Abstract
Background
Glaucoma is the international leading cause of irreversible blindness. Intraocular pressure (IOP) is the only currently known modifiable risk factor; it can be reduced by medications, incisional surgery, or laser trabeculoplasty (LTP). LTP reduces IOP by 25% to 30% from baseline, but early acute IOP elevation after LTP is a common adverse effect. Most of these IOP elevations are transient, but temporarily elevated IOP may cause further optic nerve damage, worsening of glaucoma requiring additional therapy, and permanent vision loss. Antihypertensive prophylaxis with medications such as acetazolamide, apraclonidine, brimonidine, dipivefrin, pilocarpine, and timolol have been recommended to blunt and treat the postoperative IOP spike and associated pain and discomfort. Conversely, other researchers have observed that early postoperative IOP rise happens regardless of whether people receive perioperative glaucoma medications. It is unclear whether perioperative administration of antiglaucoma medications may be helpful in preventing or reducing the occurrence of postoperative IOP elevation.
Objectives
To assess the effectiveness of medications administered perioperatively to prevent temporarily increased intraocular pressure (IOP) after laser trabeculoplasty (LTP) in people with open‐angle glaucoma (OAG).
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 11), MEDLINE Ovid (1946 to 18 November 2016), Embase.com (1947 to 18 November 2016), PubMed (1948 to 18 November 2016), LILACS (Latin American and Caribbean Health Sciences Literature Database) (1982 to 18 November 2016), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com); last searched 17 September 2013, ClinicalTrials.gov (www.clinicaltrials.gov); searched 18 November 2016 and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en); searched 18 November 2016. We did not use any date or language restrictions.
Selection criteria
We included randomized controlled trials (RCTs) in which participants with OAG received LTP. We included trials which compared any antiglaucoma medication with no medication, one type of antiglaucoma medication compared with another type of antiglaucoma medication, or different timings of medication.
Data collection and analysis
Two review authors independently screened records retrieved by the database searches, assessed the risk of bias, and abstracted data. We graded the certainty of the evidence using GRADE.
Main results
We included 22 trials that analyzed 2112 participants and identified no ongoing trials. We performed several comparisons of outcomes: one comparison of any antiglaucoma medication versus no medication or placebo, three comparisons of one antiglaucoma medication versus a different antiglaucoma mediation, and one comparison of antiglaucoma medication given before LTP to the same antiglaucoma medication given after LTP. Only one of the included trials used selective laser trabeculoplasty (SLT); the remaining trials used argon laser trabeculoplasty (ALT). Risk of bias issues were primarily in detection bias, reporting bias, and other potential bias due to studies funded by industry. Two potentially relevant studies are awaiting classification due to needing translation.
In the comparison of any medication versus no medication/placebo, there was moderate‐certainty evidence that the medication group had a lower risk of IOP increase of 10 mmHg or greater within two hours compared with the no medication/placebo group (risk ratio (RR) 0.05, 95% confidence interval (CI) 0.01 to 0.20). This trend favoring medication continued between two and 24 hours, but the evidence was of low and very low‐certainty for an IOP increase of 5 mmHg or greater (RR 0.17, 95% CI 0.09 to 0.31) and 10 mmHg or greater (RR 0.22, 95% CI 0.11 to 0.42). Medication was favored over placebo/no medication with moderate‐certainty in reducing IOP from the pre‐LTP measurements for both within two hours and between two and 24 hours. At two hours, the mean difference (MD) in IOP between the medication group and the placebo/no medication group was ‐7.43 mmHg (95% CI ‐10.60 to ‐4.27); at between two and 24 hours, the medication group had a mean reduction in IOP of 5.32 mmHg more than the mean change in the placebo/no medication group (95% CI ‐7.37 to ‐3.28). Conjunctival blanching was an ocular adverse effect that was more common when brimonidine was given perioperatively compared with placebo in three studies.
In our comparison of brimonidine versus apraclonidine, neither medication resulted in a lower risk of increased IOP of 5 mmHg or greater two hours of surgery; however, we were very uncertain about the estimate. There may be a greater mean decrease in IOP within two hours after LTP. We were unable to perform any meta‐analyses for other review outcomes for this comparison.
In our comparison of apraclonidine versus pilocarpine, we had insufficient data to perform meta‐analyses to estimate effects on either of the primary outcomes. There was moderate‐certainty evidence that neither medication was favored based on the mean change in IOP measurements from pre‐LTP to two hours after surgery.
In the comparison of medication given before LTP versus the same medication given after LTP, we had insufficient data for meta‐analysis of IOP increase within two hours. For the risk of IOP increase of 5 mmHg or greater and 10 mmHg or greater at time points between two and 24 hours, there was no advantage of medication administration before or after LTP regarding the proportion of participants with an IOP spike (5 mmHg or greater: RR 0.82, 95% CI 0.25 to 2.63; 10 mmHg or greater: RR 1.55, 95% CI 0.19 to 12.43). For an IOP increase of 10 mmHg or greater, we had very low‐certainty in the estimate, it would likely change with data from new studies.
Authors' conclusions
Perioperative medications are superior to no medication or placebo to prevent IOP spikes during the first two hours and up to 24 hours after LTP, but some medications can cause temporary conjunctival blanching, a short‐term cosmetic effect. Overall, perioperative treatment was well tolerated and safe. Alpha‐2 agonists are useful in helping to prevent IOP increases after LTP, but it is unclear whether one medication in this class of drugs is better than another. There was no notable difference between apraclonidine and pilocarpine in the outcomes we were able to assess. Future research should include participants who have been using these antiglaucoma medications for daily treatment of glaucoma before LTP was performed.
Plain language summary
Medicines given before, during, or after surgery to prevent short spikes of eye pressure after laser surgery for glaucoma
What is the aim of this review? The aim of this Cochrane Review was to find out whether medicines given before, during, or after laser trabeculoplasty (LTP), a surgical method to reduce eye pressure, can prevent increased eye pressure shortly after surgery.
Key messages People who received medicines to reduce eye pressure as part of their LTP surgery had a lower risk of increased eye pressure after surgery than people who did not receive medicines. We are moderately certain that medicine helped reduce spikes in eye pressure. There were no serious side effects, but they could cause conjunctival blanching (a whitening or lightening of the eye), a noticeable cosmetic difference, in the eye that received the eye drops.
What was studied in this review? Glaucoma is a leading cause of irreversible blindness worldwide, but treatment usually can prevent or slow visual loss. Pressure within the eye, known as intraocular pressure (IOP), is the only risk factor for glaucoma that can be treated or controlled. During a type of glaucoma surgery called LTP, the doctor uses a laser to make small cuts that drain fluid out of the front of the eye. This can lower IOP. 'Spikes' of increased IOP after LTP are common, but the spikes in IOP usually stop without treatment after the operation. However, even short periods of increased IOP can lead to further damage and permanent blindness. Research has suggested that medicines given before, during, or after surgery may prevent spikes in IOP after LTP.
What are the main results of the review? We included 22 studies with 2112 people, which compared the effects of medicine versus no medicine before, during, or after LTP, and one type of medicine versus another type of medicine before or after LTP.
In the studies that compared medicine versus no medicine, people who received medicine had a lower risk of increased IOP than people who had not received medicine. This was at both two hours and up to 24 hours after the operation. The group that received medicine also had a greater reduction of IOP after surgery than people who had not received medicine. We were unable to determine whether it was better to administer medicines before or after LTP. The medicines might cause temporary conjunctival blanching.
Based on this review, people who received medicine before or after LTP had a lower risk of increased IOP afterward. It is unclear which medicines gave the best results. Treatment was safe for patients.
How up‐to‐date is the review? Cochrane researchers searched for studies that had been published up to 18 November 2016.
Summary of findings
Summary of findings for the main comparison. Medication compared with placebo for preventing temporarily increased IOP after laser trabeculoplasty.
| Medication compared with placebo for preventing temporarily increased IOP after LTP | ||||||
|
Participant or population: people with glaucoma receiving LTP Intervention: IOP‐lowering medication (apraclonidine, acetazolamide, brimonidine, pilocarpine) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Medication | |||||
|
IOP increase of ≥ 5 mmHg within 2 hours |
See comment | ‐ | 273 (2 RCTs) | ⊕⊕⊝⊝1,2 Low | Medications in this comparison were apraclonidine and brimonidine. 2 studies reported on this outcome and 1 favored the alpha‐2 agonists while the other favored placebo. Due to significant statistical heterogeneity (I2 = 70%), we did not perform a meta‐analysis. | |
|
IOP increase of ≥ 10 mmHg within 2 hours |
195 per 1000 | 10 per 1000 (2 to 39) | RR 0.05 (0.01 to 0.20) | 446 (4 RCTs) | ⊕⊕⊕⊝1 Moderate | Medications in this comparison were acetazolamide, apraclonidine, and brimonidine. |
|
Mean change in IOP from pre‐LTP within 2 hours |
The mean change in IOP ranged across control groups from0.4 mmHg to 4.40 mmHg, for 3 included studies | The mean change in IOP in the intervention groups was 7.43 mmHg lower (10.60 lower to 4.27 lower) | ‐ | 151 (4 studies) | ⊕⊕⊕⊝1 Moderate | Each of the studies included in this outcome compared apraclonidine vs placebo. |
|
IOP increase of ≥ 5 mmHg between 2 and 24 hours |
280 per 1000 | 48 per 1000 (25 to 87) | RR 0.17 (0.09 to 0.31) | 634 (5 studies) | ⊕⊕⊝⊝3 Low |
Medications in this comparison were apraclonidine and brimonidine. |
|
IOP increase of ≥ 10 mmHg between 2 and 24 hours |
202 per 1000 | 44 per 1000 (22 to 85) | RR 0.22 (0.11 to 0.42) | 817 (9 studies) | ⊕⊝⊝⊝3,4 Very low | Medications in this comparison were apraclonidine, brimonidine, dorozolamide, and pilocarpine. |
|
Mean change in IOP from pre‐LTP between 2 and 24 hours |
The mean change in IOP ranged across control groups from‐2.0 mmHg to 0.63 mmHg, for 3 included studies | The mean change in IOP in the intervention groups was 5.32 mmHg lower (7.37 lower to 3.28 lower) | ‐ | 151 (4 studies) | ⊕⊕⊕⊝1 Moderate | Each of the studies included in this outcome compared apraclonidine vs placebo. |
|
Adverse events ‐ conjunctival blanching during study period |
See comment | ‐ | 319 (2 studies) |
⊕⊕⊕⊝1 Moderate | 2 studies reported on conjunctival blanching; however, due to significant statistical heterogeneity (I2 = 95%), we did not perform a meta‐analysis. In both studies, conjunctival blanching was reported in more participants in the group that received an alpha‐2 agonist compared with participants who received placebo. 1 other study that reported only the range of participants who had conjunctival blanching also reported that this adverse event was more frequent in the groups receiving brimonidine vs placebo. Other adverse events reported for the comparison of medication vs placebo were lid retraction and conjunctival hyperemia, reported in 1 study each. | |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; LTP: laser trabeculoplasty; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
1 The certainty of the evidence was downgraded due to concerns of risk of bias: masking of outcomes assessors was difficult and in one study, the authors of some studies worked with the company making the study drug.
2 The certainty of the evidence was downgraded due to inconsistency of the outcome measurements in the individual studies: one favored medication and one favored placebo.
3 The certainty of the evidence was downgraded two levels due to concerns of very serious plausible bias: some studies in these analyses had issues with masking of outcomes assessors, high risk of selective reporting, and authors associated with the manufacturer of the study drug.
4 The certainty of the evidence was downgraded due to imprecision: there is a small number of events in the medication groups.
Summary of findings 2. Brimonidine compared with apraclonidine for preventing temporarily increased IOP after laser trabeculoplasty.
| Brimonidine compared with apraclonidine for preventing temporarily increased IOP after LTP | ||||||
|
Participant or population: people with glaucoma receiving LTP Intervention: brimonidine Comparison: apraclonidine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Apraclonidine | Brimonidine | |||||
|
IOP increase of ≥ 5 mmHg within 2 hours |
29 per 1000 | 67 per 1000 (9 to 471) | RR 2.28 (0.32 to 16.03) | 71 (2 RCTs) | ⊕⊝⊝⊝1,2,3 Very low | 1 other study reported this outcome but found that no participants in either study group had an IOP increase of ≥ 5 mmHg. This study was not included in the meta‐analysis. |
|
IOP increase of ≥ 10 mmHg within 2 hours |
See comment | ‐ | ‐ | ‐ | 1 study reported that no participants given either medication had an IOP increase of ≥ 10 mmHg. Another study reported that only 1 eye that had received apraclonidine had an IOP spike > 10 mmHg, but this was not statistically significant given the size of the study (RR 0.33, 95% CI 0.02 to 7.32). | |
|
Mean change in IOP from pre‐LTP within 2 hours |
The mean change in IOP ranged across control groups from‐4.29 to ‐5.00 mmHg | The mean change in IOP in the intervention groups was 0.69 mmHg lower (2.56 lower to 1.17 higher) | ‐ | 71 (2 RCTs) | ⊕⊕⊕⊝3 Moderate | ‐ |
|
IOP increase of ≥ 5 mmHg between 2 and 24 hours |
This outcome was not reported for this comparison. | |||||
|
IOP increase of ≥ 10 mmHg between 2 and 24 hours |
This outcome was not reported for this comparison. | |||||
|
Mean change in IOP from pre‐LTP between 2 and 24 hours |
See comment | ‐ | ‐ | ‐ | 1 study reported that participants randomized to receive brimonidine had a mean (± SD) IOP reduction of 2.6 ± 3.6 mmHg, while participants randomized to receive apraclonidine had a mean IOP reduction of 2.3 ± 3.7 mmHg (MD ‐0.30 mmHg, 95% CI ‐2.41 to 1.81). | |
|
Adverse events ‐ during study period |
This outcome was not reported for this comparison. | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; LTP: laser trabeculoplasty; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
1 The certainty of the evidence was downgraded two levels due to imprecision: our effect measurement had a very wide confidence interval.
2 The certainty of the evidence was downgraded due to inconsistency: the RRs of the individual trials were very different.
3 The certainty of the evidence was downgraded due to concerns of risk of bias: masking of participants and personnel was unclear, and in one study, both eyes of the participants were included in the study and received different medications but the authors did not report if and how they took into account the interdependability of eyes.
Summary of findings 3. Apraclonidine compared with pilocarpine for temporarily increased IOP after laser trabeculoplasty.
| Apraclonidine compared with pilocarpine for temporarily increased IOP after LTP | ||||||
|
Participant or population: people with glaucoma receiving LTP Intervention: apraclonidine Comparison: pilocarpine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Pilocarpine | Apraclonidine | |||||
|
IOP increase of ≥ 5 mmHg within 2 hours |
See comment | ‐ | ‐ | ‐ | 1 study reported that 8.8% of the apraclonidine group had an increase of ≥ 5 mmHg vs 4.4% of the pilocarpine group. These were not statistically different (RR 2.00, 95% CI 0.71 to 5.67). | |
|
IOP increase of ≥ 10 mmHg within 2 hours |
This outcome was not reported for this comparison. | |||||
|
Mean change in IOP from pre‐LTP within 2 hours |
The mean change in IOP was only reported in 1 study: ‐3.6 mmHg. The second study reported only the mean IOP at a time point rather than the mean change | The mean change in IOP in the intervention groups was 0.61 mmHg higher (0.44 lower to 1.66 higher) | ‐ | 277 (2 RCTs) | ⊕⊕⊕⊝1 Moderate | ‐ |
|
IOP increase of ≥ 5 mmHg between 2 and 24 hours |
See comment. | ⊕⊕⊝⊝1, 2 Low | 2 studies reported on this outcome and 1 favored apraclonidine while the other favored pilocarpine. Due to significant statistical heterogeneity (I2 = 91%), we did not perform a meta‐analysis. | |||
|
IOP increase of ≥ 10 mmHg between 2 and 24 hours |
13 per 1000 | 12 per 1000 (2 to 75) | RR 0.87 (0.14 to 5.63) | 390 (2 RCTs) | ⊕⊕⊝⊝2,3 Low | 1 additional study reported on this outcome but found that no participants in either study group had an IOP increase of ≥ 10 mmHg. This study was not included in the meta‐analysis. |
|
Mean change in IOP from pre‐LTP between 2 and 24 hours |
See comment. | ‐ | 277 (2 RCTs) | ⊕⊕⊝⊝1, 2 Low | 2 studies reported on this outcome and 1 favored apraclonidine while the other favored pilocarpine. Due to significant statistical heterogeneity (I2 = 92%), we did not perform a meta‐analysis. | |
|
Adverse events ‐ during study period |
This outcome was not reported for this comparison. | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; LTP: laser trabeculoplasty; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
1 The certainty of the evidence was downgraded due to concerns of plausible bias: one study included in the analyses had issues with masking of their participants due to the nature of the study design, and additionally the authors were employees of the company that manufactured the study drug.
2 The certainty of the evidence was downgraded due to inconsistency: in each outcome analysis, one study favored pilocarpine while the other favored apraclonidine.
3 The certainty of the evidence was downgraded due to imprecision: our effect measurement had a very wide confidence interval.
Summary of findings 4. Medication given before LTP compared with the same medication given after LTP for temporarily increased IOP after LTP.
| Medication given before LTP compared with the same medication given after LTP for temporarily increased IOP after LTP | ||||||
|
Participant or population: people with glaucoma receiving LTP Intervention: medication before LTP Comparison: medication after LTP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Medication after LTP | Medication before LTP | |||||
|
IOP increase of ≥ 5 mmHg within 2 hours |
See comment | ‐ | ‐ | ‐ | 1 study comparing apraclonidine given before and after surgery reported no participants had an increase of ≥ 5 mmHg. Another study reported that 5.3% of participants given brimonidine before surgery had an IOP increase of ≥ 5 mmHg, compared with 7.5% of participants given brimonidine after surgery (RR 0.70, 95% CI 0.16 to 2.97). | |
|
IOP increase of ≥ 10 mmHg within 2 hours |
See comment | ‐ | ‐ | ‐ | 1 study comparing apraclonidine given before and after surgery reported no participants had an increase of ≥ 10 mmHg. Another study reported that only 1 study participant had this high of an increase, and they had been randomized to brimonidine before surgery. | |
|
Mean change in IOP from pre‐LTP within 2 hours |
See comment | ‐ | ‐ | ‐ | Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP was not reported in any study, but 1 study did report the mean IOP for the 2 study arms within 2 hours and it was not statistically different between the 2 groups. | |
|
IOP increase of ≥ 5 mmHg between 2 and 24 hours |
38 per 1000 | 31 per 1000 (9 to 100) | RR 0.82 (0.25 to 2.63) | 319 (4 RCTs) | ⊕⊕⊕⊝1 Moderate | ‐ |
|
IOP increase of ≥ 10 mmHg between 2 and 24 hours |
6 per 1000 | 10 per 1000 (1 to 79) | RR 1.55 (0.19 to 12.43) | 319 (4 RCTs) | ⊕⊝⊝⊝1,2 Very low | ‐ |
|
Mean change in IOP from pre‐LTP between 2 and 24 hours |
The mean change in IOP was only reported in 1 study: ‐3.4 mmHg. The other 2 studies reported only the mean IOP at a time point rather than the mean change: range was13.0 mmHg to 18.6 mmHg | The mean change in IOP in the intervention groups was 1.07 mmHg lower (2.51 lower to 0.37 higher) | ‐ | 198 (3 RCTs) | ⊕⊕⊕⊝3 Moderate | ‐ |
|
Adverse events ‐ during study period |
This outcome was not reported for this comparison. | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; LTP: laser trabeculoplasty; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
1 The certainty of the evidence was downgraded due to concerns of plausible bias: masking of outcomes assessors was difficult and the authors of two studies work with the company making the study drug; one study had a high risk of selective reporting bias.
2 The certainty of the evidence was downgraded two levels due to imprecision: the included studies for which data were available had very wide confidence intervals.
3 The certainty of the evidence was downgraded due to inconsistency: of the three included studies, two favored medication given before surgery, and the other favored medication given after surgery.
Background
Description of the condition
Glaucoma is the leading cause of irreversible blindness internationally (Tham 2014). Intraocular pressure (IOP) is the only currently known modifiable risk factor for treating glaucoma. The most common algorithms for treatment of open‐angle glaucoma (OAG) start with medications to lower IOP, followed by laser trabeculoplasty (LTP). Incisional glaucoma surgery is used whenever the prior interventions are not successful in controlling IOP (American Academy of Ophthalmology 2015). LTP has been shown in several large clinical trials and one Cochrane Review to be effective in lowering IOP (Ederer 2004; GLT 1990; Rolim de Moura 2007), and can be performed as a first‐line therapy or in conjunction with medical therapy. On average, LTP reduces IOP by 25% to 30% from baseline (Stein 2007). Medical therapy is plagued by frequent difficulties with nonadherence (Tsai 2006), the reasons for which are multifactorial. Nonadherence can limit the effectiveness of glaucoma eyedrops in reducing IOP. LTP can potentially minimize these issues by reducing or eliminating the need for glaucoma medications.
LTP is a procedure in which laser treatment is applied to the trabecular meshwork to improve drainage of aqueous humor from the eye. Argon laser trabeculoplasty (ALT) and selective laser trabeculoplasty (SLT) are the most commonly used forms of LTP. Other types of LTP used include diode, micropulse diode, krypton, and titanium‐sapphire lasers (Chung 1998; Englert 1997; Samples 2011; Spurny 1984). The mechanism of action by which LTP reduces IOP is poorly understood. The three most common theories of how LTP works include:
mechanical theory: thermal energy causes collagen shrinkage and tissue contraction, leading to mechanical stretching of uveoscleral tissues and decreased resistance to outflow into Schlemm's canal (Chandler 1997; Melamed 1986);
biologic theory: thermal energy stimulates cytokine activity, recruitment of macrophages into the trabecular meshwork, and upregulation of matrix metalloproteinase expression with subsequent remodeling of the extracellular matrix of the trabecular meshwork (Bradley 2000; Melamed 1985);
repopulation theory: laser energy stimulates cell division and repopulation of the trabecular meshwork (Bylsma 1988).
Early acute IOP elevation is a common adverse effect of anterior segment laser procedures, including LTP, that can cause pain, discomfort, and nausea. Studies have shown that the incidence of postoperative IOP rise varies from 24% to 70% after ALT (Chen 2001), and from 0% to 37.5% after SLT (Barkana 2007). Most of these IOP elevations are transient, occurring within a few hours of LTP and resolving within 24 hours with either observation or additional antiglaucoma medications. Temporarily elevated IOP, especially with an already compromised optic nerve, may cause further optic nerve damage, worsening of glaucoma requiring additional therapy, and permanent vision loss. One case review of 224 eyes revealed that 3.1% of eyes developed loss of visual acuity or visual field after ALT and 1.8% of eyes required emergency glaucoma surgery to reduce IOP (Levene 1983). Another study reported on a person who experienced substantial IOP increase immediately after ALT and subsequently noticed marked visual field loss within 24 hours of ALT (Weinreb 1983). Finally, one case series reported four occurrences of persistently elevated IOP after SLT of which three eyes required subsequent incisional surgical management of IOP (Harasymowycz 2005).
Description of the intervention
Antihypertensive prophylaxis and post‐treatment IOP monitoring (including post‐LTP medical therapy, if necessary) are recommended to blunt and treat the postoperative IOP spike (Barkana 2007). Perioperative glaucoma medications that have been tried for this purpose include acetazolamide, apraclonidine, brimonidine, dipivefrin, pilocarpine, and timolol (Robin 1987; Robin 1991). There is no standard protocol for preventing postoperative IOP increases, but of these medications, alpha agonists, such as iopidine or brimonidine, are the most commonly used (Chen 2001; Chen 2005).
How the intervention might work
Although the exact mechanism is unknown, IOP spikes after LTP are thought to be related to physical blockage of the trabecular meshwork by cellular debris, inflammatory cells, or pigment dispersion (Krupin 1992; Meyer 1990). Using perioperative topical or oral glaucoma medications may blunt this IOP spike through various mechanisms. Apraclonidine and brimonidine are alpha‐2 agonists that reduce the ciliary body blood supply and aqueous production. Applied topically, both prevent IOP spikes after LTP (Chen 2001; David 1993; Robin 1987). Topical pilocarpine, a cholinergic agonist that mechanically opens the trabecular meshwork to reduce IOP, also prevents IOP spikes after LTP (Ren 1999). Timolol, a topical beta‐adrenergic antagonist, and acetazolamide, an oral carbonic anhydrase inhibitor, both reduce aqueous production and prevent IOP elevation after LTP (Robin 1991). Finally, dipivefrin is a prodrug of epinephrine which decreases aqueous production by its alpha‐agonist properties. Topical dipivefrin reduces IOP spikes after LTP (Robin 1991), but this medication is no longer available in the US.
