Epidemiol. 2009;169:642–52. doi: 10.1093/aje/kwn361. [DOI] [PubMed] [Google Scholar]
- 13.Kuller L, Tracy R, Belloso W, et al. for the INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2005;5:e 203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dao CN, Patel P, Overton ET, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 16.Espinoza SE, Hazuda HP. Frailty in older Mexican-American and European American adults. Is there an ethnic disparity? J Am Geriatr Soc. 2008;56:1744–1749. doi: 10.1111/j.1532-5415.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 17.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. CID. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 18.Escota GV, Önen NF, Brooks JT, et al. Five-year-all-cause mortality risk according to frailty status in the SUN Study. 17th International Workshop on HIV Observational Databases; April 2013; Cavtat, Croatia. [Google Scholar]
- 19.Reiner AP, Aragaki AK, Gray SL, et al. Inflammation and thrombosis biomarkers and incident frailty in post-menopausal women. Am J Med. 2009;122:947–954. doi: 10.1016/j.amjmed.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branson B, Handsfield H, Lampe M, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 22.Hirsch C, Anderson ML, Newman A, Kop W, Jackson S, Gottdiener J, et al. The association of race with frailty: the cardiovascular health study. Ann Epidemiol. 2006;16:545–553. doi: 10.1016/j.annepidem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Losina E, Schackman BR, Sadownik SN, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. CID. 2009;49:1570–8. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong MC, Nahata MC, Lacombe VA, Seiber EE, Balkrishnan R. Association between race, depression, and antiretroviral therapy adherence in a low-income population with HIV infection. J Gen Intern Med. 2012;27:1159–1164. doi: 10.1007/s11606-012-2043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nurutdinova D, Chrusciel T, Zeringue A, et al. Mental health disorders and the risk of AIDS-defining illness and death in HIV-infected veterans. AIDS. 2012;26:229–234. doi: 10.1097/QAD.0b013e32834e1404. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher NF, McKeating JA. Hepatitis C virus and the brain. J Viral Hepat. 2012;19:301–306. doi: 10.1111/j.1365-2893.2012.01591.x. [DOI] [PubMed] [Google Scholar]
- 27.Hajjar I, Yang F, Sorond F, et al. A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risk. J Gerontol A Biol Sci Med Sci. 2009;64:994–1001. doi: 10.1093/gerona/glp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mezuk B, Lohman M, Dumenci L, Lapane KL. Are depression and frailty overlapping syndromes in mid- and late-life? A latent variable analysis. Am J Geriatr Psychiatry. 2013 Feb;:6. doi: 10.1016/j.jagp.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pence BW, Gaynes BN, Williams Q, et al. Assessing the effect of Measurement-Based Care depression treatment on HIV medication adherence and health outcomes: Rationale and design of the SLAM DUNC Study. Contemp Clin Trials. 2012;33:828–838. doi: 10.1016/j.cct.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acharya JN, Pacheco VH. Neurologic complications of hepatitis C. Neurologist. 2008;14:151–156. doi: 10.1097/NRL.0b013e31815fa594. [DOI] [PubMed] [Google Scholar]
- 31.Louie KS, St Laurent S, Forrsen UM, Mundy LM, Pimenta JM. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infec Dis. 2012;12:1–11. doi: 10.1186/1471-2334-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreoni M, Giacometti A, Maida I, Meraviglia P, Ripamonti D, Sarmati L. HIV-HCV co-infection: epidemiology, pathogenesis and therapeutic implications. Eur Rev Med Pharmacol Sci. 2012;16:1473–83. [PubMed] [Google Scholar]
- 33.Althoff K, Jacobson LP, Cranson RD, et al. Comorbidities and AIDS predict the frailty phenotype in men with treated HIV-1 infection. 3rd International Workshop on HIV and Aging; November 5-6, 2012; Baltimore. Abstract O_12. [Google Scholar]


