Abstract
Introduction
Certain personality traits are associated with higher risk of Alzheimer's disease, similar to cognitive impairment. The identification of biological markers associated with personality in mild cognitive impairment could advance the early detection of Alzheimer's disease.
Methods
We used hierarchical multivariate linear models to quantify the interaction between personality traits, state of cognitive impairment, and MRI biomarkers (gray matter brain volume, gray matter mean water diffusion) in the medial temporal lobe (MTL).
Results
Over and above a main effect of cognitive state, the multivariate linear model showed significant interaction between cognitive state and personality traits predicting MTL abnormality. The interaction effect was mainly driven by neuroticism and its facets (anxiety, depression, and stress) and was associated with right-left asymmetry and an anterior to posterior gradient in the MTL.
Discussion
Our results support the hypothesis that personality traits can alter the vulnerability and pathoplasticity of disease and therefore modulate related biomarker expression.
Keywords: Alzheimer's disease, Brain, Personality, Neuroimaging, Psychiatry, Prognosis
1. Background
Translational research in Alzheimer's disease (AD) has relied mostly on tests based on the assessment of cognitive state to detect individuals at risk, the individuals with mild cognitive impairment (MCI), and to identify the corresponding disease signatures such as medial temporal lobe (MTL) atrophy [1], [2], [3]. Looking beyond the unidimensional concept of MCI, current research aims to identify other important risk factors (genetic, personality traits) that would improve the prognostic accuracy of current tests and explain the high degree of individual variability in MTL atrophy that is not associated with cognitive decline.
In AD, personality changes, like cognitive decline, are also salient features of the disease [2], [3], [4], [5], [6], [7], [8], [9]. In previous studies, interest in personality traits, particularly neuroticism (tendency to feel negative emotions such as stress, depression) and conscientiousness (tendency to be self-disciplined), and AD [10], [11], [12], [13] was motivated by the fact that personality traits are stable into adulthood [14], have genetic-environmental underpinnings [15], brain anatomy correlates [16], and are also predictive of late-life developments such as cognitive dysfunction [7] or psychiatric symptoms [17]. However, the influence of personality traits on disease causation and their biological manifestations still remain unclear [6], [8], [18].
Our study aims to test whether morbid personality traits relate to individual differences within the MTL independent of cognitive state. To further improve the discrimination between the two groups in term of brain anatomical changes, we aim to identify the personality profile that minimizes the variance within each group (MCI and non-cognitively impaired [NCI]) and maximizes the difference between them. We predict that neuroticism and its underlying facets, anxiety, depression, and stress will have the greatest exacerbating effects on disease stages. We also expect that identified personality profile will correlate with known functional organization within the MTL.
To quantify MTL atrophy, we used structural magnetic resonance imaging and derived measures of gray matter volume (GMV) and gray matter mean diffusivity (GMMD). GMMD is considered a more sensitive marker than GMV to detect MTL abnormalities in MCI [19]. We used a multivariate strategy [20] to provide a comprehensive test of the association between personality traits, cognitive state, and brain anatomy. The method (Fig. 1) is data driven, unbiased, takes into account the multidimensional and hierarchical nature of the five-factor model of personality, and uses anatomical constraints to decompose the different sources of variability.
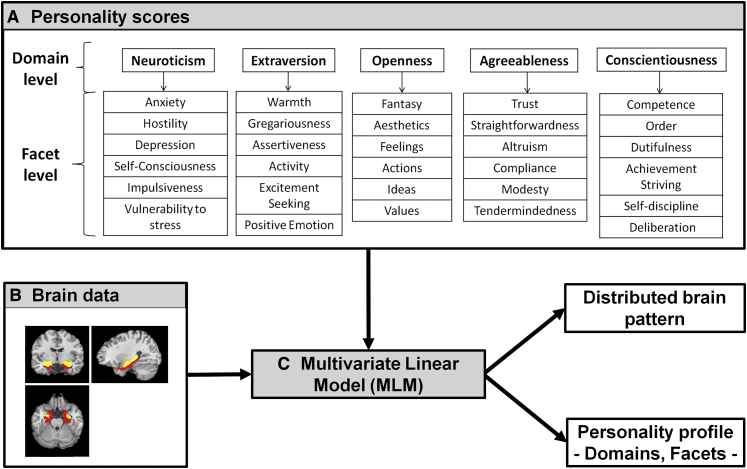
Fig. 1.
(A) NEO Personality inventory (NEO-Pi-R) is hierarchical construct composed of 5 domains and 6 facets for each domain. (B) Search volume of interest with the hippocampus in yellow and parahippocampal cortex in red. (C) Multivariate Linear Model (MLM) identified the personality profile and the brain distributed pattern that best explain the covariance between personality scores and anatomical measures.