Why it is important to do this review
LTP is a common intervention performed to lower IOP in eyes with ocular hypertension or glaucoma, and often is associated with IOP rise immediately after the procedure. In glaucomatous eyes with already compromised optic nerves, even transient elevations of IOP can cause permanent damage (Levene 1983). Several studies have shown that perioperative use of topical glaucoma medications can prevent or blunt the post‐LTP IOP spike (Chen 2001; David 1993; Ren 1999; Robin 1987; Robin 1991). However, other researchers have observed that early postoperative IOP rise happened regardless of whether people received perioperative glaucoma medications (Barkana 2007). Thus, it is unclear whether perioperative administration of antiglaucoma medications is helpful in preventing or reducing the occurrence of postoperative IOP elevation.
Objectives
To assess the effectiveness of medications administered perioperatively to prevent temporarily increased intraocular pressure (IOP) after laser trabeculoplasty (LTP) in people with open‐angle glaucoma (OAG).
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs).
Types of participants
We included all participants undergoing LTP who had a diagnosis of OAG, including primary open‐angle glaucoma (POAG) and several types of secondary OAG, namely corticosteroid‐induced, exfoliation, and pigmentary glaucoma. Participants were included regardless of age or sex.
Types of interventions
We included trials of LTP in which:
any form of perioperative antiglaucoma medication (topical or oral) was compared to no perioperative medication or placebo;
one form of perioperative antiglaucoma medication (topical or oral) was compared with another form of perioperative antiglaucoma medication (topical or oral) (we grouped topical antiglaucoma medications according to classes of medications, not dosages of medications. When oral medications were used, we grouped trials by classes of medications, not dosages of medications);
the timing of perioperative medications was compared (immediately prior to LTP, immediately after LTP, or both).
We included studies of all types of LTP (argon, selective, diode, micropulse diode, krypton, titanium) that met the above criteria. Whenever enough trials existed for a specific type of laser, we compared the efficacy of perioperative medications for each type of laser.
Types of outcome measures
Primary outcomes
Proportion of participants with IOP elevation within two hours after LTP. IOP elevation was defined as:
IOP increase of 5 mmHg or greater from pre‐LTP measurement;
IOP increase of 10 mmHg or greater from pre‐LTP measurement.
Secondary outcomes
Mean change in IOP measurements from pre‐LTP to within two hours after LTP.
Proportion of participants with IOP elevation (as described in Primary outcomes) more than two hours but within 24 hours after LTP.
Mean change in IOP measurements from pre‐LTP to more than two hours but within 24 hours after LTP.
Proportion of participants with IOP greater than preoperative baseline by 5 mmHg or greater 24 hours after LTP.
Proportion of participants with ocular or systemic adverse events related to post‐LTP‐related IOP elevation.
Percentage of participants who required additional antiglaucoma therapy or surgical glaucoma intervention to reduce post‐LTP‐related IOP elevation.
Percentage of participants with worsened vision, defined as loss of two lines or more in Snellen visual acuity within three months after LTP that could not be attributed to any ocular process other than glaucomatous damage, and in whose eyes a measured post‐LTP IOP spike (IOP 5 mmHg or greater from pre‐LTP IOP) occurred within 24 hours of LTP.
Percentage of participants with adverse events occurring within 24 hours, seven days, and 30 days after LTP.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for RCTs. There were no language or publication year restrictions. The date of the search was 18 November 2016.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11) (which contains the Cochrane Eyes and Vision Trials Register)(searched 30 November 2016) (Appendix 1);
MEDLINE Ovid (1946 to 18 November 2016) (Appendix 2);
Embase.com (1947 to 18 November 2016) (Appendix 3);
PubMed (1948 to 18 November 2016) (Appendix 4);
LILACS (Latin American and Caribbean Health Science Information database (1982 to 18 November 2016) (Appendix 5);
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com; last searched 14 November 2014) (Appendix 6);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 18 November 2016) (Appendix 7);
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp; searched 18 November 2016) (Appendix 8).
Searching other resources
We searched the reference lists of included trials to identify additional trials.
Data collection and analysis
Selection of studies
Two review authors independently reviewed the titles and abstracts generated from the searches. We assessed each record as potentially relevant or not relevant. We obtained full‐text copies of publications from potentially relevant trials. We assessed all full‐text reports according to the inclusion criteria as stated above. At this stage, two review authors independently assessed each study report as include, unclear, or exclude. We resolved disagreements by discussion. We identified colleagues to assist with classifying articles published in languages not read by the review authors; we had articles eligible for the review translated into English when possible or read by a native speaker. When a native speaker was unavailable, we placed the study in the Studies awaiting classification section. We documented studies excluded after full‐text review and the reasons for exclusion in the Characteristics of excluded studies table. We contacted the investigators of studies classified as unclear to obtain information needed to include or exclude studies. Whenever no response was received within four weeks, we included or excluded the study based on the information available in the published article.
Data extraction and management
Two review authors independently extracted data from included studies using forms developed by the Cochrane Eyes and Vision Group. We extracted data describing study characteristics (i.e. study methods, participants, interventions, and outcomes), as well as qualitative and quantitative outcome data. We gave particular attention to the range of laser power used, amount of the angle treated, type of laser, and types of glaucoma treated. We resolved discrepancies by discussion. One review author entered data into Review Manager 5 and a second review author verified the data entry (RevMan 2014).
Assessment of risk of bias in included studies
For each included study, two review authors independently assessed the risk of bias according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved discrepancies through discussion. We considered the following risk of bias domains and methods used to reduce the risk.
Selection bias: how was the randomization sequence generated and was the allocation concealed before randomization?
Performance bias: were participants and study personnel masked?
Detection bias: were outcome assessors masked?
Attrition bias: was there missing data and how much; was the amount and type of missing data similar between treatment groups; did the authors use an intention‐to‐treat analysis?
Reporting bias: was there selective reporting of outcomes?
Other sources of bias: for example, were sources of funding from industry, was the investigator or author affiliated with a sponsor or funding source, were the data analysis appropriate given the study design in paired‐eye studies?
For each study, we assessed the methods reported regarding each risk of bias domain as conferring low risk, unclear risk, or high risk of bias. When information was not sufficient to assess risk of bias, we contacted the investigators of studies. When no response was received within four weeks, we assessed the study based on the information available.
Measures of treatment effect
The primary outcome of the review, the proportion of participants with IOP elevation within two hours after LTP, is a dichotomous outcome; the intervention effect was estimated as a risk ratio (RR) with 95% confidence intervals (CIs). We also estimated a RR for the proportion of participants with IOP greater than baseline after two hours but within 24 hours after laser; the proportion of participants requiring antiglaucoma medication to lower IOP immediately postoperatively (in addition to the medication received as part of the study treatment); the proportion of participants requiring additional medical therapy or surgical intervention beyond 24 hours; the proportion of participants with sustained increased IOP post‐laser with post‐LTP vision worsened within three months after LTP.
We estimated intervention effects for continuous outcomes, including mean changes from baseline in IOP within two hours and from two to 24 hours post‐laser treatment, as mean differences (MD) with 95% CIs.
Unit of analysis issues
The primary unit of analysis was the individual participant. Standard of care with LTP is that laser is performed on only one eye at a time; however, this review included one study in which LTP was performed in both eyes. We planned to treat studies that randomized participants to treatment groups but analyzed eyes as the unit of analysis, as cluster‐randomized studies; however, we found no studies that used this method. We considered the unit of analysis to have been eyes for studies in which eyes of participants received different interventions (i.e. intra‐individual or paired‐eye studies). We included and documented studies that used eyes as the unit of analysis using the methods described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
We attempted to contact trial investigators to obtain missing outcome or data. We set the response time at four weeks and documented all communications. When no response was received, we used the data available assuming data were missing at random. We did not exclude studies from the review based on missing data.
Assessment of heterogeneity
We assessed both clinical and statistical heterogeneity among studies as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We examined forest plots for indications of statistical heterogeneity; we considered an I2 statistic greater than 60% to represent significant statistical heterogeneity. We compared details about included studies for indications of clinical heterogeneity, which we defined as intervention or participant characteristics that may have affected the estimated treatment effect among different studies and participant populations. When clinical or statistical heterogeneity were detected, we conducted subgroup analyses when possible.
Assessment of reporting biases
We assessed reporting bias by documenting study outcomes reported or not reported by included studies by comparing methods with results reported. We planned to use funnel plots to assess reporting bias if 10 or more studies were included in a meta‐analysis.
Data synthesis
Whenever two or more studies were clinically homogeneous and provided sufficient data for the same review outcome, we performed a meta‐analysis of that outcome. We used a random‐effects model when findings from three or more studies could be included in meta‐analysis; otherwise we used a fixed‐effect model. Whenever significant statistical heterogeneity (I2 statistic greater than 60%) was detected, we did not conduct a meta‐analysis; instead, we reported individual study outcomes in a narrative form. For the continuous outcomes 'Mean change in IOP from pre‐LTP to measurements taken within two hours after LTP' and 'Mean change in IOP from pre‐LTP to measurements taken more than two hours but within 24 hours after LTP' some of the included studies reported the actual mean change, while others reported only the IOP at a time point for each study arm. In meta‐analyses where we wanted to combine these two types of data, we used the generic inverse variance method.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses based on the type of medication used. We had planned to conduct subgroup analyses based on the type of laser used in the studies; however, since each comparison only included a small number of studies, the potential for subgroup analyses based on type of laser used was limited. We had also planned to conduct subgroup analyses based on types of pretreatment glaucoma medications used; however, there was not enough information on the use of pretreatment glaucoma medications to conduct these analyses.
Sensitivity analysis
We planned to conduct sensitivity analyses to assess the influence of industry‐funded studies, studies with missing data, and studies assessed as having a high risk of selection or attrition bias, but selection and attrition bias were not major concerns among our included studies. We identified issues with detection and reporting bias. We did not conduct a sensitivity analysis removing industry‐funded studies because many of our included studies had this problem (six (27%) studies), and these studies provided most of the data. Instead, we address the implications of high risk of bias from the influence of industry‐funded studies in the discussion and conclusions. We had also planned to conduct a sensitivity analysis to assess the influence of unpublished studies, but our search methods identified none.
'Summary of findings' tables
We created 'Summary of findings' tables for each of our comparisons (Table 1; Table 2; Table 3; Table 4). We used the GRADE classification to judge the certainty of the evidence for each outcome (GRADEpro 2014). Each table included the following seven outcomes: our primary outcomes, IOP increase of 5 mmHg or greater within two hours and IOP increase of 10 mmHg or greater within two hours, plus five of our secondary outcomes: mean change in IOP from pre‐LTP within two hours, IOP increase of 5 mmHg or greater between two and 24 hours, IOP increase of 10 mmHg or greater between two and 24 hours, mean change in IOP from pre‐LTP between two and 24 hours after LTP, and adverse events. The GRADE approach takes into account five factors that may affect the certainty and our confidence in the results of our meta‐analyses. The certainty of the evidence was graded as high, moderate, low, or very low, and each outcome could be individually downgraded from high (by one level for each issue) whenever that outcome was subject to any of the five factors: study limitations including a high risk of bias, inconsistency of the effect, imprecision in the estimated treatment effect, indirectness of the evidence, and publication bias.
Results
Description of studies
Results of the search
Databases were originally searched on 17 September 2013 and the searches were updated on 18 November 2016. The electronic search identified 3688 reports and registry records. One additional report was identified from the references of another included study. After removal of duplicate reports, the search identified 2948 reports of trials and registry records, from which we selected 69 potentially relevant studies and obtained full copies of reports (Figure 1). We included 32 reports (from 22 studies) and excluded 35 reports (from 31 studies); two reports from two studies await classification until translations of the full‐text become available to assess eligibility. No ongoing trials were identified.
1.
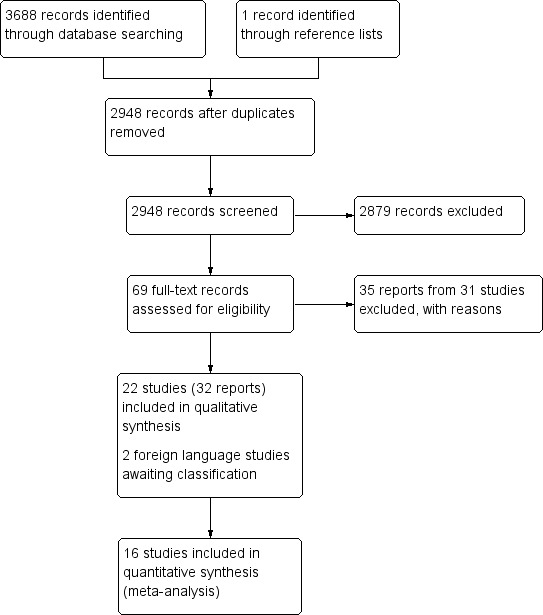
Study flow diagram.
Included studies
Of the 69 possibly relevant studies that were identified, 22 met the inclusion criteria (Barnebey 1993; Barnes 1999; Birt 1995; Brown 1988; Carassa 1992; Chevrier 1999; Dapling 1994; David 1993; Donnelly 2006; Elsas 1991; Hartenbaum 1999; Holmwood 1992; Karlik 1997; Karlik 1998; Kitazawa 1990; Ma 1999; Metcalfe 1989; Raspiller 1992; Ren 1999; Robin 1987; Robin 1991; Yalvaç 1996) (see Characteristics of included studies table). Four of these were published abstracts that were not linked to full‐length publications (Donnelly 2006; Karlik 1997; Karlik 1998; Kitazawa 1990). Two studies were in languages other than English and are reported in Characteristics of studies awaiting classification until foreign language data abstractors are available (Božić 2011; Ha 1991). Ten of the 69 potentially relevant studies that we had identified were conference abstracts that were linked to a full publication that we have included in this review. This review includes 22 studies published in 32 reports.
Types of participants
Table 5 describes the types of participants in each study included in this review. Twelve studies reported that enrolled participants had OAG. Five others reported enrolling simply "glaucoma patients;" one study each specified "advanced" and "uncontrolled" glaucoma. All participants were receiving LTP to treat glaucoma; all but one included study used ALT. Some studies included participants who were receiving other types of surgery to treat their glaucoma, but we included these studies only when the results were reported separately for each treatment.
1. Study Comparison.
| Study ID | Types of participants | Number of treatment groups | Type of trabeculoplasty | Degree of laser | Comparison (baseline IOP in mmHg) |
| Barnebey 1993 | Uncontrolled glaucoma | 4 | ALT | 360 | Brimonidine before and after vs brimonidine before and vehicle after vs vehicle before and brimonidine after vs vehicle before and after (individual baseline IOPs not reported; range 23.4 ± 0.6 to 24.3 ± 0.7) |
| Barnes 1999 | POAG, pigmentary glaucoma, pseudoexfoliation syndrome, or ocular hypertension | 2 | ALT | 360 | Brimonidine (19.6 ± 4.5) vs apraclonidine (20.5 ± 4.6) |
| Birt 1995 | POAG | 3 | ALT | 180 | Apraclonidine before and after (22.2 ± 3.6) vs apraclonidine before (23.9 ± 5.3) vs apraclonidine after (22.1 ± 3.2) |
| Brown 1988 | Inadequately controlled IOP despite maximum‐tolerated medical therapy | 2 | ALT | 360 | Apraclonidine before and after vs placebo before and after (IOPs not available) |
| Carassa 1992 | Advanced glaucoma on maximal tolerated medical therapy with inadequate IOP control | 2 | ALT | 360 | Apraclonidine before and after (19.20 ± 5.95) vs placebo before and after (19.80 ± 5.23) |
| Chevrier 1999 | Candidates for ALT, peripheral iridectomy, or posterior capsulotomy | 2 | ALT | 180 | Brimonidine before (20.3 ± 6) vs apraclonidine before (20.0 ± 5.1) *the reported IOPs included participants who received other types of glaucoma surgery besides ALT |
| Dapling 1994 | OAG | 3 (1 combination group not of interest in this study) | ALT | 180 | Apraclonidine before and after vs pilocarpine after ("all eyes had...an IOP greater than 21mmHg") |
| David 1993 | Participants undergoing ALT | 4 | ALT | 360 | Brimonidine before and after (23.3) vs brimonidine before, placebo after (23.9) vs placebo before, brimonidine after (24.1) vs placebo before and after (24.0) |
| Donnelly 2006 | POAG | 2 (opposite eyes) | SLT | 360 | Brimonidine before vs apraclonidine after (right eyes: 18, left eyes: 18.4) |
| Elsas 1991 | Exfoliative glaucoma and simple glaucoma | 2 | ALT | 360 | Pilocarpine before (34.9 ± 8.1) vs no treatment (33.3 ± 5.6) |
| Hartenbaum 1999 | OAG requiring ALT | 2 | ALT | 180 | Dorzolamide before and after (18.3 ± 0.57) vs placebo before and after (19.6 ± 0.72) |
| Holmwood 1992 | OAG | 2 | ALT | 360 | Apraclonidine before and after (22.6 ± 0.9) vs apraclonidine after (22.6 ± 0.6) |
| Karlik 1997 | Glaucoma | 2 | ALT | 180 | Latanoprost before (24.1) vs apraclonidine before (23.2) |
| Karlik 1998 | Glaucoma | 2 | ALT | 180 | Latanoprost before vs apraclonidine before |
| Kitazawa 1990 | POAG | 2 | ALT | 180 | Apraclonidine before and after (24.2 ± 9.0) vs placebo before and after (23.2 ± 6.8) |
| Ma 1999 | Glaucoma | 4 | ALT | 180 | Brimonidine before and after (24.9) vs brimonidine before, placebo after (24.8) vs placebo before, brimonidine after (24.1) vs placebo before and after (24.6) |
| Metcalfe 1989 | Uncontrolled OAG | 2 | ALT | 180 | Acetazolamide before (23.6 ± 6.1) vs placebo before (23.7 ± 6.5) |
| Raspiller 1992 | POAG | 2 | ALT | 360 | ALO 2145 (apraclonidine) before and after (20.1 ± 4.07) vs placebo before and after (25.0 ± 5.47) |
| Ren 1999 | POAG | 2 | ALT | 180 | Apraclonidine before (23.2 ± 4.5) vs pilocarpine before (21.7 ± 3.5) |
| Robin 1987 | OAG | 2 | ALT | 360 | ALO 2145 (apraclonidine) before and after (26.4 ± 3.0) vs placebo before and after (27.9 ± 6.9) |
| Robin 1991 | OAG with disc and visual field damage | 5 | ALT | 360 | Apraclonidine before and after (27.2 ± 5.1) vs timolol before and after (27.6 ± 4.1) vs pilocarpine before and after (27.1 ± 5.1) vs dipivefrin before and after (25.9 ± 3.0) vs acetazolamide before and after (25.7 ± 3.9) |
| Yalvaç 1996 | POAG | 3 | ALT | 360 and 180 | Apraclonidine before and after, 180° ALT (26.1 ± 5.1) vs placebo before and after, 180° ALT (25.6 ± 3.4) vs apraclonidine before and after, 360° ALT (26.4 ± 3.1) |
ALT: argon laser trabeculoplasty; IOP: intraocular pressure; OAG: open‐angle glaucoma; POAG: primary open‐angle glaucoma; SLT: selective laser trabeculoplasty.
Types of interventions
The studies included in this review were a mix of trials of antiglaucoma medication versus no medication, one antiglaucoma medication versus another antiglaucoma medication, and medications and placebos given at different time points before or after LTP. We grouped together studies that had more than one medication arm for a medication versus placebo analysis, but we separated the arms when timing was taken into account. For example, Barnebey 1993 had four study arms (group 1: brimonidine before and after LTP, group 2: brimonidine before and placebo after LTP, group 3: placebo before and brimonidine after LTP, and group 4: placebo before and after LTP) but in our analysis of medication versus placebo regardless of timing, we combined data for brimonidine from groups 1 to 3 because participants in these groups had received brimonidine at some point during the study and were being compared with participants who received only placebo.
Types of outcomes
Ten studies reported on our primary outcome, proportion of participants with IOP increase of 5 mmHg or greater or 10 mmHg or greater within two hours after LTP (Barnebey 1993; Barnes 1999; Birt 1995; Chevrier 1999; Donnelly 2006; Holmwood 1992; Metcalfe 1989; Ren 1999; Robin 1987; Yalvaç 1996). Whenever increases in IOP were reported by grouping the data as 6 mmHg to 10 mmHg increase and 11 mmHg to 15 mmHg increase, the former was included in our data as 5 mmHg or greater and the latter as 10 mmHg or greater. Ten studies reported on the mean change in IOP from pre‐LTP to measurements taken within two hours after LTP, though some reported the calculated mean change, while others reported the IOP measurement both at the baseline and at a specified time point (Birt 1995; Carassa 1992; Chevrier 1999; Dapling 1994; Donnelly 2006; Holmwood 1992; Raspiller 1992; Ren 1999; Robin 1987; Yalvaç 1996). To combine the data of different formats in meta‐analysis, we used the generic inverse variance method. We also used this method for the secondary outcome mean change in IOP from pre‐LTP to measurements taken more than two hours but within 24 hours after LTP, which 10 studies reported (Barnebey 1993; Barnes 1999; Birt 1995; Carassa 1992; Dapling 1994; Ma 1999; Raspiller 1992; Ren 1999; Robin 1987; Yalvaç 1996). Thirteen studies reported proportion of participants with IOP elevation of 5 mmHg or greater and 10 mmHg or greater more than two hours but within 24 hours after LTP (Barnebey 1993; Birt 1995; Brown 1988; Carassa 1992; Dapling 1994; David 1993; Elsas 1991; Hartenbaum 1999; Ma 1999; Raspiller 1992; Ren 1999; Robin 1987; Robin 1991).
Excluded studies
We excluded 31 studies (published in 35 reports (see Characteristics of excluded studies table). In 15 studies the medication studied was not an antiglaucoma medication (Ascaso 1992; Bucci 1987; Champagne 2015; De Keyser 2017 (identified as a journal article and a trial record); Diestelhorst 1995; Gelfand 1985; Herbort 1992; Herbort 1993; Jinapriya 2014; Kim 1998; Pappas 1985; Realini 2010; Shin 1996; Weinreb 1983; West 1992). Four studies included participants who also received laser peripheral iridotomies and neodymium:YAG laser capsulotomy without separating the results based on the type of laser performed (Chen 2001; Chen 2005; Patel 1998; Stingu 2001). One study included only participants who received laser iridotomy, which was not of interest for this review (Ottaiano 1989). Three other studies were dosage comparison studies (Hurvitz 1994; Rosenberg 1995; Threlkeld 1996). Six studies were not RCTs (Bergamini 1997 and León‐Alcántara 1995 were non‐comparative studies, and Krupin 1992; Leung 1986; Swendris 1991a; and Swendris 1991b were not randomized) and we were unable to determine if it was randomized (Ottaiano 1996). In one ongoing study, one study arm received only medication without LTP (Vickerstaff 2015). Four abstracts that we identified were linked to studies that we excluded.
Risk of bias in included studies
Summaries of our 'Risk of bias' judgment are shown in Figure 2, which presents the percentage judgment across all included studies, and Figure 3, which shows the individual judgments for each domain in each included study.
2.
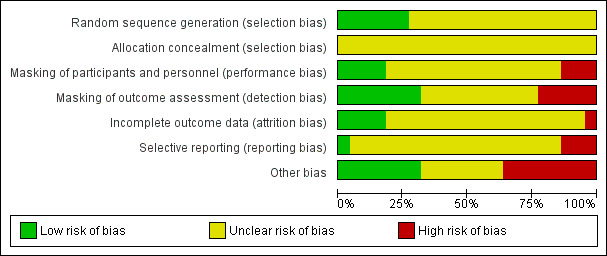
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
3.
