Abstract
Purpose
The optimal dose of radiation in high-risk neuroblastoma is unknown. We sought to evaluate local control following 21-Gy radiotherapy (RT) to the primary site in patients with high-risk neuroblastoma.
Patients and Methods
After receiving dose-intensive chemotherapy and gross total resection (GTR), 246 patients (ages 1.2–17.9, median 4.0 years) with high-risk neuroblastoma underwent RT to the primary site at xxx from 2000–2014. RT consisted of 21 Gy in twice-daily fractions of 1.5 Gy each. Local failure (LF) was correlated with biologic prognostic factors and clinical findings at the time of diagnosis and start of RT.
Results
Median follow-up of surviving patients was 6.4 years. Cumulative incidence of LF was 7.1% at 2 years post-RT and 9.8% at 5 years post-RT. The isolated LF rate was 3.0%. Eighty-six percent of all local failures were within the RT field. Local control was worse in patients who required more than one surgical resection to achieve GTR (22.4% vs 8.3%, p=0.01). There was also a trend towards inferior local control with MYCN-amplified tumors or serum LDH ≥1500 U/L (p=0.09 and p=0.06 respectively).
Conclusion
After intensive chemotherapy and maximal surgical debulking, hyperfractionated RT with 21 Gy in high-risk neuroblastoma results in excellent local control. Given the young patient age, concern for late effects, and local control >90%, dose-reduction may be appropriate for patients without MYCN amplification who achieve GTR.
Introduction
Neuroblastoma is the most common extracranial solid tumor in children, with 650 new cases (50% deemed high-risk) per year in the USA. Therapy for patients with high-risk neuroblastoma (HR-NB) involves induction, local control, consolidation, and treatment of minimal residual disease with biologic agents. Studies in the early 1990’s showed a benefit of radiation therapy (RT) to the primary site for local control in high-risk disease.1 Since then, there have been several single-institution studies reporting excellent local control rates with radiation, confirming the role of RT after surgical resection in high-risk disease.1–5 Since 1986, xxx has been using 21-Gy hyperfractionated RT to the primary site after chemotherapy and surgical resection.6 Among 99 patients treated with this regimen from 1986 to 2000, the incidence of local failure at 1 year was found to be 4.1%, and at 3 years was 10.1%.5 Other studies utilizing similar regimens have found low local relapse rates ranging from 0–17%.4,7–9
Substantial numbers of HR-NB patients achieve long-term survival with contemporary treatment programs.10–12 As patients with HR-NB are living longer, late effects from radiation are more of a concern. Recent reports have described an increased risk of late effects such as diabetes mellitus and growth abnormalities in neuroblastoma patients treated with radiation.5,13–18 Additionally, as over 90% of patients with neuroblastoma are ≤5 years old at diagnosis, this population is particularly susceptible to late effects, especially on linear growth. Despite reports of excellent local control rates, there has never been a clinical trial specifically evaluating the optimal dose of radiation in HR-NB. Thus, it is unknown if 21 Gy is the optimal dose that balances efficacy and toxicity. We sought to update the rate of local control with 21 Gy for HR-NB at a single institution with a large number of patients, with the secondary goal of evaluating whether dose reduction for a select subset of patients may be appropriate.
Methods and Materials
Patients
This is a single-institution, retrospectively ascertained cohort of neuroblastoma patients treated with 21-Gy hyperfractionated RT to the primary site during frontline therapy between 2000 and 2014 at xxx. All patients were classified as high-risk: stage 4 diagnosed at age >18 months or MYCN-amplified stage 2/3/4/4s at any age.19 Patients with macroscopic residual disease at the primary site at the time of RT were excluded. In addition, patients who did not receive standard 21-Gy hyperfractionated RT to the primary site were excluded. A total of 246 patients met criteria. Disease status was assessed every 3 months for ≥24 months by histology of bone marrow (BM) aspirates and biopsies, 123I-meta-iodobenzylguanidine (123I-MIBG) scans, and computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis.20 Disease status was defined by International NB Response Criteria (INRC), modified to incorporate 123I-MIBG findings. Complete response (CR): no neuroblastoma by BM histology and radiologic studies. Very good partial response (VGPR): volume of primary mass reduced >90%, normal 123I-MIBG scan, BM negative by histology. Partial response (PR): 123I-MIBG scan improved in all lesions and <1 BM-positive site by histology. Mixed response: 123I-MIBG scan improved in some but not all sites. No response (NR): 123I-MIBG scan unchanged. Progressive disease (PD): new lesion or >25% increase in existing lesion(s).21 The study was approved by the xxx Institutional Review Board/Privacy Board.
