 Subcomponent self-assembly generates dynamic combinatorial libraries of Zn4L6 cages whose composition is strongly affected by catenation and encapsulation.
Subcomponent self-assembly generates dynamic combinatorial libraries of Zn4L6 cages whose composition is strongly affected by catenation and encapsulation.
Abstract
Two new Zn4L6 cages composed of diamine subcomponents containing either naphthalene diimide (NDI) or porphyrin moieties are described. Their structural differences allow these cages to exhibit distinct interactions with different chemical stimuli, yielding different supramolecular products. The electron-poor NDI subunits of the first cage were observed to thread through electron-rich aromatic crown-ether macrocycles, forming mechanically-interlocked species up to a [3]catenane, whereas the porphyrin ligands of the second cage interacted favourably with C70, causing it to be bound as a guest. When mixed, the two cages were observed to form a dynamic combinatorial library (DCL) of seven constitutionally distinct mixed-ligand Zn4L6 cages. The DCL was observed to reconstitute in opposing ways when treated with either the crown ether or C70: the electron-rich macrocycle templated the formation of heteroleptic catenanes, whereas C70 caused the DCL to self-sort into homoleptic structures.
Introduction
Living systems respond in complex ways to different stimuli. The study of synthetic chemical systems that reconstitute and respond to different stimuli can offer insight into the signalling events that underpin biological systems,1 as well as identifying new means to synthesize useful stimuli-responsive systems2 and materials.3
One approach to investigating stimuli-responsive behaviour is to design dynamic combinatorial libraries (DCLs)4 of molecules that exchange components under thermodynamic control, and thus are inherently responsive to changes in physical conditions and the addition of different stimuli.5 This approach has been used to identify protein inhibitors,6 design mixtures of compounds that self-sort into different host–guest complexes7 and to engineer separations of mixtures of molecules held together by dynamic covalent bonds.8 In some cases, the response of a DCL to a stimulus has led, through reconstitution of the system, to the promotion of library members that may otherwise not be observed.9
As part of our investigations into stimuli responsive systems, we sought to design an adaptive library of different metallo-supramolecular cages that could respond to templates that are either bound inside the central cavity of a cage,10 or interact specifically with the edges of an assembly (i.e. catenation).11 To this end, it was necessary to design a library comprised of M4L6 cages containing two different kinds of diamine subcomponent, each of which engendered a response to a distinct kind of stimulus. It was also essential that the different ligands in such a library possess near-identical lengths to prevent sterically-driven self-sorting behaviour.12
Our DCL is based upon two similarly-sized Zn4L6 tetrahedral cages, built using ligands that contain naphthalene diimide (NDI) or Zn–porphyrin moieties, respectively. Combining these cages in solution yielded a DCL of seven different Zn4L6 cages, differing in their NDI : porphyrin ligand ratios. By utilizing different molecular recognition events, either the threading of the NDI components through a crown ether, or the encapsulation of the fullerene C70, we explored the responsive nature of this DCL. The formation of either homoleptic or heteroleptic M4L6 structures was favoured, depending on the chemical stimulus that was employed. Our study thus represents a singular example of a multi-stimuli-responsive DCL of three-dimensional metallo-supramolecular assemblies. The challenge of analysing and characterising the members of this complex DCL was met by employing electrospray ionization Fourier-transform ion cyclotron mass spectrometry (ESI-FTICR MS).13
Results and discussion
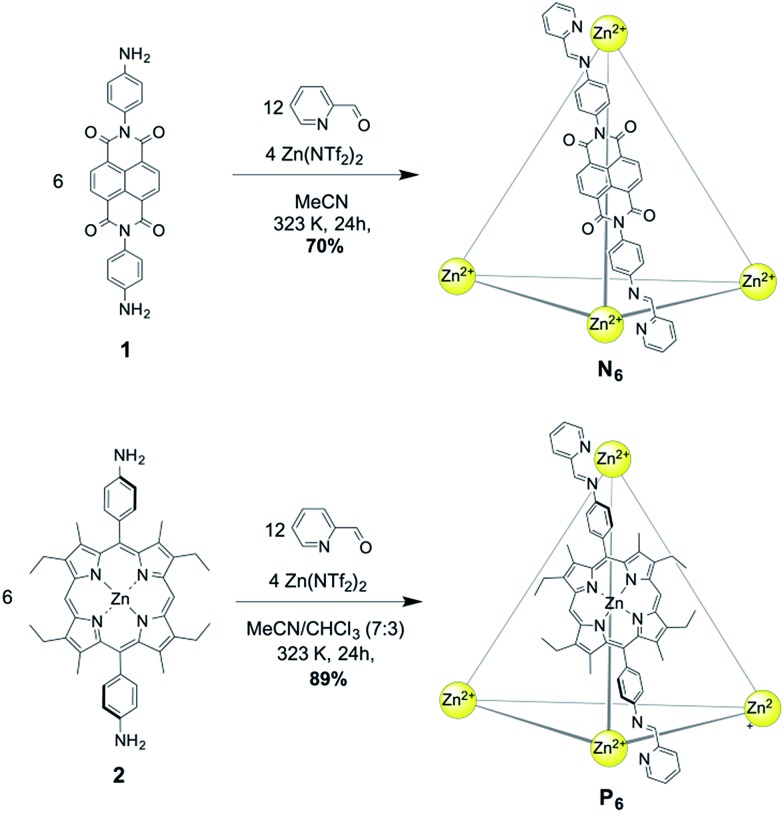
NDI cage N6 was synthesized through the subcomponent self-assembly14 of NDI-containing diamine 1 (6 equiv.), 2-formylpyridine (12 equiv.) and zinc triflimide (Zn(NTf2)2, 4 equiv.) in MeCN (Fig. 1). In similar fashion, Zn–porphyrin cage P6 was prepared using Zn–porphyrin diamine 2 (6 equiv.), 2-formylpyridine (12 equiv.) and zinc triflimide (4 equiv.) in MeCN : CHCl3 (7 : 3). Both assemblies consisted of Zn4L6 tetrahedra, with the bis(imine) condensation products of 2-formylpyridine and the respective diamine acting as bis-bidentate ligands linking adjacent metal centres.
