Abstract
Objective:
We aimed to evaluate the relationship of micronucleus (MN) frequency and nuclear division index (NDI) with SYNTAX and Gensini scores and thrombolysis in myocardial infarction (TIMI) frame counts of coronary arteries in patients undergoing coronary angiography.
Methods:
In a single-center prospective observational study, a total of 63 individuals, 48 consecutive patients with coronary artery disease (CAD) and 15 healthy people were included. Before coronary angiography (exposure to X-ray), blood samples were collected for lymphocyte cultures, MN and NDI measurements. According to the SYNTAX and Gensini scores, patients were allocated into two groups. Group 1 and 2 included the patients with SYNTAX scores <22 and ≥22 points, respectively. Similarly, groups according to Gensini scores included the ones <23 and ≥23 points. MN test was used for in vitro studies in human peripheral lymphocytes. Binucleated lymphocytes were calculated for each patient.
Results:
MN frequency was significantly higher in group 2 than group 1 and in group 1 than control group (p<0.001). NDI was significantly higher in control group than group 1 and in group 1 than group 2 (p=0.003). MN frequency had positive but moderate correlation with SYNTAX and Gensini scores and TFCs of left anterior descending (LAD), circumflex and right coronary arteries (r=0.394, p=0.003; r=0.458, p<0.001; r=0.425, p<0.001; r=0.469, p<0.001; and r=0.475, p<0.001, respectively).
Conclusion:
We can conclude that as the degree of atherosclerosis increases and coronary flow worsens, MN frequency increases and NDI decreases. Our results may help to elucidate the relationship of DNA damage in pathophysiology of atherosclerosis and endothelial dysfunction in patients with stable CAD.
Keywords: Micronucleus, nuclear division index, SYNTAX, Gensini, TIMI frame
Introduction
As one of the most common reason of mortality and morbidity throughout the world, cardiovascular (CV) disease has great importance. To define pathophysiology and elucidation of intracellular mechanisms is crucial. The presence of DNA damage in patients with CAD by means of micronucleus (MN) frequency was previously reported (1). Inflammation is one of most common mechanisms in pathophysiology of coronary artery disease (CAD) (2). The cells are monotypic in atherosclerotic plaque and this may confirm the role of a mutation or viral hit which leads smooth muscle cells transforming into the progenitor of a proliferative clone similar to a benign tumor (1–3). The MN is a reliable biomarker in genetic and cancer risk evaluation and shows the chromosomal damage (4). The nuclear division index (NDI) is an index of cellular mitotic division which abnormally elevates or decreases according to the proliferative capability of the cell (5). The Gensini score is used to define the severity and extent of coronary artery disease (CAD) (6). The SYNTAX score was produced to estimate the surgical risk of CAD patients and help us to decide either for percutaneous coronary intervention or bypass surgery (7). Thrombolysis in myocardial infarction (TIMI) frame count is used to evaluate the flow in coronary arteries and is found to be related to the endothelial function (8). All of these parameters are markers of severity of CAD. The DNA damage was previously studied in patients with acute coronary syndromes (9, 10). In our study, we included patients without known CAD and use some more specific parameters such as SYNTAX and Gensini scores and TIMI frame counts (TFC) and additionally, by using NDI we have compared the division capability of the lymphocytes. The role of DNA damage as a major cause of inflammation among pathophysiological mechanisms of atherosclerosis is observed to be more commonly emphasized in recent studies. This study is planned to test the hypothesis of possible relationship between commonly used clinical CAD severity scores and the markers of DNA damage. The rationale to conduct this study is that our findings may emphasize the genetic basis of atherosclerosis and its reflection into the clinically and angiographically validated scoring systems.
Herein we aimed to evaluate the relationship of MN frequency and NDI with SYNTAX and Gensini scores and TFCs of the coronary arteries in patients undergoing coronary angiography.