Treatment
Radiation
All patients received 21-Gy consolidative RT to their primary site at a median time of 7.8 months from initiation of induction chemotherapy. The clinical tumor volume (CTV) consisted of the post-induction, pre-surgical tumor volume and involved regional lymph nodes. The planning target volume (PTV) consisted of a 5 mm expansion on the CTV. Hyperfractionation with 1.5 Gy twice a day was utilized in all patients. Using an a/β ratio of 10, the biologically effective dose (BED) of our regimen was 24.2 Gy, comparable to the BED of 25.5 Gy seen with 21.6 Gy given in 1.8 Gy daily fractions.22 Eight-five patients (35%) underwent intensity-modulated radiation therapy (IMRT), while the rest were treated with 3-dimensional planning and opposed anteroposterior fields (AP/PA). IMRT was used over AP/PA in patients with bulky tumors and/or tumors surrounding critical organs. No patients received protons. One hundred and forty patients (57%) required anesthesia with propofol to undergo treatment. Sites of metastatic disease that remained MIBG avid were also treated with a median of 21 Gy. In total, 157 patients (64%) underwent additional consolidative RT to sites of metastatic disease, occurring at the same times as RT to the primary site in approximately 90%.
Surgery
Gross total resection (GTR, determined via operative notes and postoperative scans) was performed in all patients included in our cohort. Twenty-nine patients (11.7%) required more than 1 operation to achieve GTR, most commonly due to bulky initial tumor size. Over 75% of patients underwent primary surgical resection at xxx. A total of 29 patients underwent initial surgical resection at the time of diagnosis, prior to chemotherapy. The other patients underwent surgical resection at a median time of 3.4 months after chemotherapy initiation.
Chemotherapy and other treatment
All but 3 patients underwent chemotherapy on or according to an institutional or national protocol. One hundred and seventeen patients were treated on or according to xxx induction protocols, xx (n=30) and xx (n=87).23 One hundred and twenty-six patients were treated on or according to COG, POG, SIOP, or other institutional protocols. The remaining 3 patients received a combination of standard chemotherapy agents off protocol. Seventy-five patients underwent stem cell transplant in addition to the above therapy. Twenty-nine patients received therapeutic MIBG with 131-I, which occurred after RT in all patients but 2. The majority of patients also received 3F8 plus granulocyte-macrophage colony-stimulating factor and isotretinoin.
Statistical Methods and Design
Local failure (LF) was defined as relapse in the primary tumor bed, including regional nodes. RT treatment plans and imaging at the time of failure were reviewed to determine if the recurrence occurred within the RT field. LF was correlated with such factors as age at RT, tumor site, serum LDH ≥1500 U/L, serum ferritin ≥142ng/mL, MYCN amplification, Shimada histopathology, number of resections required to achieve GTR, response to chemotherapy, and time from initiation of chemotherapy to RT. Progression-free survival (PFS) was calculated as the time from initiation of induction to disease progression, including local and/or distant relapse. Overall survival (OS) was calculated as the time from induction to death from any cause. Patients without an event were censored at the time of last follow-up. A competing-risks analysis was used to assess the cumulative incidence of LF. The Kaplan-Meier method was used to assess the PFS and OS. Cumulative incidence curves among different subgroups of patients were compared with Gray’s method, with p≤0.05 considered significant. The small number of local failures precluded the use of multivariate analysis.
Results
Patient characteristics
See Table 1 for patient characteristics. Median age at RT was 4.0 years (range, 1.2–17.9). Among the 246 study patients (57% male, 43% female), 235 had stage 4, 9 had MYCN-amplified stage 3, and 2 had MYCN-amplified stage 2 disease. Primary location was abdomen in 94% and thorax/mediastinum in 6%. Metastatic involvement was common in the bone/bone marrow (n=204). Twelve patients with stage 4 disease (5%) had metastases in soft tissue alone (stage 4N). Median follow-up of surviving patients was 6.4 years (range, 1.3–15.6) from diagnosis and for all patients was 4.6 years (range, 0.7–15.6).
Table 1.