Fig. 1. Synthesis of the Zn4L6 cages N6 (top) from NDI-containing diamine 1, and P6 (bottom) from Zn–porphyrin containing diamine 2.
Cages N6 and P6 were characterised in solution using NMR spectroscopy and ESI-FTICR MS. In both cases, 1H NMR spectra showed multiple resonances for each proton environment, consistent with the presence of the three possible diastereomers of tetrahedral M4L6 assemblies: homochiral T (ΔΔΔΔ/ΛΛΛΛ), heterochiral C3 (ΔΔΔΛ/ΛΛΛΔ), and achiral S4 (ΛΛΔΔ).15 Analysis of the integrated signal intensities of the 1H NMR spectrum of P6 indicated a diastereomer distribution of 32 : 48 : 20 (T : C3 : S4) at 298 K (see ESI, Fig. S10†). The diastereomer distribution of N6 could not be determined from its 1H NMR spectrum (Fig. S3†) due to the greater degree of signal overlap.
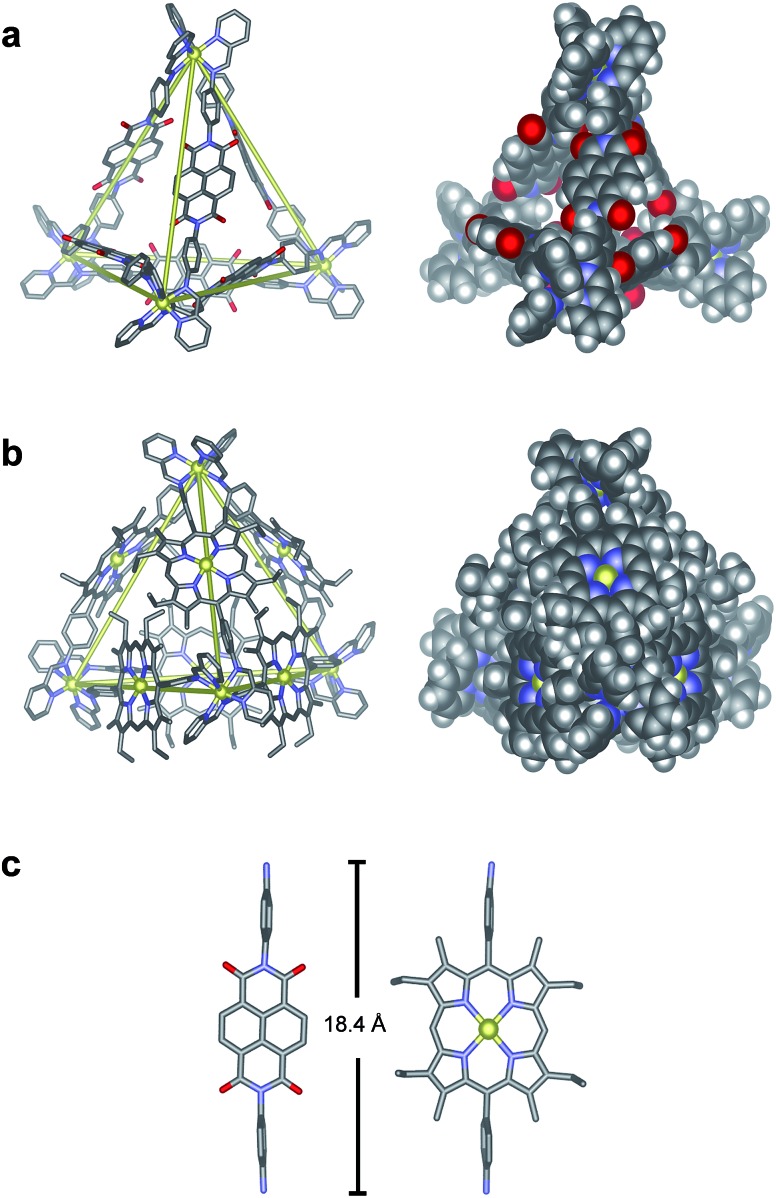
The structures of N6 and P6 were confirmed in the solid state by single-crystal X-ray analysis (Fig. 2). Crystals of N6 were obtained by vapour diffusion of diethyl ether into a solution of N6 in MeCN containing excess KPF6 (10 equiv.). The crystals were found to contain only the S4-symmetric diastereomer. Of the six ligands that bridge the four octahedral zinc centres, four thus displayed a syn-conformation, bridging zinc centres of opposing handedness, and two adopted an anti-conformation, linking zinc centres of identical handedness. The metal–metal separations are 20.6–20.8 Å and 21.0 Å for the syn- and anti-ligands respectively.
Fig. 2. Crystal structures of Zn4L6 tetrahedra (a) N6 and (b) P6. Yellow lines connect ZnII centres to highlight the tetrahedral frameworks. (c) Comparison of the two diamine residues, highlighting their similar lengths. Hydrogen atoms, disorder, solvent molecules and anions are omitted for clarity. Zn = yellow, C = grey, H = white, N = blue, O = red.
Suitable crystals of P6 were obtained through vapour diffusion of diethyl ether into a solution of its triflate salt in MeCN. In this case, only the T-symmetric diastereomer was observed, with both enantiomers (ΔΔΔΔ/ΛΛΛΛ) present in the unit cell. The metal–metal separations range from 20.7–21.4 Å, similar to those observed for N6. Axial water ligands were observed to bind to three porphyrin–ZnII centres, with all three water ligands directed inside the tetrahedral cage (Fig. S32†). The remaining three porphyrin–ZnII axial ligands were MeCN molecules that point outwards from the edges of the tetrahedron. Three of the faces of the Zn4L6 tetrahedron are almost completely enclosed by the Zn–porphyrin ligands that define the edges of the assembly. The remaining face is more open due to crystal packing effects, where a corner of each tetrahedron is observed to protrude slightly into the face of another.
