 A highly tunable visible-light-promoted reaction system for the radical-mediated functionalization of tertiary aliphatic C–H bonds of complex substrates has been developed.
A highly tunable visible-light-promoted reaction system for the radical-mediated functionalization of tertiary aliphatic C–H bonds of complex substrates has been developed.
Abstract
A highly tunable radical-mediated reaction system for the functionalization of tertiary aliphatic C–H bonds was developed. Reactions of various substrates with the Zhdankin azidoiodane reagent 1, Ru(bpy)3Cl2, and visible light irradiation at room temperature gave C–H azidated or halogenated products in an easily controllable fashion. These reactions are efficient, selective, and compatible with complex substrates. They provide a potentially valuable tool for selectively labeling tertiary C–H bonds of organic and biomolecules with tags of varied chemical and biophysical properties for comparative functional studies.
Introduction
C–H bonds are the most prevalent chemical bonds on the surface of organic and biomolecules. Better means for the selective and controllable functionalization of C–H bonds could greatly facilitate a broad range of applications such as tagging organic and biomolecules, drug development and mapping ligand–receptor interactions.1,2 Despite their tremendous potential, the low reactivity of C–H bonds and the difficulty of achieving selectivity pose a significant challenge for the realization of a C–H labeling strategy, especially for more inert aliphatic C–H bonds. Radical reactions could provide a simple yet powerful approach to selectively target specific aliphatic C–H bonds due to their inherent ability to differentiate aliphatic C–H bonds based on the bond dissociation energy.3–5 While various radical aliphatic C–H functionalization reactions have been well-studied, new methods with better reactivity, versatility and biocompatibility are necessary for the selective and efficient C–H labeling of complex substrates.
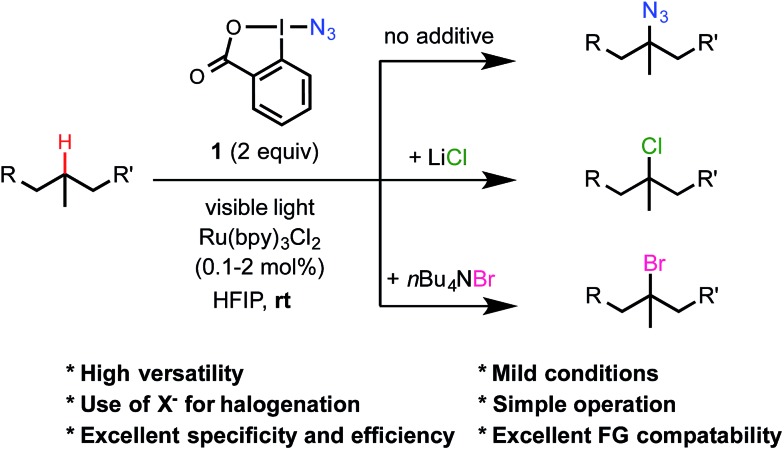
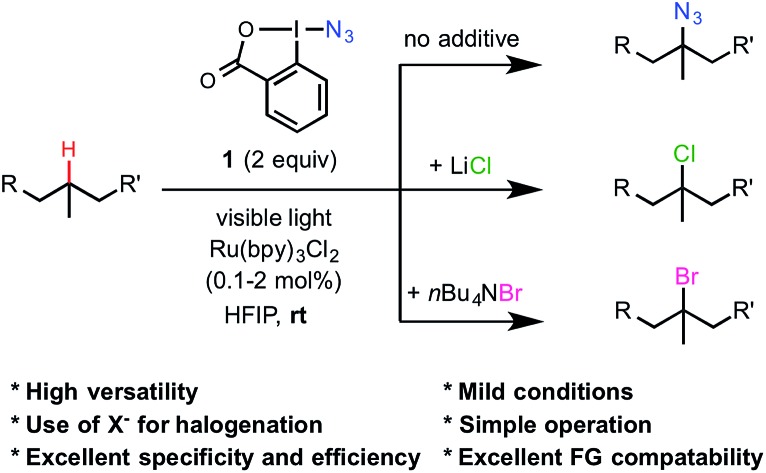
Among reported aliphatic C–H functionalization reactions, C–H azidation has proven to be particularly useful due to the unique photophysical and chemical reactivity of the azido group.6–14 In 1996, Zhdankin8 reported that the reaction of simple hydrocarbons with azidoiodane 1 in the presence of benzoyl peroxide led to the selective azidation of 3° and activated 2° C–H bonds in moderate to good yield.9–11 Hartwig recently discovered that Fe/PyBOX catalysts promote aliphatic C–H azidation with 1 under milder conditions, allowing the labeling of complex natural products with high selectivity.12,13 Herein, we report a new strategy to affect the azidation of 3° C–H bonds of complex substrates using the Zhdankin reagent 1, a photosensitizer and visible-light irradiation at room temperature (Scheme 1). Furthermore, this visible-light-promoted radical reaction system can be conveniently modulated by the addition of nucleophilic halides to achieve aliphatic C–H chlorination and bromination with high efficiency and chemospecificity.
