Abstract
Introduction
The global shortage of neurosurgeons demands tools to geographically extend the reach of expert surgeons. Technology allowing a remote, experienced surgeon to provide real-time guidance to local surgeons has great potential for training and capacity building in medical centers worldwide. Virtual interactive presence and augmented reality (VIPAR), an iPad®-based tool, allows surgeons to provide long-distance virtual assistance wherever a wireless internet connection is available. Local and remote surgeons view a composite image of video feeds at each station, allowing for intraoperative telecollaboration in real time.
Methods
Local and remote stations were established in Ho Chi Minh City, Vietnam, and Birmingham, Alabama, as part of an ongoing neurosurgical collaboration. An endoscopic third ventriculostomy with choroid plexus coagulation (ETV/CPC) utilizing VIPAR was used for subjective and objective evaluation of system performance.
Results
VIPAR allowed both surgeons to engage in complex visual and verbal communication during the procedure. Analysis of five video clips revealed video delay of 237msec (range: 93–391msec) relative to the audio signal. Excellent image resolution allowed the remote neurosurgeon to visualize all critical anatomy. The remote neurosurgeon could gesture to structures with no detectable difference in accuracy between stations, allowing for sub-millimeter precision. Both local and remote neurosurgeons felt the system improved procedural safety and efficacy.
Conclusion
Evolving technologies allowing long-distance, intra-operative guidance and knowledge transfer hold great potential for highly efficient international neurosurgical education. VIPAR is one example of an inexpensive, scalable platform for increasing global neurosurgical capacity. Efforts to create a network of Vietnamese neurosurgeons using VIPAR for collaboration are underway.
Keywords: Neurosurgery, Pediatrics, Telecommunications, Global Health
Introduction
In much of the world, subspecialty surgical care is not readily available. (11–13,28,48,55,58,67,71) The absence of local subspecialty care has a demonstrable impact on morbidity and mortality,(53,35) and time to surgical intervention is critical in many conditions.(18,53,59) Hands-on training of local surgeons in their home country is the optimal method for increasing global surgical capacity, and technology allowing a remote, experienced surgeon to provide real-time guidance to local surgeons has great potential for training and capacity building.(8,30,38)
Telesurgery, the use of robotic actuators allowing a geographically remote surgeon to perform a procedure, has attracted growing interest over the past two decades,(2,3,5,8,9,14,25,27,29,32–34,42,44–46,50,51,54,62–64,69) and robotic tools have been used in multiple subspecialties and across long distances.(8,10,25,30,31,33,37,38,42,45,46,63,65) However, the adaptation of telesurgical systems to developing countries is hampered by issues of cost,(8,17,44) connectivity,(51,52,62) and the continued need for skilled operators at the surgical site. Additionally, most neurosurgical procedures are not amenable to existing robotic technology, and the cost of complex systems has limited the role of robotic tools in neurosurgery.(20,41)
Telepresence involves nonrobotic tools to support interactive video and audio telecollaboration in which a remote surgeon provides guidance and training without directly performing the procedure. Telepresence systems have grown in popularity alongside telesurgical tools,(69) but prior systems were limited to providing assistance through verbal exchange or use of a pointer tool.(21,56)
Virtual Interactive Presence and Augmented Reality (VIPAR) is a recently developed tool which allows surgeons to provide real-time virtual assistance and training wherever a standard internet connection is available.(60,61) The technology provides a hybrid perspective of local and remote video feeds, allowing a remote surgeon to digitally “reach into the surgical field”, highlighting anatomic structures and providing visual demonstration of complex operative techniques.
VIPAR can be rapidly deployed under sterile conditions,(47) and has been used in orthopedic surgery for training of resident surgeons with an attending surgeon immediately available in an adjoining room.(49) VIPAR has been shown to be feasible for long-distance telecollaboration in neurosurgical studies on cadaveric specimens,(61) but the use of long-distance VIPAR has never been reported in neurosurgical patients or for international collaboration.
Here we describe the performance, utility, and feasibility of implementing VIPAR as a tool for global surgical education and telecollaboration between neurosurgeons in the United States and Vietnam.
Materials and methods
Overview
Neurosurgeons from the Children’s of Alabama Hospital in Birmingham, Alabama, USA traveled to Children’s Hospital #2 in Ho Chi Minh City, Vietnam, to provide lectures, in-clinic instruction, and intra-operative training to local neurosurgeons on advanced techniques in pediatric neurosurgery. The VIPAR system was implemented and trialed in neuroendoscopy and cases requiring use of the operative microscope, and used for international telecollaboration and continuing education following the return of the visiting team to Children’s of Alabama. Institutional Review Board approval was obtained from both the University of Alabama at Birmingham as well as the Ethical Review Committee at Children’s Hospital #2.
VIPAR
The VIPAR system consists of a local station and a remote station connected over a local wireless or 3G mobile connection, providing worldwide point-to-point connectivity. Local and remote stations were established at Children’s Hospital #2 and Children’s of Alabama Hospital, respectively.
Both local and remote surgeons view a composite image of video feeds at each station, allowing for visual demonstration and tele-collaboration. The proprietary software performs real-time calibrations to spatially match the local and remote visual feeds, and uses a merging feature to overlay the two images. The distant station image appears as a semitransparent overlay on the local station image,(60) and a single hybrid image is displayed to both parties. While early iterations required complex video capture and display systems,(60,61) newer versions run on iPad devices, and use a commercially available app, Lime™, downloaded onto the device. The forward-facing camera on each iPad provides video and audio capture, while the iPad screen provides video display. An iPad Air 2 was used at both local and remote stations to provide 1080p HD video recording (30 frames per second). A schematic of the VIPAR system is presented in Figure 1.
Figure 1. Diagram of the VIPAR system.