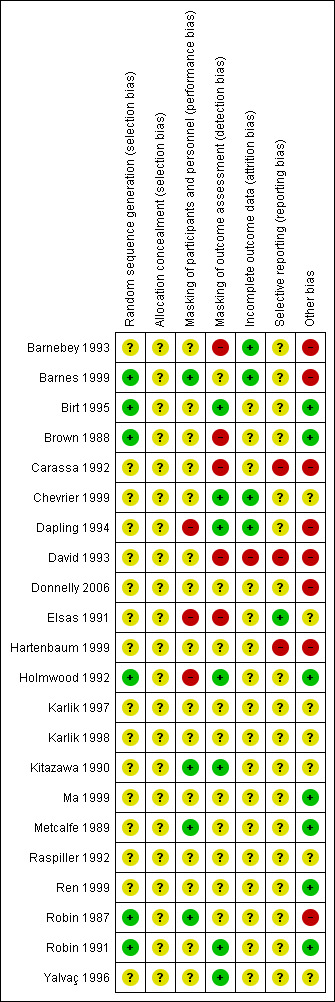
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
In the majority of the included studies, there was no mention of how participants were randomized or how allocation was concealed, and therefore, the risks of selection biases in these studies were unclear (Barnebey 1993; Carassa 1992; Chevrier 1999; Dapling 1994; David 1993; Donnelly 2006; Elsas 1991; Hartenbaum 1999; Karlik 1997; Karlik 1998; Kitazawa 1990; Ma 1999; Metcalfe 1989; Raspiller 1992; Ren 1999; Yalvaç 1996). We judged the studies that reported their method for randomizing participants to have low risk of selection bias (Barnes 1999; Birt 1995; Brown 1988; Robin 1987; Robin 1991). No studies reported how they concealed the random allocations before assignment; all were judged to be at unclear risk of selection bias for allocation concealment. We judged no study to be at high risk of bias regarding randomization but only four studies were judged at low risk of bias for this domain.
Masking (performance bias and detection bias)
Three studies were at high risk of performance bias due to issues related to masking of the participants (Dapling 1994; Elsas 1991; Holmwood 1992). Four studies were deemed to have a low risk of performance bias due to additional steps that were taken to minimize these types of bias (Barnes 1999; Kitazawa 1990; Metcalfe 1989; Robin 1987). Fifteen other studies did not present sufficient information to permit an assessment of risk of performance bias.
Studies that administer medications to the eye can be subject to a high risk of detection bias because the ocular medications administered can have visible adverse effects (i.e. conjunctival blanching/erythema, miosis, lid retraction) that easily may reveal the random assignment to the recorder of the postprocedure data unless additional steps were taken to reduce the risk. Five studies were at high risk of detection bias due to no or inadequate masking of outcome assessors (Barnebey 1993; Brown 1988; Carassa 1992; David 1993; Elsas 1991). Seven studies had undertaken additional steps to minimize such risks and we judged them at low risk of detection bias (Birt 1995; Chevrier 1999; Dapling 1994; Holmwood 1992; Kitazawa 1990; Robin 1991; Yalvaç 1996). There was not enough information to assess risk of detection bias in 10 studies.
Incomplete outcome data
One study had a high risk of incomplete outcome data reporting (David 1993). Participants with unacceptably high IOP after LTP were treated and removed from the study and not included in the final analysis. Based on comparisons of study methods and results section of reports, we deemed four studies to have complete reporting of data with minimal concerns for any attrition bias (Barnebey 1993; Barnes 1999; Chevrier 1999; Dapling 1994). Seventeen of the remaining studies did not report methods in enough detail to judge whether there was incomplete outcome data.
Selective reporting
There were three studies that were judged to have substantial concerns regarding selective reporting bias (Carassa 1992; David 1993; Hartenbaum 1999). In the David 1993 study, participants with unacceptably high IOPs were removed from the study and their results were not included in the final analysis. In the Carassa 1992 study, data for the one‐week time point was not reported as indicated in the methods section. The Hartenbaum 1999 study did not provide baseline characteristics of the study population, and did not provide data at the 24‐hour post‐LTP time point as indicated in the methods section. One study was judged to have complete reporting of all data with a low risk of selective reporting bias (Elsas 1991). The remaining 18 studies could not be judged regarding selective reporting bias.
Other potential sources of bias
Eight studies were at high risk of other types of biases (Barnebey 1993; Barnes 1999; Carassa 1992; Dapling 1994; David 1993; Donnelly 2006; Hartenbaum 1999; Robin 1987). Several studies had conflicts of interest where the study medication was made by the company providing the funding or had collaborating authors who were employed by the company that made the study medication (Barnebey 1993: Allergan; Barnes 1999: Allergan; Dapling 1994: Alcon; David 1993: Allergan; Hartenbaum 1999: Merck; Robin 1987: Alcon). One study used two types of surgery, and small sample sizes by type of surgery of did not allow for statistical analysis of the data for each surgery alone (Carassa 1992). Donnelly 2006 was a small study that included both eyes of each participant and the eyes received different medications; however, the authors did not report if and how they took into account the association and interdependency of two eyes within the same participant. Seven studies had a low risk of other types of biases (Birt 1995; Brown 1988; Holmwood 1992; Ma 1999; Metcalfe 1989; Ren 1999; Robin 1991). We judged the remaining seven studies to have unclear risk of other types of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Comparisons included in this review
A perioperative medication can be a treatment that is given before, during, or after LTP treatment. The studies included in this review had many combinations of treatments, doses, timing of treatments, and timing of doses. There was also a wide range of laser power used, amount of the angle treated, and types of glaucoma treated (Table 5). There were several levels of comparison included in this review: we first compared medications versus placebo and medications versus other medications, regardless of dose or timing. We combined data without regard to strength of the dose or when the medication was given to provide a more robust meta‐analysis to compare types of medications without restricting ourselves to each study's very specific timing/dosage pattern. For the purposes of this review, we did not compare different dosages of a study medication; we excluded studies that compared only different doses of the same medication. Therefore, a comparison may include a number of studies that each compared a medication 'A' to a medication 'B,' though each of the studies contributing data to that particular meta‐analysis may have a slightly different dosing schedule or plan.
We analyzed data for four comparisons that we performed without regard to dose or timing. In our tables, we noted 'regardless of timing.' The first comparison was any medication versus a placebo, vehicle, or no treatment. This comparison was repeated within subgroups defined by the active medication: brimonidine, apraclonidine, acetazolamide, pilocarpine, and dorzolamide. In a set of three comparisons, we compared outcomes from one medication to another. The medications compared are brimonidine versus apraclonidine, apraclonidine versus latanoprost, and apraclonidine versus pilocarpine. Comparisons involving brimonidine versus apraclonidine and apraclonidine versus pilocarpine had sufficient data to conduct meta‐analyses. The only outcome data for apraclonidine versus latanoprost were reported on in two abstracts from the same center that did not provide enough data for a meta‐analysis.
For the final comparison in this review, we analyzed data from trials in which the same medication was given to some participants before LTP and to some participants after LTP. For this comparison, participants must have received the medication either before or after LTP; we did not include data from or compare participants who received the medication both before and after LTP.
Some studies provided data for outcomes assessed at more than one time point within the period of assessment. For example, a study that provided data at one hour and at two hours post‐LTP, data from both time points would fit into the "IOP increase of 5 mmHg within two hours"; outcomes from a study that provided data collected at six and 12 hours would qualify measurements taken more than two hours but within 24 hours post‐LTP. For these cases, we chose data acquired at one time point to include the time point closer to two hours in the first example and closer to 24 hours in the second example.
Medication versus placebo
In this comparison, we included data from studies that compared a medication with a placebo or vehicle or no treatment. Of 12 trials that compared a medication with a placebo, 11 reported data for one or more of the review outcomes that could be included in meta‐analyses. For this comparison, we combined the data for apraclonidine and brimonidine because they are both alpha‐2 agonists. This comparison included data from all trials regardless of timing; participants in the trial arms could have received the medication or placebo before, during, or after LTP, or at more than one of these times.
Primary outcomes
Proportion of participants with IOP increase of 5 mmHg or greater within two hours after LTP
Two studies that involved treatment with alpha‐2 agonists examined the percentage of participants who had an IOP increase of 5 mmHg or greater within two hours after LTP: one compared brimonidine versus placebo (Barnebey 1993), and one compared apraclonidine versus placebo (Yalvaç 1996). In the study comparing brimonidine versus placebo, participants who received brimonidine had an 83% lower risk of a 5 mmHg or greater IOP increase compared with participants who received placebo (RR 0.17, 95% CI 0.07 to 0.40). In the study that compared apraclonidine versus placebo, 9.4% of participants in the apraclonidine group and 6.3% of the placebo group had an IOP increase of 5 mmHg or greater within two hours after LTP (RR 1.50, 95% CI 0.17 to 13.30). Due to the inconsistency in the direction of the estimated treatment effect, there was significant heterogeneity in the outcome estimates from the two studies (I2 = 70%) and we did not perform a meta‐analysis (Analysis 1.1). The certainty of the evidence was downgraded because of problems with risk of bias and inconsistency. Barnebey 1993 included several authors who were employees of the company that manufactured the study drug. Additionally, brimonidine is known to cause lid retraction and conjunctival blanching, so an outcome assessor would know which participants received brimonidine versus who received placebo.
1.1. Analysis.
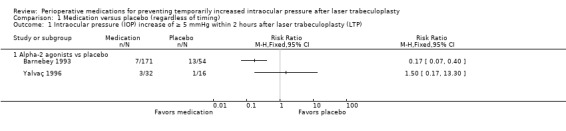
Comparison 1 Medication versus placebo (regardless of timing), Outcome 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP).
Proportion of participants with IOP increase of 10 mmHg or greater within two hours after LTP
Four studies reported on the percentage of participants who had an IOP increase of 10 mmHg or greater within two hours after LTP (Barnebey 1993; Metcalfe 1989; Robin 1987; Yalvaç 1996). Each comparison showed that fewer participants who took the medication had an IOP increase of 10 mmHg or greater compared with participants who received placebo (RR 0.05, 95% CI 0.01 to 0.20). One study compared acetazolamide versus placebo and reported that participants who received acetazolamide had a lower risk of having an IOP increase of 10 mmHg or greater (RR 0.03, 95% CI 0.00 to 0.52) (Metcalfe 1989). Three studies compared alpha‐2 agonists versus placebo; participants who received the medication had a 94% lower risk of an IOP increase 10 mmHg or greater compared with placebo (RR 0.06, 95% CI 0.01 to 0.27; Analysis 1.2) (Barnebey 1993; Robin 1987; Yalvaç 1996). The certainty of the evidence was graded as moderate; we downgraded one level due to judgments of high risk of bias for two studies wherein study authors had associations with the companies that manufactured the study drugs.
1.2. Analysis.
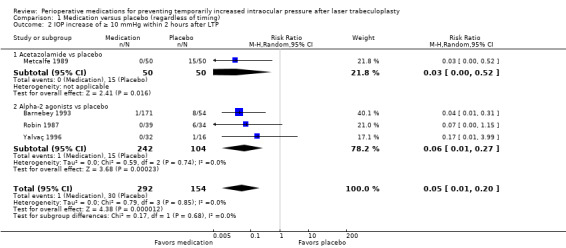
Comparison 1 Medication versus placebo (regardless of timing), Outcome 2 IOP increase of ≥ 10 mmHg within 2 hours after LTP.
Secondary outcomes
Mean change in IOP measurements from pre‐LTP to within two hours after LTP
Four studies reported IOP measurements within two hours after LTP. Three reported the change from baseline measurements, and one reported the mean for each group at the follow‐up time point; thus, we used the inverse variance method to analyze the MD. All four studies compared apraclonidine versus placebo (Carassa 1992; Raspiller 1992; Robin 1987; Yalvaç 1996). We assigned moderate‐certainty to the evidence that use of apraclonidine resulted in more IOP reduction compared with placebo (MD ‐7.43 mmHg, 95% CI ‐10.60 to ‐4.27; Analysis 1.3). The certainty of the evidence was downgraded from high to moderate due to high risk of detection bias, reporting bias, and other potential bias due association of study report authors with companies that manufactured the study drugs.
1.3. Analysis.
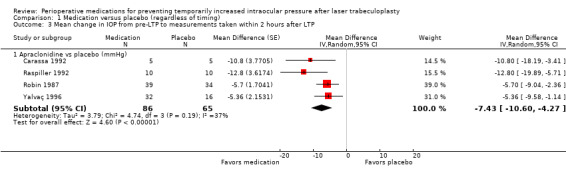
Comparison 1 Medication versus placebo (regardless of timing), Outcome 3 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP.
Proportion of participants with IOP increase of 5 mmHg or greater and 10 mmHg or greater two to 24 hours after LTP
Nine studies reported the proportion of participants who had elevated IOP at time points more than two hours but within 24 hours after LTP. Five studies reported on an increase of 5 mmHg or greater; all had a treatment group that was given an alpha‐2 agonist (two compared apraclonidine versus placebo (Brown 1988; Carassa 1992), and three compared brimonidine versus placebo (Barnebey 1993; David 1993; Ma 1999)). In this comparison, fewer participants who received the alpha‐2 agonist had IOP elevated by 5 mmHg or greater from two to 24 hours after LTP compared with participants who received placebo (RR 0.17, 95% CI 0.09 to 0.31; Figure 4; Analysis 1.4). We graded the estimate from this meta‐analysis as providing low‐certainty evidence due to substantial concerns about the risk of bias in four of the five studies.
4.
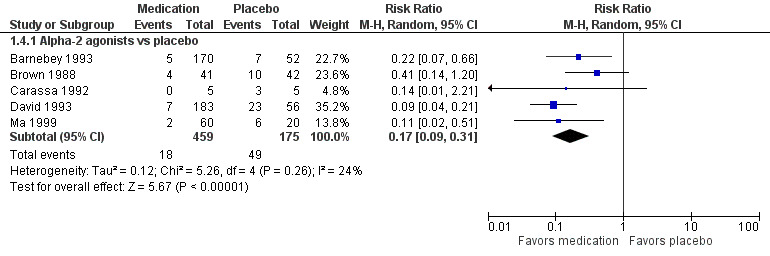
Forest plot of comparison: 1 Medication versus placebo (regardless of timing), outcome: 1.4 Intraocular pressure (IOP) increase ≥ 5 mmHg two to 24 hours after laser trabeculoplasty (LTP).
1.4. Analysis.
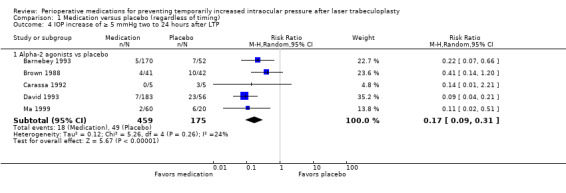
Comparison 1 Medication versus placebo (regardless of timing), Outcome 4 IOP increase of ≥ 5 mmHg two to 24 hours after LTP.
Nine studies reported on an increase of 10 mmHg or greater, seven with a comparison of an alpha‐2 agonist versus placebo (Barnebey 1993; Brown 1988; Carassa 1992; David 1993; Ma 1999; Raspiller 1992; Robin 1987), and one each comparing dorzolamide versus placebo (Hartenbaum 1999), and pilocarpine versus no treatment (Elsas 1991). In the alpha‐2 agonist versus placebo comparison, the group of participants who were given an alpha‐2 agonist had a lower risk of IOP elevation by 10 mmHg or greater compared with the group given placebo (RR 0.19, 95% CI 0.07 to 0.50), similar to the estimated effect for IOP elevation by 5 mmHg or greater. In the study that compared pilocarpine versus no treatment, fewer participants who received pilocarpine experienced IOP elevation by 10 mmHg or greater compared with participants in the no treatment group (RR 0.23, 0.07 to 0.71). For the outcome of IOP elevation of 10 mmHg or greater between two and 24 hours after surgery, participants who received any medication had a lower risk of an increase compared with participants who received placebo or no treatment (RR 0.22, 95% CI 0.11 to 0.42; Analysis 1.5). We graded the certainty of this estimate to be very low, as the majority of the included studies were subject to high risk of bias based on several bias domains, including detection bias and reporting bias, and due to imprecision; several of the studies had risk estimates with wide CIs that lead to uncertainty as to whether medication or placebo or neither was favored.
1.5. Analysis.
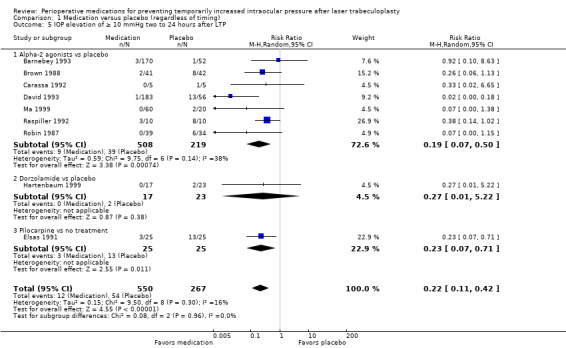
Comparison 1 Medication versus placebo (regardless of timing), Outcome 5 IOP elevation of ≥ 10 mmHg two to 24 hours after LTP.
Mean change in IOP measurements from pre‐LTP to two to 24 hours after LTP
The four studies that compared apraclonidine versus placebo and reported the mean change in IOP within two hours also reported on mean change in IOP measurements from pre‐LTP to between two and 24 hours after LTP (Carassa 1992; Raspiller 1992; Robin 1987; Yalvaç 1996). Participants in the apraclonidine group had greater IOP reduction after LTP than participants in the placebo group (MD ‐5.32 mmHg, 95% CI ‐7.37 to ‐3.28; Analysis 1.6). We graded the evidence as being of moderate‐certainty due to a high risk of bias in some domains.
1.6. Analysis.
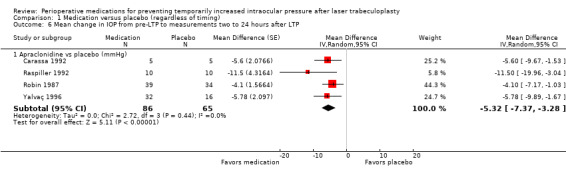
Comparison 1 Medication versus placebo (regardless of timing), Outcome 6 Mean change in IOP from pre‐LTP to measurements two to 24 hours after LTP.
Some of the included studies reported mean change in IOP from two to 24 hours after surgery, but we were unable to include the data in the meta‐analysis because the IOP data were reported in figures from which we were unable to extract data (Brown 1988; David 1993; Robin 1991). Generally, from the figures provided in the three studies, it appeared that treatment with medication resulted in greater reduction of IOP compared with treatment with placebo.
Proportion of participants with IOP greater than preoperative baseline by 5 mmHg or greater 24 hours after LTP
None of the studies that compared medication versus placebo reported proportion of participants with IOP greater than preoperative baseline by 5 mmHg or greater 24 hours after LTP.
Proportion of participants who required additional antiglaucoma therapy or surgical glaucoma intervention to reduce post‐LTP‐related IOP elevation
Two studies that compared a medication versus a placebo reported on the need for additional treatment to reduce IOP after LTP. Robin 1987 reported that two eyes in the placebo‐treated group but no eyes in the apraclonidine group required oral glycerin for an unacceptably high IOP elevation. The authors of Brown 1988 reported that of the participants with post‐laser IOP elevations, 10 participants, two in the apraclonidine group and eight in the placebo group, were given additional hypotensive therapy after the one‐hour IOP measurement but before the three‐hour measurement. As the investigators of this study reported outcomes for participants who had trabeculoplasty, iridotomy, or posterior capsulotomy, it is unclear which surgery applied to the participants who required additional therapy.
Percentage of participants with worsened vision after LTP
None of the studies that compared medication versus placebo reported data regarding worse vision after LTP.
Medication versus medication: brimonidine versus apraclonidine
In this comparison, we examined outcomes from studies that compared specific alpha‐2 agonists. Three included studies that compared brimonidine versus apraclonidine as perioperative treatment provided data for this comparison (Barnes 1999; Chevrier 1999; Donnelly 2006).
Primary outcomes
Proportion of participants with IOP increase of 5 mmHg or greater within two hours after LTP
Three studies compared brimonidine versus apraclonidine and reported on the proportion of participants who had an IOP increase of 5 mmHg or greater within two hours after LTP. It remains unknown whether brimonidine or apraclonidine or neither was better in preventing IOP increases within two hours after surgery (RR 2.28, 95% CI 0.32 to 16.03; Analysis 2.1). Barnes 1999 reported that no participants in either group had an IOP increase of 5 mmHg or greater. The certainty of the evidence was downgraded to very low due to high imprecision of the estimated effect (downgraded two levels), inconsistency (one trial had an RR of 6.25 and the other had an RR of 1.00), and concerns about risk of bias for the included studies.
2.1. Analysis.
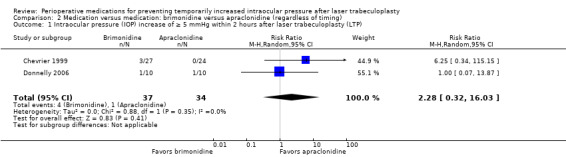
Comparison 2 Medication versus medication: brimonidine versus apraclonidine (regardless of timing), Outcome 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP).
Proportion of participants with IOP increase of 10 mmHg or greater within two hours after LTP
Barnes 1999 reported that no participant in the study had a significant IOP increase, regardless of whether they received brimonidine or apraclonidine. In another smaller study in which participants received brimonidine in one eye and apraclonidine in the other, one eye (10%) that received apraclonidine drops had an IOP increase of 10 mmHg or greater, though the estimated effect is consistent with no statistically significant difference in risk of increased IOP in either treatment group (RR 0.33, 95% CI 0.02 to 7.32) (Donnelly 2006).
Secondary outcomes
Mean change in IOP measurements from pre‐LTP to within two hours after LTP
Two studies reported on mean change in IOP from baseline to a time point within two hours after LTP (Chevrier 1999; Donnelly 2006). Based on our analysis of the data they reported, it is unknown whether brimonidine or apraclonidine or neither produced a greater reduction in IOP measurements within two hours after LTP (MD ‐0.69 mmHg, 95% CI ‐2.56 to 1.17; Analysis 2.2). We downgraded the evidence due to risk of bias concerns in the included studies; thus, we have moderate‐certainty that neither of these two alpha‐2 agonists resulted in a greater reduction in the mean IOP following LTP than the other.
2.2. Analysis.
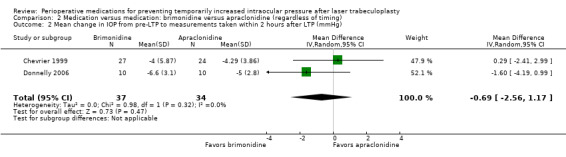
Comparison 2 Medication versus medication: brimonidine versus apraclonidine (regardless of timing), Outcome 2 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP (mmHg).
Proportion of participants with IOP increase of 5 mmHg or greater and 10 mmHg or greater two to 24 hours after LTP
None of the studies that compared brimonidine versus apraclonidine reported on IOP elevations between two and 24 hours after LTP.
Mean change in IOP measurements from pre‐LTP to time points two to 24 hours after LTP
Only one study reported on the mean change in IOP from a baseline measurement to a time more than two hours after surgery (Barnes 1999). In this study, participants in the brimonidine group had a mean reduction in IOP of 2.6 mmHg (standard deviation (SD) = 3.6), while the participants in the apraclonidine group had a mean reduction in IOP of 2.3 mmHg (SD = 3.7). The MD in IOP reduction was consistent with no clinically important difference in the effects of the two medications on this outcome (MD ‐0.30 mmHg, 95% CI ‐2.41 to 1.81).
Proportion of participants with IOP greater than preoperative baseline by 5 mmHg or greater 24 hours after LTP
None of the studies that compared brimonidine versus apraclonidine reported proportion of participants with IOP greater than preoperative baseline by 5 mmHg or greater 24 hours after LTP.
Proportion of participants who required additional antiglaucoma therapy or surgical glaucoma intervention to reduce post‐LTP‐related IOP elevation
Donnelly 2006 used a paired‐eye study design in which brimonidine was randomly administered in one eye and apraclonidine in the other. This small study reported that one participant needed additional supplemental topical medication to help reduce post‐LTP‐related IOP elevation: the participants had an 8 mmHg IOP increase in the brimonidine‐treated eye and a 15‐mmHg increase in the apraclonidine‐treated eye. The two other studies that compared brimonidine with apraclonidine did not report on the proportion of participants who required additional antiglaucoma therapy.
Percentage of participants with worsened vision after LTP
None of the studies that compared brimonidine versus apraclonidine reported on worsened vision after LTP.