Based upon the known affinity of electron-poor NDI moieties for the electron-rich macrocycle bis-1,5-(dinaphtho)-38-crown-10 (C),16 we anticipated that the NDI edges of cage N6 would thread through C to yield a library of catenated cages. An excess of C (10 equiv.) was thus added to a CD3CN/CDCl3 (1 : 1) solution of N6, and the mixture left to equilibrate at 298 K for 24 h. The resultant 1H NMR spectrum showed the presence of new resonances consistent with C threaded around the NDI groups of N6 (Fig. S7†), and the ESI-FTICR mass spectrum exhibited signals consistent with N6 associated with up to four molecules of C (Fig. 3b). In our previously studied system based on a larger NDI-edged tetrahedron, adducts of the cage with up to 12 molecules of C were detected by ESI-FTICR MS. However, infrared multiphoton dissociation (IRMPD) tandem MS analysis demonstrated that the higher adducts were the result of non-specific interactions in the gas phase.16
Fig. 3. ESI-MS spectra of (a) Zn4L6 NDI cage N6, (b) N6 + C (4 equiv.), yielding N6C2, (c) Zn4L6 porphyrin cage P6 and (d) P6 + C70 (5 equiv.), yielding C70 ⊂ P6.
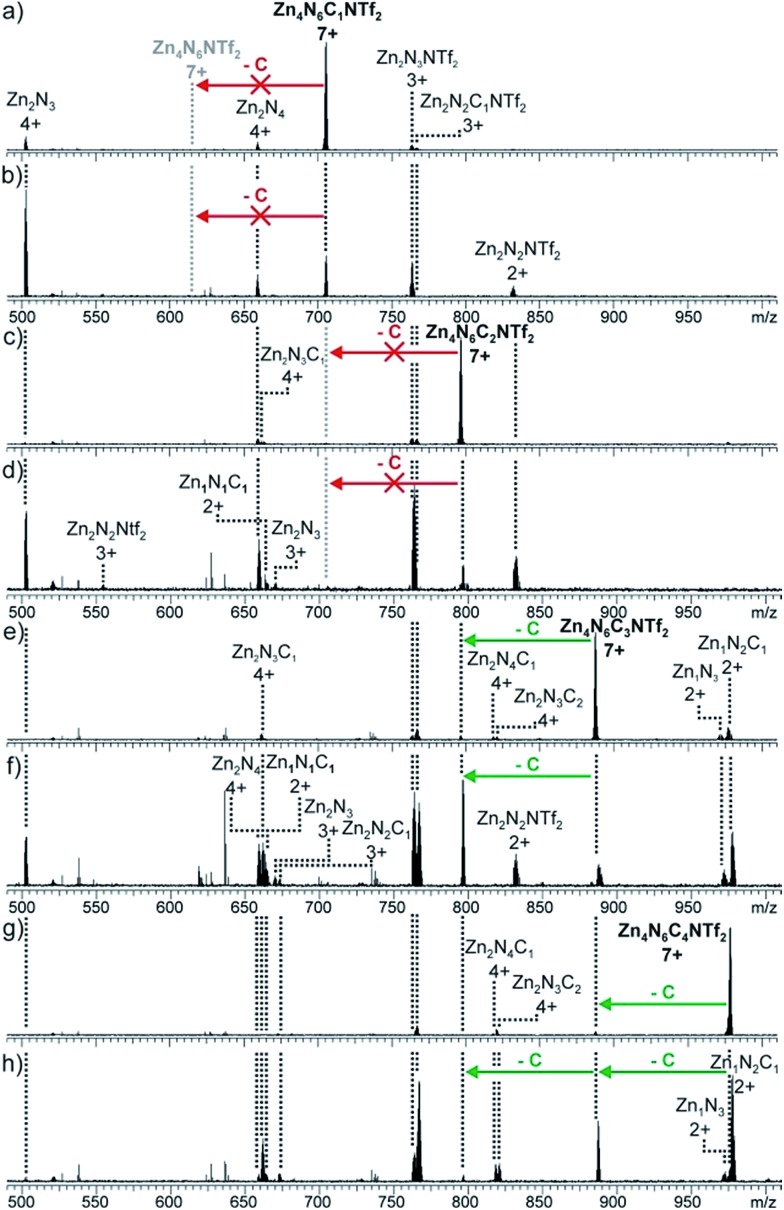
Using a similar approach, the number of catenations per cage between N6 and C was investigated by IRMPD analysis, in which mass-selected parent ions were irradiated with a CO2 laser to induce fragmentation (Fig. 4, S19–22†). The [Zn4N6C(NTf2)]7+ and [Zn4N6C2(NTf2)]7+ ions both underwent symmetrical fragmentation into ‘half-cage’ species [Zn2N3]4+ and [Zn2N3C]4+, which further decomposed into smaller fragments (e.g. [ZnN]2+ and [ZnNC]2+, Fig. 4a–d). When [Zn4N6C3(NTf2)]7+ was isolated and irradiated, [Zn4N6C2(NTf2)]7+ (the product of the non-cage-destructive loss of C), [Zn2N3]4+ and [Zn2N3C]4+ were observed (Fig. 4e and f). Furthermore, the non-symmetric [Zn2N3C2]4+ fragment was not detected, indicating that the [Zn4N6C3(NTf2)]7+ ion present in the ESI-FTICR mass spectrum does not correspond to a [4]catenane, but results from the non-specific gas-phase interactions between N6C2 and C. Similar conclusions can be drawn from the fragmentation of the [Zn4N6C4(NTf2)]7+ ion, which shows successive loss of two molecules of C without destruction of the cage framework (Fig. 4g and h).