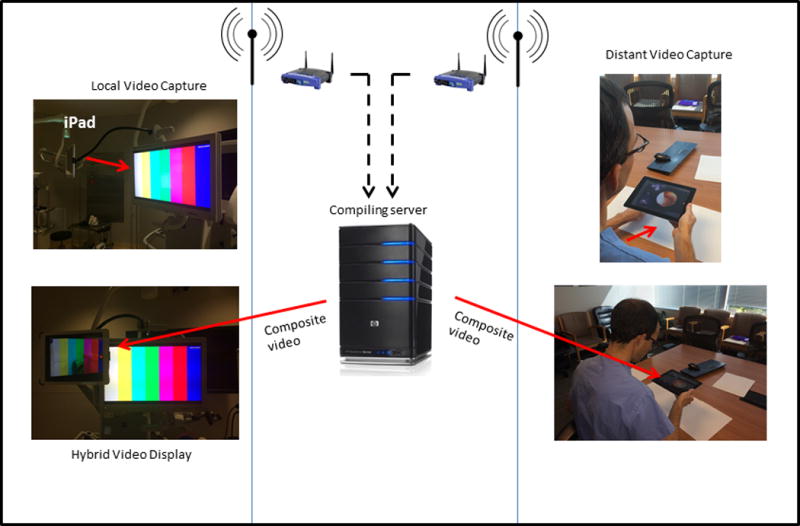
Local and distant video and audio feeds are compiled to create a single composite with each surgeon viewing a common field. The distant video feed is seen as a semitransparent overlay on the background of the local video feed
VIPAR runs on iOS6.0 or later. Information is transmitted between users using AES 128 encryption. Servers record the instance of the communication, including the start and end times of the connection. No data about content of the communication is known or recorded by the vendor servers, and neither video nor audio may be directly recorded using the VIPAR software, allowing for secure data transfer.
Local station at Children’s Hospital #2
The local station was constructed in the neurosurgery operating room at Children’s Hospital #2 in Ho Chi Minh City, Vietnam, using an iPad Air 2 and locally available internet connection. The local device was fixated to either the endoscopy tower or the operative microscope using a commercially available flexible support arm (Hoverbar 3®). Positioning of the device entails directing the camera toward the endoscopic or microscope video projection, while the iPad screen is left visible to the operating surgeon, and located outside of the surgical field. The local station setup is shown in Figure 2, left.
Figure 2. Setup of local and distant stations for neuroendoscopy.
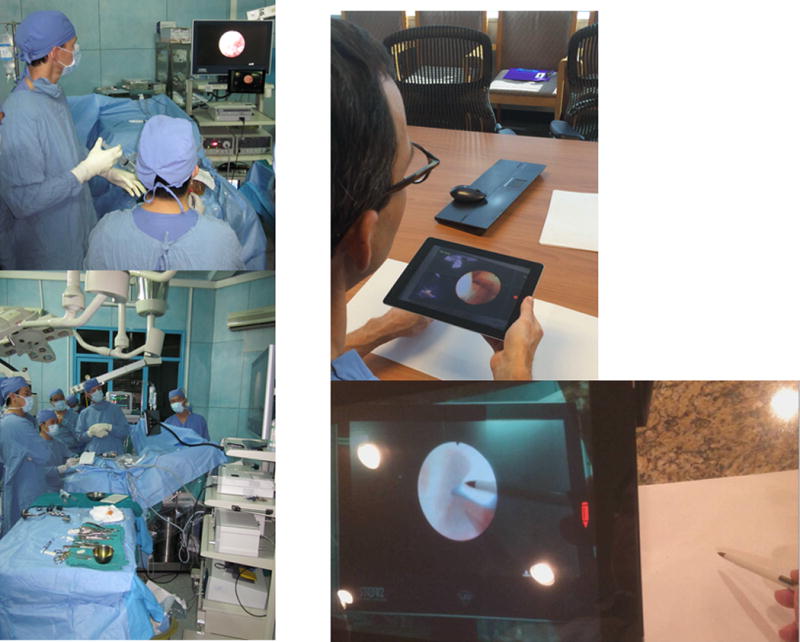
The local station within the operative suite is depicted on the left, while the setup for the distant station is shown on the right.
Distant station at Children’s of Alabama Hospital
The distant station was set up in a conference room at Children’s of Alabama Hospital in Birmingham, Alabama, USA, using a separate iPad Air 2 and local wireless internet connection. A pediatric neurosurgeon directed the forward-facing iPad camera at a white background, and placed their hands and instruments into the camera capture field. The distant station also carries a telestration feature on the iPad screen which allows the expert surgeon to freeze the screen or draw on the image using a two-dimensional pen tool. The distant station setup is shown in Figure 2, right.
Connectivity
While early VIPAR models required high-speed fiber-based local connectivity, the latest iteration allows the system to function with upload and download speeds within the throughput capacity of wireless network and 3G mobile internet connectivity. Connection between stations uses commercial codecs (Polycom vsx7000, and Tanberg, Cisco Systems).
Both local area wireless network and 3G mobile internet connectivity at the local station were evaluated. A Linksys WRT54GL Wi-Fi Wireless-G Broadband Router installed in the operating theater provided connectivity to the local internet service provider. The XCom Global Mobile Wi-Fi Hotspot®, which uses a local 3G mobile phone network to deliver internet connectivity, was also evaluated. A local area wireless network was used at the distant station for both trials. Upload speeds, download speeds, and mean transit times were measured for each method of connectivity using Network Analyzer™ a commercially available application downloadable onto iOS devices.