Patient characteristics
| N (%) | |
|---|---|
| Gender | |
| Female | 106 (43) |
| Male | 140 (57) |
| Race | |
| White | 198 (80) |
| Black | 20 (8) |
| Asian | 14 (6) |
| Other | 14 (6) |
| Age at RT (years) | |
| Median | 4.0 |
| Range | 1.2–17.9 |
| Site of primary tumor | |
| Adrenal | 189 (77) |
| Central abdominal compartment | 43 (17) |
| Thorax | 14 (6) |
| Stage | |
| 2 | 2 (1) |
| 3 | 9 (4) |
| 4 | 235 (96) |
| MYCN | |
| Non-amplified | 131 (53) |
| Amplified | 96 (39) |
| Unknown | 19 (8) |
| LDH | |
| <1500 U/L | 113 (46) |
| ≥1500 U/L | 65 (26) |
| Unknown | 68 (28) |
| Ferritin | |
| <142 ng/mL | 36 (15) |
| ≥142 ng/mL | 88 (36) |
| Unknown | 122 (50) |
| Shimada histopathology | |
| Favorable | 11 (4) |
| Unfavorable | 173 (70) |
| Unknown | 62 (25) |
| Skeletal metastases | |
| Present | 195 (79) |
| Absent | 51 (21) |
| Stem cell transplant | |
| Yes | 75 (30) |
| No | 171 (70) |
| Response to chemotherapy | |
| CR/VGPR | 135 (55) |
| PR/MR/PD | 111 (45) |
Abbreviations: RT = radiation therapy; CR/VGPR = complete remission/very good partial remission; PR/MR/PD = partial remission/mixed response/progressive disease
Local Control
Twenty-two patients (8.9%) relapsed at the primary site. Of these, 7/22 relapsed in the primary site alone as site of first failure (Table 2); 7/22 relapsed simultaneously at a distant site and in the primary site; and 8/22 relapsed at the primary site only after prior distant relapse. Primary tumor site of patients who failed locally included adrenal gland (n=16), retroperitoneum (n=4), and mediastinum (n=2). Among all patients, median time to LF was 10 months from RT (see Figure E1 for a complete breakdown of LF timing). All LF occurred within 4.5 years from RT. Cumulative incidence of LF was 5.4% (95% confidence interval [CI] 3.2%-9.1%) at 1 year, 7.1% (95% CI 4.5%-11.3%) at 2 years, and 9.8% (95% CI 6.5%-14.5%) at 5 years. The isolated local recurrence rate was 3.0% at 5 years (95% CI 1.4%-6.1%).
Table 2.
Patients with primary site failure alone at first relapse
| Patient No./Sex | Age at RT (Years) | MYCN Amplified | LDH | Ferritin | Primary Site | Chemo | No. of surgeries to achieve GTR | Time from RT to LF (years) | LF in RT field | Treatment of LF | Current Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/M | 1.5 | Yes | 872 | 448 | L adrenal | N8 | 1 | 0.4 | Yes | Surgery | Deceased |
| 2/F | 3.3 | Yes | 2983 | n/a | L adrenal | ANBL0532 | 1 | 1.1 | Inferior | Surgery, IORT, EBRT | Deceased |
| 3/M | 6.3 | No | 489 | 469 | L adrenal | ANBL0532 | 2 | 1.8 | Yes | Surgery, IORT | Alive, AD |
| 4/F | 5.3 | Yes | n/a | 258 | RP | * | 1 | 0.5 | Yes | Surgery, EBRT | Alive, NED |
| 5/F | 2.6 | Yes | 2000 | 258 | L adrenal | N8 | 2 | 0.4 | Lateral | Surgery, EBRT | Deceased |
| 6/M | 3.2 | Yes | 1511 | 149 | R adrenal | N8 | 1 | 1.0 | Lateral | EBRT | Deceased |
| 7/F | 5.1 | No | 600 | n/a | R adrenal | N8 | 1 | 0.8 | Yes | Surgery, IORT | Deceased |
Abbreviations: No. = number; M = male; F = female; L = left; R = right; GTR = gross total resection; RT = radiation therapy; LF = local failure; RP = retroperitoneal; IORT = intraoperative radiation therapy; EBRT = external beam radiation therapy; AD = active disease; NED = no evidence of disease; n/a = not applicable.