Medication versus medication: apraclonidine versus latanoprost
One abstract reported a study that enrolled 50 participants who received either apraclonidine or latanoprost prior to ALT. Though the abstract authors did not report data for our time points of interest, they reported that there was no statistically significant difference between the IOP measurements of the two groups at 24 hours or at six weeks post‐ALT. In each group, three participants had an IOP spike of 5 mmHg or greater, but the times of these spikes was not reported (Karlik 1998). Mean IOP at 1.5 hours after surgery was 20.7 mmHg and at 24 hours after surgery was 17.3 mmHg in the apraclonidine group and at 1.5 hours after surgery was 24.2 mmHg and at 24 hours after surgery was 17.5 mmHg in the latanoprost group (SDs were not provided). The same authors reported in another abstract from a study of apraclonidine versus latanoprost among 37 participants that the IOP at two hours after ALT was 19.7 mmHg in the apraclonidine group and 25.1 mmHg in the latanoprost group (Karlik 1997).
Medication versus medication: apraclonidine versus pilocarpine
Primary outcomes
Proportion of participants with IOP increase of 5 mmHg or greater within two hours after LTP
Only one study that compared apraclonidine versus pilocarpine reported the proportion of participants who had an IOP increase of 5 mmHg or greater within two hours of surgery. In the apraclonidine group, 8.8% of participants had an increase of 5 mmHg or greater versus 4.4% of participants in the pilocarpine group. However, estimates were not sufficiently precise to conclude that apraclonidine, pilocarpine, or neither were more effective for preventing IOP spikes (RR 2.00, 95% CI 0.71 to 5.67).
Proportion of participants with IOP increase of 10 mmHg or greater within two hours after LTP
None of the studies that compared apraclonidine versus pilocarpine reported proportion of participants with IOP increase of 10 mmHg or greater within two hours after LTP.
Secondary outcomes
Mean change in IOP measurements from pre‐LTP to within two hours after LTP
Two studies reported on the mean change in IOP from pre‐LTP to a time point within two hours after LTP (Dapling 1994; Robin 1991). Neither apraclonidine nor pilocarpine was favored for better reduction in IOP (MD 0.61 mmHg, 95% CI ‐0.44 to 1.66; Analysis 3.1). The small size of the MD and the width of the CI are consistent with no difference in the effect of the two treatments on this outcome. The certainty of the evidence was graded as moderate because one of the included studies was at high risk of bias due to the association of study authors with the company that manufactured one of the study medications.
3.1. Analysis.
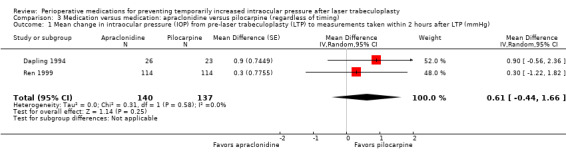
Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 1 Mean change in intraocular pressure (IOP) from pre‐laser trabeculoplasty (LTP) to measurements taken within 2 hours after LTP (mmHg).
Proportion of participants with IOP increase of 5 mmHg or greater and 10 mmHg or greater two to 24 hours after LTP
Two studies reported on the proportion of participants with a 5 mmHg or greater increase in IOP and three studies reported on the proportion of participants with a 10 mmHg or greater IOP increase at time points between two and 24 hours after LTP. In one study data for both outcomes were reported to have been collected within the first three hours after LTP; we included those data in the two‐ to 24‐hour outcome period because some of the assessments were after two hours (Robin 1991). The same study also reported on IOP increases of 6 mmHg to 10 mmHg or greater than 10 mmHg. We included the outcome data for a 6 mmHg to 10 mmHg increase within increased IOP 5 mmHg or greater and greater than 10 mmHg with increased IOP 10 mmHg or greater. For post‐LTP IOP elevation of 5 mmHg or greater, one study favored apraclonidine and one favored pilocarpine, resulting in high heterogeneity (I2 = 91%). Due to this inconsistency/heterogeneity, we did not perform a meta‐analysis (results for the individual studies are shown in Figure 5; Analysis 3.2). The certainty of the evidence for the individual study results were downgraded twice. One of the studies had a treatment regimen that could serve to unmask participants because vehicle drops were not used. Dapling 1994 compared administering apraclonidine before LTP in one group with administering pilocarpine after LTP in a second group, or both; since vehicle drops were not used participants who received drops both before and after might be able to identify that they had been randomized to receive both medications, and if they received drops only before or only after, to which medication they had been randomized. We were able to perform a meta‐analysis of data on IOP elevation of 10 mmHg or greater, in which one study reported that no participant in either group had an IOP increase of that magnitude (Dapling 1994), while of the two others, one favored apraclonidine (Ren 1999) and the other favored pilocarpine (Robin 1991). There was statistical uncertainty as to which medication, if either, better prevented IOP spikes of 10 mmHg or more after surgery (RR 0.87, 95% CI 0.14 to 5.63; Analysis 3.3). The evidence was graded as moderate due to inconsistency in direction of the treatment effect in the three studies.
5.

Forest plot of comparison: 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), outcome: 3.2 Intraocular pressure (IOP) increase of ≥ 5 mmHg two to 24 after laser trabeculoplasty (LTP).
3.2. Analysis.
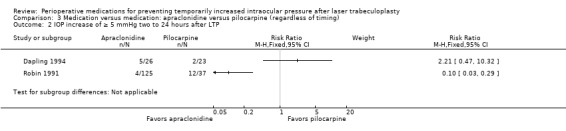
Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 2 IOP increase of ≥ 5 mmHg two to 24 hours after LTP.
3.3. Analysis.
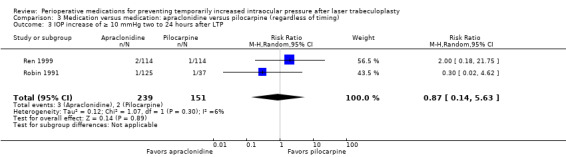
Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 3 IOP increase of ≥ 10 mmHg two to 24 hours after LTP.
Mean change in IOP measurements from pre‐LTP to two to 24 hours after LTP
Two studies reported the mean change in IOP from pre‐LTP to time points between two and 24 hours after surgery (Dapling 1994; Ren 1999). The two studies in this analysis had high statistical heterogeneity (I2 = 92%) because the effect estimate from the study with fewer participants favored apraclonidine while the effect estimate from the study with more than four times as many participants favored pilocarpine; thus, we did not perform a meta‐analysis but the results for the individual studies are shown in Analysis 3.4). This inconsistency, along with issues with risk of bias due to author associations with industry, caused us to downgrade the certainty of the evidence to low.
3.4. Analysis.
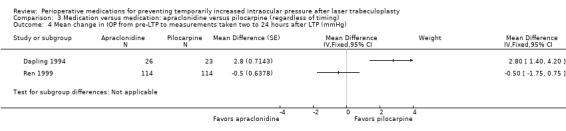
Comparison 3 Medication versus medication: apraclonidine versus pilocarpine (regardless of timing), Outcome 4 Mean change in IOP from pre‐LTP to measurements taken two to 24 hours after LTP (mmHg).
Proportion of participants with IOP greater than preoperative baseline by 5 mmHg or greater 24 hours after LTP
None of the studies that compared apraclonidine versus pilocarpine reported proportion of participants with IOP greater than preoperative baseline by 5 mmHg or greater 24 hours after LTP.
Proportion of participants who required additional antiglaucoma therapy or surgical glaucoma intervention to reduce post‐LTP‐related IOP elevation
None of the studies that compared apraclonidine versus pilocarpine reported proportion of participants requiring additional antiglaucoma therapy or surgical glaucoma intervention to reduce post‐LTP‐related IOP elevation.
Percentage of participants with worsened vision after LTP
None of the studies that compared apraclonidine versus pilocarpine reported worsened vision after LTP.
Timing comparison: medication before LTP versus medication after LTP
A question of interest for surgeons is whether the timing of administration of IOP‐reducing medications. In this comparison, we included the studies that compared treatment with any antiglaucoma medication given before LTP with treatment with that same medication given after LTP. This comparison included four studies (Barnebey 1993; Birt 1995; David 1993; Ma 1999). We excluded from this comparison studies that compared one medication given before LTP to a different medication given after LTP.
Primary outcomes
Proportion of participants with IOP increase of 5 mmHg or greater within two hours after LTP
Two studies, both treating with alpha‐2 agonists (one apraclonidine and one brimonidine), reported on an IOP increase of 5 mmHg or greater within two hours after LTP (Barnebey 1993; Birt 1995). Birt 1995 reported that no participants in either study arm had an IOP increase of 5 mmHg or greater, while Barnebey 1993 reported that 3/57 (5.3%) participants who were given the medication only before LTP group had an IOP increase of 5 mmHg or greater compared with 4/53 (7.5%) participants in the medication only after LTP group. The estimated treatment effect was too imprecise to conclude whether medication only before or medication only after LTP resulted in a lower percentage of participants with IOP increases of 5 mmHg or greater within two hours after LTP (RR 0.70, 95% CI 0.16 to 2.97).
Proportion of participants with IOP increase of 10 mmHg or greater within two hours after LTP
Two studies, both treating with alpha‐2 agonists (one apraclonidine and one brimonidine), reported on IOP increase 10 mmHg or greater (Barnebey 1993; Birt 1995). No participants in the Birt 1995 study had an increase of 10 mmHg or greater. In Barnebey 1993, just one participant in the medication only before group had an increase of 10 mmHg or greater. The number of participants with this outcome was inadequate to estimate RRs.
Secondary outcomes
Mean change in IOP measurements from pre‐LTP to within two hours after LTP
One study treating with apraclonidine compared medication only before versus medication only after LTP reported data for mean change in IOP measurements from pre‐LTP to within two hours after LTP (Birt 1995). The mean change from baseline was not reported but the mean (± SD) IOP at a time point within two hours was not statistically different in the group receiving apraclonidine before (13.8 ± 5.4 mmHg) compared with the group receiving apraclonidine after (14.4 ± 3.6 mmHg) (MD ‐0.60 mmHg, 95% CI ‐3.20 to 2.00).
Proportion of participants with IOP increase of 5 mmHg or greater and 10 mmHg or greater two to 24 hours after LTP
Four studies that compared medication before surgery to the same medication after surgery reported on the proportion of participants with IOP increases of 5 mmHg or greater and 10 mmHg or greater between 2 and 24 hours after surgery. In three studies, the medication given was brimonidine (Barnebey 1993; David 1993; Ma 1999); in the fourth study, it was apraclonidine (Birt 1995). Birt 1995 reported that no participants in either study group had an increase of 5 mmHg or greater, and Ma 1999 reported that no participant had an increase 10 mmHg or greater. For both degrees of IOP increases, neither medication only before LTP nor medication only after LTP was clearly superior (for IOP increase of 5 mmHg or greater: RR 0.82, 95% CI 0.25 to 2.63; Figure 6; Analysis 4.1; for IOP increase of 10 mmHg or greater: RR 1.55, 95% CI 0.19 to 12.43; Analysis 4.2). The certainty of the evidence was downgraded for each of these outcomes due to concerns about a high risk of bias for masking in two of the studies included in this analysis. Additionally, the same two studies had authors who were affiliated with the company that manufactured the study drug. Although this comparison was only for timing and both groups received the same study drug, the larger study population also had a study group which did not receive any study drug. The evidence for IOP increase of 10 mmHg or greater between two and 24 hours was downgraded further to low‐certainty due to imprecision in the relative effect estimate.
6.
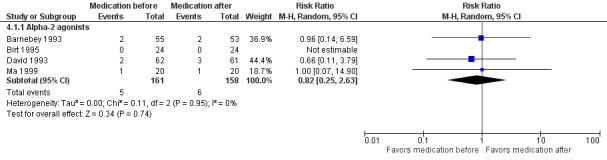
Forest plot of comparison: 4 Timing comparison: medication before versus medication after, outcome: 4.1 Intraocular pressure (IOP) increase of ≥ 5 mmHg two to 24 hours after laser trabeculoplasty (LTP).
4.1. Analysis.
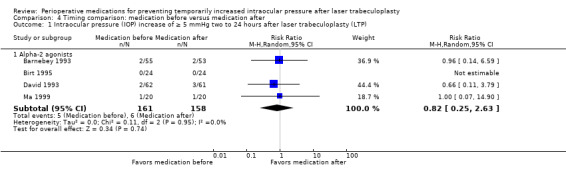
Comparison 4 Timing comparison: medication before versus medication after, Outcome 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg two to 24 hours after laser trabeculoplasty (LTP).
4.2. Analysis.
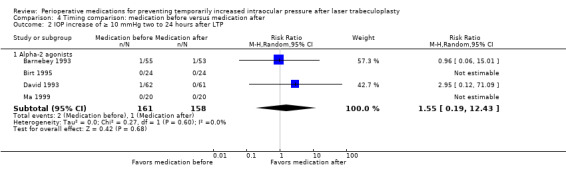
Comparison 4 Timing comparison: medication before versus medication after, Outcome 2 IOP increase of ≥ 10 mmHg two to 24 hours after LTP.
Mean change in IOP measurements from pre‐LTP to two to 24 hours after LTP
Three studies, two that treated participants before or after LTP with apraclonidine and one that treated participants before or after LTP with brimonidine, reported on IOP changes between two and 24 hours after LTP. When the three studies treating with alpha‐2 agonists were combined, neither treatment with medication only before LTP or treatment with medication only after LTP resulted in a greater IOP reduction (MD ‐1.07 mmHg, 95% CI ‐2.51 to 0.37; Analysis 4.3), although the estimated effect favored pre‐LTP treatment. Despite some statistical heterogeneity in this meta‐analysis (I2 = 64%), we did not consider it to be substantial enough to exclude this meta‐analysis from the review. However, we downgraded the certainty of the evidence to moderate based on the inconsistent findings: outcome data from two trials favored medication only before LTP and from one trial favored medication only after LTP.
4.3. Analysis.
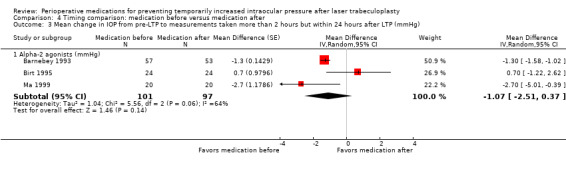
Comparison 4 Timing comparison: medication before versus medication after, Outcome 3 Mean change in IOP from pre‐LTP to measurements taken more than 2 hours but within 24 hours after LTP (mmHg).
Proportion of participants with IOP greater than preoperative baseline by 5 mmHg or greater 24 hours after LTP
None of the studies that compared treatment before or after LTP reported the proportion of participants with IOP greater than preoperative baseline by 5 mmHg or greater 24 hours after LTP.
Proportion of participants who required additional antiglaucoma therapy or surgical glaucoma intervention to reduce post‐LTP‐related IOP elevation
None of the studies that compared treatment before or after LTP reported proportion of participants requiring additional antiglaucoma therapy or surgical glaucoma intervention to reduce post‐LTP‐related IOP elevation.
Percentage of participants with worsened vision after LTP
None of the studies that compared treatment before or after LTP reported worsened vision after LTP.
Adverse events
We examined the proportion of participants with ocular or systemic adverse events. Although we planned to report adverse events occurring within different time intervals after LTP, the studies that reported adverse events did not provide details regarding timing of the event and instead reported those that occurred any time during the entire follow‐up period. Three studies compared brimonidine versus placebo and all three reported conjunctival blanching (Barnebey 1993; David 1993; Ma 1999). Two of the studies reported the percentage of participants who presented with this adverse event; however, there was significant statistical heterogeneity (I2 = 95%; Analysis 5.1) (David 1993; Ma 1999). The third study reported only on the range of participants who had conjunctival blanching within the three groups that received brimonidine at some point during treatment and found that conjunctival blanching was more frequent in the groups receiving brimonidine (49% to 60%) compared with the group that received only vehicle (8%, P < 0.001) (Barnebey 1993). Other adverse events reported in this comparison were lid retraction (14/183 participants (7.6%) treated with brimonidine and 3/56 participants given vehicle (5.4%) in David 1993 and more frequently in groups receiving brimonidine (26% to 39%) compared with the group receiving only vehicle (12%, P < 0.001) in Barnebey 1993) and conjunctival hyperemia with itching in Ma 1999 (one participant in the brimonidine and one in the placebo group).
5.1. Analysis.
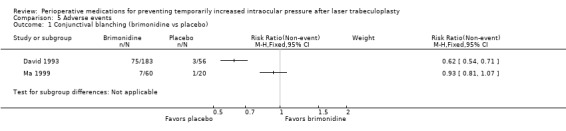
Comparison 5 Adverse events, Outcome 1 Conjunctival blanching (brimonidine vs placebo).
Three studies reported that no systemic/nonocular symptoms or event had been observed in any eye (Chevrier 1999; Donnelly 2006; Robin 1987). Carassa 1992 reported that although no adverse experience was reported during the study for either treatment group, there were a few ocular adverse effects, such as mild bleeding postsurgery. Since this study included participants who received other types of anterior segment laser procedures in addition to LTP, we do not know which surgery preceded these ocular adverse effects, but it was clear that they were due to the laser procedures rather than to the post‐LTP IOP elevation. Similarly, Brown 1988 reported on few ocular and systemic adverse events but did not separate the adverse events in participants who received trabeculoplasty from those who received iridotomy or capsulotomy.
Discussion
Summary of main results
LTP has an important role in the management of OAG. Early acute IOP elevation is a relatively a common adverse effect of LTP (Barkana 2007; Chen 2001). Studies have shown that transient IOP increase after LTP resulted in loss of vision (Levene 1983) and loss of visual field (Weinreb 1983). Therefore, post‐LTP IOP increase is an important and potentially dangerous adverse effect of LTP, especially in a population of people who already have compromised optic nerve function. Although glaucoma medications are sometimes administered perioperatively when performing LTP, evidence for this practice is debated. Individual studies using perioperative glaucoma medications to reduce the frequency of post‐LTP IOP spikes have yielded varying estimates of efficacy.
We evaluated 22 RCTs of glaucoma medications administered perioperatively when performing LTP. We chose the primary outcomes, the proportions of participants with IOP spikes of 5 mmHg or greater or 10 mmHg or greater within the first two hours after LTP, because even small, acute IOP elevations of 5 mmHg to 10 mmHg can result in permanent damage to the optic nerve in people with glaucoma. Most of the trials had relatively small sample sizes; we combined results from trials studying the alpha‐2 agonists, brimonidine and apraclonidine, that have statistically equivalent aqueous humor dynamics (Schadlu 1998).
We analyzed outcomes for several comparisons. First, we compared the use of any IOP‐lowering medication to placebo. We found that participants who had received perioperative administration of glaucoma medications (namely alpha‐2 agonists) had a lower risk of an IOP elevation of 10 mmHg or greater within the first two hours after LTP compared with participants who received placebo (Analysis 1.2). The risk of an IOP increase of 10 mmHg or more during the first two hours after LTP was about 95% lower in the group that was assigned to medication compared with the group assigned to placebo. The effect of perioperative glaucoma medications in blunting IOP spikes was sustained for the two‐ to 24‐hour period after LTP (Analysis 1.4; Analysis 1.5). In the majority of the trials, three hours post‐LTP was the last IOP data point recorded within the first 24 hours. The natural course of post‐LTP IOP peaks occur within the first two hours, but occasionally the timing of the IOP peak may be delayed, and therefore monitoring of IOP for four hours after LTP has been recommended (Weinreb 1983). Since most glaucoma medications have half‐lives of two to four hours, missing delayed IOP spikes initially blunted by glaucoma medication is a possibility that we were unable to explore using the data we assessed.
Secondary outcomes of mean change in IOP from pre‐ to post‐LTP measurements corroborated the efficacy of perioperative glaucoma drops in reducing post‐LTP IOP spikes compared with placebo. Perioperative apraclonidine resulted in reduction of IOP within two hours after LTP by 7.4 mmHg lower than the reduction in the placebo group (Analysis 1.3) and was 5.3 mmHg lower between two and 24 hours (primarily three hours) after LTP.
After establishing the benefit of using IOP‐lowering medication perioperatively to prevent IOP spikes after LTP, we focused on comparisons of individual medications for the same set of outcomes to better understand which glaucoma medication is most effective at lowering post‐LTP IOP spikes. The first comparison in this set contrasted two alpha‐2 agonists, brimonidine and apraclonidine. We found no statistically significant difference between the two medications in the proportion of participants with IOP spikes of 5 mmHg or more within the first two hours (Analysis 2.1) or in change of IOP from pre‐LTP to two hours post‐LTP (Analysis 2.2). This is not a surprising finding, since both medications are alpha‐2 agonists. Additional analyses between brimonidine and apraclonidine for other outcomes could not be performed due to lack of data from the included trials, but we would not expect to uncover any clinically or statistically significant differences between these medications for the outcomes examined.
The third comparison in this review was apraclonidine versus pilocarpine. In these comparisons, we found no statistically significant difference in the mean change from pre‐ to post‐LTP IOPs within the first two hours after LTP when medicating with either apraclonidine or pilocarpine perioperatively (Analysis 3.1). Although we did not have data for direct comparisons between brimonidine and pilocarpine, these findings suggest that both apraclonidine and brimonidine may be effective at preventing IOP spikes after LTP. However, our interpretations were limited due to the overall small number of trials that reported the comparisons and outcomes targeted, and the small number of participants who provided data from available trials for each analysis.
We intended to examine comparisons of the timing of administration of glaucoma medications, but such comparisons were difficult or impossible due to the overall number of variables (types of medication, timing of administration, frequency and strength of dose) that should be accounted for but were prevented from doing so by the available sample size. One comparison, the fifth comparison in this review, could be made: administration of alpha‐2 agonists before and after LTP. We found no statistically significant difference in the proportion of participants with IOP spikes of 5 mmHg or greater between two and 24 hours after LTP (Analysis 4.1). Data for IOP spikes occurring within two hours after LTP were not available. Likewise, there was no difference in the percentage of participants who had IOP elevations of 10 mmHg or greater between two and 24 hours after LTP (Analysis 4.2), or in the mean change in pre‐ and post‐LTP IOPs between two and 24 hours.
Post‐LTP IOP increases that result in vision or visual field loss are rare, but important complications (Levene 1983; Weinreb 1983). In this review, there were no reports of IOP spikes that required chronic escalation of glaucoma therapy or resulted in worsened vision, so we were unable to analyze the effect of perioperative mediations on this outcome. The included trials did not always report the stage of glaucoma. One study excluded people with advanced glaucoma due to the treating ophthalmologist's assessment that they could not tolerate IOP spikes from a laser procedure (Hartenbaum 1999). One study included people with advanced glaucoma who were uncontrolled on maximally tolerated medications (Carassa 1992); however, there were no further details about the severity of the glaucoma. This study included only 10 participants; post‐LTP IOP spikes occurred only in the placebo‐treated group and did not result in worse vision. Vision or visual field loss due to IOP spikes in people with less severe glaucoma may not be immediately noticeable by the person, and may have been overlooked or not evaluated in the trials included in this review. Ocular adverse effects were noted, including conjunctival blanching and eyelid retraction, which are known temporary adverse effects of some of the glaucoma medications.
Overall completeness and applicability of evidence
The most important outcome from this review was that perioperative glaucoma medications do help to reduce the risk of acute IOP spikes from LTP. The evidence appears the strongest for the alpha‐2 agonists, brimonidine and apraclonidine; however, comparisons for other drugs were limited. Due to the small number of eligible trials, the small sample sizes of many of the trials, and the multiple medications studied, we could not draw conclusions about other classes of glaucoma medications. We also could not assess the impact that a number of other factors may have on post‐LTP IOP evaluations, including the timing of administering the glaucoma medications, the location of laser burn spots, and the amount of energy delivered during LTP (Robin 1988). Most of this information was not reported in the studies we reviewed. All studies in the review reported the degree of the angle treated by LTP (180° versus 360°), but there were too few studies in each comparison to perform subgroup analyses. One study reported that LTP of a heavily pigmented trabecular meshwork was more likely to induce post‐laser IOP spikes (Robin 1988). We were unable to assess this outcome because the degree of pigmentation of the trabecular meshwork was either not provided or not well defined in the studies included in this review. Although argon LTP was the primary type of LTP performed in these RCTs, the results should be generalizable to IOP spikes experienced with other types of LTP, such as SLT. The types of glaucomas treated by LTP in this review appear to be a good sampling of what is included within OAGs (American Academy of Ophthalmology 2015). Caution should be exercised when applying the evidence to people with advanced glaucoma, in whom even mild IOP spikes may not be tolerated, as perioperative glaucoma drops reduce but do not eliminate acute IOP spikes from LTP. Even though many of these studies were conducted in the 1980s and 1990s, these medications are still in use to treat postoperative IOP spikes from LTP. Because LTP continues to have an important role in lowering IOP in people with OAG, (Samples 2011), the results of this review are clinically relevant. In the included trials, there was a lack of patient‐reported outcomes and outcomes related to disease progression and visual loss. This may be due to the fact that the focus of the trials in this group of studies was to establish the IOP‐lowering effects of the medications or because of the relatively short follow‐up time periods set by the study investigators.