Audio and video composite latency and accuracy analysis
Time difference between images depends on local processing times, which are typically fixed, and internet transmission delay, which can fluctuate. Delays in internet transmission and image compilation were assessed through off-line video analysis. An endoscopic third ventriculostomy and choroid plexus coagulation (ETV/CPC) utilizing VIPAR was used for evaluation of system performance. Independent videos of the local and remote composite fields were recorded. Video clips were synchronized to audio and identifiable movements at each station, and the delay between each video assessed in milliseconds. Composite accuracy was assessed by each surgeon touching the same indicated point and providing verbal confirmation they see the other surgeon touching the same point.
Clinical utility analysis
Both the local and distant surgeons were queried on overall utility of the tele-collaboration experience via questionnaire using a five-point Likert scale. Both surgeons were asked to rate the VIPAR system on the following criteria, where 1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, and 5 = strongly agree:
Use of the tele-communication system:
changed the course of the procedure (1–5)
resulted in a safer procedure (1–5)
resulted in a more effective procedure (1–5)
was useful overall (1–5)
resulted in increased fatigue (1–5)
Cost analysis
Assessment of both direct and indirect costs associated with institution of the VIPAR system was performed. Expense data were subdivided as follows: visiting team expenses, local station hardware, distant station hardware, proprietary software, internet connection, technical support.
Source of funding
Use of proprietary software was provided by Vipaar, LLC. This work was additionally supported by a grant from the Children’s of Alabama Global Health Program Initiative and the Kaul Foundation.
Results
Successful implementation and trial of the VIPAR telecollaboration system took place as part of ongoing neurosurgical collaboration between Children’s of Alabama Hospital and Children’s Hospital #2 in Ho Chi Minh City, Vietnam. A strong relationship exists between these institutions, with regular exchange of general surgery and neurosurgery teams. Cases requiring either the endoscope or operative microscope were performed using VIPAR assistance. Following return of the visiting team to their home institution, VIPAR was effective in providing transnational intraoperative assistance.
Local hospital
All cases were performed at Children’s Hospital #2 in Ho Chi Minh City. Five pediatric neurosurgeons provide care for the full spectrum of pediatric neurosurgical disease, and train one pediatric neurosurgeon per year. Southern Vietnam, with a population of nearly fifty million, is served by ten pediatric neurosurgeons (personal communication, 2015, D. Can), with varying levels of subspecialty training. In certain cases, pediatric neurosurgery training is distinct from adult neurosurgery residency, and consists of a three-year program started immediately following completion of medical school, which lasts either four or six years. There are two pediatric neurosurgery training programs for all of Vietnam, one located in Ho Chi Minh City, and the second located in Hanoi. For the calendar year 2014, 613 total pediatric neurosurgical procedures were performed at Children’s Hospital #2 (breakdown of cases provided in Table 1).
Table 1.
Neurosurgical cases performed at Children’s Hospital #2 during 2014
| Case Type | Number of Cases |
|---|---|
| Craniotomy for trauma | 96 |
| Craniotomy for tumor or biopsy | 123 |
| Craniotomy for infection* | 38 |
| Ventricular shunt† | 127 |
| ETV/CPC | 44 |
| Craniosynostosis correction | 18 |
| Craniotomy for vascular pathology‡ | 14 |
| Craniotomy for other§ | 49 |
| Diagnostic cerebral angiogram | 44 |
| Neuroendovascular intervention | 1 |
| Spine for trauma | 2 |
| Spine for tumor or vascular lesion | 14 |
| Spine for neural tube defects | 43 |
includes primary brain abscess, empyema
includes placement of new ventricular shunts, revisions, exploration, removal, and replacement
includes evacuation of spontaneous intracranial hemorrhage, encephaloduroarteriosynangiosis
incudes Chiari malformation repair, encephaloceles, wound washout, and other miscellaneous cases
VIPAR local trial
Initial trials took place while the visiting team was present to provide immediate hands-on intraoperative assistance if needed.
Endoscopic trials
Case 1
An ETV/CPC was performed in a seven-month old male with hydrocephalus and a Dandy-Walker malformation variant. A STORZ 2.2mm flexible endoscope was used with display on a high-definition 26-inch, 16:9 HD format, 1920 × 1200 pixel resolution digital monitor. The local station was set up as described above (Figure 2). One expert neurosurgeon remained scrubbed throughout the case, while a second visiting neurosurgeon set up the distant station in an adjacent room. Stations were connected over the same local area wireless network. One episode of dropped call occurred, requiring less than 1 minute to correct. Several sub-second episodes of noticeable transient video delay occurred. No audio delay was detected. Excellent registration was observed. VIPAR was used for a total of 2 hours and 11 minutes, with 16% battery usage over that time.
Case 2
ETV/CPC with biopsy of a third ventricular mass was performed on a 2 year-old male using the set-up described above. No episodes of dropped call, audio or detectable video delay occurred during a 40 minute run period.
Operative microscope trial
Case 3
A right pterional craniotomy was performed for biopsy of an enhancing infundibular mass in a five year-old female who presented with diabetes insipidus. The local iPad was fixated to the operative microscope and directed at the display screen, while still viewable by the operating surgeon (Figure 3). Resolution was adequate to allow the remote surgeon to identify all relevant microsurgical anatomy.
Figure 3. Setup of local station for cases requiring use of the operative microscope.
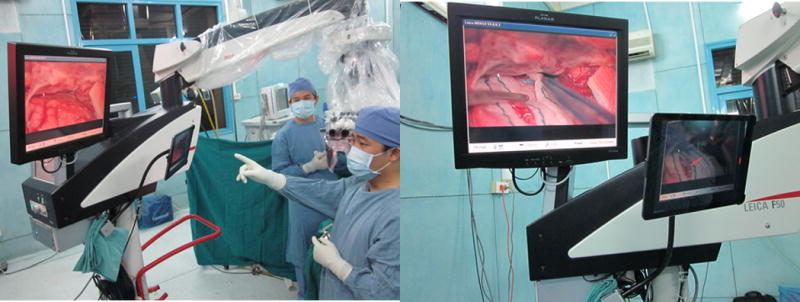
The local iPad is pointed toward the microscope display, while the screen is directed toward the surgeon, outside of the operative field.