2. Methods
2.1. Participants
The study included older adults selected from a longitudinal cohort recruited in the psychogeriatric and geriatric memory clinics of the Lausanne University hospital. The local ethics committee gave permission for the research protocol, and all participants gave written informed consent before taking part in the study. All participants completed comprehensive clinical, psychiatric, and cognitive assessments at the time of MRI scanning. Participants with psychiatric or neurological central nervous system disorders (stroke, tumor), dementia, and alcohol or drug abuse were excluded. The 97 participants included in the study were divided into two groups, MCI and NCI, according to the conventional Winblad criteria [2] where MCI is defined as abnormal but does not fulfill the diagnostic criteria for dementia. A total of 29 participants were MCI (8 males, aged 68 ± 8 years, Mini–Mental State Examination [21] [MMSE]: 27.7 ± 1/range [25–29], Clinical Dementia Rating [22] [CDR] = 0.5, with MCI amnestic 23, nonamnestic 6) and 68 were NCI (18 males, aged 66 ± 6 years, MMSE: 29.1 ± 1/range [26–30], CDR = 0).
2.2. Personality and neuropsychological/psychiatric assessments
To obtain reliable measures of current personality profiles, we asked relatives of participants to complete the 240-item NEO Personality Inventory Revised (NEO-PI-R) [23]. This questionnaire, rated on a five-point agreement scale, is based on the five-factor model of personality derived from statistical factor analysis of various personality lexical inventories [23]. It is hierarchically divided into five broad domains (Fig. 1A): neuroticism (a tendency to feel negative affects and to be susceptible to psychological distress), extraversion (a tendency to be sociable and lively), openness (a tendency to be open to new experiences), agreeableness (a tendency to be cooperative, altruistic, and trusting), and conscientiousness (a tendency to be careful, dutiful, and responsible). Each domain contains six facets (Fig. 1A). The facets of the neuroticism domain are anxiety, anger, hostility, depression, self-consciousness, impulsiveness, and vulnerability to stress. The NEO-PI-R has a high test-retest reliability in the elderly [23] and high inter-rater reliability in patients with AD [24].
Internal reliability of NEO-PI-R scores was estimated with Cronbach's alpha. In our sample, the values ranged from 0.63 to 0.68 for the NEO-PI-R domains and from 0.79 to 0.87 for the facets of neuroticism (a nominal value of 0.7 denotes internal consistency [25]). Other rating scales and tests used included the Hospital Anxiety and Depression Scale (HADS-A [anxiety] and HADS-D [depression], respectively) [26] and the RL-RI-48 memory item task [27].
2.3. Neuroimaging data
Data was acquired using whole-brain MRI T1-weighted (T1w) structural images (structural magnetic resonance imaging protocol: 1-mm isotropic resolution with a matrix of 256×256 voxels, repetition time 2.3 seconds, echo time 2.91 seconds) and diffusion-weighted MRI (1.8×1.8×2 mm3 resolution, with a matrix of 128×128 voxels, 30 directions, high b-value of 1000 seconds/mm2) on a 3T MRI scanner (Siemens Trio).
We applied a standard data preprocessing pipeline using a statistical parametric mapping package [28], [29] (SPM8-Matlab toolbox, www.fil.ion.ucl.ac.uk/spm) to the T1w images with a bias field correction and unified segmentation into white and gray matter tissue classes. In addition, we applied a standard preprocessing pipeline using FreeSurfer software [30] (FSL; http://www.fmrib.ox.ac.uk/fsl/) with correction for eddy currents and head movement distortion before extracting mean diffusion images. Mean diffusion and T1W images were spatially realigned and normalized to Montreal Neurological Institute space with the DARTEL procedure contained in the Voxel-Based Quantification toolbox [31]. The final outputs were restricted to the gray matter segment to obtain voxel-wise estimations of GMV and GMMD. Finally, we smoothed the images with an isotropic gaussian kernel of 8 mm full width at half maximum. Anatomical labeling was based on the Automated Anatomical Labeling atlas [32].
2.4. Statistical analyses
2.4.1. Anatomical differences explained by cognitive state stratification
We first conducted a univariate regression analysis to test for differences in the brain measures (GMV and GMMD) between MCI and NCI groups. The model included the cognitive state stratification factor, age, and total intracranial volume as confounding variables.
