Abstract
Cell therapy has the potential to treat different pathologies, including myocardial infarctions (heart attacks), although cell engraftment remains elusive with most delivery methods. Biological sutures composed of fibrin have been shown to effectively deliver human mesenchymal stem cell (MSC) to infarcted hearts. However, human MSCs rapidly degrade fibrin making cell seeding and delivery time sensitive. To delay the degradation process, we propose using Aprotinin, a proteolytic enzyme inhibitor that has been shown to slow fibrinolysis. Human MSCs seeded on fibrin sutures and incubated with Aprotinin demonstrated similar cell viability, examined using a LIVE/DEAD stain, to controls. No differences in proliferation, as determined by Ki-67 presence, were observed. Human MSCs incubated in Aprotinin differentiated into adipocytes, osteocytes, and chondrocytes, confirming multipotency. The number of cells adhered to fibrin sutures increased through Aprotinin supplementation at 2, 3, and 5 day time points. Uniaxial tensile testing was used to examine the effect of Aprotinin on suture integrity. Sutures exposed to Aprotinin had higher ultimate tensile strength and modulus when compared to sutures exposed to standard growth media. Fibrin sutures incubated in Aprotinin had larger diameters and less fibrin degradation products compared to the controls, confirming decreased fibrinolysis. These data suggest that Aprotinin can reduce degradation of fibrin sutures without significant effects on MSC function, providing a novel method for extending the implantation window and increasing the number of cells delivered via fibrin sutures.
Keywords: biological sutures, stem cells, tissue regeneration
INTRODUCTION
Cardiovascular disease is currently the leading cause of death in the United States.1 Annually, approximately 920,000 people in the United States suffer from myocardial infarction (MI), with coronary heart disease costing over $320 billion a year.2,3 MI can affect up to a billion heart cells, which decreases the maximum work potential of the heart.4 Cellular therapies aim to use cells to repair and restore function to damaged tissue. In an effort to restore heart function following an MI, cellular therapies utilizing mesenchymal stem cells (MSCs) are often used. Isolated from bone marrow, MSCs are a multipotent cell type having the ability to differentiate into bone, cartilage, and fat. They have several characteristics which make them appealing in regenerative medicine. These include relative ease of isolation, high expansion potential in vitro, and genetic stability.5–7 Specifically in cardiac regeneration, human MSCs can induce angiogenesis and can be used allogenically without an immune response.6,8,9 MSCs delivered to the heart have improved cardiac function in terms of an increase in ventricular pressure while also a reduction in infarction size.10–13 A common route for administration of MSCs to an infarcted heart is intramyocardial (IM) injections, where a cellular suspension is injected directly into the heart; however, there are several limitations with IM delivery including low cellular engraftment and retention, poor localization, low cell survival rates, and no matrix for cell adhesion, which often leads to cell death.4,8,14–16
Our lab has developed a novel method to deliver cells in a targeted location.17 Threads can be constructed from fibrin, which can be bundled together to create biological sutures. When seeded with MSCs, biological sutures showed significant improvement in engraftment rates compared to IM injections.17 Despite having an improved engraftment and more localized delivery, this method has limitations in regards to the quantity of cells that are seeded onto sutures. Increasing cell quantity on sutures may lead to increased suture degradation due to fibrinolytic enzymes secreted by MSCs.18 These enzymes result in a weaker suture that may not have the mechanical strength to be sutured through the heart for MSC delivery. In previous studies performed by our lab, seeding and culturing of human MSCs on sutures could not be extended past 5 days, at which point sutures lost structural integrity and were not strong enough for implantation.17,19 There is currently a need to increase the time that MSCs can be cultured on the fibrin sutures and also keep the mechanical stability of the sutures.
Aprotinin is a protease inhibitor that can be isolated from bovine lung that has been used clinically to inhibit trypsin, plasmin, and kallikreins. Herein we propose using Aprotinin as a method to decrease human MSC-seeded fibrin suture degradation. The goals of this study were to determine the effect of Aprotinin on the degradation and mechanical properties of cell loaded fibrin sutures, determine the effect of Aprotinin on human MSC viability, proliferation, and differentiation capacity, and establish the effect of Aprotinin on quantity of cells seeded on fibrin sutures.