Scheme 1. A highly tunable radical-mediated reaction system for the selective functionalization of 3° C(sp3)–H bonds under the promotion of visible light.
Results and discussion
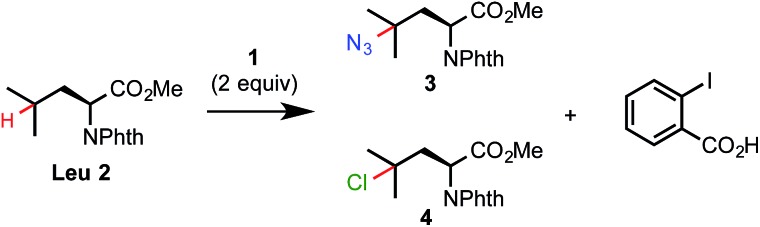
Our research in radical C–H functionalization chemistry stems from our interest in the synthetic and biological studies of peptides.15 Compared with α-amino acid (αAA) residues bearing polar or aromatic side chains, the structural and functional roles of hydrophobic aliphatic αAA residues such as leucine and valine in peptides and proteins are much more difficult to probe due to the lack of suitable chemical handles in these residues. Following the report of Hartwig's Fe-catalyzed aliphatic C–H azidation,12 we wondered whether this azidation chemistry could potentially provide a long sought-after method for selectively labeling the aliphatic αAA residues of peptides and proteins. As shown in Table 1, we commenced our investigation with the azidation of N-phthaloyl leucine methyl ester 2 with 1 under various reported conditions (Table 1). The reaction of 2 under Fe/iPr-PyBOX-catalyzed conditions in CH3CN provided the desired azidated product 3 in low yield (entry 1). The reaction under benzoyl peroxide-promoted conditions also gave 3 in moderate yield (entry 4). Interestingly, the azidation of Leu 2 proceeded in good yield without any catalyst or additive at 80 °C in hexafluoroisopropanol (HFIP) under an air atmosphere, forming ortho-iodobenzoic acid as the main byproduct (entry 9).
Table 1. 3° C–H azidation and chlorination of Leu 2.
| ||||||
| Entry | Reagents (equiv.)/atmosphere | Solvent | T (°C)/time (h) | Yield
a
(%) |
||
| 3 |
4 | |||||
| 1 | Fe(OAc)2 (0.1), iPr-PyBOX (0.11), air | CH3CN | 50/24 | 27 | 0 | |
| 2 | Fe(OAc)2 (0.1), air | CH3CN | 50/24 | 30 | 0 | |
| 3 | Air | CH3CN | 50/24 | 37 | 0 | |
| 4 | Benzoyl peroxide (0.2), air | DCE | 50/24 | 41 | 0 | |
| 5 | Benzoyl peroxide (0.2), air | HFIP | rt/24 | 7 | 0 | |
| 6 | Air | CH3CN | 80/24 | 42 | 0 | |
| 7 | Air | H2O | 80/24 | 30 | 0 | |
| 8 | Air | DCM/H2O (1 : 9) | 80/24 | 51 | 0 | |
| 9 | Air | HFIP | 80/24 | 70 | 0 | |
| 10 | Ir(ppy)3 (0.05), WL b , Ar | HFIP | rt/24 | <2 | 0 | |