Vincristine, cisplatin, cyclophosphamide, doxorubicin in Greece followed by COJEC with carboplatin
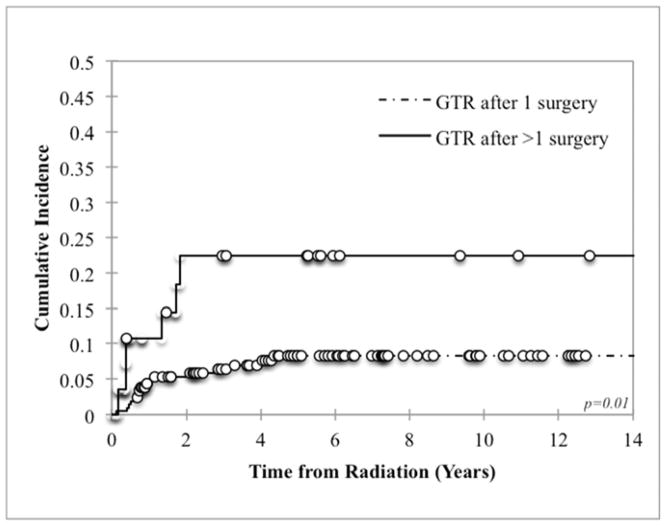
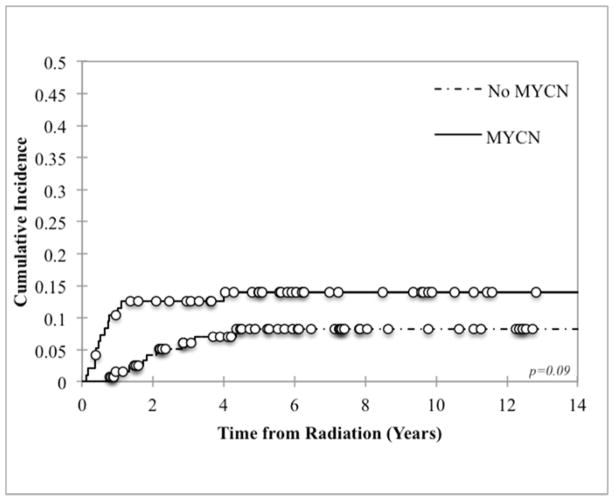
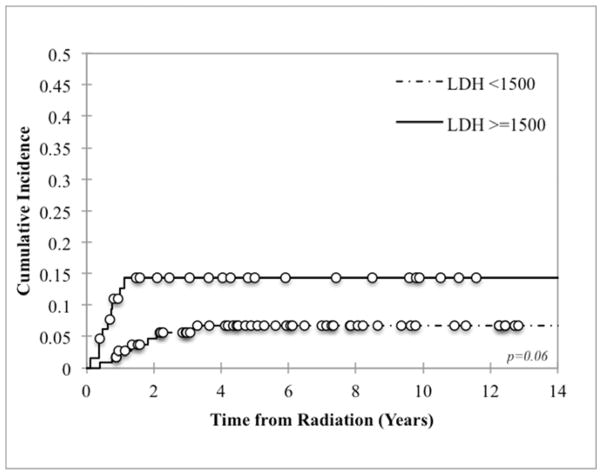
Eighty-six percent (19/22) of all LF were inside the RT field (defined as within the PTV). Of the 3 patients who failed locally outside of the RT field but within the site of the pre-induction primary tumor bed, 2 failures were lateral to the field and 1 was inferior. Patients who required more than 1 surgical resection to achieve GTR had worse local control than those in whom GTR was accomplished in 1 surgery: 22.4% vs 8.3% (p=0.01, Figure 1). There was also a trend towards worse local control with MYCN amplification and LDH ≥1500 U/L (p=0.09 and p=0.06 respectively, Figure 2). LF did not differ by age, tumor site (abdominal versus non-abdominal), time from chemotherapy to RT, response to chemotherapy, or stem cell transplant (Table 3). Local control was 100% among patients with favorable Shimada histopathology, although histology was favorable in only 6% of assessable cases, limiting statistical analysis. Of note, the presence of skeletal metastases at diagnosis was associated with a decrease in LF at the primary site (p=0.003). However, it was also associated with a significant increase in the incidence of distant failures (p=0.009).
Figure 1.
Local control based on number of surgeries required to achieve gross total resection (GTR)
Figure 2.
Local control based on (a) MYCN amplification and (b) LDH
Table 3.