MATERIALS AND METHODS
Fibrin Microthreads, Suture Production, and Cell Seeding
Fibrin microthreads were coextruded using solutions of fibrinogen and thrombin, in a manner previously described.20 In brief, thrombin isolated from bovine plasma (Sigma, St. Louis, MO T4648) and fibrinogen (Sigma, St. Louis, MO F4753) were coextruded through polyethylene tubing (0.38 mm inner diameter, Beckton Dickinson, Frank-lin Lakes, NJ) into a bath of room temperature 10 mM HEPES at a pH of 7.4. After 15 min, the fibrin microthreads were removed from the bath and allowed to air dry.
Twelve microthreads were placed parallel to each other, adhered together using a drop of Dulbecco’s phosphate-buffered saline (DPBS, Mediatech Inc, Manassas, VA) twisted together to form an entwined bundle. Microthread bundles were cut to 4 cm lengths. Each microthread bundle was threaded through the eye of a surgical suture needle (size #20, 3/8″ circle, tapered; Securos Surgical, Fiskdale, MA) and hydrated for 20 min in DI water. After hydration, the ends of the bundle were folded together, twisted, and dried to form a 2 cm long suture, shown in Figure 1.
FIGURE 1.
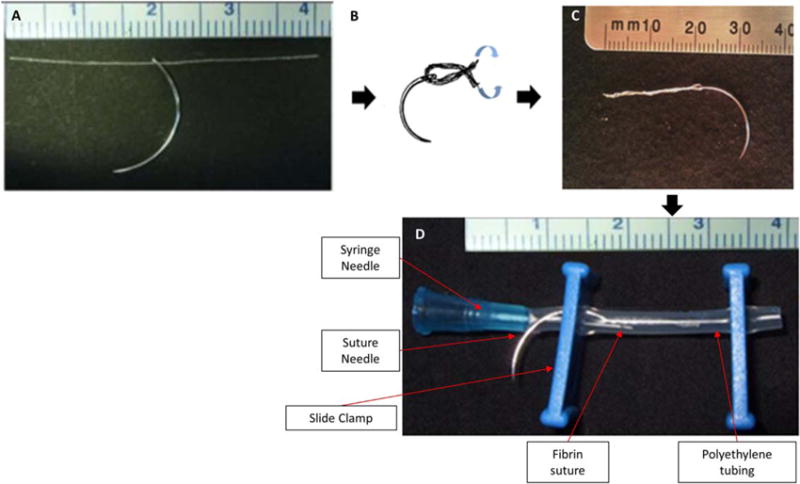
Suture construction: a 4 cm fibrin microthread bundle is threaded through the eye of a suture needle (A), hydrated and twisted with the needle at the midpoint (B) to create a 2 cm fibrin suture (C). This suture is placed inside a section of gas permeable polyethylene tubing, with a syringe needle for human MSC delivery during seeding and slide clamps to seal the tubing (D).
A seeding construct was assembled around each suture.17 A section of gas-permeable Silastic tubing (1.98-mm ID, Dow Corning, Midland, MI) was used to house the fibrin suture and a slide clamp was used to secure a 27 G needle and the suture needle inside the bioreactor tubing. Sutures were sterilized using a 12-h cycle of ethylene oxide gas.
Following sterilization, the sutures were rehydrated in sterile DPBS. Human MSCs (Lonza, Walkersville, MD; passage 5–8) were cultured in Mesenchymal Stem Cell Growth Medium (MSCGM; Lonza PT-3001, Walkersville, MD) in compliance with the manufacturer’s instructions. Sutures were seeded with human MSCs at a concentration of 1 × 106 cells/mL of MSCGM and at 50,000 cells/cm of suture. A syringe was attached to the 27 G needle of the bioreactor and used to inject the human MSCs. Seeding constructs were loaded into vented 50 mL conical tubes and placed on a rotator in a 37°C incubator (5% CO2, atmospheric gas concentrations) for 24 h.
Fibrin Degradation Product ELISA
A fibrin degradation product (FDP) ELISA kit (NeoBioLab, Cambridge, MA) was used to quantify FDP present in media used to culture seeded sutures. After cells were seeded, sutures were placed in a 12-well plate and cultured in the following concentrations of Aprotinin in MSCGM: 100, 50, 10, 5, 1, and 0 μg/mL (Aprotinin-100, −50, −10, −5, −1, and −0, respectively), with controls being unseeded sutures in Aprotinin-0 and Aprotinin-100. Media changes occurred every 3 days for 9 days and removed media was stored at −80°C until all samples were collected. Samples and components of the FDP ELISA were allowed to thaw to room temperature. Dilutions of FDP (0, 0.5, 1, 2.5, 5, and 10 mg/mL) were used to create a standard curve. Supplied conjugates were added to samples in the microtiter plate and incubated for 1 h. NeoBioLab substrates A and B were added to wells and incubated at room temperature for 15 min in the dark. A stop solution was added and the optical density (OD) at 450 nm was then measured on a Victor3 1420 Plate Reader. From the concentration at each time point, cumulative FDP mass was calculated.