2.4.2. Anatomical differences explained by personality domains and cognitive state
Secondly, we used a multivariate model [20] to address the question whether, beyond cognitive factors, there are specific personality traits that could explain anatomical MTL differences between MCI and NCI. In the literature, multivariate factorial analysis has often been used in studies of personality to extract significant factorial structures [23], [33], [34]. We used a variant of this method, the multivariate linear method (MLM) that is similar to standard multivariate factorial analysis but additionally integrates anatomical information with the cognitive variables and any confounds. The MLM procedure is based on singular value decomposition, which summarizes covariance between personality scores (Fig. 1A) and anatomical data (Fig. 1B). The output of the MLM (Fig. 1C) consists of pairs of spatially distributed brain patterns associated with a set of linear combinations of personality traits that are maximally correlated with them. The significance of the personality profiles is assessed by a multivariate F-test (based on partial averages of the eigenvalues) that defines the spaces of interest for the five personality domains beyond those of the cognitive and other confounding factors. Post hoc univariate analyses were then performed with identified profiles to determine their mapping at the voxel level.
2.4.3. Anatomical differences explained by personality facets and cognitive state
We performed the MLM analysis at the facet level within the whole search volume of interest.
3. Results
3.1. Demographic, personality traits, and neuropsychological/psychiatric results
We found (see details in Table 1) no statistical differences between MCI and NCI groups for all demographic variables (age, gender). As expected, the MMSE was significantly different between the two groups, and the memory scores measured by the RL-RI-48 memory item task were significantly lower in the MCI group. There were no statistical differences in HADS-D for depressive symptoms and HADS-A for anxiety symptoms scores. Neuroticism scores were also significantly higher in MCI patients (Table 1).
Table 1.
Demographic variables and neuropsychological scores
| Non-cognitively impaired (mean ± SD) | MCI (mean ± SD) | T- or χ2-statistic (d.o.f: degree)T- or χ2-statistic (d.o.f) | P value | |
|---|---|---|---|---|
| Demographic variables | ||||
| n | 68 | 29 | ||
| CDR | 0 | 0.5 | ||
| MMSE | 29.1 ± 1 | 27.7 ± 1 | 5.4 (95) | 0 |
| Age | 66 ± 6 | 68 ± 8 | −1.6 (95) | .1 |
| Gender (Female/Male) | 0.7 | 0.7 | 0.01 (95) | .9 |
| Education level | 2 | 2 | 0.4 (2) | .8 |
| Personality: domain scores (NEO PI-R) | ||||
| Neuroticism | 77.6 ± 23 | 88.8 ± 27 | −2 (95) | .04 |
| Extraversion | 105.7 ± 18 | 94.72 ± 17 | 2.7 (95) | .007 |
| Openness | 109.9 ± 19 | 98.9 ± 16 | 2.6 (95) | .01 |
| Agreeableness | 135.2 ± 17 | 123.2 ± 17 | 3 (95) | .003 |
| Conscientiousness | 134.5 ± 19 | 111.5 ± 22 | 5 (95) | 0 |
| Other neuropsychological scores | ||||
| Cued recall (RL-RI-48) | 29.2 ± 4 | 27.27 | −40.83 | 0 |
| Depression score (HADS-D) | 2.1 ± 2 | 3.3 ± 3 | −1.8 (95) | .07 |
| Anxiety score (HADS-A) | 4.7 ± 3 | 5 ± 3 | −0.3 (95) | .7 |
Abbreviations: CDR, Clinical Dementia Rating; HADS, Hospital Anxiety and Depression Scale; MCI, mild cognitive impairment; MMSE, Mini–Mental State Examination; NEO PI-R, NEO Personality Inventory Revised.
3.2. Anatomical differences related to cognitive state stratification
Using a whole-brain familywise error correction, we found no significant differences in GMV. However, with the same stringent level of correction for multiple comparisons, GMMD was significantly different between the two groups in several regions. In the MTL, local maxima for these differences (Table 2) were located in both parahippocampal and hippocampal subregions (cornu ammonis, dentate gyrus, and subiculum, according to the probabilistic cytoarchitectonic map [35]). At the whole-brain level, GMMD differences were significant in the left middle temporal cortex (Z = 5.06, xyz = [−57,−13.5,−3]), right superior temporal cortex (Z = 4.74, xyz = [54,0,−3], Z = 3.83, xyz = [−32,−23,−5]), left insula (Z = 4.84, xyz = [−41,12,−14]; Z = 4.83, xyz = [−41,3,−9]), left lingual cortex (Z = 4.65, xyz = [−12,−36,0]), and right postcentral gyrus (Z = 4.79, xyz = [53,−24,56]). Inclusion of education level and gender did not add contributive information to the brain measures.
Table 2.