| 11 | Ru(bpy)3Cl2 (0.05), VL, Ar | HFIP | rt/24 | 46 | <5 | |
| 12 | Ru(bpy)3Cl2 (0.001), VL, Ar | HFIP | rt/24 | 91 (86 c ) | <3 | |
| 13 | Ru(bpy)3Cl2 (0.0001), VL, Ar | HFIP | rt/24 | 70 | <3 | |
| 14 | Ru(bpy)3Cl2 (0.2), VL, Ar | HFIP | rt/24 | 15 | 35 | |
| 15 | Ru(bpy)3Cl2 (0.001), MgCl2 (2), VL, Ar | HFIP | rt/24 | 5 | 30 | |
| 16 | Ru(bpy)3Cl2 (0.001), TMSCl (2), VL, Ar | HFIP | rt/24 | <2 | 87 | |
| 17 | Ru(bpy)Cl2 (0.001), LiCl (4), VL, Ar | HFIP | rt/24 | <2 | 95 (77 c ) | |
| 18 | LiCl (4), VL, Ar | HFIP | rt/24 | 0 | 0 | |
| 19 | Ru(bpy)3Cl2 (0.001), TEMPO (2), VL, Ar | HFIP | rt/24 | <2 | 0 | |
| 20 | Ru(bpy)3Cl2 (0.001), Ar, in darkness | HFIP | rt/24 | <2 | 0 | |
| 21 | VL, Ar (no Ru(bpy)3Cl2) | HFIP | rt/24 | 6 | 0 | |
aYields are based on 1H-NMR analysis on a 0.2 mmol scale.
bVL: 12 W fluorescent bulb.
cIsolated yield (see ESI).
In order to achieve better biocompatibility, we next investigated whether the azidation reaction could proceed at room temperature. While the use of various electron transfer reagents such as zinc metal and cerium ammonium nitrate failed, we were delighted to find that the application of a photosensitizer (Ru(bpy)3Cl2) and visible light irradiation (VL: 12 W household fluorescent light bulb) formed 3 in moderate yield under an Ar atmosphere (entry 11).16 Interestingly, the use of 0.1 mol% of Ru(bpy)3Cl2 gave significantly improved results than when using 5 mol% of Ru(bpy)3Cl2 (entry 12). Furthermore, we were surprised to find that the use of 20 mol% of Ru(bpy)3Cl2 produced only a small amount of 3 but instead produced a 35% yield of chlorinated product 4 (entry 14). This result prompted us to evaluate different Cl donors to improve the yield of the chlorinated product (see ESI† for more screening results). A number of nucleophilic Cl sources worked, LiCl or TMSCl gave 4 in the highest yield and selectivity (entries 16 and 17). The addition of 2 equiv. of TEMPO shut down the reaction with little conversion of 2 (entry 19). The reaction in darkness gave little product (entry 20). Interestingly, visible light irradiation in the absence of Ru(bpy)3Cl2 also gave a small amount of 3 (entry 21).
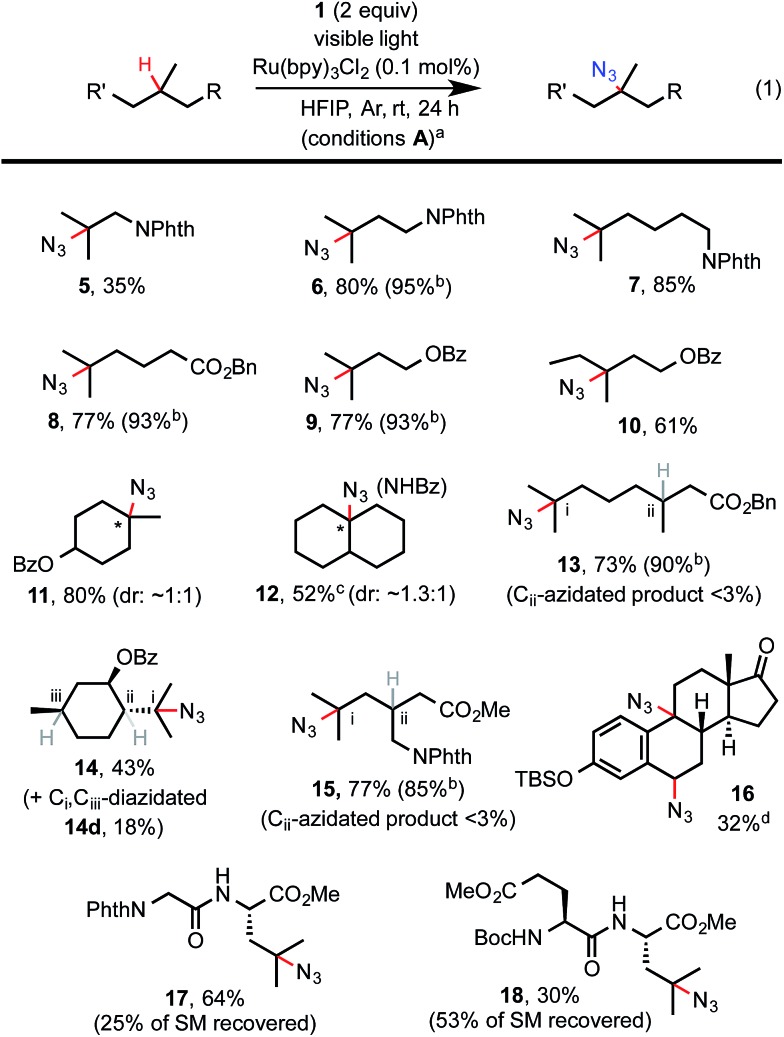