Fig. 4. IRMPD ESI-MS spectra of [N6C1–4NTf2]7+ ions. (a) N6C1, 30% laser intensity (b) N6C1, 70% (c) N6C2, 30% (d) N6C2, 50% (e) N6C3, 25% (f) N6C3, 35% (g) N6C4, 20% (h) N6C4, 28%.
The inhibition of further catenation once two equivalents of C have been threaded onto the N6 framework contrasts with the statistical non-cooperative binding behaviour observed between C and our previously reported larger NDI-containing tetrahedron. In the larger system, a [7]catenane was obtained, in which up to six molecules of C were threaded onto a tetrahedral framework. The more limited catenation propensity of N6 appears to arise from its smaller overall size, leading to a sterically-controlled, inhibitive binding process.17 We postulate that the degree of catenation is limited to two crown ethers, because the binding of a third molecule would require two macrocycles to thread around adjacent NDI ligands, generating steric strain within the resulting assembly.
C70 was identified as a potential guest molecule for both N6 and P6 due to their large internal cavities and the potential for π-interactions between C70 and the ligands of the hosts.18 C70 (5 equiv.) was thus added to an MeCN solution of either N6 or P6 and the mixtures were heated to 323 K for 24 h. Analysis of the mixture of N6 and C70 provided no evidence for host–guest interaction by either 1H NMR or ESI-MS. We infer that the large pores in the faces of N6 provide limited screening of C70 from the bulk solvent; an inner phase is thus not differentiated, in contrast with more enclosed NDI-containing assemblies that have been shown to bind fullerenes.18a In contrast, NMR analysis of P6 after the addition of C70 provided clear evidence of guest binding (Fig. S15†).
The 13C NMR spectrum of the solution showed resonances consistent with the presence of C70, despite its limited solubility in MeCN. ESI-MS of the solution also showed signals corresponding to the C70 adduct of P6 (Fig. 3d). We infer from these observations that C70 is encapsulated within the cavity of P6 (Fig. 5). The behaviour of P6 contrasted with the analogous porphyrinato–NiII assembly, which was found to rearrange into new architectures in the presence of C70.19 We attribute this differing behaviour to the planar conformation adopted by Zn–porphyrin 2 (as observed in its crystal structure, Fig. 2c and S28†), whereas the Ni–porphyrin congener adopts a saddled conformation.
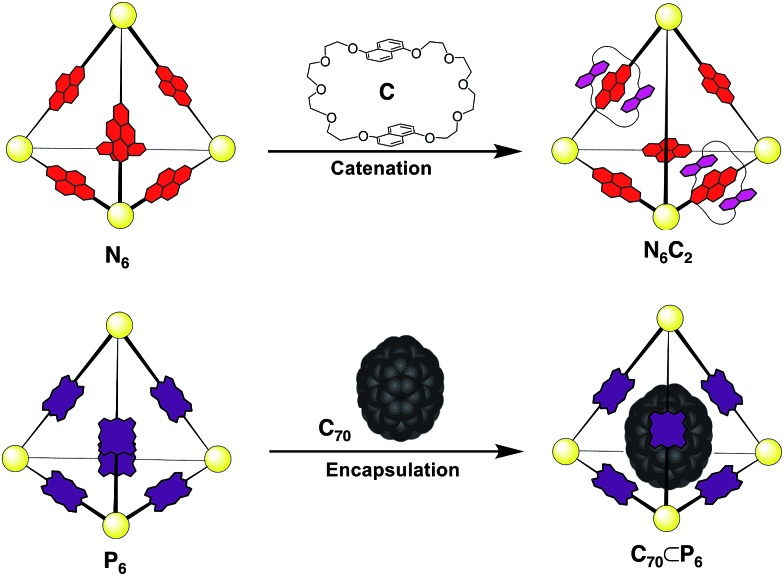
Fig. 5. Summary of the host–guest of the homoleptic cages N6 and P6: catenation of N6 with crown C yielding N6C2 (top), encapsulation of fullerene C70 in porphyrin cage P6, yielding P6 ⊂ C70, (bottom).
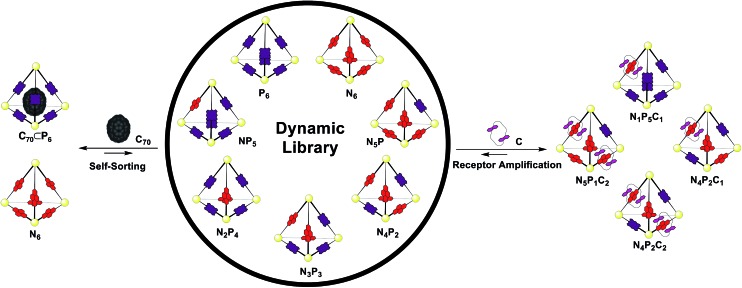
Having established the responses of N6 and P6 to the chemical stimuli of crown C and fullerene C70 (Fig. 5), the responses of a mixed-ligand DCL of tetrahedra were investigated. Separate solutions of pre-formed N6 and P6 (CD3CN/CDCl3, 1 : 1, 1.0 mM) were mixed in a 1 : 1 ratio at 298 K. Attempts to confirm the equilibrium state by an independent synthesis from a mixture of all building blocks failed due to incompatible solubilities. As no further changes were observed after 24 hours, we inferred that equilibrium had been reached.
