Abstract
Objective:
Left ventricular (LV) rotation and twist play an important role in LV contraction and relaxation. Left bundle branch block (LBBB) deteriorates both diastolic and systolic functions. We evaluated the LV twist in patients with LBBB and preserved ejection fraction (EF) (>50%) to determine twist as a potential marker for subtle myocardial dysfunction.
Methods:
This observational cross-sectional study included 34 LBBB patients with preserved EF who were free from ischemic and valvular disease (Group 1) and 36 healthy controls (Group 2). All patients underwent 2-D Doppler and 2-D speckle tracking echocardiography. LV apical, basal rotation, and twist were evaluated in both groups and compared accordingly. In addition, subjects were dichotomized considering the median twist value of the study population. Binary logistic regression analysis was performed to determine the independent variables associated with inframedian twist.
Results:
Baseline clinical characteristics were similar in LBBB patients and controls. Mean apical rotation (2.5°±1.9° vs. 4.4°±2.9°; p=0.002), basal rotation (-2.9°±2.3° vs. -4.1°±2.7°; p=0.05), and twist (5.4°±3° vs. 8.6°±3.3°; p<0.001) were decreased in group 1. Parameters related to intra- and interventricular mechanical dyssynchrony, such as longitudinal left ventricular dyssynchrony index (LVdys) and preejection interval of LV, interventricular mechanical delay (IVMD), and left posterior wall contractions (SPMWD) were significantly higher in the LBBB group. The median twist value of the studied population was 6.65°. Binary logistic regression analysis showed that only presence of LBBB was independently associated with inframedian twist (OR=6.250; 95% CI: 2.215–17.632; p<0.001).
Conclusion
The LBBB might have induced the reduction of LV twist by diminishing the LV rotation before inducing a prominent effect on the left ventricular ejection fraction (LVEF). Therefore, twist may be considered as a marker for subtle LV dysfunction in LBBB with substantially normal EF.
Keywords: speckle-tracking imaging, left ventricular function, left ventricular twist, left bundle branch block
Introduction
Left bundle branch block (LBBB) is, in general, linked to an underlying heart disease and it has been reported to affect approximately 25% of all heart failure (HF) patients (1). Alternatively, LBBB can also be isolated and may diminish cardiac performance in the absence of structural defects with a high prevalence rate of 1.5% in the general population (2). Chronic HF is a major ongoing problem in clinical medicine and one of the crucial issues in treating HF is its detection in the subclinical phase.
LBBB causes widening of the QRS complex, deteriorates both systolic and diastolic functions, and constitutes a risk factor for the development and progression of HF (3–5). The deteriorations are associated with a shortening of LV diastole by prolongation of preejection and relaxation times, paradoxical septal motion with an associated decrease in regional ejection fraction (3–6). Moreover, LBBB causing intra- and interventricular dyssynchrony leads to uncoordinated contraction of the ventricle and alterations in LV mechanical activity; it is also associated with cardiac remodeling (3, 5, 6).
LV twist is defined as the wringing motion of the heart caused by opposite rotations of the LV apical and basal segments. The magnitude of LV twist is dependent on the fibres’ architecture, relaxation, and contractility of the myocardium. Measurement of LV twist might play an important role in determining the LV diastolic and systolic dysfunctions. Alterations in the LV twist have been reported in various conditions and the potential clinical applications of twist are still under research (7–10). The changes in ventricular geometry due to cardiac remodeling in LBBB may influence the LV twist (5, 6).
Conventional echocardiographic methods may be insufficient to show cardiac functional changes earlier in patients with LBBB. Speckle tracking echocardiography (STE), which is a noninvasive image-processing method that analyzes myocardial deformation, independent of cardiac translation and angle, has been extensively used in many studies for twist analysis (8–11).