As shown in Scheme 2, C–H azidation reactions with azidoiodane 1 worked well for a variety of aliphatic substrates with excellent 3° C–H selectivity under the optimized light-promoted conditions A.17 Common functional groups e.g. Boc, Bn, TBS, ester and amide were well tolerated. Steric hindrance and electron-withdrawing functional groups generally diminish the reactivity of the neighbouring C–H bonds, allowing preferential labeling of certain 3° C–H bonds of complex substrates.1b,e For instance, azidation selectively took place at the more distal 3° carbon of 13 and N-phthaloyl pregabalin methyl ester 15. Moreover, we were delighted to find that the C–H azidation of dipeptides under the standard light-promoted conditions also provided good yield and excellent selectivity (see 17 and 18).
Scheme 2. Substrate scope of aliphatic C–H azidation. [a] Isolated yield on a 0.2 mmol scale. [b] 1H-NMR yield. Isolated yield was occasionally compromised by the volatility of the product and/or difficulties in purification. [c] Isolated yield of its amide derivative (see ESI†). [d] As the only major product.
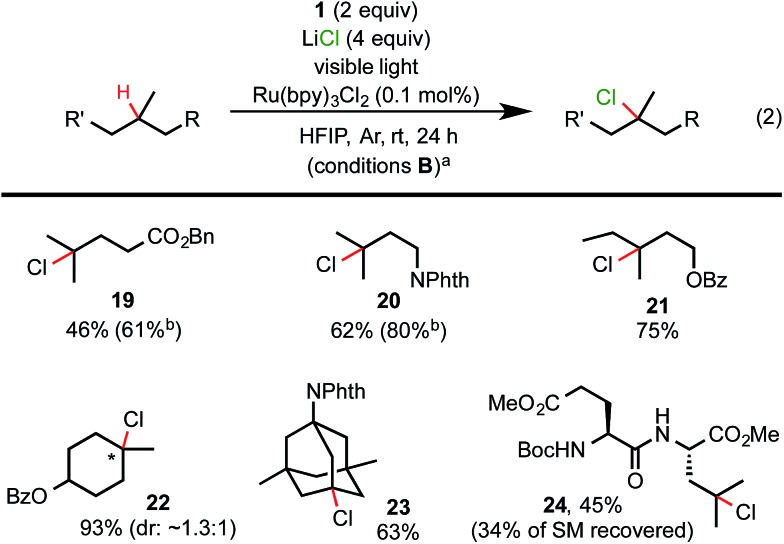
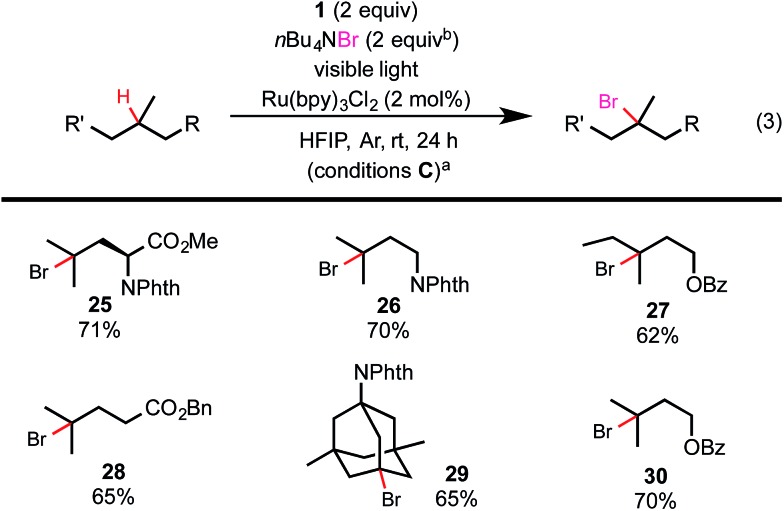
As shown in Scheme 3, visible light-promoted 3° C–H chlorination reactions with azidoiodane 1 and LiCl as the Cl donor also demonstrated excellent efficiency, site-selectivity and functional group tolerance.18 The reactivity of these C–H chlorination reactions was slightly higher than the corresponding azidation reaction (see 21vs.10 and 24vs.18). Additionally, we were pleased to find that this reaction system can be further modulated to form 3° C–H brominated products by the addition of a bromide donor.19,20 As shown in Scheme 4, the reaction of various aliphatic substrates with 2 equiv. of nBu4NBr, 2 equiv. of 1, and 2 mol% of Ru(bpy)3Cl2 under visible light irradiation (conditions C) gave 3° C–H brominated products in good yield and with excellent selectivity. It is worth noting that the competing C–H azidation process was completely suppressed in both chlorination and bromination reaction systems.
Scheme 3. Substrate scope of C–H chlorination. (a) Isolated yield on a 0.2 mmol scale and (b) 1H-NMR yield.