Methods
Our study was a single-center prospective observational study in Erzurum Trainining and Research Hospital between October 2015 and January 2016. The study population consisted of 48 consecutive patients admitted to our cardiology department who were considered for coronary angiography and 15 age, sex and smoking status matched control subjects. At hospitalization, the blood samples were taken to evaluate whole blood count, serum glucose, lipid profile, and renal function (blood urea nitrogen and creatinine) tests by using Abbott Architect C16000 auto analyzer (Abbott Laboratories, Abbott park, IL, USA). The total and differential leukocyte counts were measured by an automated hematology analyzer (Beckman Coulter Ireland Inc. Mervue, Galway, Ireland). Absolute cell counts were used for analysis. The neutrophil to lymphocyte ratio was calculated as the ratio of the neutrophils to lymphocytes. The body mass index (BMI) was calculated by division of weight in kilograms by square of height in meters.
At admission, the detailed physical examination of all patients was performed and the current smoking status, history of CAD, previous myocardial infarction, hypertension (defined as systolic blood pressure >140 mm Hg and diastolic blood pressure >90 mm Hg in more than one measurement or being under antihypertensive drug treatment), diabetes mellitus, and non-cardiac diseases such as active or chronic infection, cancer, chronic obstructive pulmonary disease, chronic autoimmune and systemic inflammatory disease, chronic kidney or liver pathology. Among 410 individuals admitted for coronary angiography in the study period, 362 patients with known CAD, acute coronary syndrome at admission, prior ST elevation myocardial infarction, history of coronary intervention or bypass grafting, known congestive heart failure and/or severe valvular disease, renal failure, autoimmune disease, systemic inflammatory conditions, cancer, hematological disorders, acute or chronic infection of any organ system, alcohol abuse and any drug therapy that may affect the MN frequency were excluded from the study. The control group included the healthy age and sex matched volunteers who had none of these exclusion criteria.
Transthoracic echocardiography was performed at admission to determine left ventricular ejection fraction and the presence of valvular disease (Vivid 7, GE Medical Systems, USA).
The coronary angiography was performed using the Judkins technique. Greater than 50% stenosis in one of the major coronary arteries was assumed to be significant. The synergy between percutaneous coronary intervention with TAXUS and cardiac surgery (SYNTAX) score of the patients were calculated using a web-based computer program (http://www.syntaxscore.com) by two invasive cardiologists in order to determine the severity and complexity of the CAD (7). According to angiographic stenosis degree and scoring system designed by Gensini, 1 point is given for a stenosis of 0%–25%, 2 points for a stenosis of 25%–50%, 4 points for a stenosis of 50%–75%, 8 points for a stenosis of 75–90, 16 points for a stenosis of 90%–99% and 32 points for a narrowing of 100%. Later on the scores obtained according to the degree of angiographic stenosis are multiplied by the coefficients pre-defined for each main coronary artery segment and the sums are added (6).
A SYNTAX score <22 indicates mild and less complex disease, score between 22 and 32 indicates moderate and score >32 represents the more complex and severe CAD. In a similar manner, Gensini scores <23 show mild, scores between 24 to 53 indicate moderate and scores greater than 53 points indicate the more severe CAD. In our study, according to the SYNTAX and Gensini scores, the patients were allocated into two groups. The group 1 and 2 included the patients with SYNTAX scores <22 and ≥22 points, respectively. Similarly, the groups according to the Gensini scores included the ones <23 and ≥23 points.
TFC is an objective index of coronary flow in which the number of cine-frames required for contrast to first reach standardized distal coronary landmarks in the artery is measured. The first frame in TFC is the first frame in which dye fully enters the artery. Three criteria are required: The entire width of the origin of the artery must be fully concentrated by the contrast material; both borders of the origin of the artery must be touched by the dye, and an antegrade motion to the dye must be seen. The last frame is defined as the frame when dye first enters the distal landmark branch. The TFC of LAD artery is multiplied by 1.7 for correction (8).