Most studies evaluating the impact of LBBB on LV systolic function examined the effect of cardiac resynchronization therapy and evaluated the effect of dyssynchrony on LV torsion essentially in advanced HF patients (12–14). In this study, the influence of isolated LBBB on LV rotation and LV twist was investigated by 2-D-STE to identify subclinical LV dysfunction in patients with preserved EF (>50%).
Methods
Patient selection
We screened all of the patients who were admitted to our hospital’s outpatient cardiology clinic for a general check-up with electrocardiogram (ECG) in this observational cross-sectional study. Any symptomatic patient with a history of systemic diseases, ischemic heart disease, abnormal systolic function, global or regional wall motion abnormality, valvular chronic heart disease, sinus node dysfunction, atrial fibrillation, pacemaker implantation, and consuming antiarrhythmic medication was excluded. Initially, 81 patients were enrolled in the study. After excluding 11 patients from the study due to inadequate image quality, we recruited 70 volunteers in total. Among the screened subjects; 34 patients (mean age 66±11 years, 23 females) had LBBB on the ECG with normal systolic function (Group 1). Age and sex matched 36 asymptomatic patients (mean age 64±12 years, 22 females) with normal ECG and LV function were selected as a control group (Group 2). All patients gave their voluntary and informed consent for data collection and the study was approved by the local ethics committee of the Sakarya University.
Electrocardiogram
The QRS duration was measured on 12-lead surface ECG from the first deflection of the QRS complex to its terminal isoelectric component. The diagnostic criteria for LBBB included QRS duration over 120 ms; increased intrinsicoid deflection time (80–120 ms); absence of septal Q-waves in the left precordial leads; presence of wide, notched, or slurred R-waves on the left precordial leads; and presence of monophasic QS on leads V1 and V2.
Echocardiography
Conventional echocardiography was implemented with a (Philips Medical Systems, Amsterdam, Netherlands) using an X5 transducer. M-mode/2-D echocardiography and Doppler study were performed to evaluate the LVEF and diastolic function. The early transmitral (E) and late transmitral (A) inflow velocities were obtained from the mitral inflow Doppler signals. Mitral annulus velocities were achieved from the septal annulus of the LV by tissue Doppler imaging (TDI).
A pulsed-wave Doppler was used to evaluate the interventricular mechanical delay (IVMD). The right and left ventricular preejection intervals were measured from the onset of the QRS on the ECG to the onset of pulmonary and aortic outflow; the IVMD was calculated by subtracting the preejection intervals of the right ventricles from the left ventricles.
Intraventricular dyssynchrony was evaluated by calculating the time delay between the motion of the septum and the left posterior wall contractions (SPMWD) on M-mode images from the parasternal short-axis view at papillary muscle level.
To evaluate the longitudinal LV dyssynchrony, apical 4-chamber views were used and the regions of interest (ROIs) were in the basal segments of the left ventricle’s lateral and septal walls. LV dyssynchrony index (LVdys), standard deviation of the time from cardiac cycle onset to minimum systolic volume, was assessed quantitatively as the maximal time delay between the two basal segments and calculated as the time interval from the onset of QRS to the peak systolic velocity (Sm).
Apical and basal short-axis rotations were measured by 2-D STE. Sector width and image depth were optimized to maintain an adequate frame rate of 60–110 frames/s and probe frequency was in the range of 1.7–4 mHz. Short-axis basal images were defined from the mitral ring level and apical images were defined from the LV cavity without any papillary muscles and any visualization of the RV in sight. In particular, efforts were taken to make the LV cross-section as circular as possible. For short axis basal images and apical images, four sequential cardiac cycles were obtained and transferred to a QLAB workstation (Philips) for off-line analysis. Using the commercially available 2D strain software, the endocardial border of each short-axis plane in the end-systolic frame was manually traced. The software algorithm then automatically segmented the LV short-axis plane into six segments and searched for speckles in the ROIs on a frame-by-frame basis using the sum of the absolute difference algorithm. Furthermore, the software defined the ventricular centroid for the mid-myocardial line on a frame-by-frame basis during one cardiac cycle and calculated the time-domain LV rotation and radial displacement profiles for each segment in both short-axis planes. The peak LV twist was calculated by subtracting the peak basal rotation from the peak apical rotation (15).