Scheme 4. Substrate scope of C–H bromination. (a) Isolated yield on a 0.2 mmol scale; (b) added in 2 portions (1 + 1 equiv.).
The azidation reactions under the newly developed conditions are consistent with the radical chain mechanisms proposed by Zhdankin8 (Scheme 5A). The radical chain mechanism is supported by the large quantum yield (Φ ∼ 18, measured by Yoon's method21) of the C–H azidation reaction of 32 with 1 (see ESI†). Under thermally promoted conditions (see entry 9, Table 1), the weak I–N3 bond of 1 presumably undergoes homolytic cleavage to form an iodanyl radical I and an azido radical.8 Because of the relative weak reactivity the of N3 radical,22I likely serves as the H abstractor23 to convert the C–H substrate to a C-centered radical intermediate II. II then attacks 1 to form the azidated product and regenerates I, which propagates the radical chain reaction. Under visible-light irradiation in the presence of Ru(bpy)3Cl2, 1 may be activated by electron transfer24 to generate radical I, which can trigger the same chain reaction at rt. The chlorination reactions likely share the same mechanism for the azidoiodane-mediated radical initiation step (formation of I) as the azidation reactions. However, chloroiodane 31 25 formed via in situ substitution of 1 with Cl– probably overrides azidoiodane in the subsequent chain propagation step to selectively form the C–H chlorinated products (Scheme 5B). Our control experiments indicated that both 1 and 31 are involved in the chlorination reaction. As shown in eqn 4, mixing of 1 and LiCl in HFIP at rt in the absence of light can form 31 (42% yield in 12 h). Subjecting substrate 32 and 2 equiv. of pre-made 31 to the standard light-promoted conditions did not give any reaction (eqn 5). Interestingly, the addition of 1 equiv. of 1 together with 31 cleanly restored the chlorination reaction (see more details in ESI†).26 This suggests that chloride 31 cannot be directly activated to generate the iodanyl radical I for the initial H-abstraction, but is more reactive than azidoiodane toward nucleophilic attack by the radical intermediate II. C–H bromination with nBu4NBr may follow a similar pathway as chlorination.
Scheme 5. Plausible mechanisms for C–H azidation and chlorination.