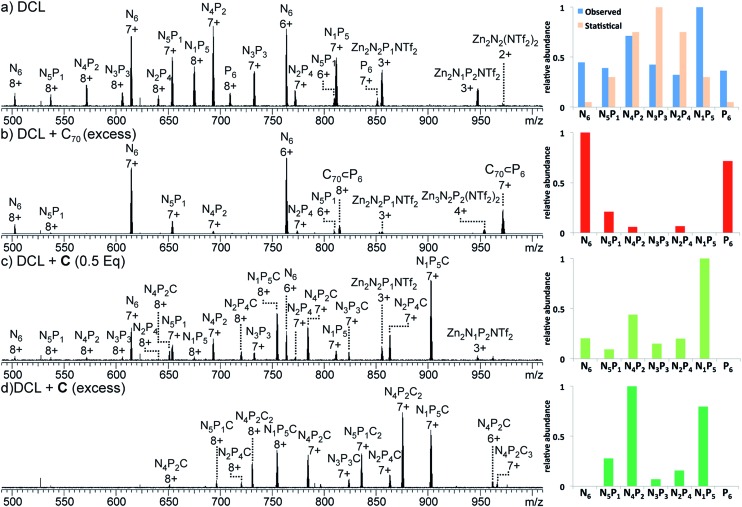
1H NMR spectra of the mixture showed many resonances, corresponding to the numerous magnetically distinct environments of the mixed-ligand DCL members. The challenge of performing meaningful NMR analysis on this DCL was further complicated by the presence of resonances corresponding to the different regio- and stereo-isomers of the library members. The complicated nature of the spectra rendered DCL analysis by NMR impractical, even with the use of two-dimensional and diffusion-ordered experiments. Therefore, ESI-FTICR MS analysis was employed to gain insight as to the composition of the library. Mass spectra revealed signals which corresponded to all seven of the possible Zn4L6 assemblies, hereby labelled NxP(6–x) where N and P represent the NDI and porphyrin-based ligands (Fig. 6a).
Fig. 6. ESI-MS spectra (left) and corresponding charts (right) that show the relative abundances summed for all observed charge states: (a) 1 : 1 DCL of N6 and P6 after 24 h of equilibration, no template, (blue) and statistical distribution (orange) (b) 1 : 1 DCL of N6 and P6 containing C70 (4 equiv.), (c) 1 : 1 DCL of N6 and P6 with C (0.5 equiv.), and (d) 1 : 1 DCL of N6 and P6 with C (4 equiv.).
Strictly speaking, a quantitative analysis of the changes in DCL composition using mass spectrometry requires that all species analysed possess similar ESI-response factors.20 As the homoleptic cages N6 and P6 appeared with similar intensities in the mass spectrum of the DCL (Fig. 6a), we infer that this requirement is met to such a degree that changes in peak intensity between mixed ligand species correlate to the degree of change in their solution concentrations in the DCL.21
All analysed DCLs were prepared under similar conditions, where solutions of N6 and P6 (CD3CN/CDCl3, 1 : 1, 1.0 mM) were allowed to equilibrate over 24 h at 298 K before template addition. The template (C or C70) was added to DCLs in excess, at a ratio of 4 : 1 template/cage because this ratio was found to yield the strongest ESI-MS signals. Following template addition, the library was allowed to equilibrate at room temperature for a further 24 h, by which time no further changes to the library were observed. The MS signals observed in each experiment were processed to establish the relative abundance of each NxP(6–x) cage species (summing the detected m/z signal intensities of the +7 and +8 charge states for each ligand ratio, including those signals that comprise additional C or C70, see the ESI†). In the case of the non-templated DCL (Fig. 6a), each one of the seven possible NxP(6–x) species is observed, with N4P2 and N1P5 occurring in highest abundance.
This distribution deviates from a statistical ratio of species which would follow the 7th level of Pascal's triangle (1 : 6 : 15 : 20 : 15 : 6 : 1). We infer this deviation to result from a thermodynamic preference for the homoleptic cages N6 and P6. Minor differences between the lengths of subcomponents 1 and 2 could give rise to slight strain when both ligands are incorporated into the same assembly,22 thereby favouring the formation of homoleptic over heteroleptic assemblies.21 Given the high abundance of N1P5 observed from a 1 : 1 mixture of N6 and P6, attempts were made to form N1P5 exclusively in similar experiments using a 1 : 5 mixture of N6 and P6. These experiments also yielded a NxP(6–x) library, which was enriched with N1P5, rather than a single species (Fig. S28†).
Having generated a NxP(6–x) DCL, we sought to investigate its response to C and fullerene C70, which interacted with the individual homoleptic cages N6 and P6, respectively. Following addition of C70 to the DCL and equilibration for 24 h at 298 K (Fig. 6b), the composition shifted to give almost entirely N6 and C70 ⊂ P6. ESI-MS analysis of the library showed that only P6 was interacting with C70 to form C70 ⊂ P6, indicating that this self-sorting behaviour can be attributed to the thermodynamic stability of the C70 ⊂ P6 host–guest complex, which removes the porphyrin ligands from the system.23 We observed no evidence for a mixed-ligand assembly that encapsulates C70. We infer that the larger surface area of the porphyrin ligands relative to the NDIs (Fig. 2c) renders P6, the most enclosed cage, uniquely capable of binding C70.
While the addition of C70 causes the library distribution to shift almost completely towards the formation of the species with an optimized binding pocket for the template, we anticipated that the addition of C would have a less pronounced effect on DCL composition. The recognition events of C are limited to interactions with the NDI components and thus can be expected to affect the library distribution in a way that will maximize the favourable NDI-napthalene π-interactions.
In the experiment where excess C (4 equiv.) was added to the NxP(6–x) DCL (Fig. 6d), ESI-MS of the equilibrated library showed signals corresponding to mixed ligand assemblies N5P1, N4P2, N3P3, N2P4, and N1P5. These mixed-ligand cages were observed to interact with 1 or 2 units of C.
No signals for the homoleptic assemblies N6 or P6 were observed. Only one signal in the ESI-MS is consistent with a species interacting with three units of C (N4P2C3). Given that this stoichiometry exceeds the degree of catenation previously observed for N6 (Fig. 4), we infer that the third crown ether is not likely to be catenated.16,24
In the similar experiment conducted with a sub-stoichiometric amount of C (0.5 equiv., Fig. 6c), the ESI-MS shows a distribution of all possible tetrahedral library members except P6. In these experiments a maximum of one equivalent of C was shown to interact with each tetrahedron, and signals were also observed for tetrahedra that are not associated with C, as expected given the limited amount of C added in the experiment.