Certainty of the evidence
The certainty of the evidence for the outcomes we were able to estimate was graded mainly as moderate or low. Our judgments regarding certainty of estimates for individual outcomes must be tempered by the lack of independence among the outcomes analyzed. Six of the 10 outcomes considered were based on comparison of pre‐ and post‐LTP measurements of IOP. Many of the outcome estimates were downgraded for high or uncertain risk of bias; several studies had issues with masking of outcomes assessors. In one study that contributed data for several of our analyses, participants were not masked due to the design of the study. Downgrades due to risk of bias also were frequently required due to inclusion of data from studies in which the authors were employees of, or had a relationship with, the manufacturer of at least one of the study drugs. Additionally, some studies had a high risk of reporting bias. For some of the outcomes, there was substantial statistical heterogeneity that precluded performing a meta‐analysis, but we graded the quality of the evidence and downgraded certainty due to inconsistency of the estimated effects from individual studies. We downgraded a few studies due to imprecision. There were very few instances of IOP increases 10 mmHg or greater in the medication versus placebo comparison and very wide CIs in the analyses of IOP increase of 5 mmHg or greater within two hours in the apraclonidine versus pilocarpine comparison and of IOP increase of 10 mmHg or greater between two and 24 hours outcome in the timing of medication administration comparison.
Potential biases in the review process
There may be some inherent biases in the trials that may limit the strengths of our conclusions. Previous studies have reported mixed evidence of efficacy for timolol, acetazolamide and pilocarpine as prophylaxis for IOP spike after LTP (Robin 1988). One rationale is that people who are already on these medications may be at the top of the dose response curve for that particular medication, and additional drops would not enhance the effect (Robin 1988). Several of the trials excluded people who were previously on a class of ocular or systemic medications similar to the glaucoma medication in question. Excluding such people likely would enhance the IOP‐lowering estimates; thus, our findings and estimates may not apply to people who are already on multiple glaucoma medications. Known ocular side effects of the tested medications, such as conjunctival blanching, eyelid retraction, and pupillary miosis, are difficult to mask, and may cause detection bias. More importantly, there may be some reporting biases because several of the studies had financial or editorial support from industry sponsors who made the drugs used in the trials. An example of likely selective reporting bias is David 1993; the study report lists three authors who are employees of the industry sponsor. Participants who had an "unacceptably high" IOP elevation were treated, removed from the study, and their data were excluded from the final analysis. In another industry‐sponsored trial of dorozolamide, baseline characteristics, attrition of study subjects, and 24 hour IOP measurements were not reported although these data were collected according to the methods section (Hartenbaum 1999).
Agreements and disagreements with other studies or reviews
To date, no other similar meta‐analysis evaluating perioperative glaucoma drops for preventing or ameliorating IOP spikes associated with LTP has been published. The largest study on the subject combined the results of two RCTs (Barnebey 1993; David 1993), which had 471 participants undergoing ALT in one eye randomly assigned to receive brimonidine or vehicle (or both) before and after ALT according to one of four treatment regimens (The Brimonidine‐ALT Study Group). Their conclusion that brimonidine was effective in reducing IOP spikes after ALT was congruent with our observations, which is not surprising since the two studies were included in our review. Data from participants of these two studies made up the largest participant samples and contributed a notable portion of the data in some of our meta‐analyses; therefore, these studies had a large effect on our review conclusions. It is important to note that reports from both studies included authors who worked for the makers of brimonidine. Wong and colleagues performed a systematic review and meta‐analysis on the efficacy of SLT in OAG with a descriptive review of adverse events associated with SLT (Wong 2015). The prevalence of transient IOP rise was 0% to 62%, and the statistic dropped to 0% to 29% when participants were treated with prophylactic/empirical treatment. The definition of IOP spike ranged from 1 mmHg to 10 mmHg one to two hours post‐laser, to IOP greater than 30 mmHg or a 30% increase within four weeks. No additional information was given about which medication was administered prophylactically. There are other reports on the IOP‐lowering effects of LTP that provided narrative reviews of the prevalence and treatment of perioperative IOP spikes. One review concluded that apraclonidine or brimonidine before and immediately after SLT and ALT can be used to prevent IOP spikes based on what was used in seven of the RCTs evaluated for effectiveness of SLT in lowering IOP (De Keyser 2017). Another review remarked that there were early postoperative IOP spikes in some participants regardless of whether the participants received perioperative antihypertensive treatment (brimonidine 2%, apraclonidine 0.5% or 1%, pilocarpine 1%) (Barkana 2007).
Authors' conclusions
Implications for practice.
Perioperative medications are superior to no medication or placebo to prevent intraocular pressure (IOP) spikes during the first two hours and up to 24 hours after laser trabeculoplasty (LTP). Apraclonidine or brimonidine given in the perioperative period leads to a greater reduction in IOP than no medication or placebo. There is insufficient evidence to draw conclusions regarding the relative efficacy of other antiglaucoma medications. A cautionary note regarding alpha‐2 agonists is that these medications can cause conjunctival blanching and eyelid retraction, adverse events that are temporary and not usually substantially problematic, but they do not occur when no medication is used. Alpha‐2 agonists are useful in helping to prevent IOP increases after LTP, but it is unknown whether one medication in this class of drugs is better than the other. The available evidence was sufficient to contrast the effect of apraclonidine versus brimonidine in curbing IOP spikes. These two alpha‐2 agonists show little difference in their effects on IOP. We found that there was no notable difference between apraclonidine and pilocarpine in the outcomes we were able to assess (mean change in IOP, and IOP increase of 10 mmHg or greater); however, only three studies reported on this comparison and the outcomes that were judged with moderate‐ and low‐certainty could be impacted by further research.
Implications for research.
Future research on this topic could be with participants who have been using these antiglaucoma medications for daily treatment of glaucoma before having LTP to see whether there is a difference in their response to the medication given perioperatively. Such participants may not have a noticeable reduction of IOP after using the same medication regularly. Studies conducted and reported by investigators who are free of conflicts of interest relating to the treatments being evaluated are preferred. As SLT is more commonly used than ALT in current practice, future studies on participants receiving SLT surgery would be most relevant.
Acknowledgements
We acknowledge the Cochrane Eyes and Vision (CEV) US Project for providing methodological support and for developing and conducting the search strategies. We also thank Barbara Hawkins, Stefano Miglior, and Augusto Azuara‐Blanco for comments to the review drafts.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Trabeculectomy] explode all trees #2 (Trabeculectom* or Trabeculoplast* or Trabeculotom* or Goniotom* or Microtrabeculectom*) #3 (trabeculopuncture or "trabeculo puncture" or "trabecular puncture" or goniopuncture) #4 MeSH descriptor: [Trabecular Meshwork] explode all trees and with qualifiers: [Surgery ‐ SU] #5 "Trabecular meshwork" #6 MeSH descriptor: [Glaucoma] explode all trees and with qualifiers: [Surgery ‐ SU] #7 Glaucoma* near/5 (surg* or filter* or filtrat*) #8 MeSH descriptor: [Filtering Surgery] explode all trees #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 MeSH descriptor: [Lasers] explode all trees #11 (Laser* or LTP or ALT or SLT or MDLT or YLT) #12 (Neodymium YAG or Nd YAG or Neodymium*YAG or Nd*YAG) #13 MeSH descriptor: [Laser Therapy] explode all trees #14 #10 or #11 or #12 or #13 #15 #9 and #14
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Trabeculectomy/ 13. (Trabeculectom* or Trabeculoplast* or Trabeculotom* or Goniotom* or Microtrabeculectom*).tw. 14. (trabeculopuncture or trabeculo puncture or trabecular puncture or goniopuncture).tw. 15. Trabecular Meshwork/su 16. Trabecular meshwork.tw. 17. exp Glaucoma/su 18. (Glaucoma* adj5 (surg* or filter* or filtrat*)).tw. 19. exp filtering surgery/ 20. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. exp Lasers/ 22. (Laser* or LTP or ALT or SLT or MDLT or YLT).tw. 23. (Neodymium YAG or Nd YAG or Neodymium*YAG or Nd*YAG).tw. 24. exp Laser Therapy/ 25. 21 or 22 or 23 or 24 26. 11 and 20 and 25
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'trabeculectomy'/exp #34 trabeculectom*:ab,ti OR trabeculoplast*:ab,ti OR trabeculotom*:ab,ti OR goniotom*:ab,ti OR microtrabeculectom*:ab,ti #35 trabeculopuncture:ab,ti OR 'trabeculo puncture' OR' trabecular puncture' OR goniopuncture:ab,ti #36 'trabecular meshwork'/exp #37 'trabecular meshwork':ab,ti #38 'glaucoma surgery'/exp #39 (glaucoma* NEAR/5 (surg* OR filter* OR filtrat*)):ab,ti #40 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 #41 'laser'/exp #42 laser*:ab,ti OR ltp:ab,ti OR alt:ab,ti OR slt:ab,ti OR mdlt:ab,ti OR ylt:ab,ti #43 "neodymium yag" OR "nd yag":ab,ti OR neodymium*yag:ab,ti OR nd*yag:ab,ti #44 'low level laser therapy'/exp #45 #41 OR #42 OR #43 OR #44 #46 #32 AND #40 AND #45
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 (Trabeculectom*[tiab] OR Trabeculoplast*[tiab] OR Trabeculotom*[tiab] OR Goniotom*[tiab] OR Microtrabeculectom*[tiab]) NOT Medline[sb] #3 (trabeculopuncture[tiab] OR trabeculo puncture[tiab] OR trabecular puncture[tiab] OR goniopuncture[tiab]) NOT Medline[sb] #4 Trabecular meshwork[tiab] NOT Medline[sb] #5 (Glaucoma*[tiab] AND (surge*[tiab] OR surgi*[tiab] OR filter*[tiab] OR filtrat*[tiab])) NOT Medline[sb] #6 #2 OR #3 OR #4 OR #5 #7 (Laser*[tiab] OR LTP[tiab] OR ALT[tiab] OR SLT[tiab] OR MDLT[tiab] OR YLT[tiab]) NOT Medline[sb] #8 (Neodymium YAG[tiab] OR Nd YAG[tiab] OR Neodymium:YAG[tiab] OR Nd:YAG[tiab]) NOT Medline[sb] #9 #7 OR #8 #10 #1 AND #6 AND #9
Appendix 5. LILACS search strategy
(Trabeculectom$ or MH:E04.540.450.700$ or Trabeculoplast$ or Trabeculotom$ or Goniotom$ or Microtrabeculectom$ or trabeculopuncture or "trabeculo puncture" or "trabecular puncture" or goniopuncture or "Trabecular Meshwork" or "Malla Trabecular" or "Malha Trabecular" or MH:A09.371.060.932$ or "Filtering Surgery" or "Cirugía Filtrante" or "Cirurgia Filtrante" or MH:E04.540.450$ or glaucoma/sugery or glaucoma/cirugía or glaucoma/cirurgia or (glaucoma$ and (surg$ or filter$ or filtrate$))) and (Laser$ or Láser or MH:E07.632.490$ or MH:E07.710.520$ or MH:SP4.011.087.698.384.075.166.027$ or MH:VS2.006.002.009$ or LTP or ALT or SLT or MDLT or YLT or "Neodymium YAG" or "Nd YAG" or NeodymiumYAG or NdYAG or MH:E02.594$ or MH:E04.014.520$)
Appendix 6. metaRegister of Controlled Trials search strategy
(Trabeculectomy or Trabeculoplasty or Trabeculotomy or Goniotomy or "Trabecular meshwork" or Glaucoma) and (Laser or Lasers)
Appendix 7. ClinicalTrials.gov search strategy
(Trabeculectomy OR Trabeculoplasty OR Trabeculotomy OR Goniotomy OR "Trabecular meshwork" OR Glaucoma) AND (Laser)
Appendix 8. ICTRP search strategy
Trabeculectomy AND Laser OR Trabeculoplasty AND Laser OR Trabeculotomy AND Laser OR Goniotomy AND Laser OR "Trabecular meshwork" AND Laser OR Glaucoma AND Laser
Data and analyses
Comparison 1. Medication versus placebo (regardless of timing).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Alpha‐2 agonists vs placebo | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 IOP increase of ≥ 10 mmHg within 2 hours after LTP | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.20] |
| 2.1 Acetazolamide vs placebo | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.52] |
| 2.2 Alpha‐2 agonists vs placebo | 3 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.27] |
| 3 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Apraclonidine vs placebo (mmHg) | 4 | 151 | Mean Difference (Random, 95% CI) | ‐7.43 [‐10.60, ‐4.27] |
| 4 IOP increase of ≥ 5 mmHg two to 24 hours after LTP | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Alpha‐2 agonists vs placebo | 5 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.09, 0.31] |
| 5 IOP elevation of ≥ 10 mmHg two to 24 hours after LTP | 9 | 817 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.42] |
| 5.1 Alpha‐2 agonists vs placebo | 7 | 727 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.50] |
| 5.2 Dorzolamide vs placebo | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.01, 5.22] |
| 5.3 Pilocarpine vs no treatment | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.07, 0.71] |
| 6 Mean change in IOP from pre‐LTP to measurements two to 24 hours after LTP | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 6.1 Apraclonidine vs placebo (mmHg) | 4 | 151 | Mean Difference (Random, 95% CI) | ‐5.32 [‐7.37, ‐3.28] |
Comparison 2. Medication versus medication: brimonidine versus apraclonidine (regardless of timing).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg within 2 hours after laser trabeculoplasty (LTP) | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [0.32, 16.03] |
| 2 Mean change in IOP from pre‐LTP to measurements taken within 2 hours after LTP (mmHg) | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐2.56, 1.17] |
Comparison 3. Medication versus medication: apraclonidine versus pilocarpine (regardless of timing).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in intraocular pressure (IOP) from pre‐laser trabeculoplasty (LTP) to measurements taken within 2 hours after LTP (mmHg) | 2 | 277 | Mean Difference (Random, 95% CI) | 0.61 [‐0.44, 1.66] |
| 2 IOP increase of ≥ 5 mmHg two to 24 hours after LTP | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 IOP increase of ≥ 10 mmHg two to 24 hours after LTP | 2 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.14, 5.63] |
| 4 Mean change in IOP from pre‐LTP to measurements taken two to 24 hours after LTP (mmHg) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only |
Comparison 4. Timing comparison: medication before versus medication after.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intraocular pressure (IOP) increase of ≥ 5 mmHg two to 24 hours after laser trabeculoplasty (LTP) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Alpha‐2 agonists | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.25, 2.63] |
| 2 IOP increase of ≥ 10 mmHg two to 24 hours after LTP | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Alpha‐2 agonists | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.19, 12.43] |
| 3 Mean change in IOP from pre‐LTP to measurements taken more than 2 hours but within 24 hours after LTP (mmHg) | 3 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Alpha‐2 agonists (mmHg) | 3 | 198 | Mean Difference (Random, 95% CI) | ‐1.07 [‐2.51, 0.37] |
Comparison 5. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Conjunctival blanching (brimonidine vs placebo) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barnebey 1993.
| Methods |
Study design: vehicle‐controlled, double‐masked, multicenter, parallel‐group RCT Country: US Number randomized: Total: 232 Per group: brimonidine/brimonidine = 62, brimonidine/vehicle = 57, vehicle/brimonidine = 53, vehicle/vehicle = 60 Exclusions after randomization: none reported Number analyzed: Total: 232 Per group: brimonidine/brimonidine = 62, brimonidine/vehicle = 57, vehicle/brimonidine = 53, vehicle/vehicle = 60 Unit of analysis (participants vs eyes): eye Losses to follow‐up: 10 eyes had unacceptably high IOPs within the first 3 hours after surgery (brimonidine/brimonidine = 1, brimonidine/vehicle = 1, vehicle/vehicle = 8). These participants were released from the study and treated at the discretion of the investigator. How was missing data handled?: data from the released participants included in the analysis Reported power calculation: power calculation not reported but the authors stated, "this study had an 80% success rate in detecting a difference between treatments in the incidence of IOP elevation of approximately 21%." Unusual study design (any issues with study design)?: no |
|
| Participants |
Age (mean ± SD; years): brimonidine/brimonidine = 69.3 ± 1.4, brimonidine/vehicle = 63.9 ± 1.8, vehicle/brimonidine = 66.8 ± 1.5, vehicle/vehicle = 66.9 ± 1.4 Females: brimonidine/brimonidine = 47%, brimonidine/vehicle = 58%, vehicle/brimonidine = 58%, vehicle/vehicle = 53% Inclusion criteria: people with uncontrolled glaucoma whose IOPs were inadequately controlled despite maximal tolerated medication, and in whom 360° ALP was indicated Exclusion criteria: people with active ocular infection or inflammation, contraindications to alpha‐agonist treatment or hyposensitivity to alpha‐agonists or other components of the formulation, women of childbearing potential or who were nursing, people taking topical or systemic alpha‐agonists 2 weeks prior to study entry or who took systemic clonidine 4 weeks before study entry Equivalence of baseline characteristics: yes, "No significant differences were noted between treatments or sites in demographic data." |
|
| Interventions |
Intervention 1: brimonidine 0.5%, 30 to 45 min before and immediately after ALT Intervention 2: brimonidine 0.5%, 30 to 45 min before but vehicle immediately after ALT Intervention 3: vehicle, 30 to 45 min before but brimonidine 0.5% immediately after ALT Intervention 4: vehicle, 30 to 45 min before and immediately after ALT Length of follow‐up: Planned: 1, 2, and 3 hours, 1 to 2, and 4 to 6 weeks after ALT Actual: 1, 2, and 3 hours, 1 to 2, and 4 to 6 weeks after ALT |
|
| Outcomes |
Primary outcome: mean IOP Secondary outcomes: mean IOP lowering in contralateral eye, mean systolic BP after treatment, mean heart rate after treatment Adverse events reported: yes Intervals at which outcomes assessed: hourly for 3 hours; 1 to 2 weeks, and 4 to 6 weeks |
|
| Notes |
Trial registration: not reported Funding sources: not reported Disclosures of interest: 3 authors were employees of Allergan Pharmaceuticals, who make Alphagan, a brimonidine tartrate ophthalmic solution Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were assigned to one of four treatment groups in a randomized, double‐masked fashion..." States randomization was done but not the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | "Patients were assigned to one of four treatment groups in a randomized, double‐masked fashion..." Authors reported that the study was double‐masked, but did not say who was masked: participants, surgeons, or outcome assessors. |
| Masking of outcome assessment (detection bias) | High risk | No information provided about outcome assessors, but lid retraction and conjunctival blanching is a known adverse effect of brimonidine, which would have been obvious to a clinician at the time of IOP measurements at post‐ALT checkpoints as to whether the vehicle or study medication was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants with "unacceptably high IOPs" at 3 hours were released from further study participation but data from these participants was still included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | High risk | 3 of the authors were employees of Allergan, the company that manufactures brimonidine. |
Barnes 1999.
| Methods |
Study design: parallel‐group RCT Country: US Number randomized: Total: 56 eyes of 41 participants Per group: brimonidine = 29 eyes, apraclonidine = 27 eyes Exclusions after randomization: 10/15 participants who required bilateral ALT had randomization to receive the identical medication for each eye, and for these participants, only the first eye was included in the study. Number analyzed: Total: 46 eyes of 41 participants Per group: brimonidine = 23 eyes, apraclonidine = 23 eyes Unit of analysis (participants vs eyes): eyes Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: yes, power of 80% Unusual study design (any issues with study design)?: the unit of measurement was the eye and not the participant. The second eye was treated 2 to 6 weeks after the first. |
|
| Participants |
Age (mean; years): brimonidine = 69.9, apraclonidine = 62.4 Females: brimonidine = 48%; apraclonidine = 30% Inclusion criteria: aged ≥ 21 years with diagnosis of POAG, pigmentary glaucoma, pseudoexfoliation syndrome, or ocular hypertension. Participants had IOP too high for their level of optic nerve cupping, no glaucoma medications were used 12 to 24 hours before ALT Exclusion criteria: people with active ocular inflammation, contraindications to treatment with alpha‐agonists, or known hypersensitivities to alpha‐agonists, women of childbearing potential, current use of either of the study medications, previous experience with ALT Equivalence of baseline characteristics: no statistical difference in the baseline IOP levels, number of laser applications, or energy level used in either group. Statistically significant difference in mean age (P = 0.008) of each group and the distribution of gender in each group; however, these were likely not clinically significant differences. |
|
| Interventions |
Intervention 1: brimonidine 0.2%, 30 to 45 min before and immediately after 360° ALT Intervention 2: apraclonidine 1.0%, 30 to 45 min before and immediately after 360° ALT Length of follow‐up: Planned: 4 hours after surgery Actual: 4 hours after surgery |
|
| Outcomes |
Primary outcome: maximum IOP change (from baseline to the highest postoperative IOP) Secondary outcomes: none reported Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 2, and 4 hours after ALT |
|
| Notes |
Trial registration: not reported Funding sources: research grant provided by Allergan, Inc Disclosures of interest: "The authors have no proprietary interest in the products described in this study." Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random‐number generator assigned patients to a treatment group before ALT." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Low risk | "Both patient and physician were masked as to which agent the patient received" "…and a technician would give the appropriate medication without the physician or patients' knowledge." |
| Masking of outcome assessment (detection bias) | Unclear risk | Details about outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some participants' randomization caused them to receive the same medication for each eye, so only the first eye was included in the study to account for the intra‐dependability of eyes and to prevent skewed results. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | High risk | Study reported a research grant provided by Allergan, Inc, which makes the brimonidine 0.2% ophthalmic solution. |
Birt 1995.
| Methods |
Study design: parallel‐group RCT Country: US Number randomized: Total: 72 Per group: 2 doses apraclonidine = 24, 1 dose apraclonidine before surgery = 24, 1 dose apraclonidine after surgery = 24 Exclusions after randomization: none Number analyzed: Total: 72 Per group: 2 doses apraclonidine = 24, 1 dose apraclonidine before surgery = 24, 1 dose apraclonidine after surgery = 24 Unit of analysis (participants vs eyes): participants Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design (any issues with study design)?: potentially not double‐masked, as there was no mention of a vehicle drop given, so participant could have known which group he or she was in based on when the drops were given. |
|
| Participants |
Age (mean ± SD; years): 2 doses apraclonidine = 70.4 ± 10.6, 1 dose apraclonidine before surgery = 69.3 ± 10.9, 1 dose after surgery = 69.2 ± 9.6 Females: 2 doses apraclonidine = 75%, 1 dose apraclonidine before surgery = 75%, 1 dose apraclonidine after surgery = 54% Inclusion criteria: POAG, including an elevated IOP in the setting of either characteristic glaucomatous optic nerve damage on stereoscopic biomicroscopic exam or glaucomatous visual field defects on Humphrey automated field testing, or both Exclusion criteria: previous treatment over 360° of the angle, unable to return for the 24‐hour IOP check Equivalence of baseline characteristics: yes, no significant difference between the groups in the mean power setting used or in the mean number of burns, or the mean IOP at baseline and between‐group differences were not significant for race, gender, vision, age, and number of medications |
|
| Interventions |
Intervention 1: 2 doses apraclonidine 1.0%, 15 min before and immediately after the laser procedure Intervention 2: 1 dose apraclonidine 1.0%, 15 min before the laser procedure Intervention 3: 1 dose apraclonidine 1.0%, immediately after the laser procedure Length of follow‐up: Planned: 24 hours Actual: 24 hours |
|
| Outcomes |
Primary outcomes: IOP at 1 hour and 24 hours after surgery Secondary outcomes: none reported Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 24 hours |
|
| Notes |
Trial registration: not reported Funding sources: none reported Disclosures of interest: not reported Study period: 1 September to 30 November 1994 Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly assigned with the use of a random number table to one of three treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Personnel appear to be masked, "…a second investigator (D.H.S.), who was unaware of the group, assignment, performed the laser treatment…"; however, the report did not mention a vehicle drop given, so the participant could have known to which group (before or after surgery or both) they were assigned. |
| Masking of outcome assessment (detection bias) | Low risk | "…a third investigator (B.M.), who was also unaware of group assignment, measured the IOP." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Low risk | None |
Brown 1988.