Conclusions
In summary, we have developed a uniquely tunable radical-mediated reaction system for the azidation and halogenation of tertiary aliphatic C–H bonds of various substrates with azidoiodane 1 and visible light irradiation at room temperature. These reactions provide a simple and powerful method for selectively labeling tertiary aliphatic C–H bonds of organic molecules with diverse tags. In addition to a mild protocol for radical aliphatic C–H functionalization, this study demonstrated a novel strategy to use more easily available nucleophilic halide reagents for radical C–H halogenation reactions. Further mechanistic studies and expansion of this reaction system to achieve other types of aliphatic C–H functionalization and applications in labeling biomolecules are currently under investigation.
Supplementary Material
Acknowledgments
We gratefully thank the State Key Laboratory of Elemento-Organic Chemistry at Nankai University for financial support of this work.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc04169d
References
- For selected reviews on C–H activation for organic synthesis and labeling: ; (a) Godula K., Sames D. Science. 2006;312:67. doi: 10.1126/science.1114731. [DOI] [PubMed] [Google Scholar]; (b) Newhouse T., Baran P. S. Angew. Chem., Int. Ed. 2011;50:3362. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yamaguchi J., Yamaguchi A. D., Itami K. Angew. Chem., Int. Ed. 2012;51:8960. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; (d) Wencel-Delord J., Glorius F. Nat. Chem. 2013;5:369. doi: 10.1038/nchem.1607. [DOI] [PubMed] [Google Scholar]; (e) White M. C. Science. 2012;335:807. doi: 10.1126/science.1207661. [DOI] [PubMed] [Google Scholar]; (f) Noisier A. F. M., Brimble M. A. Chem. Rev. 2014;114:8775. doi: 10.1021/cr500200x. [DOI] [PubMed] [Google Scholar]
- (a) Sletten E. M., Bertozzi C. R. Angew. Chem., Int. Ed. 2009;48:6974. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Takaoka Y., Ojida A., Hamachi I. Angew. Chem., Int. Ed. 2013;52:4088. doi: 10.1002/anie.201207089. [DOI] [PubMed] [Google Scholar]
- For selected examples on aliphatic C–H oxygenation, thiolation, and amination via non-organometallic mechanisms: ; (a) Chen M. S., White M. C. Science. 2007;318:783. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]; (b) Michaudel Q., Thevenet D., Baran P. S. J. Am. Chem. Soc. 2012;134:2547. doi: 10.1021/ja212020b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Amaoka Y., Kamijo S., Hoshikawa T., Inoue M. J. Org. Chem. 2012;77:9959. doi: 10.1021/jo301840e. [DOI] [PubMed] [Google Scholar]; (d) Litvinas N. D., Brodsky B. H., Du Bois J. Angew. Chem., Int. Ed. 2009;48:4513. doi: 10.1002/anie.200901353. [DOI] [PubMed] [Google Scholar]; (e) Guo S., Zhang X., Tang P. Angew. Chem., Int. Ed. 2015;54:4065. doi: 10.1002/anie.201411807. [DOI] [PubMed] [Google Scholar]
- For selected examples on radical-mediated aliphatic C–H fluorination: ; (a) Xia J.-B., Zhu C., Chen C. J. Am. Chem. Soc. 2013;135:17494. doi: 10.1021/ja410815u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Amaoka Y., Nagatomo M., Inoue M. Org. Lett. 2013;15:2160. doi: 10.1021/ol4006757. [DOI] [PubMed] [Google Scholar]; (c) Halperin S. D., Fan H., Chang S., Martin R. E., Britton R. Angew. Chem., Int. Ed. 2014;53:4690. doi: 10.1002/anie.201400420. [DOI] [PubMed] [Google Scholar]; (d) Kee C. W., Chin K. F., Wong M. W., Tan C.-H. Chem. Commun. 2014;50:8211. doi: 10.1039/c4cc01848f. [DOI] [PubMed] [Google Scholar]