After coronary angiography, all of the patients were treated either by percutaneous intervention or bypass surgery or managed medically according to their coronary lesion severity and characteristics.
Before selective coronary angiography (before exposure to X-ray), blood samples were collected from each patient for lymphocyte cultures, MN, and NDI measurements.
Micronucleus test is a widely used technique to determine structural and numerical DNA damage of chemical and physical agents (11). The micronuclei are formed as a result of retardation of chromosome material during anaphase or failure of chromosomes to detach from the mitotic spindles and migrate to the poles (12). During telophase, the remnant chromosomes and fragments are covered by nuclear membrane and smaller micronuclei are formed (Fig. 1). MN test is an economic and short-term test which is widely used in genetic toxicology studies (13). The sensitivity of MN test was increased by implementation of cytokinesis-block (14). The MN test has an acceptable sensitivity (80%) and specificity (73%) as reported previously (15). However, in its clinical use, it may have some limitations such as inter-individual variability of MN determination and need for a laboratory capable of performing cell cultures. In our study, the micronuclei were determined and counted by a single biologist.
Figure 1.
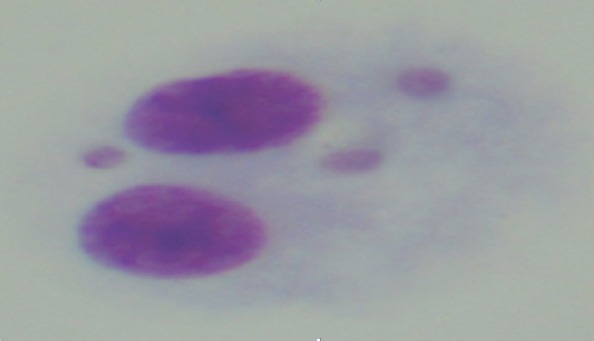
A binucleated cell with three micronuclei
We prepared 6 mL sterile culture tubes including chromosome medium B. A 0.5 mL 1/10 heparinized blood from each donor was added into each tube and they are incubated at 37°C±1°C for 72 hours. At 48th hour of incubation, 3 µg/mL cytochalasin-B was added to the tubes to stop cytokinesis. The distilled water and blood samples from healthy individuals were used as negative control and 2 mM ethyl methane sulfonate (EMS) was used as positive control. After 72 hours of incubation, the tubes were centrifuged at 1.000 rpm for 10 minutes and the supernatant material was removed. After addition of a 0.075 M hypotonic solution, which was prepared previously using 0.5592 g potassium chloride, this procedure was repeated three times by using fixation solution. The fixation solution was prepared using glacial acetic acid and methanol solutions in a 1:3 concentration to fix lymphocytes and hydrolyze other cells. The supernatant after these procedures was removed again and the remaining pellet was mixed. The pellet in the pipet was poured onto the lams which were previously cleaned and treated in the fixation solution at -20°C and these lams were left drying at room temperature. The dried preparations were treated by fresh Giemsa solution for 15 minutes. The Giemsa solution was prepared by addition of 5 mL Giemsa dye into 95 mL Sorenson’s buffer. After this procedure, the preparations were washed with tap water and stored for drying. The dry lams were closed by lamellae and fixed examination preparations were formed. The fixed preparations were examined under light microscopy. A 10 x10 magnification was used at first for general assessment of the field and the counting of micronuclei individually was performed at 40 x 10 magnification. During light microscopic examination, a total of 1.000 binucleated cells were counted for each preparation. The ones with micronuclei among these 1.000 binucleated cells were identified and recorded. The counting of micronuclei was performed according to the pre-defined criteria (16, 17). Additionally, another 1.000 cells for each preparation were recorded randomly and the number of cells with one, two, three or four nuclei was determined. These numbers were used for calculation of NDI (12). The calculation formula is as follows:
NDI=[(1´N1)+(2´N2)+(3´N3)+(4´N4)]/1000 (N1: the number of cells with one nucleus; N2: the number of cells with two nuclei; N3: the number of cells with three nuclei; N4: the number of cells with four nuclei).