Regardless of the amount of C added, catenated cages with ligand ratios of N4P2 and N1P5 were the most abundant species observed in the DCL. This observation follows the trend of speciation in the non-templated DCL, where N4P2 and N1P5 were also the most abundant library members. However, the addition of C significantly increases the preference for N4P2 and N1P5 at the expense of N6 and P6 (Fig. 6c and d).
The shift in library composition towards mixed-ligand tetrahedra upon addition of C appears to maximize the number of interactions between the NDI ligands and C. In N6, a maximum of two molecules of C can thread the six NDI ligands that are available. Therefore, shifting the library towards mixed-ligand assemblies increases the total number of NDI sites within the system that are available to thread through C. This hypothesis is supported by the absence of N6 in the DCL when an excess of C is present and the observation that all of the species observed by ESI-MS are catenated one or two times.
In order to achieve the maximum degree of catenation between the NDI ligands and C, the optimal tetrahedra to form are N4P2, N3P3 and N2P4 because these species are each able to accommodate two molecules of C and would thus maximize the number of NDI-napthalene π-interactions. That N3P3 and N2P4 are only minor species in the DCL suggests that there are other factors influencing the distribution of the library. This inference is supported by the observation that the non-templated DCL also deviates from the purely statistical distribution, suggesting that the strain leading to a non-statistical distribution of the non-templated DCL also plays a role in controlling the DCL composition in the presence of C.
Experiments in which both templates, C and C70, were added to the DCL either simultaneously or sequentially yielded poor ESI-MS data. We infer that aggregation may be occurring between C and C70, which has been observed in other systems,25 precluding us from probing possible synergistic effects of these templates upon the DCL.
Conclusions
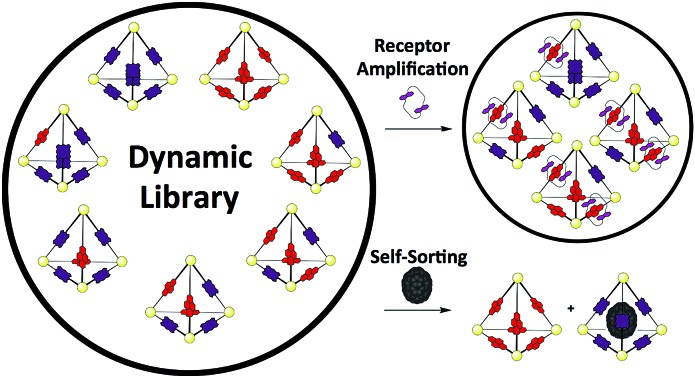
The difference in the shape and functionality of the NDI and porphyrin moieties of the ligands of N6 and P6 manifests in the form of distinct host–guest interactions with either C or C70. The similarity in lengths of the edges of N6 and P6 allows for ligand exchange between the two assemblies, yielding a dynamic library of seven different tetrahedral cages NxP(6–x). This DCL was shown to respond to the addition of C or C70, with the different templates influencing the composition of the DCL in distinct ways. A point of interest in our study is the use of both encapsulation and catenation (Fig. 7) as templation mechanisms within a DCL of three-dimensional receptors. Unlike systems derived from dynamic covalent species, the labile nature of these Zn4L6 tetrahedra precluded analysis by standard chromatographic separation4b and instead a methodology was employed where information as to the distribution of the library could be extracted from ESI-MS. This approach allowed us to decipher the behaviour of a complex mixture of seven different cage species, each comprising 22 individual components, and each of which was shown to form additional host–guest interactions. The multiple-stimuli responsiveness thus engendered could be of use in modulating guest uptake within more complex chemical networks that include DCLs of cages.
Fig. 7. A schematic overview of the different templation effects observed in the 1 : 1 DCL formed upon mixing of N6 and P6. Addition of C70 leads to the self-sorting of ligands into their homoleptic cages, as the library shifts to form the C70 receptor porphyrin cage P6. Addition of macrocycle C leads to the formation of heteroleptic cages, in which the total number of N–C interactions is maximized, given that only two such interactions per cage are possible.
Supplementary Material
Acknowledgments
This work was supported by the UK Engineering and Physical Sciences Research Council (EPSRC), the Deutsche Forschungsgemeinschaft (SFB 765), a Marie Curie Fellowship for J. J. H. (ITN-2010-26465), and a Beilstein-Institut PhD fellowship for F. B. S. We thank Diamond Light Source for synchrotron beamtime on I19 (MT8464).