- For selected examples on enzyme-catalyzed aliphatic C–H functionalization: ; (a) Zhang K., Shafer B. M., Demars M. D., Stern H. A., Fasan R. J. Am. Chem. Soc. 2012;134:18695. doi: 10.1021/ja3073462. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Matthews M. L., Chang W.-C., Layne A. P., Miles L. A., Krebs C., Bollinger Jr J. M. Nat. Chem. Biol. 2014;10:209. doi: 10.1038/nchembio.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Brase S., Gil C., Knepper K., Zimmermann V. Angew. Chem., Int. Ed. 2005;44:5188. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]; (b) Song W., Kozhushkov S. I., Ackermann L. Angew. Chem., Int. Ed. 2013;52:6576. doi: 10.1002/anie.201302015. [DOI] [PubMed] [Google Scholar]
- For selected examples of sp2 C–H azidation reactions: ; (a) Kita Y., Tohma H., Inagaki M., Hatanaka K., Yakura T. Tetrahedron Lett. 1991;32:4321. [Google Scholar]; (b) Lubriks D., Sokolovs I., Suna E. J. Am. Chem. Soc. 2012;134:15436. doi: 10.1021/ja305574k. [DOI] [PubMed] [Google Scholar]; (c) Tang C., Jiao N. J. Am. Chem. Soc. 2012;134:18924. doi: 10.1021/ja3089907. [DOI] [PubMed] [Google Scholar]; (d) Xie F., Qi Z., Li X. Angew. Chem., Int. Ed. 2013;52:11862. doi: 10.1002/anie.201305902. [DOI] [PubMed] [Google Scholar]
- Zhdankin V. V., Krasutsky A. P., Kuehl C. J., Simonsen A. J., Woodward J. K., Mismash B., Bolz J. T. J. Am. Chem. Soc. 1996;118:5192. [Google Scholar]
- For pioneering work on azidation with hypervalent iodine reagents: ; (a) Magnus P., Lacour J., Weber W. J. Am. Chem. Soc. 1993;115:9347. [Google Scholar]; (b) Kita Y., Tohma H., Takada T., Mitoh S., Fujita S., Gyoten M. Synlett. 1994:427. [Google Scholar]
- For selected examples on radical aliphatic C–H functionalization with hypervalent iodine(iii) reagents: ; (a) Ochiai M., Miyamoto K., Kaneaki T., Hayashi S., Nakanishi W. Science. 2011;332:448. doi: 10.1126/science.1201686. [DOI] [PubMed] [Google Scholar]; (b) Maruoka K. Angew. Chem., Int. Ed. 2013;52:8657. doi: 10.1002/anie.201304359. [DOI] [PubMed] [Google Scholar]; (c) Antonchick A. P., Burgmann L. Angew. Chem., Int. Ed. 2013;52:3267. doi: 10.1002/anie.201209584. [DOI] [PubMed] [Google Scholar]; (d) Zhao Y., Yim W. L., Tan C. K., Yeung Y.-Y. Org. Lett. 2011;13:4308. doi: 10.1021/ol2016466. [DOI] [PubMed] [Google Scholar]; (e) Kita Y. ChemCatChem. 2014;6:76. [Google Scholar]
- For selected reviews on hypervalent iodine chemistry: ; (a) Zhdankin V. V., Stang P. Chem. Rev. 2002;102:2523. doi: 10.1021/cr010003+. [DOI] [PubMed] [Google Scholar]; (b) Tohma H., Kita Y. Adv. Synth. Catal. 2004;346:111. [Google Scholar]; (c) Narayan R., Manna S., Antonchick A. P. Synlett. 2015;26:1785. [Google Scholar]
- Sharma A., Hartwig J. F. Nature. 2015;517:600. doi: 10.1038/nature14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a Mn-based catalytic system that uses NaN3 as an azido source and PhIO as an oxidant to achieve the azidation of 3° and unactivated 2° C–H bonds of drug molecules at rt: Huang X., Bergsten T. M., Groves J. T., J. Am. Chem. Soc., 2015, 137 , 5300 . [DOI] [PubMed] [Google Scholar]
- For two recent examples of transition-metal-free aliphatic C–H azidation reactions: ; (a) Zhang X., Yang H., Tang P. Org. Lett. 2015;17:5828. doi: 10.1021/acs.orglett.5b03001. [DOI] [PubMed] [Google Scholar]; (b) Kamijo S., Watanabe M., Kamijo K., Tao K., Murafuji T. Synthesis. 2015;48:115. [Google Scholar]
- (a) Feng Y., Chen G. Angew. Chem., Int. Ed. 2010;49:958. doi: 10.1002/anie.200905134. [DOI] [PubMed] [Google Scholar]; (b) He G., Zhang S.-Y., Nack W. A., Pearson R., Rabb-Lynch J., Chen G. Org. Lett. 2014;16:6488. doi: 10.1021/ol503347d. [DOI] [PubMed] [Google Scholar]