Mechanical Testing and Thread Diameters
Following the fibrin degradation study, only Aprotinin-100 (Aprotinin) and Aprotinin-0 (MSCGM) were used; unseeded sutures were also tested. Sutures were created as described above, using 4 cm suture lengths to allow room for gripping while mechanical testing, and seeded with 200,000 human MSCs per suture. After seeding, the sutures were placed in 6-well plates with either Aprotinin or MSCGM for 3 days, after which sutures were removed from the media for diameter measurements and mechanical testing.
Thread diameters
Before mechanical testing, sutures were placed on a glass slide and three images were acquired on Leica (Leica, Wetzlar, Germany) DM LB2 microscope .at 5× magnification. Three images were taken of each suture and the average diameter was determined across the three regions.
Mechanical testing
Mechanical testing was performed on an Instron E1000 (Norwood, MA), using a 1 N load cell (Instron, Norwood, MA). A 10 mN tare load was applied to the suture, and a tensile test was performed until failure, with data acquisition occurring every 0.1 s. The initial gauge length and cross-sectional area was used to create a stress/strain curve and determine ultimate tensile strength (UTS) and modulus (E).
Cell Viability
To confirm human MSC viability, a Viability/Cytoxicity LIVE/DEAD Kit for mammalian cells (Invitrogen L-3244, Carlsbad, CA) was used. Calcein, ethidium, and Hoechst were used to stain live cells, dead cells, and nuclei, respectively. Human MSCs were seeded on glass coverslips at a seeding density of 5000 cells/cm2 and cultured in either standard MSCGM (n = 6) or Aprotinin (n = 6). Media was changed every 3 days for 7 days.
After 7 days, the coverslips were rinsed in PBS and incubated at 37°C with dilutions of 1:1000 ethidum, 1:1000 calcein, and 1:2000 Hoechst in Serum Free Dulbecco’s Modified Eagle Medium for 30 min. Coverslips were mounted on glass slides, and imaged using a Leica DM LB2 microscope at 20× magnification. Images were imported into ImageJ to quantify the numbers of live and dead cells.
Ki-67 Proliferation
To determine if Aprotinin affected human MSC proliferation, cells were stained for Ki-67. Ki-67 is a protein expressed during cellular proliferation.21 Human MSCs were seeded on glass coverslips at a seeding density of approximately 5000 cells/cm2 and cultured in MSCGM (n = 6) or Aprotinin (n = 6). After 3 days, the coverslips were rinsed with PBS, fixed in paraformaldehyde. A 0.25% Triton-X100 solution was used to permeabilize human MSCs. Coverslips were blocked with 5% goat serum in PBS for 30 min and then incubated at 4°C with rabbit anti Ki-67 monoclonal (Sigma, AB1667) at a dilution of 1:100 in 3% goat serum for 10 h. Alexa-fluor 568 goat antirabbit (Invitrogen A11036) at a dilution of 1:400 in 3% goat serum was used as the secondary antibody and incubated at room temperature for 1 h. The coverslips were rinsed with PBS then counterstained with Hoechst at a concentration of 1:6000 for 5 min and mounted. Fluorescent images were acquired using a Leica DM LB2 microscope. Two images were acquired from each coverslip and number of Ki-67-positive cells was counted using ImageJ.
Differentiation Assay
A differentiation assay was used to determine the effects of Aprotinin on human MSC multipotency. Human MSCs were differentiated down the following lineages: adipogenic, osteogenic, and chondrogenic. For human MSC differentiation, cells were seeded at 5000 cells/cm2 in either Aprotinin or MSCGM in standard culture conditions until confluence, with media changes occurring every 3 days.
Human MSCs were differentiated into adipocytes using 3 cycles of 3 days with Adipogenic Induction Medium and 3 days with Adipogenic Maintenance Medium (Lonza, Walkersville, MD; Adipogenic Differentiation Medium, PT-3004). After the 3 cycles of Maintenance and Induction media, all cells were cultured for an additional week in Adipogenic Maintenance Medium (25 days).
To assess lipid vacuole formation, adipogenic cells were stained with Oil Red O. Cells were fixed using paraformaldehyde, stained with 0.3% Oil Red O (Sigma Aldrich, St. Louis, MO), and counterstained with Mayer’s hematoxylin (Sigma Aldrich, St. Louis, MO). Stained images were acquired with a Leica DMIL microscope.