Neuroimaging results
| Cluster (voxels) | Region (label) | X | Y | Z | Z statistic |
|---|---|---|---|---|---|
| Summary of VBM results | |||||
| GMMD: MCI > NCI | |||||
| 621 | Left hippocampus | −14 | −35 | 2 | 4.57 |
| −29 | −27 | −11 | 4.27 | ||
| −15 | −39 | 5 | 4.17 | ||
| −17 | −33 | −1 | 4.07 | ||
| Left parahippocampal | −23 | −36 | −6 | 3.72 | |
| 96 | Left parahippocampal | −23 | 7 | −23 | 4.26 |
| −18 | 4 | −20 | 4.08 | ||
| 1645 | Right hippocampus | 38 | −24 | −7 | 3.94 |
| 30 | −6 | −14 | 3.93 | ||
| 38 | −8 | −20 | 3.85 | ||
| 27 | −32 | −3 | 3.84 | ||
| 17 | −33 | 3 | 3.77 | ||
| Right parahippocampal cortex | 17 | −35 | −4 | 3.31 | |
| 33 | −21 | −24 | 3.25 | ||
| 112 | Right parahippocampal cortex | 27 | 11 | −23 | 3.77 |
| Post hoc MLM analysis on the five domains of personality | |||||
| GMV: interaction with disease: MCI < NCI | |||||
| 209 | Left parahippocampal | −24 | −22.5 | −22.5 | 3.98 |
| 77 | Right parahippocampal | 24 | −27 | −19.5 | 3.91 |
| 24 | −31.5 | −13.5 | 3.74 | ||
| GMMD: interaction with disease: MCI > NCI | |||||
| 905 | Right hippocampus | 15 | −30 | −3 | 4.1 |
| Right parahippocampal | 22.5 | −39 | −3 | 4.02 | |
| 28.5 | −31.5 | −13.5 | 3.78 | ||
| 96 | Right parahippocampal | 30 | 10.5 | −31.5 | 4.07 |
| Right hippocampus | 16.5 | −31.5 | 1.5 | 3.86 | |
| 36 | −22.5 | −9 | 3.8 | ||
| 22.5 | −31.5 | 3 | 3.71 | ||
| 28.5 | −30 | −3 | 3.69 | ||
| 15 | −27 | −6 | 3.5 | ||
| 13.5 | −34.5 | 6 | 3.35 | ||
| 90 | Right hippocampus | 18 | −9 | −13.5 | 3.79 |
| 154 | Right hippocampus | 30 | −7.5 | −12 | 3.35 |
| 28.5 | −6 | −21 | 3.33 | ||
Abbreviations: VBM, voxel-based morphometry; GMV, gray matter volume; GMMD, gray matter mean diffusivity; MCI, mild cognitive impairment; MLM, multivariate linear method.
3.3. Anatomical differences related to personality domains and cognitive state
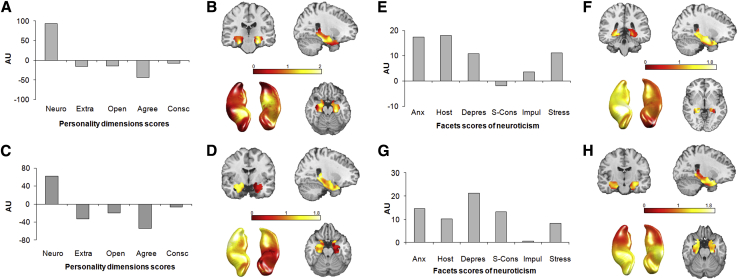
The multivariate analysis of GMV showed that there was a significant contribution of personality domains to alterations in brain structure. The first component identified related to personality traits (Fig. 2A) was significant (F = 3.77, P < e-5) and explained 54.39% of the between-group covariance in the search volume of interest (Fig. 2B). Neuroticism and agreeableness were identified as the main domains contributing to this brain component (Fig. 2A). No other regions showed significant differences between the two groups.
Fig. 2.
MLM analysis of personality profile at domain level: (A) First Eigen-component (P <0.05) and (B) the associated spatial distribution within the search volume of interest for GMV; (C) First Eigen-component (P <0.05) and (D) the associated spatial distribution within the search volume of interest for GMMD. Abbreviations: Neuro, neuroticism; Extra, extraversion; Open, openness; Agree, agreeableness; Consc, conscientiousness. Y axis is an arbitrary unit (AU).
Post hoc univariate regression analyses of GMV were performed with the first component of the MLM analysis as predictor with domain level. This revealed significant structural differences located in both parahippocampal cortices (in the entorhinal cortex and subiculum) (Table 2).