Statistical analysis
Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. Data are expressed as means±SD for normally distributed continuous variables, as median and interquartile ranges for skew-distributed continuous variables, and as frequencies for categorical variables. The Pearson chi-squared test was used to compare categorical variables. The means for normally distributed continuous variables were compared by independent-samples t-test. Skew-distributed continuous variables were compared using a Mann–Whitney U test. Pearson’s and Spearman’s correlation coefficients were used for determining the correlations between twist and other parameters. LV median twist was identified. Binary logistic regression analysis was performed to determine the independent variables associated with inframedian twist. A two-tailed p<0.05 was considered statistically significant. Statistical analyses were performed with the SPSS software (IBM SPSS Statistics 20, SPSS Inc., IBM Corp., Armonk, NY).
Results
General characteristics of the study population
Age and sex distributions, heart rate, BMI, hypertension, diabetes mellitus, and smoking incidence were similar between the two groups. The baseline clinical characteristic values of all subjects were summarized in Table 1.
Table 1.
Baseline clinical characteristics of the patients
| LBBB | Control | P | |
|---|---|---|---|
| Age, years | 66±11 | 64±12 | 0.582 |
| Men/women | 11/23 | 14/22 | 0.682 |
| Heart rate, beats/min | 74±12 | 72±9 | 0.523 |
| BMI, kg/m2 | 29.7±5.7 | 29.6±5.2 | 0.889 |
| Hypertension | 20 (57.1%) | 17 (48.6%) | 0.632 |
| DM | 7 (20%) | 16 (17.1%) | 0.540 |
| Smoking | 5 (14.3%) | 10 (28.6%) | 0.244 |
| QRS duration, ms* | 138/143/155 | 82/90/93 | 0.001 |
| PR interval, ms | 168.4±28.3 | 168.3±27.4 | 0.994 |
BMI - body mass index; DM - diabetes mellitus; LBBB - left bundle branch block.
Data are presented as median and interquartile ranges
Echocardiographic parameters of the study population
Although LVEF was preserved in all patients, EF was slightly lower in group 1 (58.8±7 vs. 65.7±7%; p=0.042). Variables representing the LV diastolic function such as E, A, and E/A ratios were similar. E’ (5.6±1.3 vs. 7±1.9 cm/sn; p<0.001) and E/E’ ratios (9.95±3.4 vs. 7.7±2.2; p=0.002) were distinctly different between the two groups. Parameters related to intra- and interventricular mechanical dyssynchrony, such as longitudinal LVdys, preejection interval of LV, IVMD, and SPMWD were significantly different in the two groups (Table 2). The LVdys was found to be longer in group 1 (38.9±28.3 vs. 18.5±17.1; p=0.009); however, there were no significant correlations between LVdys and the apical, basal rotation, and LV twist. The SPWMD was higher in group 1 (71.7±26 ms vs. 22.08±12.8 ms; p<0.001), and it negatively correlated with the apical rotation (r=-0.308; p=0.01), basal rotation (r=-0.158; p=0.188), and LV twist (r=-0.448; p<0.001). Additionally, the SPWMD was found to be positively correlated with the QRS duration (r=0.786; p<0.001) in univariate analysis. LV preejection intervals were longer in group 1 (226.9±25.7 ms vs. 180.6±25.7 ms; p=0.001) but right ventricular preejection intervals were similar between the two groups (163.9±22.6 ms vs. 156.9±27.2 ms; p=0.247). IVMD was consequently longer in group 1 (63±22.5 ms vs. 23.7±16.7 ms; p=0.001). The correlation was not obvious between IVMD and LV twist (OR=0.988; 95% CI 0.960–1.017; p=0.416). The absolute values of IVMD and QRS duration were positively correlated (r=0.568; p<0.001).