Prognostic factors for local failure (LF)
| Factor | No. of patients | 5-year LF (%) | p |
|---|---|---|---|
| Age at diagnosis | |||
| <4 years | 123 | 9.8 | 0.92 |
| ≥4 years | 123 | 9.5 | |
| Site of primary tumor | |||
| Abdominal | 232 | 9.7 | 0.77 |
| Non-abdominal | 14 | 12.5 | |
| MYCN | |||
| Non-amplified | 131 | 8.2 | 0.09 |
| Amplified | 96 | 14.0 | |
| LDH | |||
| <1500 | 113 | 6.7 | 0.06 |
| ≥1500 | 65 | 14.4 | |
| Ferritin | |||
| <142 | 36 | 6.0 | 0.32 |
| ≥142 | 88 | 11.9 | |
| Shimada histopathology | |||
| Favorable | 11 | 0.0 | 0.27 |
| Unfavorable | 173 | 12.6 | |
| Skeletal metastases | |||
| Present | 195 | 7.0 | 0.003 |
| Absent | 51 | 20.1 | |
| No. of surgeries to achieve GTR | |||
| 1 | 212 | 8.3 | 0.01 |
| >1 | 29 | 22.4 | |
| Time from chemotherapy to RT | |||
| <7.8 months | 121 | 11.2 | 0.60 |
| ≥7.8 months | 125 | 8.5 | |
| Response to chemotherapy | |||
| CR/VGPR | 135 | 11.5 | 0.29 |
| PR/MR/PD | 111 | 7.0 | |
| Stem cell transplant | |||
| Yes | 75 | 6.7 | 0.29 |
| No | 171 | 11.5 | |
Abbreviations: LF = local failure; GTR = gross total resection; RT = radiation therapy; CR/VGPR = complete remission/very good partial remission; PR/MR/PD = partial remission/mixed response/progressive disease
Treatment of local relapse involved external beam radiation therapy (EBRT) alone (n=1), surgery with EBRT (n=3), surgery with intraoperative radiation therapy (IORT, n=3), surgery with IORT and EBRT (n=1), surgery alone (n=1), chemotherapy alone (n=6), chemotherapy and MIBG therapy (n=1), chemotherapy and EBRT (n=1), trial with an aurora kinase inhibitor (n=1), and none due to early mortality (n=5). Five patients who relapsed locally (22.7%) were alive at a median time of 5.0 years from diagnosis.
Progression-free Survival and Overall Survival
One hundred and thirty-five patients (54.9%) relapsed distantly at a median time of 1.5 years from initiation of chemotherapy. Including local failures, the 5-year PFS was 40.0% (95% CI 33.4%–46.6%). Overall survival at 5 years was 64.6% (95% CI 58.2%–71.0%) and at 10 years was 50.4% (95% CI 42.6%–58.1%).
Toxicity
Acute toxicities from RT were mostly grade 1, including nausea (n=109), emesis (n=52), fatigue (n=39), diarrhea (n=37), and dermatitis (n=20). Four patients experienced grade 2 nausea, and 6 patients grade 2 diarrhea. One patient developed grade 3 nausea. One hundred and fifty two patients (62%) did not experience any acute toxicity. Two patients experienced a 1-day break in treatment, 1 due to a respiratory viral infection and 1 because of nausea.
Recorded late effects related to RT among survivors most commonly included musculoskeletal abnormalities such as short stature, slipped capital femoral epiphysis, and scoliosis (n=32); chronic diarrhea / RT enteritis (n=9); and osteochondroma (n=6). Seven patients (2.8%) developed a second cancer at a median time of 5.0 years from diagnosis. All second cancers were hematologic malignancies.
Discussion
In a large cohort of children treated uniformly at our institution with 21-Gy hyperfractionated RT, we found local control to be >90%. Other smaller institutional studies have found similar high rates of local control with 21–24 Gy (Table 4).1–5,7,24,25 A large multi-institutional trial from the Children’s Cancer Group (CCG-3891) suggested a dose response relationship with respect to local control, with 20 Gy providing better local control than 10 Gy in patients with macroscopic residual disease.26 In CCG-3891, patients with gross residual disease after surgery were non-randomly given 10 Gy external beam radiation to the primary site and then randomly assigned to either continuation chemotherapy or myeloablative chemotherapy with 10 Gy total body irradiation; the incidence of LF was 52% vs. 22% with an additional 10 Gy RT.26 From CCG-3891 and single-institution studies, it has been extrapolated that approximately 20–24 Gy provides excellent local control for HR-NB. However, there has never been a clinical trial looking specifically at the role of radiation for local control in patients with high-risk disease. Thus, the indications, technique and dose of radiation are not standardized, and the question of the optimal dose for high-risk disease remains unanswered.
Table 4.