The MLM analysis of GMMD also showed a significant contribution of personality traits (Fig. 2C) to the first component (F = 5.32, P < .001), which explained 69.24% of the between-group covariance in the search volume of interest (Fig. 2D). The neuroticism and agreeableness domains had more weight than the three others (Fig. 2C), and a distributed spatial pattern of brain differences was revealed in the right hippocampal and parahippocampal cortices (Fig. 2D). Post hoc univariate analyses of GMMD with the first component of the MLM analysis revealed significant brain differences between MCI and NCI in the subiculum, cornu ammonis, dentate gyrus, and in a part of the hippocampal-amygdala transition area (Table 2). Outside this region, GMMD was also significantly higher in MCI compared with NCI in the right inferior temporal cortex (at two significant sites: Z = 5.77, xyz = [39,9,−43.5] and Z = 4.54, xyz = [61.5,−31.5,−16.5]), the right temporal pole (Z = 4.23, xyz = [36,18,−33]), the right temporal cortex (at two significant sites: Z = 4.21, xyz = [46.5,−51,−4.5] and Z = 4.12, xyz = [58.5,−9,−19.5]), and in the right rolandic operculum (Z = 4.52, xyz = [52.5,10,3]).
3.4. Anatomical differences related to personality facets of neuroticism and cognitive state
The MLM analysis of GMV showed contributions from the neuroticism facets profile (Fig. 2E) in the MTL region, mainly in the right hemisphere (Fig. 2F). The first component was significant (F = 8.84, P = 0) and explained 72.71% of the covariance (Fig. 2E and F).
The MLM analysis of GMMD again revealed a significant contribution of the personality facets profile (Fig. 2G), with the first component significant (F = 3.52, P < .0005) and explaining 46.72% of the variance in the MTL, mainly in the anterior part (Fig. 2H).
4. Discussion
Our multifactorial and multivariate analysis decomposes the complex relationship between three risk markers of AD, namely (1) anatomical atrophy, (2) cognitive decline, and (3) personality traits, which together reveal clinical and topographical signatures in MCI that has direct implications for refining current models of AD.
Our results are important because, with a few exceptions, there is a paucity of data-linking personality to neurobiological mechanisms of disease. A few neuropathologic studies [8], [11], [13], [36] of confirmed AD cases have provided evidence for a role of higher levels of neuroticism and depression in relation to disease symptoms (i.e., a more rapid cognitive decline in old age and higher risk of AD [11]) but provide ambiguous evidence for a direct link with lesions observed at autopsy (no link or linked with a higher level of neurofibrillary tangles and neuritic plaques) [8], [11], [36]. Neuroimaging studies of personality have been mainly conducted in healthy adults and have found significant associations between neuroticism trait and structural differences in frontal and temporal regions [16]. A recent study showed that in MCI individuals, the severity of white matter lesions in the MTL, but not the atrophy, was associated with higher neuroticism and lower conscientiousness [18]. Here, we investigate the multivariate relationship between personality and the MTL and provide a link to the vast majority of AD neuroimaging studies that report consistent effects of stress and depressive symptoms or cognitive and AD states on the hippocampus [37], [38].
We identified a spatial pattern of anatomical alterations in MCI individuals compared with NCI that extends beyond the temporal cortex to the left insula, the left lingual cortex, and the right postcentral gyrus for GMMD. We also observed that diffusion-based measures are more sensitive than volumetric ones to detect brain abnormality in MCI, which is in line with recent findings [19]. GMMD differences may be caused by modifications in the intra/extracellular space due to pre-atrophic changes.
We also identified specific anatomical patterns associated with personality traits. The MLM analysis of GMMD with personality domains revealed an asymmetry between the right and left MTL. The anatomical changes associated with the facet level of neuroticism showed a spatial gradient from the anterior to posterior parts of the MTL. For GMV this asymmetry was also observed at the facet level of neuroticism. Other studies on the effect of stress and depression on healthy and depressive individuals have also reported differences between the left and right hippocampal areas that could be explained by neurochemical and brain tissue property differences [39], [40]. The anteroposterior gradient has been related to the specific role of the anterior hippocampus in stress and emotion-related behavior [41]. The link between depression and AD has also been clinically reported, with chronic distress and depression being risk factors for cognitive decline in AD [7], [42]. Our results, in light of these studies, converge to suggest that depression and AD share biological substrates in the hippocampus that are stress related [43], [44].
Our findings highlight neuroticism, agreeableness and facets of anxiety, stress, hostility, and depression as key the explanatory variables of anatomical changes in the MTL. High scores for neuroticism are often reported to be predictive of cognitive impairment in AD [6], [7], [11], [12]. This is related to the occurrence of neuropsychiatric problems (e.g., depression and anxiety symptoms), higher comorbidity of mental disorders, lower quality of life, and shorter life expectancy [17], [45]. A premorbid agreeableness is linked to agitation and irritability in AD [4], [46]. The low level of conscientiousness also predicts conversion of MCI to AD [13]. Although personality traits such as openness and extraversion [4], [5] have been associated with early AD and MCI, we found less effect of these domains in our study. This can be due to fact that in our study, we identified the personality traits that correlate with brain changes and not just cognition stratification.