Table 2.
Comparison of echocardiographic parameters between the two groups
| LBBB | Control | P | |
|---|---|---|---|
| LVEF, % | 58.8±7 | 65.7±7 | 0.042 |
| E, cm/s | 54.1±16.6 | 52.4±16.3 | 0.666 |
| A, cm/s | 82.4±17.9 | 70.6±13.7 | 0.003 |
| E/A ratio* | 0.56/0.64/0.74 | 0.6070.65/1.10 | 0.186 |
| E', cm/s | 5.6±1.3 | 7±1.9 | 0.001 |
| A', cm/s | 8.7±2 | 9.2±2.1 | 0.403 |
| E/E' ratio | 9.95±3.4 | 7.7±2.2 | 0.002 |
| Basal rotation,°, | -2.9±2.3 | -4.1±2.7 | 0.050 |
| Apical rotation,°, | 2.5±1.9 | 4.4±2.9 | 0.002 |
| Twist,°, | 5.4±3 | 8.6±3.3 | 0.001 |
| Longitudinal, LVdys | 38.9±28.3 | 18.5±17.1 | 0.009 |
| Preejection interval of RV, ms | 163.9±22.6 | 156.9±27.2 | 0.247 |
| Preejection interval of LV, ms | 226.9±25.7 | 180.6±25.7 | 0.001 |
| IVMD, ms | 63±22.5 | 23.7±16.7 | 0.001 |
| SPMWD, ms | 71.7±26 | 22.1±12.8 | 0.001 |
A - late-diastolic mitral inflow velocity; A’ - late-diastolic mitral annular velocity; E - early mitral inflow velocity; E’ - early-diastolic mitral annular velocity; IVMD - interventricular mechanical delay; LVEF - left ventricular ejection fraction; LVdys - left ventricular dyssynchrony index; SPWMD - septal to posterior wall motion delay.
Data are presented as median and interquartile ranges
There was a significant decrease in the magnitude of the mean peak systolic apical rotation (2.5°±1.9° vs. 4.4°±2.9°; p=0.002) and a less pronounced decrease in the basal rotation (-2.9°±2.3° vs. -4.1°±2.7°; p=0.05) in the LBBB patients (Fig. 1). The mean LV twist was found to significantly decrease (5.4°±3° vs. 8.6°±3.3°; p=0.001) as a result of a predominant decrease in the apical rotation (r=0.997; p<0.001) and a slight decrease was observed in the basal rotation (r=0.639; p<0.001) in group 1. The median twist value of the studied population was 6.65°. In order to find the independent factors associated with abnormal twist (inframedian twist), binary logistic regression analysis was performed. Parameters included in logistic regression equation were; age, sex, presence of LBBB, presence of diabetes, presence of HT and LVEF, QRS duration, PR interval, E, E’, E/E’, BMI, SPWMD, LVdys, and preejection interval of LV. Binary logistic regression analysis showed that only presence of LBBB was independently associated with the inframedian twist (OR=6.250; 95% CI: 2.215–17.632; p<0.001) (Table 3).
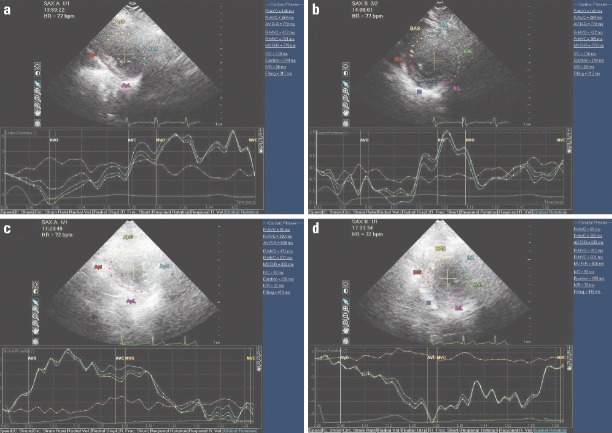
Figure 1.