Footnotes
†Electronic supplementary information (ESI) available: Full experimental procedures, NMR, ESI-FTICR MS and X-ray crystallography. CCDC 1061975–1061977. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc04906g
References
- (a) Mauro M., Aliprandi A., Septiadi D., Kehr N. S., De Cola L. Chem. Soc. Rev. 2014;43:4144–4166. doi: 10.1039/c3cs60453e. [DOI] [PubMed] [Google Scholar]; (b) Wiester M. J., Ulmann P. A., Mirkin C. A. Angew. Chem., Int. Ed. 2011;50:114–137. doi: 10.1002/anie.201000380. [DOI] [PubMed] [Google Scholar]
- (a) Foy J. T., Ray D., Aprahamian I. Chem. Sci. 2015;6:209–213. doi: 10.1039/c4sc02882a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ray D., Foy J. T., Hughes R. P., Aprahamian I. Nat. Chem. 2012;4:757–762. doi: 10.1038/nchem.1408. [DOI] [PubMed] [Google Scholar]; (c) Li H., Fahrenbach A. C., Coskun A., Zhu Z., Barin G., Zhao Y. L., Botros Y. Y., Sauvage J. P., Stoddart J. F. Angew. Chem., Int. Ed. 2011;50:6782–6788. doi: 10.1002/anie.201102510. [DOI] [PubMed] [Google Scholar]; (d) Jo J., Olasz A., Chen C. H., Lee D. J. Am. Chem. Soc. 2013;135:3620–3632. doi: 10.1021/ja312313f. [DOI] [PubMed] [Google Scholar]; (e) Dragna J. M., Pescitelli G., Tran L., Lynch V. M., Anslyn E. V., Di Bari L. J. Am. Chem. Soc. 2012;134:4398–4407. doi: 10.1021/ja211768v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Beck J. B., Rowan S. J. J. Am. Chem. Soc. 2003;125:13922–13923. doi: 10.1021/ja038521k. [DOI] [PubMed] [Google Scholar]; (b) Wong K. M., Yam V. W. Acc. Chem. Res. 2011;44:424–434. doi: 10.1021/ar100130j. [DOI] [PubMed] [Google Scholar]; (c) Tidhar Y., Weissman H., Wolf S. G., Gulino A., Rybtchinski B. Chem.–Eur. J. 2011;17:6068–6075. doi: 10.1002/chem.201003419. [DOI] [PubMed] [Google Scholar]; (d) Mitra T., Jelfs K. E., Schmidtmann M., Ahmed A., Chong S. Y., Adams D. J., Cooper A. I. Nat. Chem. 2013;5:276–281. doi: 10.1038/nchem.1550. [DOI] [PubMed] [Google Scholar]; (e) Motiei L., Lahav M., Freeman D., van der Boom M. E. J. Am. Chem. Soc. 2009;131:3468–3469. doi: 10.1021/ja900507c. [DOI] [PubMed] [Google Scholar]
- (a) Lehn J.-M., Eliseev A. V. Science. 2001;291:2331–2332. doi: 10.1126/science.1060066. [DOI] [PubMed] [Google Scholar]; (b) Corbett P. T., Leclaire J., Vial L., West K. R., Wietor J.-L., Sanders J. K. M., Otto S. Chem. Rev. 2006;106:3652–3711. doi: 10.1021/cr020452p. [DOI] [PubMed] [Google Scholar]; (c) Cougnon F. B. L., Sanders J. K. M. Acc. Chem. Res. 2012;45:2211–2221. doi: 10.1021/ar200240m. [DOI] [PubMed] [Google Scholar]; (d) Li J., Nowak P., Otto S. J. Am. Chem. Soc. 2013;135:9222–9239. doi: 10.1021/ja402586c. [DOI] [PubMed] [Google Scholar]
- (a) Belowich M. E., Stoddart J. F. Chem. Soc. Rev. 2012;41:2003–2024. doi: 10.1039/c2cs15305j. [DOI] [PubMed] [Google Scholar]; (b) Giuseppone N., Lehn J.-M. Chem.–Eur. J. 2006;12:1715–1722. doi: 10.1002/chem.200501038. [DOI] [PubMed] [Google Scholar]
- Mondal M., Hirsch A. K. H. Chem. Soc. Rev. 2014;44:2455–2488. doi: 10.1039/c4cs00493k. [DOI] [PubMed] [Google Scholar]
- (a) Mukhopadhyay P., Zavalij P. Y., Isaacs L. J. Am. Chem. Soc. 2006;128:14093–14102. doi: 10.1021/ja063390j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wu A., Isaacs L. J. Am. Chem. Soc. 2003;125:4831–4835. doi: 10.1021/ja028913b. [DOI] [PubMed] [Google Scholar]; (c) Wang F., Han C., He C., Zhou Q., Zhang J., Wang C., Li N., Huang F. J. Am. Chem. Soc. 2008;130:11254–11255. doi: 10.1021/ja8035465. [DOI] [PubMed] [Google Scholar]; (d) Dong S., Yan X., Zheng B., Chen J., Ding X., Yu Y., Xu D., Zhang M., Huang F. Chem.–Eur. J. 2012;18:4195–4199. doi: 10.1002/chem.201200016. [DOI] [PubMed] [Google Scholar]
- Osowska K., Miljanic O. S. Angew. Chem., Int. Ed. 2011;50:8345–8349. doi: 10.1002/anie.201102813. [DOI] [PubMed] [Google Scholar]
- (a) Stefankiewicz A. R., Sanders J. K. M. Chem. Commun. 2013;49:5820–5822. doi: 10.1039/c3cc41158c. [DOI] [PubMed] [Google Scholar]; (b) Ponnuswamy N., Cougnon F. B. L., Pantoş G. D., Sanders J. K. M. J. Am. Chem. Soc. 2014;136:8243–8251. doi: 10.1021/ja4125884. [DOI] [PubMed] [Google Scholar]; (c) Riddell I. A., Smulders M. M. J., Clegg J. K., Hristova Y. R., Breiner B., Thoburn J. D., Nitschke J. R. Nat. Chem. 2012;4:751–756. doi: 10.1038/nchem.1407. [DOI] [PubMed] [Google Scholar]
- (a) Lo S., Lu J., Krause L., Stalke D., Dittrich B., Clever G. H. J. Am. Chem. Soc. 2014;137:1060–1063. doi: 10.1021/ja5130379. [DOI] [PubMed] [Google Scholar]; (b) Han M., Engelhard D. M., Clever G. H. Chem. Soc. Rev. 2014;43:1848–1860. doi: 10.1039/c3cs60473j. [DOI] [PubMed] [Google Scholar]; (c) Cook T. R., Stang P. J. Chem. Rev. 2015;115:7001–7045. doi: 10.1021/cr5005666. [DOI] [PubMed] [Google Scholar]; (d) Kishi N., Akita M., Yoshizawa M. Angew. Chem., Int. Ed. 2014;53:3604–3607. doi: 10.1002/anie.201311251. [DOI] [PubMed] [Google Scholar]; (e) Hiraoka S., Harano K., Shiro M., Shionoya M. Angew. Chem., Int. Ed. 2005;44:2727–2731. doi: 10.1002/anie.200462394. [DOI] [PubMed] [Google Scholar]