VIPAR international trial
An attending pediatric neurosurgeon in the United States was contacted using VIPAR while a visiting neurosurgeon remained scrubbed during an ETV/CPC on a 6 month-old female, allowing collaboration spanning 14,904 kilometers. VIPAR was used throughout the endoscopic portion of the procedure, without noticeable interaction delay or appreciable difference in resolution between the two sites. The system allowed for discussion of procedural strategy and visual conveyance of surgical maneuvers which would not have been possible with standard video conferencing. Video 1 demonstrates the system in use at both the local and remote stations.
Audio latency
Optical fiber cables provide long distance telecommunication through the transmission of light impulses. Minimum latency time is dependent on the speed of light (299,792 kilometers per second in a vacuum) and a standard fiber delay ratio, estimated at 1.52 for the purpose of this study. Most telecommunications networks connect between multiple nodes, significantly increasing total distance a signal must travel between each station. Even if a single fiberoptic cable connected directly between the two stations in this study, a minimum lag time of 75.54 msec would be expected simply for light to travel from one station to the other. Despite the great distances involved, audio delay was not perceptible to participants at either station.
Video composite latency
Off-line analysis was performed using independent videos of the local and remote stations. Video clips which included unique movements and audio were used for synchronization and frame-by-frame analysis. The local-to-remote station video latency averaged 237 msec relative to the audio signal (range: 93–391 msec). While the surgeon at each station therefore viewed their counterparts’ field as slightly delayed relative to their own, this did not interfere with performing the procedure. The number of elapsed frames between the synchronized videos was used as the metric of latency time between the two stations.
Composite accuracy
Confirmation of accuracy between stations was performed by the distant surgeon pointing at specific anatomic structures at the request of the local surgeon, and tracing clearly identifiable borders of the local video feed. Each participant agreed the spatial accuracy was sufficient such that any difference was imperceptible. This was confirmed on off-line video analysis.
Connectivity
Local station area wireless upload speeds ranged from 7.25 to 8.24 Mbps, with download speeds from 3.97 to 5.54 Mbps (IP address 192.168.4.187). Distant station wireless upload speeds ranged from 26.39 to 27.62 Mbps, with download speeds from 31.90 to 34.43 Mbps (IP address 138.26.72.17). Round trip time ranged from 321.71 to 363.41msec over 200 test packets. 3G mobile wireless upload speeds ranged from 2.39 to 3.31 Mbps, with download speeds from 2.98 to 5.28 Mbps.
Set-up and disassembly
Setting up the local station and breakdown at the end of a case took less than 10 minutes to complete. Setup time for the distant station consists only of finding a white background toward which to direct the iPad camera. Operative times were not felt to be significantly affected by use of the VIPAR system.
Cost
Financial data collected included visiting team expenses, local station hardware, distant station hardware, proprietary software, internet connection, and technical support. Three main internet service providers in Ho Chi Minh City, Vietnam Posts and Telecommunications group, Viettel, and FPT, offer fiber-based and 3G mobile wireless connectivity. Internet costs for this study included $80 USD for placement of a Linksys WRT54GL Wi-Fi Wireless-G Broadband Router within the neurosurgery operating theater, with no additional cost incurred for use of Children’s Hospital #2 internet access. Individual subscriber internet access ranges from 260,000–2,070,000 Vietnamese Dong (12$–96$ USD) per month in Ho Chi Minh City based on connection speeds and data usage, and these rates are used for cost analysis. Total costs for establishing the VIPAR system were $14,930.39 USD for one calendar year. $12,504.60 of this was associated with the two-week visiting team experience. A breakdown of financial data is presented in Table 2.
Table 2.
Financial outlay of establishing an international telecollaboration system for one year
| Breakdown of Costs (USD) | ||
|---|---|---|
|
| ||
| Local station | 1,576.89 | |
| iPad Air 2 | 548.90 | |
| Lime™ subscription (1 subscriber, $25 per subscriber per month) | 300.00 | |
| Wireless internet access (12 months, mean $54 per month) | 648.00 | |
| Wireless Router | 79.99 | |
|
| ||
| Visiting team expenses | 12,504.60 | |
| Flights (3 participants, round-trip flights) | 7254.60 | |
| Accommodations (2 hotel rooms, 14 total days) | 4200.00 | |
| Meals (3 participants, $25 per diem) | 1050.00 | |
|
| ||
| Distant station | 848.90 | |
| iPad Air 2 | 548.90 | |
| Lime™ subscription (1 subscriber, $25 per subscriber per month) | 300.00 | |
|
| ||
| TOTAL EXPENDITURE | 14,930.39 | |
Ongoing collaboration
Following return of the visiting team, VIPAR continues to be used for intraoperative assistance and training for neuroendoscopic cases. Fifteen additional ETV/CPC procedures have been performed using VIPAR for long distance collaboration since return of the visiting surgical team, each without complication or hardware failure. As above, excellent registration and resolution were observed in fourteen cases. In one case, there was a transient loss of audio connectivity without disturbance of video connectivity. This did not interfere with the procedure as visual graphics tools were used to point out anatomy and suggestions for location of the ETV. Twelve of the fifteen patients remain shunt-free as of last follow-up. There have been no other complications observed in any cases. Over the six months immediately prior to the introduction of VIPAR, twenty seven ETV/CPCs were performed at Children’s Hospital #2, all for aqueductal stenosis. Complications prior to VIPAR included severe intraventricular bleeding requiring an external ventricular drain in two patients (7.4%), subdural hematoma in one patient (3.7%), postoperative CSF leak in one patients (18.5%), and death due to hemorrhage from a basilar artery injury in one patient (3.7%).