Examples of LV rotation in two patients with LBBB and control. The top two panels represent apical and basal rotations in patient with LBBB and the bottom panels represent the rotations in the control patients. The LV rotates counter-clockwise at the apical level and rotates clockwise at the basal level. Counter-clockwise rotation as viewed from the apex is expressed as a positive value, and clockwise rotation is expressed as a negative value. Peak LV twist can be computed as peak apical–peak basal rotation. There was a decrease in the magnitude of the peak systolic apical rotation, basal rotation and consequently an LV twist in the LBBB patient
Table 3.
Binary logistic regression analysis for inframedian twist
| P | OR (95% CI) | |
|---|---|---|
| LVEF (%) | 0.721 | 0.984 (0.901 to 1.075) |
| LBBB | 0.001 | 6.250 (2.215 to 17.632) |
| LVdys, ms | 0.667 | 1.005 (0.984 to 1.026) |
| IVMD, ms | 0.416 | 0.988 (0.960 to 1.017) |
| SPWMD, ms | 0.273 | 1.015 (0.988 to 1.043) |
| QRS duration | 0.695 | 1.006 (0.977 to 1.035) |
| E, cm/s | 0.916 | 1.003 (0.948 to 1.062) |
| E’, cm/s | 0.559 | 1.067 (0.859 to 1.325) |
| E/E’, cm/s | 0.140 | 0.910 (0.726 to 1.142) |
Discussion
In this study, the effect of the isolated LBBB on LV rotation and LV twist was evaluated to identify the subclinical LV dysfunction in patients with preserved EF (>50%). LV rotation and twist were decreased with LBBB, especially due to a decrease in the apical rotations. There was no significant relationship between the EF and the LV twist. LV rotation and twist are essential components of cardiac performance. Changes in the ventricular geometry due to cardiac remodeling that may result in a consequent loss of the specific LV architecture and a rearrangement of LV myofibers deteriorates both diastolic and systolic LV functions (5, 6). Moreover, alteration in the LV electrical activation usually causes change in the LV mechanical activity (16). LBBB may lead to uncoordinated contraction of the myofibers and may have the potential to diminish rotational mechanics and induced a significant reduction in LV twist while the LVEF was not apparently influenced. Hence, it might be expected that LBBB would affect LV twist that could be implemented as a clinical index of contractility and may serve as a potential marker of myocardial dysfunction in the isolated LBBB independent from EF. More studies are necessary to determine the most practical and useful parameters for identifying early-stage systolic dysfunction in patients with isolated LBBB.
The relationship between LV twist and LV function
According to the previously published studies, the LBBB plays an important role in inducing the onset of LV dysfunction (1, 3, 6). The presence of LBBB on routine ECG in patients without identifiable cardiac disease leads to the occurrence of significant impairment of LVEF during follow-up (1–3, 14). We cannot presume that our patients will develop clinically apparent HF during the follow-up, but close monitoring with a reliable index such as the LV twist index would be an appropriate approach.