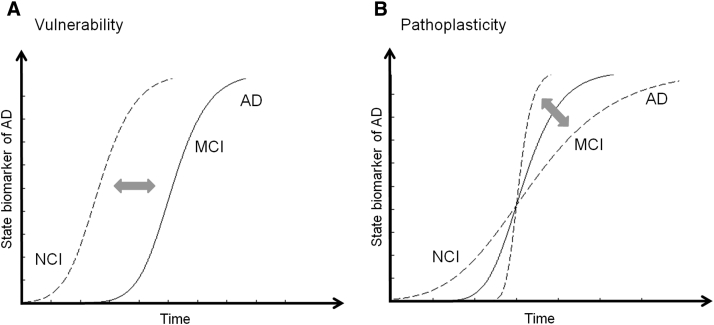
Our data suggest that personality is an important parameter that needs to be included in the modeling of the pathophysiological processes leading to AD [7], [47]. Recently, a new model has been proposed by Jack et al. (2013) [47] in which different states of the AD biomarkers (e.g., brain atrophy, tau, amyloid β, memory, and clinical function) each follow a sigmoid-shaped curve. The authors argue that for AD model, the most informative parameters are the onsets of curves on the horizontal time axis, their slopes, and their temporal ordering. We suggest adding another level to this model based on the psychopathology literature, whereby a specific personality trait, such as proneness to stress or depression, can affect the shape and the temporal ordering of biomarker state curves by two main mechanisms (Fig. 3). The first mechanism is predisposition/vulnerability, where a certain personality trait profile modulates the risk of disease and changes the onset of biomarker curves. The second exacerbating mechanism is pathoplasticity, where a personality trait has an additive (or diminishing) effect on the course of disease and hence impacts the slopes of the temporal curves.
Fig. 3.
Hypothetical model of state marker in Alzheimer's disease (AD) including personality trait. The curves show the time evolution of state marker abnormality of AD. (A) Vulnerability: Individuals characterized with a different personality profile (e.g. with lower neuroticism score) can show different disease onset. (B) Pathoplasticity: Individuals characterized with a different personality profile (e.g. with lower neuroticism score) can show different rate of decline. X axis represents the time, and Y axis, the state biomarker abnormality of AD such as cognitive or brain decline.
Therefore, other features, such as behavioral and psychological symptoms [45], pathophysiological markers of AD, genetic susceptibility factors such as serotonin [48], [49], and personality, as demonstrated here and elsewhere, may help to clarify mechanisms to explain the associations between cognitive decline and MTL atrophy in aging.
Research in Context.
-
1.
Systematic review: Most prior studies have focused on personality changes after Alzheimer's disease onset. Fewer studies reported associations between postmortem pathological markers and personality traits. A majority of studies examined the effect of one or two traits separately to dementia incidence and also reported significant contribution of neuroticism.
-
2.
Interpretation: We found a specific association between cognitive decline, personality traits, and biological changes in the medial temporal lobe. Interestingly, from disease modeling point of view, the association was explained by a low-dimensional factor, which corresponded to a personality profile dominated by neuroticism trait associated with a spatial gradient along the medial temporal lobe.
-
3.
Future directions: These findings add to the body of evidence that personality traits are stable features, which in the context of cognitive decline, lead to brain vulnerability. Multifactorial models, as proposed here, are needed to combine the different states and trait markers for accurate Alzheimer's disease risk prediction.
Acknowledgments
A.v.G. is supported by a Swiss National Science Foundation FNS 3200BO-122263. B.D. is supported by a Swiss National Science Foundation (NCCR Synapsy, project grant Nr 320030_135679 and SPUM 33CM30_140332/1), Foundation Parkinson Switzerland, Foundation Synapsis, the Novartis Foundation for medical biological research, and the German Research Council (DFG, KFO 247). Laboratoire de Recherche en Neuroimagerie is supported by generous funding from the Roger de Spoelberg and Partridge foundations. F.K. received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement number 604102 (HBP Ramp-Up Phase) and grant agreement number 720270 (HBP SGA1), the VELUX STIFTUNG and Pharnext, Paris.
The authors have declared that no conflict of interest exists.
Footnotes
A.v.G. and F.K. contributed equally to the study.