All participants were informed about the study, and written consents were taken. The local Ethics Committee of our institution approved the study protocol. The study complied with the Declaration of Helsinki.
Statistical analysis
Continuous variables were presented as mean±standard deviation and median (min–max), while categorical variables were given as percentages. The Kolmogorov–Smirnov test was used to verify the normality of the distribution of continuous variables. Statistical analysis of clinical data between two groups consisted of unpaired t-tests for parametric data and Mann-Whitney U test analysis for nonparametric data, whereas one-way analysis of variance or Kruskal-Wallis tests was used to evaluate comparisons between the 3 groups. Post hoc analysis was carried out by Bonferroni correction test. Correlations were assessed with the Spearman or Pearson’s correlation coefficient. The chi-square test was used for comparison of categorical variables. Analyses were performed with PASW 18 (SPSS/IBM, Chicago, IL, USA) software and two-tailed p-value less than 0.05 was considered statistically significant.
Results
A total of 63 individuals including 48 consecutive patients with CAD who fulfilled the inclusion criteria (mean age, 58.7±7.1 years, 52.9% male) and 15 healthy people (mean age, 57.6±9.5 years; 52% male) were included in the study. The baseline demographic, clinical, and laboratory data of the study (according to the SYNTAX score) and control groups were summarized in Table 1. The patient groups based on the SYNTAX score and the healthy individuals were similar in terms of age, BMI, gender, number of smokers, admission systolic blood pressures, serum glucose levels, LDL cholesterol level, white blood cell, and ejection fraction. The results of SYNTAX score based group 1 and 2 are as follows: The number of diabetics was significantly higher in group 2 than group 1 (p=0.004). The MN frequency was observed to be significantly higher in group 2 than group 1 and in group 1 than the control group (p<0.001). In post hoc analysis the difference between groups in terms of MN frequency had also statistical significance. NDI was significantly lower in group 2 than group 1 and in group 1 than control group (p=0.003). The SYNTAX and Gensini scores and TFCs of LAD, circumflex, and right coronary arteries were significantly higher in group 2 than group 1 (p<0.001, for all) (Table 1). The patient groups according to the Gensini score included 22 and 26 patients, respectively. In comparison of these patient groups with control group, MN frequency was found to be significantly increased in group 2 than group 1 and in group 1 than control group (p<0.001). The NDI was significantly higher in control group than the patient groups. (p=0.002) (Table 2). In post hoc analysis, the difference in NDI between groups was due mainly to the difference of groups 1 and 2 from control group individually and the difference between group 2 and 1 was insignificant (p=0.063).
Table 1.