- For selected reviews on visible light-promoted organic reactions: ; (a) Narayanam J. M. R., Stephenson C. R. J. Chem. Soc. Rev. 2011;40:102. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]; (b) Yoon T. P., Ischay M. A., Du J. N. Nat. Chem. 2010;2:527. doi: 10.1038/nchem.687. [DOI] [PubMed] [Google Scholar]; (c) Prier C. K., Rankic D. A., MacMillan D. W. C. Chem. Rev. 2013;113:5322. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xie J., Jin H., Xu P., Zhu C. Tetrahedron Lett. 2014;55:36. [Google Scholar]
- Our light-promoted C–H azidation reactions showed similar selectivity and reactivity to Hartwig’s Fe-catalyzed reaction system (ref. 12)
- Synthetically useful aliphatic C–H chlorination reactions suitable for complex substrates are still limited. These 3° C–H selective reactions provide a valuable complement to Groves’ Mn-catalyzed 2° C–H selective chlorination: ; (a) Liu W., Groves J. T. J. Am. Chem. Soc. 2010;132:2010. doi: 10.1021/ja105548x. [DOI] [PubMed] [Google Scholar]; (b) Liu W., Groves J. T. Acc. Chem. Res. 2015;48:1727. doi: 10.1021/acs.accounts.5b00062. [DOI] [PubMed] [Google Scholar]
- Intermolecular selective aliphatic C–H bromination reactions using a complex substrate as the limiting reagent are still limited. These 3° C–H bromination reactions provide a useful complement to Alexanian’s visible-light promoted 2° C–H selective bromination using N-bromoamide reagents: Schmidt V. A., Quinn R. K., Brusoe A. T., Alexanian E. J., J. Am. Chem. Soc., 2014, 136 , 14389 . [DOI] [PubMed] [Google Scholar]
- Similar iodination and fluorination reactions with nucleophilic iodide and fluoride additives have been unsuccessful by far. See ref. 4 for the recent success of light-facilitated aliphatic C–H fluorination. For the acetoxyiodane-mediated 2° C–H iodination of hydrocarbons (used as co-solvent): Barluenga J., Camos-Gomez E., Rodriguez D., Gonzalez-Bobes F., Gonzalez J. M., Angew. Chem., Int. Ed., 2005, 44 , 5851 . [DOI] [PubMed] [Google Scholar]
- For an excellent paper characterizing chain processes in visible light photoredox catalysis based on the measurements of quantum yield Φ: Crismesia M. A., Yoon T. P., Chem. Sci., 2015, 6 , 5426 , . A reaction with Φ ≫ 1 could only be consistent with a product-forming chain mechanism. Our “light/dark” experiment (see ESI) showed that the same C–H azidation reaction requires constant irradiation for product formation, which suggests a relatively short lifetime of the radical chain process .26668708 [Google Scholar]
- Alfassi Z. B., Shuler R. H. J. Phys. Chem. 1985;89:3359. [Google Scholar]
- (a) (b).The mechanism of H-abstraction using the iodanyl radical I has been proposed in a number of previous studies, see: ; (a) ref. 8 and 10e, ; Ochiai M., Ito T., Takahashi H., Nakanishi A., Toyonari M., Sueda T., Goto S., Shiro M. J. Am. Chem. Soc. 1996;118:7716. [Google Scholar]
- For the related light-induced activation of hydroxybenziodoxole to form the iodanyl radical I, see: Huang H., Zhang G., Gong L., Zhang S., Chen Y., J. Am. Chem. Soc., 2014, 136 , 2280 . [DOI] [PubMed] [Google Scholar]
- The synthesis of chloroiodane 31 was recently reported by Togni as an intermediate in the preparation of the corresponding trifluoromethyl aryliodonium compound. Matoušek V., Pietrasiak E., Schwenk R., Togni A., J. Org. Chem., 2013, 78 , 6763 , . Its use for C–H functionalization reactions was unknown . [DOI] [PubMed] [Google Scholar]
- Reaction of 32 with 2 equiv. of 31 and 0.2 equiv. of benzoyl peroxide in HFIP at either rt or 80 °C gave no chlorinated product 20
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.