| Methods |
Study design: parallel‐group RCT Country: US Number randomized: Total: 169 undergoing 1 of 3 surgeries (trabeculoplasty, iridotomy, or capsulotomy) Per group: not reported for ALT alone; for full study population apraclonidine = 85, placebo = 84 Exclusions after randomization: 4/169 could not be evaluated due to loss to follow‐up or refusal of treatment medication (apraclonidine = 2, placebo = 2) Number analyzed (total and per group): Total: 83 who underwent trabeculoplasty (out of 165 for all lasers) Per group: apraclonidine = 41, placebo = 42 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: apraclonidine = 1 (type of surgery not given) refused to wait for the follow‐up exam How was missing data handled?: authors only analyzed the 164 who had all outcomes collected Reported power calculation: no Unusual study design (any issues with study design)?: randomization was to 3 types of lasers, but for this review we used only data from the trabeculoplasty group). |
|
| Participants |
Age: not reported for ALT alone, overall cohort (mean ± SD; years): apraclonidine = 64 ± 15, placebo = 65 ± 13 Females: not reported for ALT alone, overall cohort: apraclonidine = 61%, placebo = 55% Inclusion criteria: people who were about to undergo trabeculoplasty, iridotomy, or capsulotomy*, with inadequately controlled IOP despite maximum‐tolerated medical therapy, receiving 360° of angle treatment Exclusion criteria: active ocular infection or inflammation, unstable cardiovascular disease, any abnormality preventing reliable applanation tonometry, pregnant or nursing women, women of childbearing potential, participation in any other study within the past 30 days, people with vision in 1 eye only, people taking systemic clonidine, and people whose fellow eye had been enrolled previously in the study Equivalence of baseline characteristics: yes, there were no significant differences between treatment groups with respect to demographic characteristics (P < 0.05), baseline visual acuity, preoperative IOP, pulse rate, history of glaucoma or prior surgery, or number/type of antiglaucoma medications being taken at the time of laser surgery |
|
| Interventions |
Intervention 1: 1 drop apraclonidine (para‐amino‐clonidine(PAC)) 1% before surgery and 1 drop after surgery Intervention 2: 1 drop placebo before surgery and 1 drop after surgery Length of follow‐up: Planned: 1 week Actual: 1 week |
|
| Outcomes |
Primary outcome: control of IOP in the first 3 postoperative hours after laser surgery Secondary outcomes: pulse rate, diastolic BP, systolic BP Adverse events reported: yes Intervals at which outcomes assessed: 45 min; 1, 2, 3 hours; 1 week |
|
| Notes |
Trial registration: not reported Funding sources: supported in party by an unrestricted research grant from Research to Prevent Blindness, Inc Disclosures of interest: not reported, but 1 author worked for Alcon and the study used an Alcon product Study period: not reported Reported subgroup analyses: yes, by type of surgery *Study included trabeculoplasty, peripheral iridotomy, and capsulotomy, but we used only the trabeculoplasty results |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were assigned to either the 1% ALO 2145 [apraclonidine] or placebo groups by a randomized treatment code." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Authors reported that the study was double‐masked, but did not say who was masked: participants, surgeons, or outcome assessors. |
| Masking of outcome assessment (detection bias) | High risk | Masking of outcome assessors was not reported, but apraclonidine has ocular effects which are difficult to mask such as conjunctival blanching and upper eyelid elevation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants who were randomized were not included in the analyses but that was due to refusal to wait for follow‐up measures or refusal of study drugs, and therefore unlikely to be due to the study drugs themselves. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Low risk | None |
Carassa 1992.
| Methods |
Study design: parallel‐group RCT Country: Italy Number randomized: Total: 30 total, 10 who underwent trabeculoplasty Per group: within the trabeculoplasty group, apraclonidine = 5, placebo = 5 Exclusions after randomization: none Number analyzed: Total: 30 total, 10 who underwent trabeculoplasty Per group: within the trabeculoplasty group, apraclonidine = 5, placebo = 5 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design (any issues with study design)?: baseline demographics and other characteristics not reported |
|
| Participants |
Age: not reported Females: not reported Inclusion criteria: aged ≥ 18 years, scheduled for LTP, iridotomy, or posterior capsulotomy Exclusion criteria: active ocular infection or inflammation, past or present severe ocular disease (except cataract and glaucoma, unstable cardiovascular disease, any abnormality preventing reliable applanation tonometry, pregnancy (actual or potential) or breastfeeding, 1 single seeing eye, treatment systemic clonidine, previous enrollment of the fellow eye in the study Equivalence of baseline characteristics: baseline characteristics not reported |
|
| Interventions |
Intervention 1: 1 drop apraclonidine 1%, 1 hour prior and 1 drop immediately after 360° ALT surgery Intervention 2: 1 drop placebo, 1 hour prior and 1 drop immediately after 360° ALT surgery Length of follow‐up: Planned: 1 week Actual: only up to 3 hours was reported |
|
| Outcomes |
Primary outcomes: mean IOP and IOP changes during the postoperative period, maximum IOP increases from baseline, IOP increase of 5 mmHg and 10 mmHg from baseline Secondary outcomes: heart rate, BP Adverse events reported: yes Intervals at which outcomes assessed: baseline; 1, 2, 3 hours |
|
| Notes |
Trial registration: not reported Funding sources: none reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: yes, subgroups were different laser procedures |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study was designed as a prospective, randomized, double‐masked, and placebo‐controlled trial." States randomization was done but not the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Authors reported that the study was double‐masked, but did not say who was masked: participants, surgeons, or outcome assessors. |
| Masking of outcome assessment (detection bias) | High risk | Masking of outcome assessors not reported; however, apraclonidine can cause conjunctival blanching and eyelid raising, which would have been visible to the person assessing IOP after the procedure. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | High risk | In the methods, the authors stated that, "Ocular examination, heart rate count and blood pressure measurement were repeated hourly during the first three postoperative hours and again one week post operatively;" however, no data were presented from the 1‐week assessments. |
| Other bias | High risk | Funding sources not reported, very small sample sizes within the different surgeries do not allow for statistical analyses for each surgery alone; authors stated, "Due to the low numbers of cases in each series, individual statistical analyses for each of the 3 series were considered inappropriate." |
Chevrier 1999.
| Methods |
Study design: parallel‐group RCT Country: Canada Number randomized: Total: all laser treatments = 85, trabeculoplasty = 51 Per group: all laser treatments, brimonidine = 43, apraclonidine = 42; trabeculoplasty, brimonidine = 27, apraclonidine = 24 Exclusions after randomization: none reported Number analyzed: Total: all laser treatments = 85; trabeculoplasty = 51 Per group: all laser treatments, brimonidine = 43, apraclonidine = 42; trabeculoplasty, brimonidine = 27, apraclonidine = 24 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design (any issues with study design)?: none |
|
| Participants |
Age (mean ± SD; years): (only available for all laser treatments): brimonidine = 70 ± 12.0, apraclonidine = 67.3 ± 13.7 Females: (only available for all laser treatments): brimonidine = 67%, apraclonidine = 45% Inclusion criteria: medically uncontrolled IOP, any type of glaucoma, initial or repeat ALTs 180° to any quadrant Exclusion criteria: chronic topical alpha‐2 agonist therapy, use of topical alpha‐2 agonist within the past 2 weeks, active ocular infection or inflammation, abnormality precluding reliable applanation tonometry, unable to stay for the 1‐hour follow‐up IOP check Equivalence of baseline characteristics: no, "No significant difference were found among treatment groups in terms of age, race, or baseline IOP…There was a statistically significant difference in the gender distribution between groups…" Also noted that uneven distribution of pseudoexfoliation pigmentary glaucoma/mixed glaucoma may affect post laser IOP spike numbers |
|
| Interventions |
Intervention 1: 1 drop apraclonidine hydrochloride 0.5%, 10 min prior to laser surgery Intervention 2: 1 drop brimonidine tartrate 0.2%, 10 min prior to laser surgery Length of follow‐up: Planned: 1 hour Actual: 1 hour |
|
| Outcomes |
Primary outcome: IOP Secondary outcomes: mean IOP change, IOP elevation ≥ 5 mmHg change from baseline Adverse events reported: yes, reported no systemic or localized ocular reactions and no other adverse effects Intervals at which outcomes assessed: baseline; 1 hour after surgery |
|
| Notes |
Trial registration: not reported Funding sources: not reported Disclosures of interest: "The authors hold no proprietary interest in the drugs used in this study." Study period: January 1998 to May 1998 Reported subgroup analyses: yes, subgroups were different types of anterior segment laser procedures |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | Low risk | IOP analysis performed by the same masked observer at 1 hour post laser, every effort was made to use the same tonometer as prior to surgery. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up; all participants who were randomized completed all assessments. Exclusion criteria was for people who could stay for 1 hour post laser surgery, so there was no attrition. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported. Also, this study reported subgroup analysis for the ALT group but did not report baseline demographics by subgroup. |
Dapling 1994.
| Methods |
Study design: parallel‐group RCT Country: England Number randomized: Total: 75 eyes Per group: apraclonidine = 26 eyes, pilocarpine = 23 eyes, apraclonidine/pilocarpine = 26 eyes Exclusions after randomization: none reported Number analyzed: Total: 75 eyes Per group: apraclonidine = 26 eyes, pilocarpine = 23 eyes, apraclonidine/pilocarpine = 26 eyes Unit of analysis: eyes, if both eyes required LTP, then the first eye to be treated was entered into the study. Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design (any issues with study design)?: none |
|
| Participants |
Age (mean (range); years): apraclonidine = 72.2 (53 to 84), pilocarpine = 68.4 (53 to 86), apraclonidine/pilocarpine = 71.3 (46 to 87) Females: not reported Inclusion criteria: OAG with in IOP > 21 mmHg Exclusion criteria: regular pilocarpine to either eye, active ocular infection or inflammation present, unstable cardiovascular disease, taking systemic clonidine Equivalence of baseline characteristics: yes, "There was no statistically significant difference between the groups with respect to age, eye color, type of glaucoma, or glaucoma medication. All patients had similar disease as judged by single medication, duration of disease, and cumulative treatment." |
|
| Interventions |
Intervention 1: 1 drop apraclonidine 1% 1 hour before and 1 drop immediately after 180° ALT Intervention 2: 1 drop pilocarpine 4% immediately after 180° ALT Intervention 3: 1 drop of apraclonidine 1%, 1 hour before and 1 drop of apraclonidine 1%/1 drop of pilocarpine 4%, immediately after 180° ALT Length of follow‐up: Planned: 1 week Actual: 1 week |
|
| Outcomes |
Primary outcome: IOP Secondary outcomes: heart rate, BP Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 2, 3 hours; 1 week following trabeculoplasty |
|
| Notes |
Trial registration: not reported Funding sources: Alcon Laboratories in England supported the study Disclosures of interest: no disclosures reported Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were then randomly allocated to one of the three treatment groups." Did not state how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | High risk | Masking of participants and personnel not reported but due to the nature of the interventions, participants would know whether or not they got medication before or after the surgery and that they received 2 drops of medication if in the combination group. |
| Masking of outcome assessment (detection bias) | Low risk | "The observer was masked to the study group of the patient." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available for all participants who were randomized. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | High risk | Supported by Alcon Laboratories, which makes products containing apraclonidine and pilocarpine. Additionally, a difference drop regimen without use of vehicle drops would leave participants essentially unmasked. |
David 1993.
| Methods |
Study design: parallel‐group RCT Country: Israel, US Number randomized: Total: 248 eyes Per group: not reported Exclusions after randomization: 9 were removed from the statistical analysis (2 improperly entered into the study, 7 due to protocol violations) Number analyzed: Total: 239 eyes Per group: brimonidine/brimonidine = 60, brimonidine/vehicle = 62, vehicle/brimonidine = 61, vehicle/vehicle = 56 Unit of analysis: eyes, 1 eye per participant Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design: none |
|
| Participants |
Age: not reported Females: not reported Inclusion criteria: aged ≥ 21 years with useful vision in both eyes Exclusion criteria: prior glaucoma surgery or intraocular surgery Equivalence of baseline characteristics: "The groups were similar with regard to the type of pressure‐lowering medications that the patients were receiving prior to enrollment in the study." "The four groups were similar with respect to demographics and iris color." |
|
| Interventions |
Intervention 1: brimonidine 0.5%, 30 to 45 min before and after 360° ALT Intervention 2: brimonidine 0.5%, 30 to 45 min before 360° ALT and vehicle after Intervention 3: vehicle, 30 to 45 min before and brimonidine 0.5% after ALT Intervention 4: vehicle, 30 to 45 min before and after ALT Length of follow‐up: Planned: 4 to 6 weeks Actual: 4 to 6 weeks |
|
| Outcomes |
Primary outcome: IOP Secondary outcomes: heart rate, BP Adverse events reported: yes Intervals at which outcomes assessed: baseline; 1, 2, 3 hours; 1 to 2 weeks after ALT; 4 to 6 weeks after ALT |
|
| Notes |
Type of study: published Funding sources: none reported Disclosures of interest: 3 authors were employees of Allergan Inc. The other authors had no proprietary interest in either Allergan Inc or its products. Study period: not reported Reported subgroup analyses: yes, some results were reported combining participants into those who had any brimonidine vs those in the vehicle‐only group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Description of randomization method not provided. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | High risk | Brimonidine can have ocular adverse effects of conjunctival blanching and lid retraction, which would be easy for outcome assessors to see even if they were masked. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Of the 248 patients enrolled in the study, nine were disqualified from the statistical analysis. Two subjects had been improperly entered into the study and seven were excluded due to study protocol violations." Participants with unacceptably high IOP elevations were treated and removed from the study, and not included in the final analysis, but they provided important information about which groups they initially belonged in and could alter the numbers reported of how effective brimonidine was in lowering IOP. |
| Selective reporting (reporting bias) | High risk | Participants with unacceptably high IOP elevations were treated and removed from the study, and not included in the final analysis, but they provided important information about which groups they initially belonged in and could alter the numbers reported of how effective brimonidine was in lowering IOP. |
| Other bias | High risk | 3 authors were employees of Allergan Inc, which produces ophthalmic drugs containing brimonidine. |
Donnelly 2006.
| Methods |
Study design: intra‐individual RCT Country: not reported Number randomized: Total: 20 eyes of 10 participants Per group: brimonidine = 10 eyes, apraclonidine = 10 eyes Exclusions after randomization: none reported Number analyzed: Total: 20 eyes of 10 participants Per group: brimonidine = 10 eyes, apraclonidine = 10 eyes Unit of analysis: eyes Losses to follow‐up: none reported How was missing data handled?: not reported Reported power calculation: no Unusual study design (any issues with study design)?: none |
|
| Participants |
Age: not reported Females: not reported Inclusion criteria: SLT for POAG on both eyes Exclusion criteria: not reported Equivalence of baseline characteristics: not reported |
|
| Interventions |
Intervention 1: brimonidine tartrate 0.15%, 1 hour prior to 360° LTP, 1 drop randomly assigned in the left or right eye Intervention 2: apraclonidine 0.5%, 1 hour prior to 360° LTP, 1 drop assigned in opposite eye of the brimonidine tartrate treatment Length of follow‐up: Planned: not reported Actual: 1 week |
|
| Outcomes |
Primary outcome: IOP Secondary outcomes: not reported Adverse events reported: yes, stated there were no non‐ocular clinically significant symptoms in either group Intervals at which outcomes assessed: baseline; 1 hour; 1 week post‐surgery |
|
| Notes |
Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | High risk | Both eyes of a participant were included in the study and received different medications; however, the authors did not report if and how they took into account the inter‐dependency of eyes within the same participant. |
Elsas 1991.
| Methods |
Study design: parallel‐group RCT Country: Norway Number randomized: Total: 50 Per group: pilocarpine pretreatment = 25, no pretreatment = 25 Exclusions after randomization: none Number analyzed: Total: 50 Per group: pilocarpine pretreatment = 25, no pretreatment = 25 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none reported How was missing data handled?: not reported Reported power calculation: no Unusual study design: none |
|
| Participants |
Age (mean ± SD; years): pilocarpine pretreatment = 69 ± 9.9, no treatment = 71.9 ± 7.1 Females: not reported Inclusion criteria: IOP ≥ 25 mmHg measured by applanation tonometry at the initial evaluation by 1 of the authors and just before laser treatment. The mean of these 2 was taken as prelaser IOP. Glaucomatous disk damage or visual field defects (or both), defined as cupping of the optic nerve head extending to the margin of the disc, a difference of vertical cup‐disk ratio of ≥ 0.2 between the 2 eyes, and different degrees of disk pallor in the 2 eyes with no other explanation. No earlier glaucoma treatment. Exclusion criteria: not reported Equivalence of baseline characteristics: yes, "There is no evidence of dissimilarities between the two groups." |
|
| Interventions |
Intervention 1: 2 drops pilocarpine 2%, 1 hour before LTP Intervention 2: no pretreatment Length of follow‐up: Planned: 6 months Actual: 6 months |
|
| Outcomes |
Primary outcome: IOP Secondary outcomes: number of participants with change in IOP > 10 mmHg or 20 mmHg, number of participants with peak IOP ≥ 50 mmHg Adverse events reported: no Intervals at which outcomes assessed: 1, 2, 4, 6, 8, 24 hours after treatment; 1 week; 1, 3, 6 months |
|
| Notes |
Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: September 1989 to December 1990 Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported participants were randomly assigned to a group but did not describe how randomization sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | High risk | "The study was not masked because the pilocarpine induced miosis was very obvious to the investigators." |
| Masking of outcome assessment (detection bias) | High risk | Study was not masked because of pilocarpine's induced miosis which was very obvious to the investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Low risk | IOP was the only outcome of interest and the focus of the paper; authors reported that, "visual field changes following LTP will be the subject of a separate study." |
| Other bias | Unclear risk | Funding sources not reported. |
Hartenbaum 1999.
| Methods |
Study design: parallel‐group, placebo controlled, RCT Country: US (multicenter) Number randomized: Total: 122 Per group: dorozolamide = 61, placebo = 61 Exclusions after randomization: none reported Number analyzed: Total: 122 Per group: dorozolamide = 61, placebo = 61; in ALT group: dorozolamide = 17, placebo = 23 Unit of analysis: participant (1 eye per person) Losses to follow‐up: none reported How was missing data handled?: missing data were imputed by carrying forward data from the previous time point Reported power calculation: yes, "With 60 patients per group, there was 90% power to detect such a difference at the P<0.05‐level (two‐sided)." |
|
| Participants |
Age: not reported Females: not reported Inclusion criteria: to have posterior capsular opacity requiring Nd:YAG laser capsulotomy, OAG requiring ALT or any condition requiring ALT or Nd:YAG laser iridotomy Exclusion criteria: ocular inflammation within the past 2 months, advanced visual field defects with risk of further loss if a spike in IOP were to occur, baseline IOP > 30 mmHg, use of corticosteroid, oral beta‐blocker, or oral carbonic anhydrase inhibitor therapy Equivalence of baseline characteristics: yes, "Baseline characteristics were similar in both treatment groups." |
|
| Interventions |
Intervention 1: 1 drop dorozolamide hydrochloride 2%, 1 hour before and 1 drop at the end of surgery Intervention 2: 1 drop placebo, 1 hour before and 1 drop immediately after Length of follow‐up: Planned: 24 hours Actual: 24 hours |
|
| Outcomes |
Primary outcome: percentage of participants with an increase in IOP from the baseline of ≥ 10 mmHg during the first 4 hours after surgery Secondary outcomes: heart rate, BP, incidence of adverse effects, ocular signs Adverse events reported: yes Intervals at which outcomes assessed: baseline; 1, 2, 3, 4, 24 hours |
|
| Notes |
Trial registration: not reported Funding sources: none reported Disclosures of interest: the Dorzolamide Laser Study Group was sponsored by pharmaceutical research corporation, and multiple authors work for Merck Research Laboratories, which makes dorozolamide. Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of how randomization sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Authors reported that the study was double‐masked, but did not say who was masked: participants, surgeons, or outcome assessors. |
| Masking of outcome assessment (detection bias) | Unclear risk | Authors reported that the study was double‐masked, but did not say who was masked: participants, surgeons, or outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data imputed by carrying forward data from the previous time point, but the authors did not specify how much attrition occurred. |
| Selective reporting (reporting bias) | High risk | Did not provide table of baseline characteristics. Did not discuss attrition of study participants, and did not report data at 24 hours despite mentioning it as part of methods section, no mention of specific ocular adverse events, and did not describe number of participants with systemic adverse effects other than to say there was no difference between groups. |
| Other bias | High risk | Merck is the maker of dorozolamide, and several authors were employees of Merck. |
Holmwood 1992.
| Methods |
Study design: parallel‐group RCT Country: US Number randomized: Total: 60 Per group: apraclonidine before and after = 30, apraclonidine only after = 30 Exclusions after randomization: not reported Number analyzed: Total: 60 Per group: apraclonidine before and after = 30, apraclonidine only after = 30 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no |
|
| Participants |
Age: not reported Females: not reported Inclusion criteria: people who had POAG, defined by optic disk cupping and visual field loss, and a pretreatment IOP > 21 mmHg on maximally tolerated medical therapy Exclusion criteria: previous intraocular surgical procedures or laser treatment, people who had secondary OAG (e.g. pigmentary, exfoliative, or uveitic), and aged < 40 years Equivalence of baseline characteristics: yes, "There were no statistical differences between preoperative IOP and the number of antiglaucoma medications between the two groups of patients." |
|
| Interventions |
Intervention 1: 1 drop apraclonidine 1%, 1 hour before and immediately after 360° LTP Intervention 2: 1 drop apraclonidine 1%, only after 360° LTP Length of follow‐up: Planned: 2 hours Actual: 2 hours |
|
| Outcomes |
Primary outcome: IOP Secondary outcomes: not reported Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 2 hours after treatment |
|
| Notes |
Trial registration: not reported Funding sources: "This study was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York." Disclosures of interest: none reported Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "One of the following two apraclonidine open‐label treatment regimens was determined from a random table chart…" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | High risk | Study was open‐label and no mention of vehicle drops, so participants would be aware whether they received drops before and after surgery or only after surgery. |
| Masking of outcome assessment (detection bias) | Low risk | "Intraocular pressure was measured one and two hours after treatment by an observer who was masked to the random assignment to treatment with apraclonidine." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Low risk | None |
Karlik 1997.
| Methods |
Study design: parallel‐group RCT Country: not reported Number randomized: Total: 37 Per group: apraclonidine = 21, latanoprost = 16 Exclusions after randomization: not reported Number analyzed: Total: not reported Per group: not reported Unit of analysis (participants vs eyes): participant Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: unclear whether participants had only 1 eye included or if they had both eyes included |
|
| Participants |
Age: not reported Females: not reported Inclusion criteria: people undergoing ALT for glaucoma Exclusion criteria: not reported Equivalence of baseline characteristics: no (equivalence of baseline characteristics were not fully reported, and no demographics info were provided. Only medical history and procedure reported: "Both groups had equal types of glaucoma, pigmentation and no previous surgery.") |
|
| Interventions |
Intervention 1: 1 drop apraclonidine 0.5%, 45 min prior to ALT Intervention 2: 1 drop latanoprost 0.005%, 45 min prior to ALT Length of follow‐up: Planned: not reported Actual: 6 weeks |
|
| Outcomes |
Primary outcome: IOP Secondary outcomes: not reported Adverse events reported: no Intervals at which outcomes assessed: apraclonidine group = 2 hours; 1, 6 weeks; latanoprost group = 2 hours; 1 day; 1, 6 weeks |
|
| Notes |
Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported; unclear whether the participant or the eye was analyzed. |
Karlik 1998.