Demographic, clinical and laboratory characteristics of the patient and the control groups according to the SYNTAX score
| Variables | Group 1 (SYNTAX 0-21) (N:25) | Group 2 (SYNTAX ≥22) (N:23) | Controls (N:15) | P |
|---|---|---|---|---|
| Age, years | 58.2±8.12 | 59.1±5.9 | 57.6±9.5 | 0.06 |
| Gender, male % | 52.7 | 53.0 | 52.0 | 0.062 |
| BMI | 24.2±2.1 | 23.8±2.0 | 24.1±1.9 | 0.1 |
| Number of smokers | 16 | 14 | 7 | 0.02 |
| WBC count, 103/mm3 | 8.1±1.15 | 8.1±1.25 | 6.9±1.3 | 0.690 |
| Hb, gr/L | 14.1±0.76 | 13.33±0.83 | 14.36±0.82 | 0.082 |
| Htc | 40.1±2.35 | 38.9±2.35 | 42.93±2.33 | 0.69 |
| Plt count, 103/mm3 | 192±76.2 | 220±84 | 228±84.26 | 0.53 |
| MPV, fL | 7.05±1.05 | 6.55±1.21 | 7.2±1.3 | 0.09 |
| Creatinine, mg/dL | 0.72±0.14 | 0.69±0.17 | 0.69±0.17 | 0.08 |
| AST, U/L | 24±2.15 | 20.16±3.22 | 19.69±3.2 | 0.568 |
| ALT, U/L | 28.2±4.1 | 30.1±5.02 | 26.89±4.12 | 0.784 |
| TG, mg/dL | 169.12±26.1 | 156.88±22.75 | 117.28±22.17 | 0.058 |
| TC, mg/dL | 175.8±19.2 | 188.67±15.95 | 145.62±14.75 | 0.088 |
| LDL, mg/dL | 149.1±12.4 | 153.33±11.83 | 121.92±12.06 | 0.96 |
| Glucose, mg/dL | 115.3±17.09 | 144.3±15.96 | 98.4±8.77 | 0.223 |
| Diabetics, % | 32 | 56.6 | 0 | 0.004 |
| Hypertensives, % | 48 | 43.5 | 0 | 0.051 |
| SBP, mm Hg | 121.22±10.24 | 122.32±11.84 | 113.62±8.23 | 0.258 |
| HR, beat/min | 73.98±12.55 | 72.98±12.55 | 72.23±12.43 | 0.163 |
| EF, % | 60 (55,65) | 57 (54,65) | 65 (52,68) | 0.210 |
| MN frequency, /1000 | 3.8 (1.7,8.2) | 6.2 (1.7,24.5) | 0.83 (0.7,0.9) | 0.001a |
| NDI | 1.43 (1.3,1.7) | 1.42 (1.1,1.8) | 1.54(1.5,1.6) | 0.003b |
| SYNTAX score | 14.2±3.24 | 33.5±1.45 | N/A | 0.001 |
| Gensini score | 55.66±15.32 | 69.7±17.26 | N/A | 0.001 |
| LAD TFC | 14.9±2.6 | 17.9±5.8 | N/A | 0.001 |
| Cx TFC | 12.5±4.2 | 18.3±4.6 | N/A | 0.001 |
| RCA TFC | 9.4±1.1 | 12.3±0.9 | N/A | 0.001 |
Continuous variables were presented as mean ±standard deviation and median (min-max), while categorical variables were given as percentages. The Kolmogorov-Smirnov test was used to verify the normality of the distribution of continuous variables. Statistical analysis of clinical data between two groups consisted of unpaired t-tests for parametric data and Mann-Whitney U test analysis for nonparametric data, whereas one-way analysis of variance or Kruskal-Wallis tests was used to evaluate comparisons between the 3 groups. Post-hoc analysis was carried out by Bonferroni correction test.
ALT - alanine aminotransferase; AST - aspartate aminotransferase; Cx - circumflex artery; EF - ejection fraction; Hb - hemoglobin; HDL - high density lipoprotein; HR - heart rate; Htc - hematocrit; LAD - left anterior descending artery; LDL - low density lipoprotein; MN - micronucleus; MPV - mean platelet volume; NDI - nuclear division index; Plt - platelet; RCA - right coronary artery; SBP - systolic blood pressure; TC - total cholesterol; TG - triglyceride; TFC - thrombolysis in myocardial infarction frame count; WBC - white blood count
Controls and Group 1, P=0.001; Controls and Group 2, P=0.001; Group 1 and Group 2, P=0.001.
Controls and Group 1, P=0.008; Controls and Group 2, P=0.001; Group 1 and Group 2, P=0.002.
Table 2.