Osteogenic differentiation was performed by culturing human MSCs in Complete Osteogenic Differentiation Media (Lonza, Walkersville, MD), with media changes occurring every 3 days for 21 days. Alizarin Red S was used to assess calcium deposits and confirm osteogenic differentiation. Cells were fixed in paraformaldehyde and stained using a 2% Alizarin Red S solution with a pH of 4.1–4.3. Cells were imaged using a Leica DMIL microscope.
Human MSCs (250,000 in cultured in either MSCGM or Aprotinin) were rinsed in incomplete chondrogenic media (Lonza, Walkersville, MD) and pelleted by centrifuging at 150g for 5 min. TGF-β3 (Lonza, Walkersville, MD) was added to make complete chondrogenic media, which was changed every 3 days. Chondrocyte pellets were harvested after 28 days for sectioning. Chondrocyte pellets were fixed in paraformaldehyde, paraffin embedded, sectioned to 5 μm using a Leica RM 2235 microtome and mounted to glass slides. Pellet sections were stained using Masson’s Trichrome and Alcian Blue to detect collagen and imaged using Leica DM LB2 microscope.
CyQuant Assay
The CyQuant cell proliferation assay kit (Molecular Probes, Eugene, OR) was used to determine the number of human MSCs adhered to fibrin sutures, by staining cellular DNA.19,22,23 Sutures were created, seeded, and sterilized in the manner previously described. After seeding, sutures were removed from the bioreactors and cultured in MSCGM or Aprotinin for 0, 1, 2, 3, and 5 days. Sutures were cut to 1 cm to standardize the length of suture, centrifuged at 2,000 RPM, and frozen at −80°C.
Once samples had thawed, CyQuant lysis buffer was added and the suture was removed and stained with Hoechst (1:6000 dilution in PBS for 5 min) to confirm human MSC removal. Samples were pipetted into a 96-well plate was placed in a Victor3 1420 Plate Reader to record the optical density at 480 nm. A standard curve was created using the optical densities and the known cell numbers.
Statistics
Statistical differences between groups were analyzed using a Student’s t test (for comparisons between only 2 groups; Aprotinin vs MSCGM) or an ANOVA (for comparison between >2 groups; multiple concentrations of Aprotinin, up to 100 μg/mL and multiple time points, up to 5 days). A two-way ANOVA with post-hoc Holm–Sidak test was used to determine significant differences between groups (p < 0.05). Significance was established for p < 0.05. Data are reported as means ± standard deviations, unless otherwise noted.
RESULTS
Fibrin Degradation ELISA
As expected, fibrin degradation increased over 9 days in all groups [Fig. 2(A)]. Aprotinin demonstrated a dose response with higher concentrations decreasing the degradation of the fibrin sutures as compared to control [Fig. 2(A)]. At each time point, when examining the FDP response to Aprotinin concentration [Fig. 2(A)], 100 μg/mL of Aprotinin had significantly less FDPs than all other groups (p < 0.05). Therefore, in the rest of the experiments, only 100 μg/mL of Aprotinin was tested.
FIGURE 2.
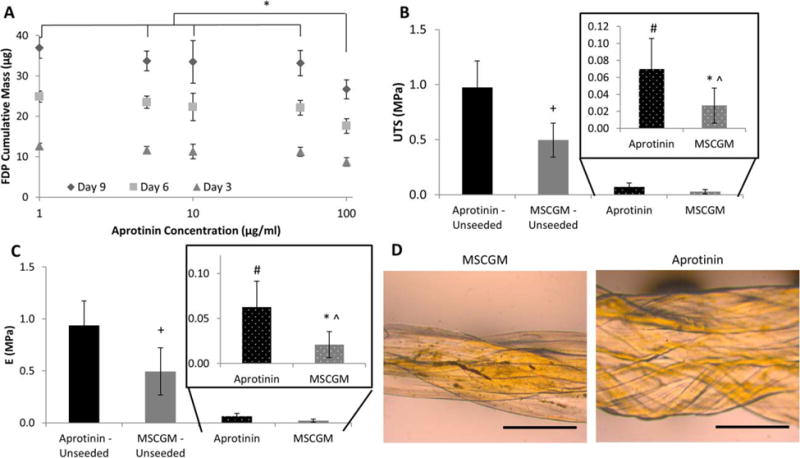
Suture degradation and mechanics: 2A depicts the cumulative mass of fibrin degradation products (FDP) versus the concentration of Aprotinin in MSCGM at days 3, 6, and 9. At each time point, there was a decrease in FDP mass compared to 100 μg/mL Aprotinin concentration (*p < 0.05 significance compared to all other Aprotinin concentrations at the same time). 2B depicts the ultimate tensile strength (UTS) of seeded and unseeded sutures in MSCGM or Aprotinin (*p < 0.05 between Aprotinin and MSCGM; +p < 0.05 between Aprotinin-unseeded and MSCGM-unseeded; #p < 0.05 between Aprotinin-unseeded and Aprotinin; and̂ p < 0.05 between MSCGM-unseeded and MSCGM). 2C depicts the modulus (E) of seeded and unseeded sutures in MSCGM or Aprotinin (*p < 0.05 between Aprotinin and MSCGM; +p < 0.05 between Aprotinin-unseeded and MSCGM-unseeded; #p < 0.05 between Aprotinin-unseeded and Aprotinin; and̂ p < 0.05 between MSCGM-unseeded and MSCGM). 2D shows the seeded suture diameters following 3 days in MSCGM or supplemented with Aprotinin, scale bar = 1 mm.