VIPAR has additionally been utilized for global telecollaboration during cases requiring use of the operative microscope, including resection of a large cerebellar tumor and clipping of a distal posterior inferior cerebellar artery aneurysm.
Clinical utility
Local and distant surgeons reported the VIPAR telecommunication system to be very useful for operating neurosurgeons in Ho Chi Minh City, Vietnam. On a 5-point Likert scale where 1=strongly disagree and 5=strongly agree, each surgeon strongly agreed that VIPAR was useful overall (5) and resulted in a more effective procedure (5). Each surgeon also agreed VIPAR changed the course of the procedure (4) and resulted in a safer procedure (4), and disagreed with the statement: “VIPAR resulted in increased fatigue” (2).
Discussion
In the coming years the global shortage of surgeons is only expected to worsen.(58,67) Surgical disease makes up one of the top fifteen causes of global disability,(40) and surgical intervention fills a crucial role in global public health.(22) This gap necessitates the development of tools to geographically extend the reach of expert surgeons. While robotic systems provide an extended geographic reach of a single surgeon, the VIPAR system allows long-distance assistance during complex cases as well as training of local surgeons. Though the VIPAR system was initially created for use through a binocular videoscope or attachment to the operative microscope, the technology has been adapted to other commercially available systems in which both video recording and display are possible, such as Google Glass or iPad. These devices are relatively inexpensive, and may prove to be valuable tools for global neurosurgical education and capacity building. Endoscopic, endovascular and microsurgical cases already rely on video projection for the critical portion of the procedure, and are ideally suited to implementation of VIPAR technology. ETV/CPC, increasingly utilized for primary treatment of infant hydrocephalus throughout the world,(64) provided an excellent example in our series.
Surgical outcomes are heavily influenced by technical acumen, and unexpected intraoperative situations may arise which would benefit from the expertise of a more experienced or specialized surgeon. Additionally, geographically remote surgeons may be called upon to assist with an emergent procedure which cannot wait for transfer to higher levels of care. In both instances, the value of a feasible paradigm permitting the digital presence of an expert surgeon within the operative field becomes clear. Telecollaboration has been demonstrated for the education of orthopedic surgery residents,(49) but has never been used for international surgical training. The VIPAR system is both practical and simple, and provides a visual adjunct to verbal description of complex surgical procedures and techniques.
Expert surgeons may have the ability to spend short periods of time providing hands-on training in developing countries, but not able to commit to longer periods. The number of short-term surgical trips has increased dramatically over the past thirty years,(68) but the lack of emphasis on training and frequent absence of skilled follow-up have led to criticisms of the short-term trip model.(16,36) While surgeons hailing from developing countries may alternatively visit the United States for longer term observerships, actual participation in surgery is largely prohibited. Immersive learning paradigms emphasizing active participation are essential for developing new skills.(6,15) As a result, the ideal method for capacity building involves hands-on training of surgeons in their home country, performing cases on their own patients. In trauma and critically ill patients, non-virtual interactive tools for extending the expertise of sub-specialists are associated with reduced morbidity and mortality.(39,70) A versatile and scalable digital telecollaboration technology to enmesh the expertise of a remote surgeon into the operative field could serve as a valuable adjunct to in-person training efforts. In this study, VIPAR allowed for ongoing skill and knowledge transfer following return of the visiting team to their own clinical practice.
The complexity of surgical execution cannot be easily conveyed by face-to-face video, and evolving technologies provide novel solutions for surgical training and remote assistance. General and orthopedic surgery programs have adopted surgical simulators for training in laparoscopic, arthroscopic and robotic techniques,(4,66) observing shortened trainee learning curves and no decline in patient outcomes.(1,19,23,24,26,43,57,72) While sophisticated, high overhead costs limit the application of simulators in the developing world, and current simulators cannot reproduce the wide range of potential complications. Additionally, surgical simulators do not presume even the most basic training. By contrast, complex and cumbersome robotic actuators still require highly skilled local surgeons to cope with unstable circumstances or system failure, limiting their application in neurosurgery (41). Interactive telecollaboration systems such as VIPAR serve as a bridge, providing new domain skills to local surgeons who already possess a functional skill set.
Such technology is not meant to replace standard neurosurgical training, but rather act as a complementary method that facilitates mentoring without physical presence of the experienced surgeon. We envision this technology as providing that last bridge of mentorship, taking a competent surgeon with fundamental neurosurgical skills and providing real-time feedback to coach them towards true expertise. While telecollaboration has great potential for capacity building, elective cases should not be performed without local expert support readily available, unless the local surgeon has adequate training to complete the case without VIPAR assistance. Technical delays or loss of internet connectivity may leave the local surgeon without expert assistance, and thus caution is warranted if use of long-distance telecollaboration tools leads a local surgeon to “over-reach” in case selection. For emergent cases, backup internet access using mobile 3G wireless internet connectivity is recommended in the event of local area wireless internet failure, to decrease the risk of losing all contact with the distant expert.
Ongoing efforts are underway to create a network of Vietnamese neurosurgeons using VIPAR technology to increase collaboration both within Vietnam and with our group in Alabama. Within the United States, VIPAR is currently under evaluation for utility in the outpatient setting as well. Issues facing the widespread adoption of digital telecollaboration tools include reimbursement and liability, as well as rigorous assessment of the impact on patient outcomes.
Conclusions
Giving remote experts the ability to guide and mentor less experienced surgeons has great potential for global surgical education and capacity building. VIPAR is one example of evolving interactive technology allowing for real-time global surgical telecollaboration and education through commercially available and inexpensive platforms. Use of such technology may increase the safety of surgical intervention, and has great potential for training, research, assessing surgical competence for maintenance of certification, and fostering relationships between geographically isolated physicians.