The comparison of MN frequency and NDI between Gensini score based groups
| Variables | Group 1 (Gensini 0-22) (22) | Group 2 (Gensini ≥23) (26) | Controls (15) | P |
|---|---|---|---|---|
| MN frequency | 4.27 (1.7,24.4) | 5.68 (1.7,10) | 0.83 (0.7,0.9) | 0.001a |
| NDI | 1.43 (1.1,1.8) | 1.41 (1.3,1.8) | 1.54 (1.52,1.57) | 0.002b |
The Kolmogorov-Smirnov test was used to verify the normality of the distribution of continuous variables. Statistical analysis of clinical data between three groups, one-way analysis of variance or Kruskal-Wallis tests was used to evaluate comparisons. Post-hoc analysis was carried out by Bonferroni correction test. MN-micronucleus; NDI-nuclear division index
Controls and Group 1, P=0.001; Controls and Group 2, P=0.001; Group 1 and Group 2, P=0.001.
Controls and Group 1, P=0.003; Controls and Group 2, P=0.001; Group 1 and Group 2, P=0.063
The MN frequency of distilled water (negative control) and EMS (positive control) was 0.70±0.38 and 5.63±1.60, respectively (p<0.05).
The results of the correlation of MN frequency and NDI with SYNTAX and Gensini scores, TFCs of LAD, circumflex, and right coronary arteries of the patients were presented in Table 3. The MN frequency was found to have a positive but moderate correlation with SYNTAX and Gensini scores and TFCs of LAD, circumflex, and right coronary arteries (r=0.394, p=0.003; r=0.458, p<0.001; r=0.425, p<0.001; r=0.469, p<0.001 and r=0.475, p<0.001, respectively). The NDI had somewhat weak but significant negative correlation with Gensini score and TFC of circumflex artery (r=-0.187, p<0.001 and r=-0.229, p=0.01, respectively). The scatter plot graphic of SYNTAX score and MN frequency was demonstrated in Figure 2. We could not find any statistically significant correlation between the other parameters and NDI.
Table 3.
The correlation of MN frequency and NDI with SYNTAX and Gensini scores and TFC of LAD, CX and RCA
| MN frequency | NDI | |||
|---|---|---|---|---|
| Variables | r | P | r | P |
| SYNTAX Score | 0.394 | 0.003 | -0.049 | 0.147 |
| Gensini Score | 0.458 | 0.001 | -0.187 | 0.001 |
| LAD TFC | 0.425 | 0.001 | 0.132 | 0.035 |
| Cx TFC | 0.469 | 0.001 | -0.229 | 0.01 |
| RCA TFC | 0.475 | 0.001 | 0.117 | 0.323 |
Correlations were assessed with the Spearman or Pearson’s correlation coefficient. Cx - circumflex artery; LAD - left anterior descending artery; MN - micronucleus; NDI - nuclear division index; RCA - right coronary artery; TFC - thrombolysis in myocardial infarction frame count
Figure 2.
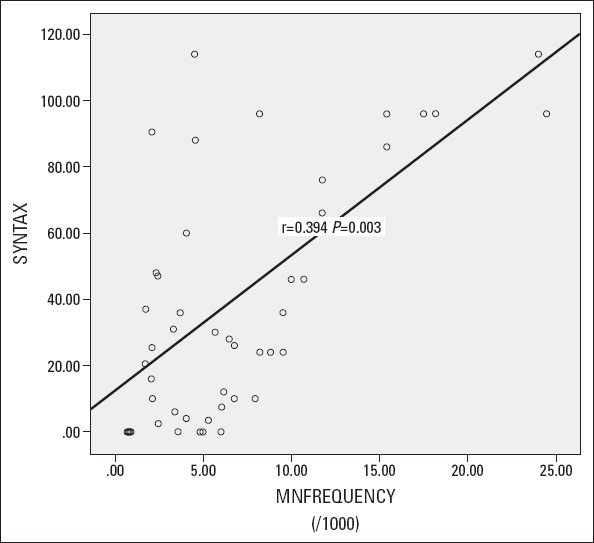
Scatter plot graphic of SYNTAX score and MN frequency
Discussion
Our findings indicate that increased peripheral levels of chromosomal damage can be related to the degree of atherosclerosis in patients without acute coronary syndrome at admission and history of previous CAD.