Suture Mechanics
Thread diameters
The diameters of sutures were measured as a marker of degradation. Figure 2(D) shows the human MSC-seeded sutures following 3 days in culture. In both the Aprotinin and MSCGM groups, the mean diameter decreased when human MSCs were seeded on the suture (Table I). Seeded sutures saw a diameter decrease of 14% when compared to unseeded sutures cultured in Aprotinin; similarly, a decrease was also observed between the human MSC seeded and MSCGM-unseeded groups. A larger decrease was observed between these two groups; the mean diameter decreased 41.1% when cells were attached to sutures exposed to MSCGM alone.
TABLE I.
Suture Diameters After 3 days in Culture (mm)
| Diameter | Standard Deviation | |
|---|---|---|
| Aprotinin | 1.29a | 0.11 |
| MSCGM | 1.00b | 0.09 |
| Aprotinin-unseeded | 1.50 | 0.07 |
| MSCGM-unseeded | 1.70 | 0.05 |
p < 0.05 significance between Aprotinin-unseeded.
p < 0.05 significance between MSCGM-unseeded.
Mechanical data
Uniaxial tensile testing was used to evaluate mechancial strength of sutures. When cultured in Aprotinin, seeded sutures had a significantly higher UTS when compared to MSCGM alone (p < 0.05) [Fig. 2(B)] and a significant decrease was observed when sutures were seeded with human MSCs. In both seeded and unseeded conditions, sutures exposed to Aprotinin had higher UTS.
A similar trend was also observed between the modulus of seeded sutures. Modulus values recorded for sutures (both seeded and unseeded) exposed to Aprotinin were significantly higher (p < 0.05) than the MSCGM counter parts [Fig. 2(C)].
Human MSC Viability, Proliferation, and Differentiation
Viability results were recorded as percent live cells and are shown in Figure 3(B). Cells cultured in media supplemented with Aprotinin showed no significant difference in cell viability when compared to MSCGM alone [Fig. 3(A)]. Aprotinin also did not significantly affect cell proliferation compared to control. The percentage of Ki-67-positive human MSCs cultured in MSCGM was similar to that in Aprotinin [Fig. 4(B)], with no significant differences observed. Human MSCs were able to differentiate into adipocytes, osteocytes, and chondrocytes when incubated with MSCGM and when supplemented with Aprotinin (Fig. 5). Differentiation of cells incubated in Aprotinin did not appear different from cells incubated without Aprotinin.
FIGURE 3.

Live/dead results: (A) live/dead stain of hMSCs cultured in Aprotinin or MSCGM, scale bar = 100 μm; (B) percentages of live hMSCs when cultured in Aprotinin or MSCGM.
FIGURE 4.

Proliferation results: (A) Ki-67 proliferation stain of hMSCs cultured in Aprotinin or MSCGM, scale bar = 100 μm; (B) Ki-67-positive hMSCs percentages when cultured in Aprotinin or MSCGM.
FIGURE 5.