References
- 1.Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. Ann Surg. 2007;246(5):771–779. doi: 10.1097/SLA.0b013e3180f61b09. [DOI] [PubMed] [Google Scholar]
- 2.Allen D, Bowersox J, Jones GG. Telesurgery. Telepresence. Telementoring. Telerobotics. Telemed Today. 1997;5(3):18–20. 25. [PubMed] [Google Scholar]
- 3.Anvari M. Telesurgery: remote knowledge translation in clinical surgery. World J Surg. 2007;31(8):1545–1550. doi: 10.1007/s00268-007-9076-5. [DOI] [PubMed] [Google Scholar]
- 4.Atesok K, Mabrey JD, Jazrawi LM, Egol KA. Surgical simulation in orthopaedic skills training. J Am Acad Orthop Surg. 2012;20(7):410–422. doi: 10.5435/JAAOS-20-07-410. [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results. Surg Endosc. 2002;16(10):1389–1402. doi: 10.1007/s00464-001-8283-7. [DOI] [PubMed] [Google Scholar]
- 6.Bloom BS, Krathwohl DR, Masia BB. Taxonomy of Educational Objectives: The Classification of Educational Goals. New York: Longman; 1984. [Google Scholar]
- 7.Brower V. The cutting edge in surgery. Telesurgery has been shown to be feasible–now it has to be made economically viable. EMBO Rep. 2002;3(4):300–301. doi: 10.1093/embo-reports/kvf083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bove P, Stoianovici D, Micali S, et al. Is telesurgery a new reality? Our experience with laparoscopic and percutaneous procedures. J Endourol. 2003;17(3):137–142. doi: 10.1089/089277903321618699. [DOI] [PubMed] [Google Scholar]
- 9.Bowersox JC, Cordts PR, LaPorta AJ. Use of an intuitive telemanipulator system for remote trauma surgery: an experimental study. J Am Coll Surg. 1998;186(6):615–621. doi: 10.1016/s1072-7515(98)00105-7. [DOI] [PubMed] [Google Scholar]
- 10.Clayman RV. Transatlantic robot-assisted telesurgery. J Urol. 2002;168(2):873–874. [PubMed] [Google Scholar]
- 11.Cobey JC. The surgeon shortage: constructive participation during health reform. J Am Coll Surg. 2010;211(4):568. doi: 10.1016/j.jamcollsurg.2010.07.005. author reply 568. [DOI] [PubMed] [Google Scholar]
- 12.Cofer JB, Burns RP. The developing crisis in the national general surgery workforce. J Am Coll Surg. 2008;206(5):790–795. doi: 10.1016/j.jamcollsurg.2007.12.017. discussion 795–797. [DOI] [PubMed] [Google Scholar]
- 13.Cohn SM, Price MA, Villarreal CL. Trauma and surgical critical care workforce in the United States: a severe surgeon shortage appears imminent. J Am Coll Surg. 2009;209(4):446–452.e4. doi: 10.1016/j.jamcollsurg.2009.06.369. [DOI] [PubMed] [Google Scholar]
- 14.Doarn CR, Hufford K, Low T, Rosen J, Hannaford B. Telesurgery and robotics. Telemed J E Health. 2007;13(4):369–380. doi: 10.1089/tmj.2007.9980. [DOI] [PubMed] [Google Scholar]
- 15.Dreyfus HL, Dreyfus SE. The Ethical Implications of the Five-Stage Skill-Acquisition Model. Bulletin of Science, Technology and Society. 2004;24(3):251–264. doi: 10.1177/0270467604265023. [DOI] [Google Scholar]
- 16.Dupuis CC. Humanitarian missions in the third world: a polite dissent. Plast Reconstr Surg. 2004;113(1):433–435. doi: 10.1097/01.PRS.0000097680.73556.A3. [DOI] [PubMed] [Google Scholar]
- 17.Eadie LH, Seifalian AM, Davidson BR. Telemedicine in surgery. Br J Surg. 2003;90(6):647–658. doi: 10.1002/bjs.4168. [DOI] [PubMed] [Google Scholar]
- 18.Eastman AB. Wherever the dart lands: toward the ideal trauma system. J Am Coll Surg. 2010;211(2):153–168. doi: 10.1016/j.jamcollsurg.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Edelman DA, Mattos MA, Bouwman DL. Value of fundamentals of laparoscopic surgery training in a fourth-year medical school advanced surgical skills elective. J Surg Res. 2012;177(2):207–210. doi: 10.1016/j.jss.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Eljamel MS. Robotic neurological surgery applications: accuracy and consistency or pure fantasy? Stereotact Funct Neurosurg. 2009;87(2):88–93. doi: 10.1159/000202974. [DOI] [PubMed] [Google Scholar]
- 21.Ereso AQ, Garcia P, Tseng E, et al. Live transference of surgical subspecialty skills using telerobotic proctoring to remote general surgeons. J Am Coll Surg. 2010;211(3):400–411. doi: 10.1016/j.jamcollsurg.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Farmer PE, Kim JY. Surgery and global health: a view from beyond the OR. World J Surg. 2008;32(4):533–536. doi: 10.1007/s00268-008-9525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Learning curves and impact of previous operative experience on performance on a virtual reality simulator to test laparoscopic surgical skills. Am J Surg. 2003;185(2):146–149. doi: 10.1016/s0002-9610(02)01213-8. [DOI] [PubMed] [Google Scholar]
- 24.Grantcharov TP, Kristiansen VB, Bendix J, Bardram L, Rosenberg J, Funch-Jensen P. Randomized clinical trial of virtual reality simulation for laparoscopic skills training. Br J Surg. 2004;91(2):146–150. doi: 10.1002/bjs.4407. [DOI] [PubMed] [Google Scholar]
- 25.Guillonneau B, Jayet C, Tewari A, Vallancien G. Robot assisted laparoscopic nephrectomy. J Urol. 2001;166(1):200–201. [PubMed] [Google Scholar]