Besides the absence of any report in the literature evaluating the effect of LBBB on LV twist in patients with preserved EF, there are conflicting studies about LV twist in patients with LBBB and reduced EF. Mornoş et al. (17) indicated that LV twist was significantly lower in patients with reduced LVEF and LBBB compared to patients without LBBB. Alternatively, Attana et al. (10) revealed that LV twist was lower in patients with HF; however, the QRS duration or LBBB had no effect on LV twist. In contrast to Attana et al. (10), our findings revealed that the net twist decreased with LBBB and apical rotation was found to be the primary contributing factor for decrease in LV twist, which is consistent with the study by Opdahl et al. (18). Alternatively, similar to results by Attana’s study, QRS duration was not found to be the independent predictor of net LV twist. There was no significant relationship between EF and LV twist in our study, similar to the results from study of Bertini et al. (13). This is perhaps because all the subjects enrolled in this study had no significant structural heart disease that would lead to the occurrence of significant LVEF impairment; however, notably, the conflicting reports in the literature about the LV twist values may result in one of the major limitations of twist studies. For example, Mornoş et al. (17) and Attana et al. (10) reported mean LV twist values in the range of 10°–13°, which are higher than the LV twist values reported in our study; however, Takeuchi et al. (8) and Saygısunar et al. (19) reported mean peak twist values in the range of 6°–8°, which are quite similar to our findings. The discrepancies in the reported LV twist values may be due to the placement and positioning of the imaging plane in the apex: it is known that rotation increases toward the apex. Hence, a more caudal transducer position is associated with increased measured LV apical rotation and twist values (20, 21). In our study, the apical level was defined as the imaging plane with no visible papillary muscles and any visualization of the RV in sight. Therefore, the apical rotation may have been measured more basally, and thus, we obtained lower rotation and twist values.
Ventricular dyssynchrony and its relationship to twist
LV mechanics and particularly LV twist are absolutely dependent on the electrical activation, and the presence of abnormal activation of the ventricles [(e.g., an LBBB) results in intra- and interventricular delays (6, 12, 22)]. This leads to a reduction in the LV apical and basal rotation (consequently, LV twist) and the dyssynchronous contraction of the LV apical and basal regions, and thus, plays an important role in inducing the onset of LV dysfunction (3–5). The effects of cardiac pacemakers and resynchronization therapy on LV torsion in advanced HF patients have been investigated in several studies (12–14). All of these findings demonstrate that LV torsion is not only a parameter of LV function but also reflects the extent of LV dyssynchrony. Consistent with these studies, patients with LBBB had a longer inter- and intraventricular delay, which were positively correlated with the QRS duration. But there was no correlation with LV twist and inter- and intraventricular delay in our study. This may be related to the normal EF in the study population.
The LVdys was longer in patients with LBBB than in the control patients. The longer interval to peak velocity suggested that myocardial segments needed more time to synchronically contract or relax and this might have resulted in a loss of mechanical work. Although the inverse correlation of LV torsion with the degree of LV dyssynchrony has been reported, we found no significant relationship between the apical rotation, basal rotation, LV twist, and the LVdys. This is possibly due to the assessment of the LVdys only via the two-segmental model and without having any clear cut-off or predictive values for LV twist (23).
Ventricular diastolic dysfunction and its relation to twist
In general, collectively with systolic dysfunction, which is reflected by less LV twist, diastolic dysfunction is also present in patients with LBBB. Only a minor degree of diastolic dysfunction was present for both groups in our study, with slightly higher estimated LV filling pressure for the LBBB patients. Although variables representing LV diastolic function, such as E’ and E/E’ ratios, were significantly different between the two groups, we found no significant correlation among these variables and LV twist; however, Park et al. (9) have shown an increase in the torsion in the early stages of diastolic dysfunction in patients with hypertension, hypertrophic cardiomyopathy, and amyloidosis. This difference may result from the inclusion criteria, that is, our patients were free from structural heart disease; however, this is an interesting research area and should be further explored to elucidate the relation between cardiac mechanic indices with different stages of diastolic dysfunction in various patient groups.
Study limitations
Our results should be considered in the context of several limitations. First, the number of patients in this study was relatively small. In addition, although STE can be a useful and additive method for transthoracic echocardiography, several limitations with speckle-tracking exist. The selection of optimal imaging planes for computation is quite challenging due to limited acoustic windows and the oblique orientation of the heart in the chest cavity. Twist is a nonlinear function of the ventricle, and its magnitude crucially depends on the measurement of the apical and basal levels, without any clear reference points reported (12, 20). The more caudal transducer position is associated with increased measured LV apical rotation and twist values (20, 21). Poor speckle-tracking can also lead to false-positive results, which is a major concern. Additionally, our primary aim did not include the evaluation of diastolic dysfunction and LV dyssynchrony. Therefore, the untwisting rate was not assessed. We assessed LV dyssynchrony using conventional methods and only with two-segmental models lacking clear cut-off and predictive values.