Institutional experiences evaluating local control in high-risk neuroblastoma
| No. of patients | Median RT dose (Gy) | Local failure | |
|---|---|---|---|
|
MSKCC5 1986–2000 |
99 | 21 | 10.1% at 3 years |
|
St. Jude25 2007–2010 |
20 | 23.4 | 0% at 2 years |
|
Emory4 2001–2007 |
34 | 22 | 6% at 3 years |
|
Texas Children’sHospital24 2006–2011 |
30 | 24 | 16% at 5 years |
|
U Washington2 1998–2002 |
21 (17 irradiated) | 21 | 7% at 2 years |
|
Dana-Farber / Children’s Hospital of Philadelphia7 1994–1998 |
52 (39 irradiated) | 22.8* | 3% at 19 months |
|
Ospedale Pediatrico Bambino Gesù3 1996–2009 |
58 (28 irradiated) | 21 | 0% at 5 years in those who got RT |
Cumulative dose including total body irradiation
In our cohort, only patients who underwent GTR and did not have residual disease at the time of RT were included. The majority of patients (>90%) who have undergone surgical resection at our institution in the last 15 years have achieved GTR. Of note, unlike our study, CCG-3891 and other protocols such as NB97 reserved RT only for those with gross residual disease.26,27 Thus, our data cannot be directly compared with these studies and cannot be generalized to patients who have macroscopic residual disease at the time of RT. While low-dose RT is highly effective in the setting of microscopic disease after GTR, RT is not as effective at controlling gross disease at the primary site. Recent protocols have used doses of 36 Gy for gross residual disease, but it is uncertain that this will be effective. In support of the need for a more aggressive approach in patients with subtotal resections, we previously found extent of resection to be prognostic of both local control and survival. In a cohort of 141 patients treated at our institution from 1979–2002, local progression was 10% in patients who underwent GTR vs. 50% in patients who did not.28
Our cohort was large enough to look at other prognostic factors of local control. The number of surgeries required to achieve GTR was prognostic of local control, likely because bulkier tumors required more extensive surgeries. There was also a trend toward worse local control with serum LDH ≥1500 U/L and tumor MYCN amplification, both of which are well-known prognostic factors of survival. We also found the presence of skeletal metastases to be associated with improved local control. However, given the incidence of distant failure of 65.6% in this subgroup, distant progression in bone is probably just a competing risk for local progression. Response to chemotherapy (at the primary and distant sites) was not associated with local control in our cohort. Similarly, primary tumor response does not seem to be predictive of survival outcomes.29
Given the large fields of RT utilized and young patient age, it is important to monitor for RT-induced toxicities. Furthermore, with the recent increase in survival and longevity of patients with HR-NB, late toxicities are now a primary concern. Late effects related to RT in our cohort included most commonly growth abnormalities. However, as our study was not specifically designed to look at late effects, the number reported is likely an underestimation of the true prevalence of long-term toxicities from RT. Among 63 patients treated at our institution previously (89% of whom received RT), late effects from any treatment modality were seen in 95%, including most commonly hearing loss and primary hypothyroidism.30 In this cohort, musculoskeletal abnormalities were seen in 19%. In a study specifically looking at RT-induced late effects among 35 patients treated in NB90 and NB94 with RT, 73% of survivors (16/22) developed RT-related sequelae, 50% of which were in-field and the most frequent of which were musculoskeletal abnormalities.31 The authors also found a dose response relationship, with those receiving >31 Gy most likely to develop an in-field toxicity.
Additionally, recent studies have shown an increased risk of metabolic abnormalities such as diabetes in neuroblastoma survivors. The Childhood Cancer Survivor Study (CCSS) showed that treatment with abdominal radiation is a significant risk factor for the development of diabetes mellitus.14 In fact, among all patients in the CCSS cohort treated with abdominal radiation, those with neuroblastoma were at the highest risk (odds ratio 6.9).14 In a large retrospective study from UK and France, there was a dose-response relationship with respect to the risk of diabetes in patients who received abdominal radiation, with a 16% cumulative incidence of diabetes after >10 Gy to the tail of the pancreas.13 Other studies specifically in survivors of neuroblastoma have confirmed an increased risk of developing diabetes and other components of metabolic syndrome.18 An xxx protocol is currently assessing the incidence of pre-diabetes and diabetes among neuroblastoma survivors treated with abdominal radiation via measurement of markers of insulin sensitivity and β cell function.
Seven patients in our cohort (2.8%) developed a second hematologic malignancy. There were no second solid tumors, although there were 6 survivors who developed an osteochondroma, consistent with previous findings at our institution of a cumulative incidence of osteochondromas of 0.6% at 5 years and 4.9% at 10 years.32 Longer follow-up is needed prior to establishing the true incidence of RT-induced cancers in this cohort.