- 26.Henn RF, Shah N, Warner JJP, Gomoll AH. Shoulder arthroscopy simulator training improves shoulder arthroscopy performance in a cadaveric model. Arthroscopy. 2013;29(6):982–985. doi: 10.1016/j.arthro.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen JF, Hill JW. Advanced telepresence surgery system development. Stud Health Technol Inform. 1996;29:107–117. [PubMed] [Google Scholar]
- 28.Kane GC, Grever MR, Kennedy JI, et al. The anticipated physician shortage: meeting the nation’s need for physician services. Am J Med. 2009;122(12):1156–1162. doi: 10.1016/j.amjmed.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Kong M, Du Z, Sun L, Fu L, Jia Z, Wu D. A robot-assisted orthopedic telesurgery system. Conf Proc IEEE Eng Med Biol Soc. 2005;1:97–101. doi: 10.1109/IEMBS.2005.1616351. [DOI] [PubMed] [Google Scholar]
- 30.Larkin M. Transatlantic, robot-assisted telesurgery deemed a success. Lancet. 2001;358(9287):1074. doi: 10.1016/S0140-6736(01)06240-7. [DOI] [PubMed] [Google Scholar]
- 31.Latifi R, Peck K, Porter JM, Poropatich R, Geare T, Nassi RB. Telepresence and telemedicine in trauma and emergency care management. Stud Health Technol Inform. 2004;104:193–199. [PubMed] [Google Scholar]
- 32.Latifi R, Weinstein RS, Porter JM, et al. Telemedicine and telepresence for trauma and emergency care management. Scand J Surg. 2007;96(4):281–289. doi: 10.1177/145749690709600404. [DOI] [PubMed] [Google Scholar]
- 33.Lee BR, Png DJ, Liew L, et al. Laparoscopic telesurgery between the United States and Singapore. Ann Acad Med Singap. 2000;29(5):665–668. [PubMed] [Google Scholar]
- 34.Lum MJH, Rosen J, Lendvay TS, Wright AS, Sinanan MN, Hannaford B. TeleRobotic fundamentals of laparoscopic surgery (FLS): effects of time delay–pilot study. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5597–5600. doi: 10.1109/IEMBS.2008.4650483. [DOI] [PubMed] [Google Scholar]
- 35.Lynge DC, Larson EH. Workforce issues in rural surgery. Surg Clin North Am. 2009;89(6):1285–1291. vii. doi: 10.1016/j.suc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Maki J, Qualls M, White B, Kleefield S, Crone R. Health impact assessment and short-term medical missions: a methods study to evaluate quality of care. BMC Health Serv Res. 2008;8:121. doi: 10.1186/1472-6963-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marescaux J, Leroy J, Gagner M, et al. Transatlantic robot-assisted telesurgery. Nature. 2001;413(6854):379–380. doi: 10.1038/35096636. [DOI] [PubMed] [Google Scholar]
- 38.Marescaux J, Leroy J, Rubino F, et al. Transcontinental robot-assisted remote telesurgery: feasibility and potential applications. Ann Surg. 2002;235(4):487–492. doi: 10.1097/00000658-200204000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marttos A, Kelly E, Graygo J, et al. Usability of telepresence in a level 1 trauma center. Telemed J E Health. 2013;19(4):248–251. doi: 10.1089/tmj.2012.0102. [DOI] [PubMed] [Google Scholar]
- 40.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendez I, Hill R, Clarke D, Kolyvas G, Walling S. Robotic long-distance telementoring in neurosurgery. Neurosurgery. 2005;56(3):434–440. doi: 10.1227/01.neu.0000153928.51881.27. discussion 434–440. [DOI] [PubMed] [Google Scholar]
- 42.Menkis AH, Kodera K, Kiaii B, Swinamer SA, Rayman R, Boyd WD. Robotic Surgery, the First 100 Cases: Where Do We Go from Here? Heart Surg Forum. 2004;7(1):1–4. [PubMed] [Google Scholar]
- 43.Modi CS, Morris G, Mukherjee R. Computer-simulation training for knee and shoulder arthroscopic surgery. Arthroscopy. 2010;26(6):832–840. doi: 10.1016/j.arthro.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 44.Moses GR, Doarn CR. Barriers to wider adoption of mobile telerobotic surgery: engineering, clinical and business challenges. Stud Health Technol Inform. 2008;132:308–312. [PubMed] [Google Scholar]
- 45.Nguan C, Miller B, Patel R, Luke PPW, Schlachta CM. Pre-clinical remote telesurgery trial of a da Vinci telesurgery prototype. Int J Med Robot. 2008;4(4):304–309. doi: 10.1002/rcs.210. [DOI] [PubMed] [Google Scholar]
- 46.Nguan CY, Morady R, Wang C, et al. Robotic pyeloplasty using internet protocol and satellite network-based telesurgery. Int J Med Robot. 2008;4(1):10–14. doi: 10.1002/rcs.173. [DOI] [PubMed] [Google Scholar]
- 47.Phillips JD, Withrow K. Virtual Interactive Presence: An Operative Feasibility Study. Otolaryngology – Head and Neck Surgery. 2012;147(2 Suppl):P143–P143. doi: 10.1177/0194599812451426a59. [DOI] [Google Scholar]
- 48.Polk HC, Vitale DS, Qadan M. The very busy urban surgeon: another face of the evermore obvious shortage of general surgeons. J Am Coll Surg. 2009;209(1):144–147. doi: 10.1016/j.jamcollsurg.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Ponce BA, Jennings JK, Clay TB, May MB, Huisingh C, Sheppard ED. Telementoring: use of augmented reality in orthopaedic education: AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(10):e84. doi: 10.2106/JBJS.M.00928. [DOI] [PubMed] [Google Scholar]