| Methods |
Study design: parallel‐group RCT Country: not reported Number randomized: Total: 50 Per group: apraclonidine = 28, latanoprost = 22 Exclusions after randomization: not reported Number analyzed: Total: not reported Per group: not reported Unit of analysis: participants Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: unclear whether the study analyzed participants or eyes |
|
| Participants |
Age: not reported Females: not reported Inclusion criteria: people undergoing ALT Exclusion criteria: not reported Equivalence of baseline characteristics: no, demographic characteristics were not discussed, only medical characteristics: "Both groups had equal types of glaucoma, pigmentation, and previous surgical histories." |
|
| Interventions |
Intervention 1: 1 drop apraclonidine 0.5%, 1 hour prior to surgery Intervention 2: 1 drop latanoprost 0.005%, 6 hours and 1 hour prior to surgery Length of follow‐up: Planned: not reported Actual: 6 weeks |
|
| Outcomes |
Primary outcome: IOP Secondary outcomes: not reported Adverse events reported: no Intervals at which outcomes assessed: 1.5 hours; 1 day; 1, 6 weeks |
|
| Notes |
Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported; unclear whether the participant or the eye were analyzed. |
Kitazawa 1990.
| Methods |
Study design: parallel‐group RCT Country: not reported Number randomized: Total: 23 eyes (17 participants) Per group: not reported Exclusions after randomization: not reported Number analyzed: Total: not reported Per group: not reported Unit of analysis: eyes Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Age: not reported Females: not reported Inclusion criteria: participants with POAG undergoing ALT Exclusion criteria: not reported Equivalence of baseline characteristics: not reported |
|
| Interventions |
Intervention 1: 1 drop apraclonidine 1%, 1 hour before and immediately after ALT Intervention 2: 1 drop placebo, 1 hour before and immediately after ALT Length of follow‐up: Planned: 24 hours Actual: 24 hours |
|
| Outcomes |
Primary outcomes: IOP, flare intensity Secondary outcomes: not reported Adverse events reported: no Intervals at which outcomes assessed: participants observed during a 24‐hour observation period, but specific time points not described. |
|
| Notes |
Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Low risk | "We studied the effects of this compound on the inflammatory reaction and the IOP responses to ALT in a randomized, double‐masked manner." |
| Masking of outcome assessment (detection bias) | Low risk | "We studied the effects of this compound on the inflammatory reaction and the IOP responses to ALT in a randomized, double‐masked manner." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported. |
Ma 1999.
| Methods |
Study design: parallel‐group RCT Country: Korea Number randomized: Total: 80 Per group: brimonidine/brimonidine = 20; brimonidine/placebo = 20, placebo/brimonidine = 20, placebo/placebo = 20 Exclusions after randomization: none reported Number analyzed: Total: 80 Per group: brimonidine/brimonidine = 20; brimonidine/placebo = 20, placebo/brimonidine = 20, placebo/placebo = 20 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design?: none |
|
| Participants |
Age (mean ± SD; years): overall = 58.4 ± 8.9, brimonidine/brimonidine = 57.7, brimonidine/placebo = 58.0, placebo/brimonidine = 60.6, placebo/placebo = 57.5 Females: brimonidine/brimonidine = 35%, brimonidine/placebo = 55%, placebo/brimonidine = 55%, placebo/placebo = 45% Inclusion criteria: none listed Exclusion criteria: people who had glaucoma or intraocular surgery, who had already received any systemic alpha‐agonist or had a hypersensitivity to any alpha‐agonist Equivalence of baseline characteristics: yes, "No significant pretreatment differences in terms of age, sex, iris color, or baseline IOP were noted among treatment groups." |
|
| Interventions |
Intervention 1: brimonidine 0.2%, 30 to 60 min before and immediately after 180° ALT Intervention 2: brimonidine 0.2%, 30 to 60 min before and placebo immediately after 180° ALT Intervention 3: placebo, 30 to 60 min before and brimonidine 0.2% immediately after 180° ALT Intervention 4: placebo, 30 to 60 min before and immediately after Length of follow‐up: Planned: 4 weeks Actual: 4 weeks |
|
| Outcomes |
Primary outcome: IOP Secondary outcomes: IOP of the contralateral eye, mean heart rate, systolic BP Adverse events reported: yes Intervals at which outcomes assessed: 1, 2, 3 hours; 1 day; 1, 4 weeks |
|
| Notes |
Trial registration: not reported Funding sources: "This study was supported by the Research Institute of Clinical Medicine, Chonnam University Hospital." Disclosures of interest: none reported Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective reporting. |
| Other bias | Low risk | None |
Metcalfe 1989.
| Methods |
Study design: prospective, randomized, double‐masked, parallel‐group RCT Country: UK Number randomized: Total: 100 Per group: acetazolamide = 50; placebo = 50 Exclusions after randomization: N/A Number analyzed: Total: 100 Per group: acetazolamide = 50; placebo = 50 Unit of analysis (participants vs eyes): eyes, 1 eye per participant, chosen if that eye needed laser. If both eyes were lasered, the first eye was chosen for inclusion. Losses to follow‐up: N/A How was missing data handled?: not reported Reported power calculation: no Unusual study design?: no |
|
| Participants |
Age (mean ± SD; years): acetazolamide = 74.0 ± 6.0, placebo = 74.6 ± 5.9 Females: acetazolamide = 54%, placebo = 52%, overall = 53% Inclusion criteria: uncontrolled OAG with IOP > 21 mmHg and progressive visual field loss, on maximum tolerated topical therapy, no previous LTP Exclusion criteria: already receiving acetazolamide Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: acetazolamide (2 × 250 mg tablets), 1 hour prior to LTP Intervention 2: placebo (2 placebo tablets), 1 hour prior to LTP Length of follow‐up: Planned: 2 months Actual: 2 months |
|
| Outcomes |
Primary outcomes: IOP in both eyes, degree of anterior segment inflammation Secondary outcomes: not reported Adverse events reported: no Intervals at which outcomes assessed: 30 min; 1, 2, 3, 24 hours; 2 months after laser treatment |
|
| Notes |
Trial registration: not reported Funding sources: none reported Disclosures of interest: none Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Low risk | "The medication selected was masked to both the patient and the physician." |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Low risk | None |
Raspiller 1992.
| Methods |
Study design: parallel‐group RCT Country: France Number randomized: Total: 38 Per group: trabeculoplasty/apraclonidine = 10, capsulotomy/apraclonidine = 8, trabeculoplasty/placebo = 10; capsulotomy/placebo = 10 Exclusions after randomization: none Number analyzed: Total: 38 Per group: apraclonidine = 18 (trabeculoplasty = 10, capsulotomy = 8); placebo n = 20 (trabeculoplasty = 10, capsulotomy = 8) Unit of analysis: eyes Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Age: not reported Females: not reported Inclusion criteria: not reported Exclusion criteria: aged < 18 years, infection or eye inflammation, severe eye disease in the past or currently, with the exception of cataract and glaucoma, non‐stabilized cardiovascular disease, an abnormality preventing reliable measure of IOP tonometry, blindness, receiving general clonidine, already participated in the study with their other eye, participated in another clinical trial during the last 30 days Equivalence of baseline characteristics: not reported |
|
| Interventions |
Intervention 1: placebo Intervention 2: apraclonidine 1% Length of follow‐up: Planned: 1 week Actual: 1 week |
|
| Outcomes |
Primary outcomes: efficacy/efficiency with IOP (i.e. mean change in IOP) Secondary outcome: incident IOP spikes (≥ 10 mmHg) Adverse events reported: yes Intervals at which outcomes assessed: 1, 2, 3 hours; 1 week |
|
| Notes |
Trial registration: not reported Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: yes, by surgical procedure, i.e. trabeculoplasty vs capsulotomy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Authors report the study was "double‐blinded" but did not describe who was masked: participants, surgeons, or outcome assessors. |
| Masking of outcome assessment (detection bias) | Unclear risk | Authors report the study was "double‐blinded" but did not describe who was masked: participants, surgeons, or outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how the authors handled missing data; missing a "table 1" study population demographics. |
| Selective reporting (reporting bias) | Unclear risk | Authors described outcomes and how they graded/collected in the methods section; same outcomes reported in results; reported both statistically significant and non‐significant data; however, there was a protocol deviation (i.e. from iridotomies to capsulotomies) and the authors dropped 2 iridotomy cases. |
| Other bias | Unclear risk | Funding sources not reported. |
Ren 1999.
| Methods |
Study design: parallel‐group RCT Country: US Number randomized: Total: 228 Per group: apraclonidine = 114; pilocarpine = 114 Exclusions after randomization: none reported Number analyzed: Total: 228 Per group: apraclonidine = 114; pilocarpine = 114 Unit of analysis (participants vs eyes): participant (1 eye per participant) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: yes Unusual study design?: none |
|
| Participants |
Age (mean ± SD; years): apraclonidine = 68.4 ± 11.4, pilocarpine = 70.3 ± 10.1 Females: apraclonidine = 62%, pilocarpine = 56% Inclusion criteria: POAG with bilateral elevation (> 21 mmHg before therapy), characteristic glaucomatous optic nerve damage on stereoscopic biomicroscopy, and glaucomatous visual field defects on Humphrey automated field testing Exclusion criteria: secondary OAG and previous intraocular surgery Equivalence of baseline characteristics: no, pre‐ALT IOP was higher in the apraclonidine group |
|
| Interventions |
Intervention 1: 1 drop apraclonidine 1%, 15 min before 180° LTP Intervention 2: 1 drop pilocarpine 4%, 15 min before 180° LTP Length of follow‐up: Planned: 24 hours Actual: 24 hours |
|
| Outcomes |
Primary outcome: IOP Secondary outcome: incidence of IOP spike Adverse events reported: yes, "There was an apparent lack of serious or longlasting side effects after single instillation of either apraclonidine or pilocarpine." Intervals at which outcomes assessed: 5 min; 1, 24 hours |
|
| Notes |
Trial registration: not reported Funding sources: "Supported in part by an unrestricted grant from Research to Prevent Blindness, Inc" Disclosures of interest: none reported Study period: not reported Reported subgroup analyses: yes, by regular medication type |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was an RCT but no description of how the randomization sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting |
| Other bias | Low risk | None |
Robin 1987.
| Methods |
Study design: parallel‐group RCT Country: US Number randomized: Total: 73 Per group: apraclonidine = 39, placebo = 34 Exclusions after randomization: none reported Number analyzed: Total: 73 Per group: apraclonidine = 39, placebo = 34 Unit of analysis (participants vs eyes): participant (1 eye per participant); if a participant required bilateral therapy, the eye treated first was selected Losses to follow‐up: none reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Age (mean ± SD; years): apraclonidine = 60.9 ± 14.3, placebo = 68.8 ± 12.4 Females: apraclonidine = 54%, placebo = 74% Inclusion criteria: pre‐existing OAG and poor IOP control despite maximum tolerated medical therapy Exclusion criteria: prior ALT Equivalence of baseline characteristics: no, "There was no statistically significant difference in any variable except for mean patient age, (P<0.25)" |
|
| Interventions |
Intervention 1: topical 1% apraclonidine, 1 hour prior and immediately after 360° ALT Intervention 2: placebo, 1 hour prior and immediately after 360° ALT Length of follow‐up: Planned: 1 month Actual: 1 month |
|
| Outcomes |
Primary outcomes: visual acuity, IOP, anterior segment inflammation Secondary outcome: heart rate Adverse events reported: authors reported that there were no adverse events Intervals at which outcomes assessed: 1, 2, 3 hours; 1 week; 1 month |
|
| Notes |
Trial registration: not reported Funding sources: "This study was funded in part by a grant from Alcon Laboratories." Disclosures of interest: "Betty House is an employee of Alcon Laboratories, Fort Worth, Tex. None of the authors as any financial, commercial, or proprietary interest in ALO 2145 [apraclonidine]." Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated random‐number table was utilized, and the selected medication was masked to both the physician and the patient." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Low risk | "A computer‐generated random‐number table was utilized, and the selected medication was masked to both the physician and the patient." |
| Masking of outcome assessment (detection bias) | Unclear risk | No information on efforts to mask the outcome assessors reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | High risk | Study funded in part by Alcon Laboratories, who manufacture the study drug apraclonidine. |
Robin 1991.
| Methods |
Study design: parallel‐group RCT Country: US Number randomized: Total: 260 Per group: apraclonidine = 125, pilocarpine = 37, timolol = 35, dipivefrin = 32, acetazolamide = 31 Exclusions after randomization: none reported Number analyzed: Total: 260 Per group: apraclonidine = 125, pilocarpine = 37, timolol = 35, dipivefrin = 32, acetazolamide = 31 Unit of analysis (participants vs eyes): participants (1 eye per participant; if both eyes had elevated IOP and required trabeculoplasty, the study included only the eye treated first) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design?: "To increase our experience with the use of apraclonidine, the randomization allowed about four times more eyes to receive topical 1% apraclonidine than either timolol 0.5%, pilocarpine 4%, dipivefrin 0.1%, or 250 mg acetazolamide." |
|
| Participants |
Age (mean ± SD; years): apraclonidine = 66.5 ± 12.2, pilocarpine = 67.6 ± 8.9, timolol = 68.4 ± 10.3, dipivefrin = 65.5 ± 14.0, acetazolamide = 63.0 ± 13.1 Females: apraclonidine = 56%, pilocarpine = 62%, timolol = 57%, dipivefrin = 50%, acetazolamide = 65% Inclusion criteria: people of legal age with various forms of glaucoma, with disk and visual field damage, poor IOP control despite maximum‐tolerated medical therapy Exclusion criteria: people with asthma, sulfa allergy, unstable cardiovascular disease, allergy to any of the test medications, and eyes that had previously undergo ALT Equivalence of baseline characteristics: yes, "There were no significant preoperative differences among the five treatment groups in terms of race, age, sex, eye color, or preoperative types of glaucoma." |
|
| Interventions |
Intervention 1: apraclonidine 1%, 1 hour before and immediately after LTP Intervention 2: pilocarpine hydrochloride, 4% 1 hour before and immediately after LTP Intervention 3: timolol maleate 0.5%, 1 hour before and immediately after LTP Intervention 4: dipivefrin 0.1%, 1 hour before and immediately after LTP Intervention 5: acetazolamide 250 mg, 1 hour before and immediately after LTP Length of follow‐up: Planned: 1 month Actual: 1 month |
|
| Outcomes |
Primary outcome: IOP changes Secondary outcomes: none reported Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 2, 3 hours; 1 week; 1 month |
|
| Notes |
Trial registration: not reported Funding sources: none reported Disclosures of interest: "The author has no proprietary interests in Alcon Laboratories, Inc, or in apraclonidine hydrochloride." Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated random‐number table was used to assign eyes to five treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Participants in the acetazolamide group would be aware they were taking a pill rather than receiving topical treatment, though they may not have known what the drug was. |
| Masking of outcome assessment (detection bias) | Low risk | "The investigator was masked to which medication each subject received." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were any missing data or how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Low risk | None |
Yalvaç 1996.
| Methods |
Study design: parallel‐group RCT Country: Turkey Number randomized: Total: 48 Per group: apraclonidine/180° ALT = 16, apraclonidine/360° ALT = 16, placebo/180° ALT = 16 Exclusions after randomization: none reported Number analyzed: Total: 48 Per group: apraclonidine/180° ALT = 16, apraclonidine/360° ALT = 16, placebo/180° ALT = 16 Unit of analysis (participants vs eyes): participant (1 eye per person) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design?: none |
|
| Participants |
Age (mean ± SD; years): apraclonidine/180° ALT = 63.3 ± 8.8, apraclonidine/360° ALT = 65.5 ± 7.4, placebo/180° ALT = 62.1 ± 8.3 Females: apraclonidine/180° ALT = 25%, apraclonidine/360° ALT = 38%, placebo/180° ALT = 31% Inclusion criteria: people with POAG, defined by optic disk cupping and visual field loss and a pretreatment IOP > 21 mmHg on maximally tolerated medical therapy Exclusion criteria: previous intraocular surgical procedures or laser treatment, secondary OAG (i.e. pigmentary, exfoliative, or uveitic), and aged < 40 years Equivalence of baseline characteristics: yes, "There were no significant differences among the three groups in terms of average age, gender, preoperative IOP, or number of antiglaucoma medications (P>0.05)." |
|
| Interventions |
Intervention 1: apraclonidine 1%, 1 hour before and immediately after 180° ALT Intervention 2: apraclonidine 1%, 1 hour before and immediately after 360° ALT Intervention 3: placebo, 1 hour before and immediately after 180° ALT Length of follow‐up: Planned: 3 hours Actual: 3 hours |
|
| Outcomes |
Primary outcomes: IOP change, frequency of IOP elevation Secondary outcomes: none reported Adverse events reported: no Intervals at which outcomes assessed: baseline; 1, 2, 3 hours after treatment |
|
| Notes |
Trial registration: not reported Funding sources: none reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | No information on masking of participants and personnel reported. |
| Masking of outcome assessment (detection bias) | Low risk | "IOP was measured preoperatively and 1, 2, and 3 hours after treatment by an observer who was blinded to the random assignment of treatment with the drugs." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study did not address whether outcome data were complete at each time point, but study only lasted 3 hours post‐ALT. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported. |
ALT: argon laser trabeculoplasty; BP: blood pressure; IOP: intraocular pressure; LTP: laser trabeculoplasty; min: minute; N/A: not applicable; Nd:YAG: neodymium‐doped yttrium aluminum garnet; OAG: open‐angle glaucoma; POAG: primary open‐angle glaucoma; RCT: randomized controlled trial; SD: standard deviation; SLT: selective laser trabeculoplasty.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ascaso 1992 | Study medication was not an IOP‐lowering drop. |
| Bergamini 1997 | Non‐comparative study. |
| Bucci 1987 | Study medications were not IOP‐lowering drops. |
| Champagne 2015 | Study medications were not IOP‐lowering drops. |
| Chen 2001 | Included participants who received laser peripheral iridotomy, ALT, and Nd:YAG laser capsulotomy; results not separated by type of surgery. |
| Chen 2005 | Included participants who received laser peripheral iridotomy, ALT, and Nd:YAG laser capsulotomy; results not separated by type of surgery. |
| De Keyser 2017 | Study medication was not an IOP‐lowering drop. |
| Diestelhorst 1995 | Study medication was not an IOP‐lowering drop. |
| Gelfand 1985 | Study medication was not an IOP‐lowering drop. |
| Herbort 1992 | Study medication was not an IOP‐lowering drop. |
| Herbort 1993 | Study medication was not an IOP‐lowering drop. |
| Hurvitz 1994 | Dosage study; no eligible comparison group. |
| Jinapriya 2014 | Study medication was not an IOP‐lowering drop. |
| Kim 1998 | Study medication was not an IOP‐lowering drop. |
| Krupin 1992 | Not an RCT. |
| Leung 1986 | Not an RCT. |
| León‐Alcántara 1995 | Non‐comparative study. |
| Ottaiano 1989 | Included only participants who received laser iridotomy. |
| Ottaiano 1996 | Unable to confirm whether study was randomized. |
| Pappas 1985 | Study medication was not an IOP‐lowering drop. |
| Patel 1998 | Unable to confirm whether study was randomized; included participants who received ALT or Nd:YAG capsulotomy; results not separated by type of surgery. |
| Realini 2010 | Study medication was not an IOP‐lowering drop. |
| Rosenberg 1995 | Dosage study; no eligible comparison group. |
| Shin 1996 | Study medication was not an IOP‐lowering drop. |
| Stingu 2001 | Unable to confirm whether study was randomized; included participants who received ALT, and Nd:YAG laser iridotomy; results not separated by type of surgery. |
| Swendris 1991a | Unable to confirm whether study was randomized; categorized by handsearchers as a CCT as no randomization was reported. |
| Swendris 1991b | Unable to confirm whether study was randomized; categorized by handsearchers as a CCT as no randomization was reported. |
| Threlkeld 1996 | Dosage study; no eligible comparison group. |
| Vickerstaff 2015 | 1 study arm received only medication without LTP; no eligible comparison group. |
| Weinreb 1983 | Study medication was not an IOP‐lowering drop. |
| West 1992 | Study medications were not IOP‐lowering drops. |
ALT: argon laser trabeculoplasty; CCT: controlled clinical trial; IOP: intraocular pressure; LTP: laser trabeculoplasty; Nd:YAG: neodymium‐doped yttrium aluminum garnet; RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Božić 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Serbian. Awaiting translation. |
Ha 1991.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Korean. Awaiting translation. |
Differences between protocol and review
We added the methods for the 'Summary of findings' tables and GRADE assessment that were not included in the original protocol. We were unable to use some methods and perform some analyses we had outlined in the protocol (Zhang 2013). For example, we had planned to conduct sensitivity analyses to assess the influence of industry‐funded studies, studies with missing data, and studies assessed as having a high risk of selection or attrition bias, but selection and attrition bias were not major concerns among our included studies. We had also planned a sensitivity analysis to remove industry‐funded studies, but the majority of the studies contributing data to our analyses were industry‐funded, and removing them would leave too few data to draw any conclusions.
Contributions of authors
The protocol for this review was written by LZ with significant contributions from JW and DM. Screening and data extraction: LZ and JW with assistance from CEV staff Elizabeth Clearfield (EC), Sueko Ng, and Nan Zhang. Data checking and entering: EC. Data reviewed: LZ. Writing: LZ and EC with contributions from JW and DM.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
Funding for methodological support from the Cochrane Eyes and Vision US Project
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
LZ none known. JW none known. DM none known.
New
References
References to studies included in this review
Barnebey 1993 {published data only}
- Barnebey HS, Robin AL, Zimmerman TJ, Morrison JC, Hersh SB, Lewis RA, et al. The efficacy of brimonidine in decreasing elevations in intraocular pressure after laser trabeculoplasty. Ophthalmology 1993;100(7):1083‐8. [DOI] [PubMed] [Google Scholar]
- Robin AL, Barnebey HS, Zimmerman TJ, Morrison JC, Hersh SB, Lewis RA. The effect of single and multiple doses of 0.5% brimonidine tartrate on IOP elevations following 360° ALT surgery. American Academy of Ophthalmology 1992:86.
- The Brimonidine‐ALT Study Group. Effect of brimonidine 0.5% on intraocular pressure spikes following 360° argon laser trabeculoplasty. Ophthalmic Surgery and Lasers 1995;26(5):404‐9. [PubMed] [Google Scholar]
Barnes 1999 {published data only}
- Barnes SD, Campagna JA, Dirks MS, Doe EA, Geanon JD. Comparison of brimonidine 0.2% to apraclonidine 1.0% for the control of intraocular pressure spikes after argon laser trabeculoplasty. American Academy of Ophthalmology 1997:174. [DOI] [PubMed]
- Barnes SD, Campanga JA, Dirks MS, Doe EA. Control of intraocular pressure elevations after argon laser trabeculoplasty. Comparison of brimonidine 0.2% to apraclonidine 1.0%. Ophthalmology 1999;106(10):2033‐7. [DOI] [PubMed] [Google Scholar]
- Barnes SD, Dirks MS, Doe EA, Campagna JA, Zimmerman T. Control of intraocular pressure spikes after argon laser trabeculoplasty: brimonidine 0.2% vs. apraclonidine 1.0%. Journal of Glaucoma 1999; Vol. 8, issue 1:S15. [DOI] [PubMed]
- Dirks M, Conner C, Barnes SD. A cost minimization analysis comparing brimonidine to apraclonidine in IOP control following ALT. Journal of Glaucoma 2000; Vol. 9, issue 1:120.
Birt 1995 {published data only}
- Birt CM, Shin DH, Reed SY, McCarty B, Kim C, Frenkel RE. One vs. two doses of 1.0% apraclonidine for prophylaxis of intraocular pressure spike after argon laser trabeculoplasty. Canadian Journal of Ophthalmology 1995;30(5):266‐9. [PubMed] [Google Scholar]
Brown 1988 {published data only}
- Brown RH, Stewart RH, Lynch MG, Crandall AS, Mandell AI, Wilensky JT, et al. ALO 2145 reduces the intraocular pressure elevation after anterior segment laser surgery. Ophthalmology 1988;95(3):378‐84. [DOI] [PubMed] [Google Scholar]
Carassa 1992 {published data only}
- Carassa R, Trabucchi G, Fontana P, Brancato R. Apraclonidine (AL 02145) reduces intraocular pressure in patients undergoing anterior segment laser surgery. Italian Journal of Ophthalmology 1992;6(4):249‐53. [Google Scholar]
Chevrier 1999 {published data only}
- Chevrier RL, Assalian A, Duperre J, Lesk MR. Apraclonidine 0.5% versus brimonidine 0.2% for the control of intraocular pressure elevation following anterior segment laser procedures. Ophthalmic Surgery and Lasers 1999;30(3):199‐204. [PubMed] [Google Scholar]
Dapling 1994 {published data only}
- Dapling RB, Cunliffe IA, Longstaff S. Influence of apraclonidine and pilocarpine alone and in combination on post laser trabeculoplasty pressure rise. British Journal of Ophthalmology 1994;78(1):30‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
David 1993 {published data only}
- David R, Spaeth GL, Clevenger CE, Perell HF, Siegel LI, Henry JC, et al. Brimonidine in the prevention of intraocular pressure elevation following argon laser trabeculoplasty. Archives of Ophthalmology 1993;111(10):1387‐90. [DOI] [PubMed] [Google Scholar]
- Spaeth G, David R, Clevenger C, Perell H, Siegel L. The effects of brimonidine tartrate on the incidence of intraocular pressure (IOP) spikes following argon laser trabeculoplasty (ALT). Investigative Ophthalmology and Visual Science 1992; Vol. 33:ARVO E‐Abstract 2340.