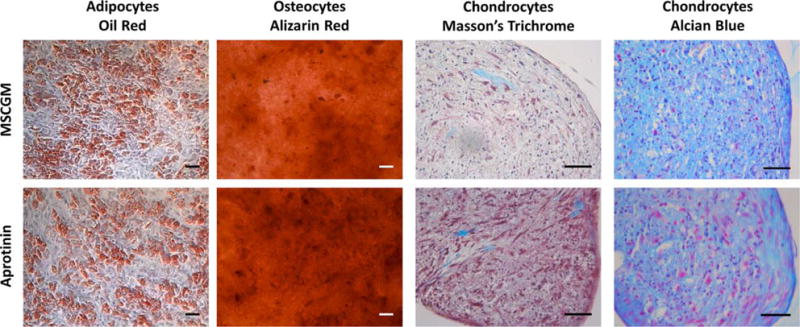
Differentiation assay: adipocytes stained with oil red—stains lipid vacuoles red; osteocytes stained with alizarin red—stains calcium deposits red; chondrocytes stained with Masson’s trichrome—stains collagen blue, nuclei black, and cytoplasm pink/red; chondrocytes stained with Alcian Blue—stains glycoaminoglycans blue and cells/nuclei red/purple. All scale bars = 50 microns.
Human MSC Quantities on Fibrin Sutures
A CyQuant assay was used to quantify the number of human MSCs adhered to the surface of sutures. In both groups, we observed an increase in the number of cells as time increased. At the later time points (days 2, 3, and 5), sutures cultured in Aprotinin had significantly more sutures adhered to the surface when compared to MSCGM alone [Fig. 6(A)].
FIGURE 6.

Cell quantities adhered to sutures: (A) depicts hMSC numbers adhered to sutures, an upward trend was observed in both groups across the 5 days (mean ± SEM) (#p < 0.05 between MSCGM and Aprotinin day 2; ^p < 0.05 between MSCGM and Aprotinin day 3; *p < 0.05 between MSCGM and Aprotinin day 5); (B) displays the percentage of proliferating hMSCs as indicated by a Ki-67 stain, an upward trend was observed with increases in the number of proliferating cells at day 2 (+p < 0.05 between Aprotinin and MSCGM at each day; *p < 0.05 between MSCGM day 2 and days 0 and 1 and; #p < 0.05 between Aprotinin day 2 and MSCGM day 2).
Looking at the number of cells attached to the sutures, a large jump (approximately 10,000) occurred between days 1 and 2. To investigate this increase in cell quantity, sutures were seeded and plated for 0, 1, or 2 days, with an n = 3 in each group, and stained of Ki-67. At day 2, sutures cultured in Aprotinin showed a significant increase in Ki-67-positive cells, when compared to sutures cultured in Aprotinin at days 0 and 1. The same trend was also observed in cells cultured in MSCGM alone, with a significant increase between day 2 and both days 0 and 1 [Fig. 6(B)]. Additionally, at day 2, sutures cultured in Aprotinin had a significant increase in the number of Ki-67-positive cells when compared to MSCGM alone sutures.
DISCUSSION
Using human MSC-seeded sutures allows cells to be delivered to a targeted location.17 Compared to IM injections, fibrin sutures allow surgeons to deliver cells to a very specific region of the heart and have a much higher engraftment rate.17 Despite these benefits, fibrin sutures have a limited “shelf-life;” once seeded, these sutures degrade very rapidly and become mechanically unstable. To expand the “shelf-life,” we propose using Aprotinin.
Aprotinin has been used in other applications for inhibiting the degradation of fibrin.19,22–24 In cardiac tissue engineering, Aprotinin has been used with the culture of human myofibroblasts onto fibrin gels22,23 as well as a culture of rat cardiomyocytes, endothelial cells, and fibroblasts onto micro templated fibrin scaffolds19. Cell-seeded fibrin scaffolds have a limited in vitro life, due to a rapid loss of mechanical integrity and structure. These studies demonstrated the addition of Aprotinin aided in the control of fibrin scaffold degradation, without effecting cell function. Herein, we demonstrate MSCs retain their ability to differentiate down multiple linages, an inherent property that is essential in the use of this cell type in many therapeutic applications.
Supplementation of MSCGM with Aprotinin allowed human MSCs to grow on sutures, while also inhibiting the degradation of the sutures. Although there was a significant decrease in the mechanical integrity of cell-seeded sutures, Aprotinin supplementation limited this response, similar to other Aprotinin studies.19,22–25 Previous research has reported that there is no statically significant increase in the number of cells adhered to fibrin sutures beyond day 3.19 Degradation was extended past the day 3 time point because we wanted to investigate the molecular degradation of the sutures over a longer time period. Degradation could be examined using an ELISA over a longer time period because there were no mechanical forces being applied over the course of the experiment and therefore handling would not break the suture, allowing the sutures to remain intact. During mechanical testing of sutures at the day 3 time point, many of the sutures cultured in MSCGM were extremely weak and broke when loading the samples for mechanical testing, a problem that did not occur in any of the Aprotinin supplemented sutures. Samples that broke during loading, before data could be collected were removed from data analysis in both mechanical testing and diameters. Extending mechanical testing past 3 days would be extremely difficult because sutures would have lost mechanical integrity, making handling and testing difficult.