Conclusion
We conclude that LBBB might affect LV twist, it can be implemented as a clinical index of systolic function, and may serve as a potential marker of subtle myocardial dysfunction in patients with isolated LBBB.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – S.Y., H.K.; Design – S.Y.; Supervision – S.Y., H.K., M.T.A.; Fundings – S.Y., N.K., R.A., H.G.; Materials – S.Y., M.B.V., S.D.; Data collection &/or processing – S.Y., M.T.A., E.E.; Analysis &/or interpretation – S.Y., H.K., M.T.A.; Literature search – S.Y., H.K., N.K.; Writing – S.Y., H.K., M.T.A.; Critical review – H.K., M.T.A., R.A., H.G.
References
- 1.Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson P, Hansson PO, Eriksson H, Dellborg M. Bundle-branch block in a general male population: the study of men born 1913. Circulation. 1998;98:2494–500. doi: 10.1161/01.cir.98.22.2494. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, McCulloch C, Mangat I, Foster E, De Marco T, Saxon LA. Isolated bundle branch block and left ventricular dysfunction. J Card Fail. 2003;9:87–92. doi: 10.1054/jcaf.2003.19. [DOI] [PubMed] [Google Scholar]
- 4.Breithardt G, Breithardt OA. Left bundle branch block, an Old-New entity. J Cardiovasc Transl Res. 2012;5:107–16. doi: 10.1007/s12265-011-9344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zannad F, Huvelle E, Dickstein K, van Veldhuisen DJ, Stellbrink C, Kober L, et al. Left bundle branch block as a risk factor for progression to heart failure. Eur J Heart Fail. 2007;9:7–14. doi: 10.1016/j.ejheart.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Van Dalen BM, Kauer F, Vletter WB, Soliman OI, van der Zwaan HB, Ten Cate FJ, et al. Influence of cardiac shape on left ventricular twist. J Appl Physiol. 2010;108:146–51. doi: 10.1152/japplphysiol.00419.2009. [DOI] [PubMed] [Google Scholar]
- 7.Enache R, Popescu BA, Piazza R, Muraru D, Călin A, Beladan CC, et al. Left ventricular shape and mass impact torsional dynamics in asymptomatic patients with chronic aortic regurgitation and normal left ventricular ejection fraction. Int J Cardiovasc Imaging. 2015;31:1315–26. doi: 10.1007/s10554-015-0684-0. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi M, Borden WB, Nakai H, Nishikage T, Kokumai M, Nagakura T, et al. Reduced and delayed untwisting of the left ventricle in patients with hypertension and left ventricular hypertrophy: A study using two-dimensional speckle tracking imaging. Eur Heart J. 2007;28:2756–62. doi: 10.1093/eurheartj/ehm440. [DOI] [PubMed] [Google Scholar]
- 9.Park SJ, Miyazaki C, Bruce CJ, Ommen S, Miller FA, Oh JK. Left ventricular torsion by two-dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction. J Am Soc Echocardiogr. 2008;21:1129–37. doi: 10.1016/j.echo.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Attanà P, Paoletti Perini A, Votta CD, Cappelli F, Pieragnoli P, Ricciardi G, et al. QRS duration in left bundle branch block does not affect left ventricular twisting in chronic systolic heart failure. Clin Physiol Funct Imaging. 2015;35:436–42. doi: 10.1111/cpf.12181. [DOI] [PubMed] [Google Scholar]
- 11.Omar AM, Vallabhajosyula S, Sengupta PP. Left ventricular twist and torsion: research observations and clinical applications. Circ Cardiovasc Imaging. 2015;8:e003029. doi: 10.1161/CIRCIMAGING.115.003029. [DOI] [PubMed] [Google Scholar]