- The Brimonidine‐ALT Study Group. Effect of brimonidine 0.5% on intraocular pressure spikes following 360° argon laser trabeculoplasty. Ophthalmic Surgery and Lasers 1995;26(5):404‐9. [PubMed] [Google Scholar]
Donnelly 2006 {published data only}
- Donnelly SJ, Brooks DB, Fechter HP III, Psolka M. Comparison of the alpha‐2 agonists for prevention of intraocular pressure elevation after selective laser trabeculoplasty. Investigative Ophthalmology and Visual Science 2006;47:ARVO E‐Abstract 4565. [Google Scholar]
- Yakopson VS, Donnelly SJ, Fechter HP, Wilson WF. Brimonidine 0.1% vs apraclonidine 0.5% for prevention of intraocular pressure elevation after selective laser trabeculoplasty. Investigative Ophthalmology and Visual Science 2008; Vol. 49:ARVO E‐Abstract 1234.
Elsas 1991 {published data only}
- Elsas T, Johnsen H, Stang O. Pilocarpine to prevent acute pressure increase following primary laser trabeculoplasty. Eye 1991;5(pt. 4):390‐4. [DOI] [PubMed] [Google Scholar]
Hartenbaum 1999 {published data only}
- Hartenbaum D, Wilson H, Maloney S, Vacarelli L, Orillac R, Sharpe E, et al. A randomized study of dorzolamide in the prevention of elevated intraocular pressure after anterior segment laser surgery. Journal of Glaucoma 1999;8(4):273‐5. [PubMed] [Google Scholar]
Holmwood 1992 {published data only}
- Holmwood P, Chase D, Ruderman I, Rosenberg L, Krupin T. Effect of apraclonidine dosage on argon laser trabeculoplasty (ALT) induced increase in intraocular pressure (IOP). Investigative Ophthalmology and Visual Science 1991; Vol. 32:ARVO E‐Abstract 1356.
- Holmwood PC, Chase RD, Krupin T, Rosenberg LF, Ruderman JM, Tallman BA, et al. Apraclonidine and argon laser trabeculoplasty. American Journal of Ophthalmology 1992;114(1):19‐22. [DOI] [PubMed] [Google Scholar]
Karlik 1997 {published data only}
- Karlik JS, Barber JC, Humphreys A, Dutt RM. The comparison of latanoprost verses apraclonidine as pretreatment in eyes undergoing argon laser trabeculoplasty. Investigative Ophthalmology and Visual Science 1997; Vol. 38:ARVO E‐Abstract 1303.
Karlik 1998 {published data only}
- Karlik JS, Baker KS, Dutt RM. Comparison of latanoprost versus apraclonidine as pretreatment in eyes undergoing argon laser trabeculoplasty. Investigative Ophthalmology and Visual Science 1998;39:ARVO E‐Abstract 1173. [Google Scholar]
Kitazawa 1990 {published data only}
- Kitazawa Y, Shimizu U. Apraclonidine hydrochloride suppresses the increase of aqueous flare induced by argon laser trabeculoplasty. Investigative Ophthalmology and Visual Science 1990; Vol. 31:ARVO E‐Abstract 2477.
Ma 1999 {published data only}
- Ma YR, Lee BH, Yang KJ, Park YG. The efficacy of 0.2% brimonidine for preventing intraocular pressure rise following argon laser trabeculoplasty. Korean Journal of Ophthalmology 1999;13(2):78‐84. [DOI] [PubMed] [Google Scholar]
Metcalfe 1989 {published data only}
- Metcalfe TW, Etchells DE. Prevention of the immediate intraocular pressure rise following argon laser trabeculoplasty. British Journal of Ophthalmology 1989;73(8):612‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raspiller 1992 {published data only}
- Raspiller A, Berrod JP. Efficacy and safety of apraclonidine (ALO 2145, 1%) in patients undergoing anterior segment laser surgery. Journal Francais d'Ophtalmologie 1992;15(12):651‐6. [PubMed] [Google Scholar]
Ren 1999 {published data only}
- Ren J, Shin DH, Chung HS, Birt CM, Glover BK, Juzych MS, et al. Efficacy of apraclonidine 1% versus pilocarpine 4% for prophylaxis of intraocular pressure spike after argon laser trabeculoplasty. Ophthalmology 1999;106(6):1135‐9. [DOI] [PubMed] [Google Scholar]
- Ren J, Tinoosh F, Chung HS, Birt CM, Glover B. Efficacy of apraclonidine 1% vs. pilocarpine 4% for prophylaxis of intraocular pressure spike after argon laser trabeculoplasty. American Academy of Ophthalmology 1997:195. [DOI] [PubMed]
Robin 1987 {published data only}
- Robin AL, Pollack IP, House B, Enger C. Effects of ALO 2145 on intraocular pressure following argon laser trabeculoplasty. Archives of Ophthalmology 1987;105(5):646‐50. [DOI] [PubMed] [Google Scholar]
Robin 1991 {published data only}
- Robin AL. Argon laser trabeculoplasty medical therapy to prevent the intraocular pressure rise associated with argon laser trabeculoplasty. Ophthalmic Surgery 1991;22(1):31‐7. [PubMed] [Google Scholar]
Yalvaç 1996 {published data only}
- Yalvaç I, Karagöz Y, Akgün Ü, Kasim R, Duman S. Apraclonidine use in argon laser trabeculoplasty [Argon laser trabeküloplasti'de apraklonidin uygulaması]. Türk Oftalmolojı Dergısı 1994;24(3):282‐5. [Google Scholar]
- Yalvaç IS, Eksioglu U, Karagoz Y, Akgun U, Kasim R, Duman S. Prophylactic use of apraclonidine for intraocular pressure increase after 180‐degree argon laser trabeculoplasty. Annals of Ophthalmology 1996;28(4):240‐3. [Google Scholar]
References to studies excluded from this review
Ascaso 1992 {published data only}
- Ascaso FJ, Castillo JS, Loras E, Minana MI, Palomar A. Effects of topical diclofenac sodium administered before argon laser trabeculoplasty: a double‐blind comparison versus dexamethasone phosphate eye drops. Lasers and Light in Ophthalmology 1992;5(1):29‐33. [Google Scholar]
Bergamini 1997 {published data only}
- Bergamini F, Vigo C, Pissarello R, Brancato C. Evaluation of apraclonidine hydrochloride effects on IOP control after argon laser trabeculoplasty or Nd:YAG laser iridotomy [Valutazione dell'apraclonidina cloridrato nel controllo della IOP dopo ALT o iridotomia Nd:YAG laser]. Annali di Ottalmologia e Clinicia Oculistica 1997;123(1):25‐8. [Google Scholar]
Bucci 1987 {published data only}
- Bucci MG, Cichetti MP, D'Andrea D, Corridore F. Evaluation of the effects of piroxicam e.d. in the intraocular pressure control after argon laser trabeculoplasty [Valutazione degli effetti del piroxicam collirio nel controllo dell pressione oculare dopo trabeculoplastica argon laser]. Annali di Ottalmologia e Clinica Oculistica 1987;113(7):643‐8. [Google Scholar]
Champagne 2015 {published data only}
- Champagne S, Anctil JL, Goyette A, Lajoie C, Marchais B. Influence on intraocular pressure of anti‐inflammatory treatments after selective laser trabeculoplasty [Impact du traitement anti‐inflammatoire post‐trabéculoplastie au laser sélectif sur la tension intraoculaire]. Journal Français d'Ophtalmologie 2015;38(7):588‐94. [DOI] [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen TC, Ang RT, Grosskreutz CL, Pasquale LR, Fan JT. Brimonidine 0.2% versus apraclonidine 0.5% for prevention of intraocular pressure elevations after anterior segment laser surgery. Ophthalmology 2001;108(6):1033‐8. [DOI] [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Chen E, Golchin S, Blomdahl S. A comparison between 90 degrees and 180 degrees selective laser trabeculoplasty. Journal of Glaucoma 2004; Vol. 13, issue 1:62‐5. [DOI] [PubMed]
- Chen TC. Brimonidine 0.15% versus apraclonidine 0.5% for prevention of intraocular pressure elevation after anterior segment laser surgery. Journal of Cataract and Refractive Surgery 2005;31(9):1707‐12. [DOI] [PubMed] [Google Scholar]
De Keyser 2017 {published data only}
Diestelhorst 1995 {published data only}
- Diestelhorst M, Thull D, Krieglstein GK. The effect of argon laser trabeculoplasty on the blood‐aqueous barrier and intraocular pressure in human glaucomatous eyes treated with diclofenac 0.1%. Graefes Archive for Clinical and Experimental Ophthalmology 1995;233(9):559‐62. [DOI] [PubMed] [Google Scholar]
Gelfand 1985 {published data only}
- Gelfand YA, Wolpert M. Effects of topical indomethacin pretreatment on argon laser trabeculoplasty: a randomised, double‐masked study on black South Africans. British Journal of Ophthalmology 1985;69(9):668‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herbort 1992 {published data only}
- Herbort C, Mermoud A, Shnyder C, Pittet N. Effect of the topical nonsteroidal anti‐inflammatory drug (NSAID) diclofenac after argon laser trabeculoplasty (ALT). Investigative Ophthalmology and Visual Science 1992; Vol. 33:ARVO E‐Abstract 2336.
- Herbort CP, Mermoud A, Schnyder C, Pittet N. Anti‐inflammatory effect of topical diclofenac sodium (Voltarène Ophta) after argon laser trabeculoplasty: preliminary results of prospective double‐blind method [Effet anti‐inflammatoire du diclofenac sodium topique (Voltarèn® Ophta) après trabéculoplastie au laser argon (TLA): résultats préliminaires d'une étude prospective en double‐insu]. Klinische Monatsblätter für Augenheilkunde 1992;200(5):358‐61. [DOI] [PubMed] [Google Scholar]
Herbort 1993 {published data only}
- Herbort CP, Mermoud A, Schnyder C, Pittet N. Anti‐inflammatory effect of diclofenac drops after argon laser trabeculoplasty. Archives of Ophthalmology 1993;111(4):481‐3. [DOI] [PubMed] [Google Scholar]
Hurvitz 1994 {published data only}
- Hurvitz LM. A randomized prospective study of apraclonidine 1/2% vs 1% in preventing post‐laser IOP rise. American Academy of Ophthalmology 1994:129.
Jinapriya 2014 {published data only}
- Jinapriya D, D'Souza M, Hollands H, El‐Defrawy S, Irrcher I, Smallman D, et al. Anti‐inflammatory therapy after selective laser trabeculoplasty. A randomized, double‐masked, placebo‐controlled clinical trial. Ophthalmology 2014;121(12):2356‐61. [DOI] [PubMed] [Google Scholar]
Kim 1998 {published data only}
- Kim YY, Glover BK, Shin DH, Lee D, Frenkel RE, Abreu MM. Effect of topical anti‐inflammatory treatment on the long‐term outcome of laser trabeculoplasty. Fluorometholone‐Laser Trabeculoplasty Study Group. American Journal of Ophthalmology 1998;126(5):721‐3. [DOI] [PubMed] [Google Scholar]
Krupin 1992 {published data only}
- Feitl M, Krupin T, Stank T. Apraclonidine pretreatment decreases the acute intraocular pressure (IOP) rise after argon laser trabeculoplasty (ALT) or laser iridectomy (LPI). Investigative Ophthalmology and Visual Science 1991; Vol. 32:ARVO E‐Abstract 1355.
- Krupin T, Stank T, Feitl ME. Apraclonidine pretreatment decreases the acute intraocular pressure rise after laser trabeculoplasty or iridotomy. Journal of Glaucoma 1992;1(2):79‐86. [Google Scholar]
León‐Alcántara 1995 {published data only}
- León‐Alcántara LM, Aguilar‐Muñoz JO, Carrillo Ruiz de Chávez R, Berges Salgado G, Harleben M, Maqueo M. Evaluation of the efficacy of apraclonidine chlorhydrate 1% in patients who underwent anterior segment laser surgery [Eficacia y seguridad de la solución oftálmica de hidrocloruro de apraclonidina al 1% en pacientes sometidos a cirugía del segmento anterior con láser]. Revista Mexicana de Oftalmología 1995;69(3):109‐16. [Google Scholar]
Leung 1986 {published data only}
- Leung KW, Gillies WE. The detection and management of the acute rise in intraocular pressure following laser trabeculoplasty. Australian and New Zealand Journal of Ophthalmology 1986;14(3):259‐62. [DOI] [PubMed] [Google Scholar]
Ottaiano 1989 {published data only}
- Ottaiano JA. The topic therapy with fluorometholone 0.1% and indomethacin 1% in iridotomy by argon laser [Estudo comparativo da terapêutica tópica com fluorometolona a 0,1 e indometacina a 1 em olhos submetidos à iridotomia por laser de argônio]. Arquivos Brasileiros de Oftalmologia 1989;52(6):211‐4. [Google Scholar]
Ottaiano 1996 {published data only}
- Ottaiano JA, Castro Moreira JB, Fudo A, Ueda EM, Bosso EP, Martin RT. Topical 1% apraclonidine in the argon laser trabeculoplasty [Apraclonidine a 1% em olhos submetidos à trabeculoplastia por laswer de argônio]. Arquivos Brasileiros de Oftalmologia 1996;59(3):299‐306. [Google Scholar]
Pappas 1985 {published data only}
- Pappas HR, Berry DP, Partamian L, Hertzmark E, Epstein DL. Topical indomethacin therapy before argon laser trabeculoplasty. American Journal of Ophthalmology 1985;99(5):571‐5. [DOI] [PubMed] [Google Scholar]
Patel 1998 {published data only}
- Patel HC, Ren J, Shin DH, Chung HS, Hong YJ, Rho SH, et al. Comparative efficacies of apraclonidine, brimonidine, pilocarpine, and latanoprost in prophylaxis of IOP spikes following ALT or YAG capsulotomy. Investigative Ophthalmology and Visual Science 1998;39(4):S 2196. [Google Scholar]
Realini 2010 {published data only}
- Realini T, Charlton J, Hettlinger M. The impact of anti‐inflammatory therapy on intraocular pressure reduction following selective laser trabeculoplasty. Ophthalmic Surgery, Lasers and Imaging 2010;41(1):100‐3. [DOI] [PubMed] [Google Scholar]
- Realini T, Charlton J, Hettlinger M. The role of prednisolone acetate in the postoperative period following selective laser trabeculoplasty. Investigative Ophthalmology and Visual Science 2007; Vol. 48:ARVO E‐Abstract 5674.
Rosenberg 1995 {published data only}
- Rosenberg LF, Krupin T, Ruderman J, McDaniel DL, Siegfried C, Karalekas DP, et al. Apraclonidine and anterior segment laser surgery. Comparison of 0.5% versus 1.0% apraclonidine for prevention of postoperative intraocular pressure rise. Ophthalmology 1995;102(9):1312‐8. [DOI] [PubMed] [Google Scholar]
Shin 1996 {published data only}
- Shin DJ, Frenkel RE, David R, Cheetham JK. Effect of topical anti‐inflammatory treatment on the outcome of laser trabeculoplasty. The Fluorometholone‐Laser Trabeculoplasty Study Group. American Journal of Ophthalmology 1996;122(3):349‐54. [DOI] [PubMed] [Google Scholar]
Stingu 2001 {published data only}
- Stîngu C, Cristescu A, Dârâban C, Ioniţă M, Serghiescu S. Iopidine in the control of intraocular pressure after glaucoma laser treatment [Iopidina în controlul cresterilor presionale după proceduri antiglaucomatoase laser]. Oftalmologia 2001;54(4):32‐5. [PubMed] [Google Scholar]
Swendris 1991a {published data only}
- Swendris RP, Shin DH, Hong YJ. Apraclonidine versus timolol pretreatment in the prevention of intraocular pressure spikes following argon laser trabeculoplasty. Investigative Ophthalmology and Visual Science 1991; Vol. 32:ARVO E‐Abstract 1358.
Swendris 1991b {published data only}
- Swendris RP, Shin DH, Khatana AK, Hong YJ. Medical pretreatment to prevent the acute intraocular pressure elevation after laser trabeculoplasty. American Academy of Ophthalmology 1991:108.
Threlkeld 1996 {published data only}
- Threlkeld AB, Assalian AA, Allingham RR, Shields MB. Apraclonidine 0.5% versus 1% for controlling intraocular pressure elevation after argon laser trabeculoplasty. Ophthalmic Surgery and Lasers 1996;27(8):657‐60. [PubMed] [Google Scholar]
Vickerstaff 2015 {published data only}
- Vickerstaff V, Ambler G, Bunce C, Xing W, Gazzard G. Statistical analysis plan for the Laser‐1st versus Drops‐1st for Glaucoma and Ocular Hypertension Trial (LiGHT): a multi‐centre randomised controlled trial. Trials 2015;16(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinreb 1983 {published data only}
- Weinreb RN, Robin AL, Baerveldt G, Drake MV, Blumenthal M, Wilensky J. Flurbiprofen pretreatment in argon laser trabeculoplasty for primary open‐angle glaucoma. Archives of Ophthalmology 1983;102(11):1629‐32. [DOI] [PubMed] [Google Scholar]
West 1992 {published data only}
- West RH. The effect of topical corticosteroids on laser‐induced peripheral anterior synechiae. Australian and New Zealand Journal of Ophthalmology 1992;20(4):305‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Božić 2011 {published data only}
- Božić M, Hentova‐Senćanić P, Kontić D, Marković V, Marjanović I. Prevention of intraocular pressure elevation after argon laser trabeculoplasty in primary open angle glaucoma [Превенција наглог повећања интраокуларногпритиска после лечења примарног глаукомаотвореног угла трабекулопластиком аргонскимласером]. Journal of the Serbian Medical Society 2011;139(1‐2):12‐7. [DOI] [PubMed] [Google Scholar]
Ha 1991 {published data only}
- Ha I, Hong J. The effects of 1% apraclonidine in preventing the intraocular pressure rise following argon laser surgery in glaucoma. Journal of the Korean Ophthalmological Society 1991;32(1):68‐73. [Google Scholar]
Additional references
American Academy of Ophthalmology 2015
- American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel. Primary Open‐Angle Glaucoma PPP ‐ 2015. Ophthalmology 2015.
Barkana 2007
- Barkana Y, Belkin M. Selective laser trabeculoplasty. Survey of Ophthalmology 2007;52(6):634‐54. [DOI] [PubMed] [Google Scholar]
Bradley 2000
- Bradley JM, Anderssohn AM, Colvis CM, Parshley DE, Zhu XH, Ruddat MS, et al. Mediation of laser trabeculoplasty‐induced matrix metalloproteinase expression by IL‐1beta and TNFalpha. Investigative Ophthalmology and Visual Science 2000;41(2):422‐30. [PubMed] [Google Scholar]
Bylsma 1988
- Bylsma SS, Samples JR, Acott TS, Buskirk EM. Trabecular cell division after argon laser trabeculoplasty. Archives of Ophthalmology 1988;106(4):544‐7. [DOI] [PubMed] [Google Scholar]
Chandler 1997
- Chandler PA, Grant WM. Chandler and Grant's Glaucoma. 4th Edition. Baltimore, MD: Williams and Wilkins, 1997. [Google Scholar]
Chung 1998
- Chung PY, Schuman JS, Netland PA, Lloyd‐Muhammad RA, Jacobs DS. Five‐year results of a randomized, prospective, clinical trial of diode vs argon laser trabeculoplasty for open‐angle glaucoma. American Journal of Ophthalmology 1998;126(2):185‐90. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ederer 2004
- Ederer F, Gaasterland DA, Dally LG, Kim J, VanVeldhuisen PC, Blackwell B, et al. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10‐year results. Ophthalmology 2004;111(4):651‐64. [DOI] [PubMed] [Google Scholar]
Englert 1997
- Englert JA, Cox TA, Allingham RR, Shields MB. Argon vs diode laser trabeculoplasty. American Journal of Ophthalmology 1997;124(5):627‐31. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GLT 1990
- The Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT). 2. Results of argon laser trabeculoplasty versus topical medicines. Ophthalmology 1990;97(11):1403‐13. [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 15 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Harasymowycz 2005
- Harasymowycz PJ, Papamatheakis DG, Latina M, Leon M, Lesk MR, Damji KF. Selective laser trabeculoplasty (SLT) complicated by intraocular pressure elevation in eyes with heavily pigmented trabecular meshworks. American Journal of Ophthalmology 2005;139(6):1110‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Levene 1983
- Levene R. Major early complications of laser trabeculoplasty. Ophthalmic Surgery 1983;14(11):947‐53. [PubMed] [Google Scholar]
Melamed 1985
- Melamed S, Pei J, Epstein DL. Short‐term effect of argon laser trabeculoplasty in monkeys. Archives of Ophthalmology 1985;103(10):1546‐52. [DOI] [PubMed] [Google Scholar]
Melamed 1986
- Melamed S, Pei J, Epstein DL. Delayed response to argon laser trabeculoplasty in monkeys. Morphological and morphometric analysis. Archives of Ophthalmology 1986;104(7):1078‐83. [DOI] [PubMed] [Google Scholar]
Meyer 1990
- Meyer E, Gdal‐On M. Ultra‐structural study of argon laser trabeculoplasty in open‐angle glaucoma. Glaucoma 1990;12(3):84‐7. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robin 1988
- Robin AL. The role of apraclonidine hydrochloride in laser therapy for glaucoma. Transactions of the American Ophthalmological Society 1988;87:729‐61. [PMC free article] [PubMed] [Google Scholar]
Rolim de Moura 2007
- Rolim de Moura CR, Paranhos A Jr, Wormald R. Laser trabeculoplasty for open angle glaucoma. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003919.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samples 2011
- Samples JR, Singh K, Lin SC, Francis BA, Hodapp E, Jampel HD, et al. Laser trabeculoplasty for open‐angle glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology 2011;118(11):2296‐302. [DOI] [PubMed] [Google Scholar]
Schadlu 1998
- Schadlu R, Maus TL, Nau CB, Brubaker RF. Comparison of the efficacy of apraclonidine and brimonidine as aqueous suppressants in humans. Archives of Ophthalmology 1998;116(11):1441‐4. [DOI] [PubMed] [Google Scholar]
Spurny 1984
- Spurny RC, Lederer CM. Krypton laser trabeculoplasty. A clinical report. Archives of Ophthalmology 1984;102(11):1626‐8. [DOI] [PubMed] [Google Scholar]
Stein 2007
- Stein JD, Challa P. Mechanisms of action and efficacy of argon laser trabeculoplasty and selective laser trabeculoplasty. Current Opinion in Ophthalmology 2007;18(2):140‐5. [DOI] [PubMed] [Google Scholar]
Tham 2014
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta‐analysis. Ophthalmology 2014;121(11):2081‐90. [DOI] [PubMed] [Google Scholar]
The Brimonidine‐ALT Study Group
- The Brimonidine‐ALT Study Group. Effect of brimonidine 0.5% on intraocular pressure spikes following 360% argon laser trabeculoplasty. Ophthalmic Surgery and Lasers 1995;26(5):404‐9. [PubMed] [Google Scholar]
Tsai 2006
- Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. Current Opinion in Ophthalmology 2006;17(2):190‐5. [DOI] [PubMed] [Google Scholar]
Wong 2015
- Wong M, Lee J, Choy B, Chan J, Lai J. Systematic review and meta‐analysis on the efficacy of selective laser trabeculoplasty in open‐angle glaucoma. Survey of Ophthalmology 2015;60(1):36‐50. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Zhang 2013
- Zhang L, Weizer JS, Musch DC. Perioperative medications for preventing temporarily increased intraocular pressure after laser trabeculoplasty. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010746] [DOI] [PMC free article] [PubMed] [Google Scholar]