Similar to other studies investigating Aprotinin, we used mechanical testing to determine the effect on seeded fibrin scaffolds.19,25 In Thomson et al., multiple cell lines were cultures on fibrin scaffolds and following 10 days of culture, patch stiffness was measured. In the Aprotinin-treated group, fibrin scaffolds retained approximately 60% of the day 0 stiffness, whereas the untreated group retained only 50% of the original stiffness at day 3, which tapered down to <20% by day 10. Another experimental group utilized by Thomson et al. was a fibrin scaffold cross-linked with zymogen factor XIII (FXIII), which covalently cross-links unstable fibrin network to form an insoluble fibrin clot. This improved further improved stiffness retention data over the 10 day study. The use of FXIII, or other cross-linking agents was not considered in our study due to the fact that fibrin cross-linking may alter the in vivo degradation. Aprotinin was also used to control degradation in fibrin-based tissue engineered blood vessels, seeded with smooth muscle cells.25 Swartz et al. cultured the seeded fibrin blood vessels for 2 weeks before evaluating mass and toughness. They observed that when Aprotinin was added to the culture media, the fibrin based blood vessels retained approximately 50% of their original mass (compared to <20% in the untreated groups). In terms of toughness, Aprotinin-treated groups had up to a threefold increase compared to untreated groups.25
The mechanical testing in our study not only served as a method to characterize cellular degradation in the presence of Aprotinin, but also to determine the potential effectiveness of the suture implantation following seeding. Our method to deliver stem cells to the infarcted heart involves stitching the suture directly to the infarcted area,17 which puts stress on the suture as it is pulled through the wall of the heart. The mechanical integrity of the fibrin suture affects not only the cellular environment of the seeded cells but also the overall performance of the suture. We have observed success with the delivery of seeded sutures after culturing cells for 24 h, although suture failure during implantation is not a rare occurrence. With the addition of Aprotinin and an increase in mechanical strength, we hope to see a decrease in the number of sutures that break during attempted delivery.
No changes in human MSC viability were observed, indicating that Aprotinin did not negatively affect human MSCs in concentrations up to 100 μg/mL. Factors produced by human MSC were not examined. Human MSCs excrete a range of factors; it is not known which of these factors are important in the healing response.26,27 We observed that human MSCs maintained their multipotency, as indicated by differentiation into adipocytes, osteocytes, and chondrocytes. However, some morphological changes in the chondrocyte pellet sections were seen. In both the Trichome and Alcian Blue stains, it appeared that human MSCs exposed to Aprotinin formed chondrocyte pellets with more extracellular material. Given that Aprotinin is a proteolytic enzyme inhibitor, it is possible that the Aprotinin present in the formation of the pellet had become trapped inside and inhibited the degradation or reconstruction of the extracellular material, resulting in a more densely packed chondrocyte pellet.
An increase in the number of proliferating cells on sutures was noted at day 2, which was not observed in the human MSCs seeded on glass coverslips. This finding may indicate that the presence of both Aprotinin and fibrin could increase the proliferative capacity of human MSCs, although more studies are required to confirm this hypothesis. In addition, an increase in the number of cells adhered to sutures at later time points (days 2, 3, and 5) were also seen. Given the terminal nature of the experimental procedures to determine proliferation, it was not possible to track proliferation over time in each suture. The increase in proliferation corresponded with an increase in the number of cell adhered to the sutures. This increase of human MSCs would allow a greater number of cells to be delivered per suture. The use of Aprotinin allows sutures to have a greater “shelf-life” and offers a greater window of delivery by reducing the degradation of fibrin.
In this study, we did not examine any potential in vivo effects; however, we hypothesize that the Aprotinin supplementation would have insignificant effects upon delivery. The human MSC-seeded sutures in this study were only exposed to Aprotinin during culture, and this was through the culture media to inhibit the degradation of the fibrin suture. Once the suture is removed from the culture media and no longer exposed to Aprotinin, it should behave like an untreated fibrin suture and rapidly degrade in vivo.