Treatment with 21 Gy provides outstanding local control rates for HR-NB, and most failures in this patient population are distant rather than local. With the excellent local control rates, young patient age, and increase in incidence of late toxicities seen with improved survival, it is essential to find the dose of RT that minimizes toxicity without compromising local control and survival outcomes. Dose-reduction has been tested and found successful in other pediatric malignancies such as Hodgkin Lymphoma33 and Wilms tumor.34,35 In fact, omission of radiation in patients with HR-NB who have undergone GTR has resulted in favorable local control rates in some studies27,36 but not all.3,37 Although there has been a decrease in life-threatening late effects with reduction in treatment exposure seen with childhood cancers such as Wilms and leukemia, there has been an increase in late mortality in neuroblastoma, attributed to the increase in therapeutic exposure.38 With the primary goal of balancing outcomes with toxicity, and with the favorable rates of local control observed in our large cohort, we recommend dose reduction on protocol in patients with favorable prognostic factors, such as those who achieve GTR and do not have adverse biologic factors like MYCN amplification or LDH ≥1500 U/L. At xxx, we are currently treating children who have undergone GTR with 18 Gy on a step-wise dose-reduction protocol.
In summary, we report local control >90% in a cohort of 246 HR-NB patients treated with GTR and dose-intensive chemotherapy. As most other single institution studies have <40 patients, the major strength of our study is the large number of patients treated within a single institution. In addition, the size of our cohort allowed for statistical comparison of prognostic factors of local control. The main limitation of our report is its retrospective nature. The results of our current prospective dose-reduction protocol should provide valuable benchmarks in determining the optimal dose of radiation for high-risk neuroblastoma.
Supplementary Material
Summary.
We report local control >90% in a cohort of 246 high-risk neuroblastoma patients treated with gross total resection (GTR), dose-intensive chemotherapy, and 21-Gy hyperfractionated radiation therapy. With the primary goal of maintaining rates of cure while minimizing late toxicity, and with the excellent rates of local control observed, radiation dose-reduction may be considered in patients with favorable prognostic factors, such as those who achieve GTR and do not have MYCN amplification.
Acknowledgments
This work was funded in part by NIH/NCATS grant KL2 TR000458 of the Clinical and Translational Science Center at Weill Cornell and the NIH/NCI Cancer Support Grant P30 CA008748.
Footnotes
Conflict of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castleberry RP, Kun LE, Shuster JJ, et al. Radiotherapy improves the outlook for patients older than 1 year with Pediatric Oncology Group stage C neuroblastoma. J Clin Oncol. 1991;9:789–95. doi: 10.1200/JCO.1991.9.5.789. [DOI] [PubMed] [Google Scholar]
- 2.Bradfield SM, Douglas JG, Hawkins DS, et al. Fractionated low-dose radiotherapy after myeloablative stem cell transplantation for local control in patients with high-risk neuroblastoma. Cancer. 2004;100:1268–75. doi: 10.1002/cncr.20091. [DOI] [PubMed] [Google Scholar]
- 3.De Ioris MA, Crocoli A, Contoli B, et al. Local control in metastatic neuroblastoma in children over 1 year of age. BMC Cancer. 2015;15:79. doi: 10.1186/s12885-015-1082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatcombe HG, Marcus RB, Jr, Katzenstein HM, et al. Excellent local control from radiation therapy for high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2009;74:1549–54. doi: 10.1016/j.ijrobp.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 5.xxx
- 6.xxx
- 7.Marcus KJ, Shamberger R, Litman H, et al. Primary tumor control in patients with stage 3/4 unfavorable neuroblastoma treated with tandem double autologous stem cell transplants. J Pediatr Hematol Oncol. 2003;25:934–40. doi: 10.1097/00043426-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda H, August CS, Goldwein JW, et al. Sites of relapse in patients with neuroblastoma following bone marrow transplantation in relation to preparatory “debulking” treatments. J Pediatr Surg. 1992;27:1438–41. doi: 10.1016/0022-3468(92)90195-d. [DOI] [PubMed] [Google Scholar]
- 9.Sibley GS, Mundt AJ, Goldman S, et al. Patterns of failure following total body irradiation and bone marrow transplantation with or without a radiotherapy boost for advanced neuroblastoma. Int J Radiat Oncol Biol Phys. 1995;32:1127–35. doi: 10.1016/0360-3016(95)00011-m. [DOI] [PubMed] [Google Scholar]