- (a) Ghosh K., Yang H. B., Northrop B. H., Lyndon M. M., Zheng Y. R., Muddiman D. C., Stang P. J. J. Am. Chem. Soc. 2008;130:5320–5334. doi: 10.1021/ja711502t. [DOI] [PubMed] [Google Scholar]; (b) Gil-Ramirez G., Leigh D. A., Stephens A. J. Angew. Chem., Int. Ed. 2015;54:6110–6150. doi: 10.1002/anie.201411619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Johnson A. M., Hooley R. J. Inorg. Chem. 2011;50:4671–4673. doi: 10.1021/ic2001688. [DOI] [PubMed] [Google Scholar]; (b) Gütz C., Hovorka R., Schnakenburg G., Lützen A. Chem.–Eur. J. 2013;19:10890–10894. doi: 10.1002/chem.201301499. [DOI] [PubMed] [Google Scholar]; (c) Jiménez A., Bilbeisi R. A., Ronson T. K., Zarra S., Woodhead C., Nitschke J. R. Angew. Chem., Int. Ed. 2014;53:4556–4560. doi: 10.1002/anie.201400541. [DOI] [PubMed] [Google Scholar]
- Marshall A. G., Hendrickson C. L., Jackson G. S. Mass Spectrom. Rev. 1998;17:1–35. doi: 10.1002/(SICI)1098-2787(1998)17:1<1::AID-MAS1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- (a) Ronson T. K., Zarra S., Black S. P., Nitschke J. R. Chem. Commun. 2013;49:2476–2490. doi: 10.1039/c2cc36363a. [DOI] [PubMed] [Google Scholar]; (b) Zhou X.-P., Wu Y., Li D. J. Am. Chem. Soc. 2013;135:16062–16065. doi: 10.1021/ja4092984. [DOI] [PubMed] [Google Scholar]; (c) Wu Y., Zhou X.-P., Yang J.-R., Li D. Chem. Commun. 2013;49:3413–3415. doi: 10.1039/c3cc39287b. [DOI] [PubMed] [Google Scholar]; (d) Dömer J., Slootweg J. C., Hupka F., Lammertsma K., Hahn F. E. Angew. Chem., Int. Ed. 2010;49:6430–6433. doi: 10.1002/anie.201002776. [DOI] [PubMed] [Google Scholar]
- (a) Meng W., Clegg J. K., Thoburn J. D., Nitschke J. R. J. Am. Chem. Soc. 2011;133:13652–13660. doi: 10.1021/ja205254s. [DOI] [PubMed] [Google Scholar]; (b) Saalfrank R. W., Demleitner B., Glaser H., Maid H., Bathelt D., Hampel F., Bauer W., Teichert M. Chem.–Eur. J. 2002;8:2679–2683. doi: 10.1002/1521-3765(20020617)8:12<2679::AID-CHEM2679>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]; (c) Beissel T., Powers R. E., Parac T. N., Raymond K. N. J. Am. Chem. Soc. 1999;121:4200–4206. [Google Scholar]
- Black S. P., Stefankiewicz A. R., Smulders M. M. J., Sattler D., Schalley C. A., Nitschke J. R., Sanders J. K. M. Angew. Chem., Int. Ed. 2013;52:5749–5752. doi: 10.1002/anie.201209708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. A., Anderson H. L. Angew. Chem., Int. Ed. 2009;48:7488–7499. doi: 10.1002/anie.200902490. [DOI] [PubMed] [Google Scholar]
- (a) Stefankiewicz A. R., Tamanini E., Pantoş G. D., Sanders J. K. M. Angew. Chem., Int. Ed. 2011;50:5725–5728. doi: 10.1002/anie.201100806. [DOI] [PubMed] [Google Scholar]; (b) Canevet D., Pérez E. M., Martín N. Angew. Chem., Int. Ed. 2011;50:9248–9259. doi: 10.1002/anie.201101297. [DOI] [PubMed] [Google Scholar]; (c) Boyd P. D. W., Reed C. a. Acc. Chem. Res. 2005;38:235–242. doi: 10.1021/ar040168f. [DOI] [PubMed] [Google Scholar]
- Wood D. M., Meng W., Ronson T. K., Stefankiewicz A. R., Sanders J. K. M., Nitschke J. R. Angew. Chem., Int. Ed. 2015;54:3988–3992. doi: 10.1002/anie.201411985. [DOI] [PubMed] [Google Scholar]
- Leize E., Jaffrezic A., Dorsselaer A. V. J. Mass Spectrom. 1996;31:537–544. [Google Scholar]
- Baxter P. N., Khoury R. G., Lehn J.-M., Baum G., Fenske D. Chem.–Eur. J. 2000;6:4140–4148. doi: 10.1002/1521-3765(20001117)6:22<4140::aid-chem4140>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Safont-Sempere M. M., Fernandez G., Würthner F. Chem. Rev. 2011;111:5784–5814. doi: 10.1021/cr100357h. [DOI] [PubMed] [Google Scholar]
- (a) Acharyya K., Mukherjee P. S. Chem.–Eur. J. 2014;20:1646–1657. doi: 10.1002/chem.201303397. [DOI] [PubMed] [Google Scholar]; (b) Samanta D., Mukherjee P. S. Chem.–Eur. J. 2014;20:12483–12492. doi: 10.1002/chem.201402553. [DOI] [PubMed] [Google Scholar]; (c) Acharyya K., Mukherjee S., Mukherjee P. S. J. Am. Chem. Soc. 2013;135:554–557. doi: 10.1021/ja310083p. [DOI] [PubMed] [Google Scholar]; (d) Northrop B. H., Zheng Y. R., Chi K. W., Stang P. J. Acc. Chem. Res. 2009;42:1554–1563. doi: 10.1021/ar900077c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N., Sun J., Kitova E. N., Klassen J. S. J. Am. Soc. Mass Spectrom. 2009;20:1242–1250. doi: 10.1016/j.jasms.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Sharma A., Nayak S. K., Chattopadhyay S., Mukherjee A. K. J. Phys. Chem. B. 2003;107:4213–4217. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.