References
- 1.Apostolova L.G., Thompson P.M. Mapping progressive brain structural changes in early Alzheimer's disease and mild cognitive impairment. Neuropsychologia. 2008;46:1597–1612. doi: 10.1016/j.neuropsychologia.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 3.Dubois B., Feldman H.H., Jacova C., Cummings J.L., Dekosky S.T., Barberger-Gateau P. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 4.von Gunten A., Pocnet C., Rossier J. The impact of personality characteristics on the clinical expression in neurodegenerative disorders–a review. Brain Res Bull. 2009;80:179–191. doi: 10.1016/j.brainresbull.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Robins Wahlin T.B., Byrne G.J. Personality changes in Alzheimer's disease: a systematic review. Int J Geriatr Psychiatry. 2011;26:1019–1029. doi: 10.1002/gps.2655. [DOI] [PubMed] [Google Scholar]
- 6.Wilson R.S., Arnold S.E., Schneider J.A., Kelly J.F., Tang Y., Bennett D.A. Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- 7.Wilson R.S., Schneider J.A., Boyle P.A. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;28:2085–2093. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 8.Terracciano A., Iacono D., O'Brien R.J., Troncoso J.C., An Y., Sutin A.R. Personality and resilience to Alzheimer's disease neuropathology: a prospective autopsy study. Neurobiol Aging. 2013;34:1045–1050. doi: 10.1016/j.neurobiolaging.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donati A., Studer J., Petrillo S., Pocnet C., Popp J., Rossier J. The Evolution of Personality in Patients with Mild Cognitive Impairment. Dement Geriatr Cogn Disord. 2013;36:329–339. doi: 10.1159/000353895. [DOI] [PubMed] [Google Scholar]
- 10.Wilson R.S., Fleischman D.A., Myers R.A., Bennett D.A., Bienias J.L., Gilley D.W. Premorbid proneness to distress and episodic memory impairment in Alzheimer's disease. Psychiatr Interpers Biol Process. 2004:191–196. [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson R.S., Begeny C.T., Boyle P.A., Schneider J.A., Bennett D.A. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry. 2011;19:327–334. doi: 10.1097/JGP.0b013e31820119da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuzma E., Sattler C., Toro P., Schönknecht P., Schröder J. Premorbid personality traits and their course in mild cognitive impairment: results from a prospective population-based study in Germany. Dement Geriatr Cogn Disord. 2011;32:171–177. doi: 10.1159/000332082. [DOI] [PubMed] [Google Scholar]
- 13.Wilson R.S., Schneider J.A., Arnold S.E., Bienias J.L., Bennett D.A. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry. 2007;64:1204–1212. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- 14.Roberts B.W., DelVecchio W.F. The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychol Bull. 2000;126:3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- 15.Van Gestel S., Van Broeckhoven C. Genetics of personality: are we making progress? Mol Psychiatry. 2003;8:840–852. doi: 10.1038/sj.mp.4001367. [DOI] [PubMed] [Google Scholar]
- 16.Deyoung C.G., Hirsh J.B., Shane M.S., Papademetris X. Testing predictions from personality neuroscience: brain structure and the big five. Psychol Sci. 2010;21:820–828. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahey B.B. Public health significance of neuroticism. Am Psychol. 2009;64:241–256. doi: 10.1037/a0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duron E., Vidal J.S., Bounatiro S., Ben Ahmed S., Seux M.L., Rigaud A.S. Relationships between personality traits, medial temporal lobe atrophy, and white matter lesion in subjects suffering from mild cognitive impairment. Front Aging Neurosci. 2014;6:1–9. doi: 10.3389/fnagi.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller M.J., Greverus D., Weibrich C., Dellani P.R., Scheurich A., Stoeter P. Diagnostic utility of hippocampal size and mean diffusivity in amnestic MCI. Neurobiol Aging. 2007;28:398–403. doi: 10.1016/j.neurobiolaging.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Kherif F. Multivariate model specification for fMRI data. Neuroimage. 2002;16:1068–1083. doi: 10.1006/nimg.2002.1094. [DOI] [PubMed] [Google Scholar]
- 21.McHugh P.R., Folstein M.F., Folstein S.E. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Morris J.C. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 23.Costa P., MacCrae R. Psychological Assessment Resources; Odessa, FL: 1992. Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor Inventory (NEO FFI): Professional Manual. [Google Scholar]
- 24.Strauss M.E., Pasupathi M., Chatterjee A. Concordance between observers indescriptions of personality change in Alzheimer's disease. Psychol Aging. 1993;8:475–480. doi: 10.1037//0882-7974.8.4.475. [DOI] [PubMed] [Google Scholar]