- 10.George RE, Li S, Medeiros-Nancarrow C, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–6. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 11.Cheung NK, Cheung IY, Kushner BH, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–70. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vathaire F, El-Fayech C, Ben Ayed FF, et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: a retrospective cohort study. Lancet Oncol. 2012;13:1002–10. doi: 10.1016/S1470-2045(12)70323-6. [DOI] [PubMed] [Google Scholar]
- 14.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med. 2009;169:1381–8. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovi L, Saarinen-Pihkala UM, Vettenranta K, et al. Growth in children with poor-risk neuroblastoma after regimens with or without total body irradiation in preparation for autologous bone marrow transplantation. Bone Marrow Transplant. 1999;24:1131–6. doi: 10.1038/sj.bmt.1702021. [DOI] [PubMed] [Google Scholar]
- 16.Roebuck DJ. Skeletal complications in pediatric oncology patients. Radiographics. 1999;19:873–85. doi: 10.1148/radiographics.19.4.g99jl01873. [DOI] [PubMed] [Google Scholar]
- 17.Sklar CA. Growth following therapy for childhood cancer. Cancer Invest. 1995;13:511–6. doi: 10.3109/07357909509024916. [DOI] [PubMed] [Google Scholar]
- 18.van Waas M, Neggers SJ, Raat H, et al. Abdominal radiotherapy: a major determinant of metabolic syndrome in nephroblastoma and neuroblastoma survivors. PLoS One. 2012;7:e52237. doi: 10.1371/journal.pone.0052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27:289–97. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushner BH, Kramer K, Modak S, et al. Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol. 2009;27:1041–6. doi: 10.1200/JCO.2008.17.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–77. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 22.Hall EJ. Molecular biology in radiation therapy: the potential impact of recombinant technology on clinical practice. Int J Radiat Oncol Biol Phys. 1994;30:1019–28. doi: 10.1016/0360-3016(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 23.xxx
- 24.Mazloom A, Louis CU, Nuchtern J, et al. Radiation therapy to the primary and postinduction chemotherapy MIBG-avid sites in high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2014;90:858–62. doi: 10.1016/j.ijrobp.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pai Panandiker AS, Beltran C, Billups CA, et al. Intensity modulated radiation therapy provides excellent local control in high-risk abdominal neuroblastoma. Pediatr Blood Cancer. 2013;60:761–5. doi: 10.1002/pbc.24350. [DOI] [PubMed] [Google Scholar]
- 26.Haas-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: a Children's Cancer Group study. Int J Radiat Oncol Biol Phys. 2003;56:28–39. doi: 10.1016/s0360-3016(02)04506-6. [DOI] [PubMed] [Google Scholar]
- 27.Simon T, Hero B, Bongartz R, et al. Intensified external-beam radiation therapy improves the outcome of stage 4 neuroblastoma in children > 1 year with residual local disease. Strahlenther Onkol. 2006;182:389–94. doi: 10.1007/s00066-006-1498-8. [DOI] [PubMed] [Google Scholar]
- 28.xxx
- 29.Bagatell R, McHugh K, Naranjo A, et al. Assessment of Primary Site Response in Children With High-Risk Neuroblastoma: An International Multicenter Study. J Clin Oncol. 2016;34:740–6. doi: 10.1200/JCO.2015.63.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.xxx
- 31.Ducassou A, Gambart M, Munzer C, et al. Long-term side effects of radiotherapy for pediatric localized neuroblastoma : results from clinical trials NB90 and NB94. Strahlenther Onkol. 2015;191:604–12. doi: 10.1007/s00066-015-0837-z. [DOI] [PubMed] [Google Scholar]
- 32.xxx
- 33.Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:640–52. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 34.D'Angio GJ, Breslow N, Beckwith JB, et al. Treatment of Wilms' tumor. Results of the Third National Wilms' Tumor Study. Cancer. 1989;64:349–60. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 35.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms' tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol. 1998;16:237–45. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 36.Matthay KK, Atkinson JB, Stram DO, et al. Patterns of relapse after autologous purged bone marrow transplantation for neuroblastoma: a Childrens Cancer Group pilot study. J Clin Oncol. 1993;11:2226–33. doi: 10.1200/JCO.1993.11.11.2226. [DOI] [PubMed] [Google Scholar]
- 37.Robbins JR, Krasin MJ, Pai Panandiker AS, et al. Radiation therapy as part of local control of metastatic neuroblastoma: the St Jude Children's Research Hospital experience. J Pediatr Surg. 2010;45:678–86. doi: 10.1016/j.jpedsurg.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016;374:833–42. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.