CONCLUSION
Mechanical stability of human MSC-seeded fibrin sutures in terms of diameter, ultimate tensile strength, and modulus can be increased when cultured with Aprotinin. Aprotinin decreased the degradation of fibrin while not significantly altering human MSCs viability and differentiation. In addition, human MSCs proliferated on fibrin sutures increasing the number of cells on the suture after 2 days. These results suggest that incubation of cell-seeded fibrin sutures in Aprotinin will extend suture life, while not affecting human MSC function.
Acknowledgments
Contract grant sponsor: National Institutes of Health; contract grant number: R01HL115282
Footnotes
One of the authors has received or will receive remuneration or other perquisites for personal or professional use from a commercial or an industrial agent in a direct or indirect relationship to their authorship.
References
- 1.Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: A review of global methodologies of mortality measurement. Circulation. 2013;127:749–756. doi: 10.1161/CIRCULATIONAHA.112.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S Heart disease and stroke statistics–2015 update: A report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 5.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 6.Barry FP, Murphy JM. Mesenchymal stem cells: Clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Tae SK, Lee SH, Park JS, Im GI. Mesenchymal stem cells for tissue engineering and regenerative medicine. Biomed Mater. 2006;1:63–71. doi: 10.1088/1748-6041/1/2/003. [DOI] [PubMed] [Google Scholar]
- 8.Reffelmann T, Kloner RA. Cellular cardiomyoplasty–cardiomyocytes, skeletal myoblasts, or stem cells for regenerating myocardium and treatment of heart failure? Cardiovasc Res. 2003;58:358–368. doi: 10.1016/s0008-6363(02)00739-3. [DOI] [PubMed] [Google Scholar]
- 9.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 10.Hou M, Yang KM, Zhang H, Zhu WQ, Duan FJ, Wang H, Song YH, Wei YJ, Hu SS. Transplantation of mesenchymal stem cells from human bone marrow improves damaged heart function in rats. Int J Cardiol. 2007;115:220–228. doi: 10.1016/j.ijcard.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56:721–734. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potapova IA, Doronin SV, Kelly DJ, Rosen AB, Schuldt AJ, Lu Z, Kochupura PV, Robinson RB, Rosen MR, Brink PR, Gaudette GR, Cohen IS. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. Am J Physiol Heart Circ Physiol. 2008;295:H2257–H2263. doi: 10.1152/ajpheart.00219.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Rane AA, Christman KL. Biomaterials for the treatment of myocardial infarction: A 5-year update. J Am Coll Cardiol. 2011;58:2615–2629. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 17.Guyette JP, Fakharzadeh M, Burford EJ, Tao ZW, Pins GD, Rolle MW, Gaudette GR. A novel suture-based method for efficient transplantation of stem cells. J Biomed Mater Res A. 2013;101:809–818. doi: 10.1002/jbm.a.34386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuss S, Schneider RK, Tietze L, Knuchel R, Jahnen-Dechent W. Secretion of fibrinolytic enzymes facilitates human mesenchymal stem cell invasion into fibrin clots. Cells Tissues Organs. 2010;191:36–46. doi: 10.1159/000215579. [DOI] [PubMed] [Google Scholar]
- 19.Thomson KS, Korte FS, Giachelli CM, Ratner BD, Regnier M, Scatena M. Prevascularized microtemplated fibrin scaffolds for cardiac tissue engineering applications. Tissue Eng Part A. 2013;19:967–977. doi: 10.1089/ten.tea.2012.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornwell KG, Pins GD. Discrete crosslinked fibrin microthread scaffolds for tissue regeneration. J Biomed Mater Res A. 2007;82:104–112. doi: 10.1002/jbm.a.31057. [DOI] [PubMed] [Google Scholar]
- 21.Lorentz KM, Kontos S, Frey P, Hubbell JA. Engineered Aprotinin for improved stability of fibrin biomaterials. Biomaterials. 2011;32:430–438. doi: 10.1016/j.biomaterials.2010.08.109. [DOI] [PubMed] [Google Scholar]
- 22.Jockenhoevel S, Zund G, Hoerstrup SP, Chalabi K, Sachweh JS, Demircan L, Messmer BJ, Turina M. Fibrin gel – Advantages of a new scaffold in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2001;19:424–430. doi: 10.1016/s1010-7940(01)00624-8. [DOI] [PubMed] [Google Scholar]
- 23.Ye Q, Zund G, Benedikt P, Jockenhoevel S, Hoerstrup SP, Sakyama S, Hubbell JA, Turina M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardio-thorac Surg. 2000;17:587–591. doi: 10.1016/s1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 24.Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27:5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451–H1460. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 26.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]