- 50.Rassweiler J, Frede T. Robotics, telesurgery and telementoring–their position in modern urological laparoscopy. Arch Esp Urol. 2002;55(6):610–628. [PubMed] [Google Scholar]
- 51.Rayman R, Croome K, Galbraith N, et al. Long-distance robotic telesurgery: a feasibility study for care in remote environments. Int J Med Robot. 2006;2(3):216–224. doi: 10.1002/rcs.99. [DOI] [PubMed] [Google Scholar]
- 52.Rayman R, Primak S, Patel R, et al. Effects of latency on telesurgery: an experimental study. Med Image Comput Comput Assist Interv. 2005;8(Pt 2):57–64. doi: 10.1007/11566489_8. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez M, Sariego J. The general surgeon shortage: causes, consequences, and solutions. South Med J. 2009;102(3):291–294. doi: 10.1097/SMJ.0b013e31818c96ac. [DOI] [PubMed] [Google Scholar]
- 54.Satava RM. Robotics in colorectal surgery: telemonitoring and telerobotics. Surg Clin North Am. 2006;86(4):927–936. doi: 10.1016/j.suc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Satiani B, Williams TE, Go MR. Predicted shortage of vascular surgeons in the United States: population and workload analysis. J Vasc Surg. 2009;50(4):946–952. doi: 10.1016/j.jvs.2009.06.056. [DOI] [PubMed] [Google Scholar]
- 56.Schlachta CM, Lefebvre KL, Sorsdahl AK, Jayaraman S. Mentoring and telementoring leads to effective incorporation of laparoscopic colon surgery. Surg Endosc. 2010;24(4):841–844. doi: 10.1007/s00464-009-0674-1. [DOI] [PubMed] [Google Scholar]
- 57.Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236(4):458–463. doi: 10.1097/01.SLA.0000028969.51489.B4. discussion 463–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheldon GF, Ricketts TC, Charles A, King J, Fraher EP, Meyer A. The global health workforce shortage: role of surgeons and other providers. Adv Surg. 2008;42:63–85. doi: 10.1016/j.yasu.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Sheldon GF. Access to care and the surgeon shortage: American Surgical Association forum. Ann Surg. 2010;252(4):582–590. doi: 10.1097/SLA.0b013e3181f886b6. [DOI] [PubMed] [Google Scholar]
- 60.Shenai MB, Dillavou M, Shum C, et al. Virtual interactive presence and augmented reality (VIPAR) for remote surgical assistance. Neurosurgery. 2011;68(1 Suppl Operative):200–207. doi: 10.1227/NEU.0b013e3182077efd. discussion 207. [DOI] [PubMed] [Google Scholar]
- 61.Shenai MB, Tubbs RS, Guthrie BL, Cohen-Gadol AA. Virtual interactive presence for real-time, long-distance surgical collaboration during complex microsurgical procedures. J Neurosurg. 2014;121(2):277–284. doi: 10.3171/2014.4.JNS131805. [DOI] [PubMed] [Google Scholar]
- 62.Smithwick M. Network options for wide-area telesurgery. J Telemed Telecare. 1995;1(3):131–138. doi: 10.1177/1357633X9500100302. [DOI] [PubMed] [Google Scholar]
- 63.Sterbis JR, Hanly EJ, Herman BC, et al. Transcontinental telesurgical nephrectomy using the da Vinci robot in a porcine model. Urology. 2008;71(5):971–973. doi: 10.1016/j.urology.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 64.Stone SS, Warf BC. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infant hydrocephalus: a prospective North American series. J Neurosurg Pediatr. 2014;14(5):439–446. doi: 10.3171/2014.7.PEDS14152. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki N, Hattori A, Ieiri S, et al. Tele-control of an endoscopic surgical robot system between Japan and Thailand for tele-NOTES. Stud Health Technol Inform. 2009;142:374–379. [PubMed] [Google Scholar]
- 66.Swanstrom LL, Fried GM, Hoffman KI, Soper NJ. Beta test results of a new system assessing competence in laparoscopic surgery. J Am Coll Surg. 2006;202(1):62–69. doi: 10.1016/j.jamcollsurg.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 67.Voelker R. Experts say projected surgeon shortage a “looming crisis” for patient care. JAMA. 2009;302(14):1520–1521. doi: 10.1001/jama.2009.1456. [DOI] [PubMed] [Google Scholar]
- 68.Warf BC. Neurosurgical humanitarian aid. J Neurosurg Pediatr. 2009;4(1):1–2. doi: 10.3171/2009.2.PEDS0928. discussion 2–3. [DOI] [PubMed] [Google Scholar]
- 69.Whitten P, Mair F. Telesurgery versus telemedicine in surgery–an overview. Surg Technol Int. 2004;12:68–72. [PubMed] [Google Scholar]
- 70.Wilcox ME, Adhikari NKJ. The effect of telemedicine in critically ill patients: systematic review and meta-analysis. Crit Care. 2012;16(4):R127. doi: 10.1186/cc11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams TE, Sun B, Ross P, Thomas AM. A formidable task: Population analysis predicts a deficit of 2000 cardiothoracic surgeons by 2030. J Thorac Cardiovasc Surg. 2010;139(4):835–840. doi: 10.1016/j.jtcvs.2009.12.004. discussion 840–841. [DOI] [PubMed] [Google Scholar]
- 72.Wilson MS, Middlebrook A, Sutton C, Stone R, McCloy RF. MIST VR: a virtual reality trainer for laparoscopic surgery assesses performance. Ann R Coll Surg Engl. 1997;79(6):403–404. [PMC free article] [PubMed] [Google Scholar]