- 12.Sorger JM, Wyman BT, Faris OP, Hunter WC, McVeigh ER. Torsion of the left ventricle during pacing with MRI tagging. J Cardiovasc Magn Reson. 2003;5:521–30. doi: 10.1081/jcmr-120025227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertini M, Marsan NA, Delgado V, van Bommel RJ, Nucifora G, Borleffs CJ, et al. Effects of cardiac resynchronization therapy on left ventricular twist. J Am Coll Cardiol. 2009;54:1317–25. doi: 10.1016/j.jacc.2009.05.063. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson P, Wilhelmsen L, Rosengren A. Bundle-branch block in middle-aged men: risk of complications and death over 28 years. The Primary Prevention Study in Goteborg, Sweden. Eur Heart J. 2005;26:2300–6. doi: 10.1093/eurheartj/ehi580. [DOI] [PubMed] [Google Scholar]
- 15.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12:167–205. doi: 10.1093/ejechocard/jer021. [DOI] [PubMed] [Google Scholar]
- 16.Nelson GS, Curry CW, Wyman BT, Kramer A, Declerck J, Talbot M, et al. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation. 2000;101:2703–9. doi: 10.1161/01.cir.101.23.2703. [DOI] [PubMed] [Google Scholar]
- 17.Mornoş C, Petrescu L, Cozma D, Pescariu S, Mornoş A, Ionac A. The ınfluence of left bundle branch-block and cardiac dyssynchrony on 2-D-strain parameters in patients with heart failure complicating ıschemic cardiomyopathy. Rom J Intern Med. 2011;49:179–88. [PubMed] [Google Scholar]
- 18.Opdahl A, Helle-Valle T, Remme EW, Vartdal T, Pettersen E, Lunde K, et al. Apical rotation by speckle tracking echocardiography: a simplified bedside index of left ventricular twist. J Am Soc Echocardiogr. 2008;21:1121–8. doi: 10.1016/j.echo.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Saygısunar U, Kılıç H, Aytürk M, Karagöz A, Gökan Vural M, Aksoy M, et al. Volume depletion provided by blood donation alters twist mechanics of the heart. Preload dependency of left ventricular torsion. Scand Cardiovasc J. 2016;50:23–7. doi: 10.3109/14017431.2015.1112028. [DOI] [PubMed] [Google Scholar]
- 20.Helle-Valle T1, Remme W, Lyseggen E, Pettersen E, Vartdal T, Opdahl A, et al. Clinical assessment of left ventricular rotation and strain: a novel approach for quantification of function in infarcted myocardium and its border zones. Am J Physiol Heart Circ Physiol. 2009;297:257–67. doi: 10.1152/ajpheart.01116.2008. [DOI] [PubMed] [Google Scholar]
- 21.van Dalen BM, Vletter WB, Soliman OI, ten Cate FJ, Geleijnse ML. Importance of transducer position in the assessment of apical rotation by speckle tracking echocardiography. J Am Soc Echocardiogr. 2008;21:895–8. doi: 10.1016/j.echo.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Wyman BT, Hunter WC, Prinzen FW, Faris OP, McVeigh ER. Effects of single- and biventricular pacing on temporal and spatial dynamics of ventricular contraction. Am J Physiol Heart Circ Physiol. 2002;282:372–9. doi: 10.1152/ajpheart.2002.282.1.H372. [DOI] [PubMed] [Google Scholar]
- 23.Sade LE, Demir O, Atar I, Müderrisoglu H, Özin B. Effect of mechanical dyssynchrony and cardiac resynchronization therapy on left ventricular rotational mechanics. Am J Cardiol. 2008;101:1163–9. doi: 10.1016/j.amjcard.2007.11.069. [DOI] [PubMed] [Google Scholar]