- 25.Gregory B.J., Gerald M., Doanld S.H. SAGE; London, UK: 2008. (The SAGE Handbook of Personality Theory and Assessment: Personality Measurement and Testing, Volume 2). [Google Scholar]
- 26.Zigmond R., Snaith A.S. The Hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 27.Buschke H., Sliwinski M.J., Kulansky G., Lipton R.B. Diagnosis of early dementia by the Double Memory Test: Encoding specificity improves diagnostic sensitivity and specificity. Neurology. 1997;48:989–997. doi: 10.1212/wnl.48.4.989. [DOI] [PubMed] [Google Scholar]
- 28.Friston K.J., Holmes A.P., Worsley K.J., Poline J.-P., Frith C.D., Frackowiak R.S.J. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 29.Ashburner J., Friston K.J. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 30.Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Draganski B., Ashburner J., Hutton C., Kherif F., Frackowiak R.S., Helms G. Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ) Neuroimage. 2011;55:1423–1434. doi: 10.1016/j.neuroimage.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 33.Roepke S., McAdams L.A., Lindamer L.A., Patterson T.L., Jeste D.V. Personality profiles among normal aged individuals as measured by the NEO-PI-R. Aging Ment Health. 2001;5:159–164. doi: 10.1080/13607860120038339. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg L.R. An alternative ‘description of personality’: the big-five factor structure. J Pers Soc Psychol. 1990;59:1216–1229. doi: 10.1037//0022-3514.59.6.1216. [DOI] [PubMed] [Google Scholar]
- 35.Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 36.Rapp M.A., Schnaider-Beeri M., Grossman H.T., Sano M., Perl D.P., Purohit D.P. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63:161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 37.Lee G.J., Lu P.H., Hua X., Lee S., Wu S., Nguyen K. Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer's disease-related regions. Biol Psychiatry. 2012;71:814–821. doi: 10.1016/j.biopsych.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gianaros P.J., Jennings J.R., Sheu L.K., Greer P.J., Kuller L.H., Matthews K.A. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spasojevic N., Jovanovic P., Dronjak S. Molecular basis of chronic stress-induced hippocampal lateral asymmetry in rats and impact on learning and memory. Acta Physiol Hung. 2013;100:388–394. doi: 10.1556/APhysiol.100.2013.4.3. [DOI] [PubMed] [Google Scholar]
- 40.Madsen K.S., Jernigan T.L., Iversen P., Frokjaer V.G., Knudsen G.M., Siebner H.R. Hypothalamic-pituitary-adrenal axis tonus is associated with hippocampal microstructural asymmetry. Neuroimage. 2012;63:95–103. doi: 10.1016/j.neuroimage.2012.06.071. [DOI] [PubMed] [Google Scholar]
- 41.Strange B.A., Witter M.P., Lein E.S., Moser E.I. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 42.Andersen K., Lolk A., Kragh-Sørensen P., Petersen N.E., Green A. Depression and the risk of Alzheimer disease. Epidemiology. 2005;16:233–238. doi: 10.1097/01.ede.0000152116.32580.24. [DOI] [PubMed] [Google Scholar]
- 43.Sotiropoulos I., Cerqueira J.J., Catania C., Takashima A., Sousa N., Almeida O.F. Stress and glucocorticoid footprints in the brain-the path from depression to Alzheimer's disease. Neurosci Biobehav Rev. 2008;32:1161–1173. doi: 10.1016/j.neubiorev.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Rothman S.M., Mattson M.P. Adverse stress, hippocampal networks, and Alzheimer's disease. Neuromolecular Med. 2010;12:56–70. doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendez Rubio M., Antonietti J.P., Donati a., Rossier J., Gunten A. Personality traits and behavioural and psychological symptoms in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2013;35:87–97. doi: 10.1159/000346129. [DOI] [PubMed] [Google Scholar]
- 46.Archer N., Brown R.G., Reeves S.J., Boothby H., Nicholas H., Foy C. Premorbid personality and behavioral and psychological symptoms in probable Alzheimer disease. Am J Geriatr Psychiatry. 2007;15:202–213. doi: 10.1097/01.JGP.0000232510.77213.10. [DOI] [PubMed] [Google Scholar]
- 47.Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Tracking pathophysiological processes in Alzheimer's disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Hara R., Schröder C.M., Mahadevan R., Schatzberg A.F., Lindley S., Fox S. Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: association and interaction with cortisol. Mol Psychiatry. 2007;12:544–555. doi: 10.1038/sj.mp.4001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Assal F., Alarcón M. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch Neurol. 2004;61:1249–1253. doi: 10.1001/archneur.61.8.1249. [DOI] [PubMed] [Google Scholar]