The cardiovascular disease is a major health problem throughout the world and the number one cause of death today and estimated to be in the near future (18–20). Myocardial infarction, CAD, stroke, peripheral artery disease, and congestive heart failure are the major manifestations of the cardiovascular disease. Atherosclerosis, as the main pathophysiological condition in most of the cases, is the progressive and inflammatory disease of the arterial wall and characterized by focal thickening and luminal obstruction (2). Genetic instability leading oxidative stress is assumed to be an important contributor of atherosclerotic plaque formation and its complications (18, 21–23). The inflammatory response to injury is the most common hypothesis regarding pathophysiology of atherosclerotic plaque formation in which a fibro-proliferative reaction to different insults to the arterial wall is detected (1, 2). During human adult life, DNA damage is common and this may probably be involved in the early phase and/or acute complications of atherosclerotic process (9). The chromosomal deletions or additions, loss of heterozygosity, microsatellite instability, DNA strand breaks, modifications of DNA, or DNA adducts are among the major types of DNA damage which can be seen during atherosclerotic process (18, 24–26). The presence of chromosomal damage in circulating cells of CAD patients using the cytokinesis-block micronucleus (CBMN) cytome assay was demonstrated previously (18). The CBMN cytome assay measures the MN frequency and shows other genomic aberrations in cultured human cells and is an efficient technique to assess cellular and nuclear malfunctions and dysfunctions (27). The increased MN frequency was reported to be correlated with the occurrence (28) and the severity of CAD (1, 18). It was also shown that chromosomal DNA damage is formed in the peripheral lymphocytes of patients undergoing repeated procedural X-rays and as a result of chronically administered drugs, such as nitrates (29–31). In previous reports, increased MN frequency in peripheral lymphocytes was shown to be related to cancer risk and cardiovascular mortality in healthy individuals (9, 32, 33) and adverse events in CAD patients on long-term follow up (9). In the experimental model reported by Helm et al. (34), the MN frequency increased in radiation-treated human umbilical vein endothelial cells. In the recent study by Dönmez–Altuntaş et al. (35) both MN and NDI had significant association with obesity, and genomic damage was reported to be increased in obese individuals. We found increased MN frequency in our patient group as compared to the control subjects. We have also demonstrated that as the severity of CAD increases the MN frequency increases. The point that discriminates our study from the previous ones is the use of clinically validated scoring systems such as SYNTAX and Gensini scores.
The evaluation of NDI in our study is another issue that its relationship with CAD was previously not discussed. NDI is a measure of general cytotoxicity and is a marker of cell proliferation (5). The rationale behind NDI is cells with greater chromosomal damage are less likely to enter cell division or cell death occurs before cell division (5). 1.0 is the lowest NDI value and it occurs in case of failure of division of the viable cells during the cytokinesis-block, as a result, all of them are mono-nucleated. The binucleated cells will be formed if all viable cells complete one division and the NDI will be 2.0. If some viable cells complete more than one nuclear division during the cytokinesis-block phase, they will contain more than two nuclei (5, 12). In a previous study, a negative correlation was observed between age and NDI, and between BMI and NDI, in obese subjects (35). Our findings showed decreased NDI in CAD patients in comparison to the healthy controls in the SYNTAX score based grouping. Although there was no significant difference between Gensini based CAD groups we showed the decrease in NDI in patients compared to the healthy controls.
Another issue to be discussed is the relationship of MN frequency and NDI with TFCs. In our SYNTAX score based patient groups MN frequency got higher as the TFC of each main coronary artery increased. Beyond the severity of the CAD, this relationship with coronary slow flow is another interesting finding of our study. Similarly, NDI was found to be lower as the coronary flow worsens. TFC as an objective quantitative index of coronary flow uses a number of cine-frames required for the injected dye to first reach the distal coronary bed (8). TFC has been extensively studied previously. It was shown that slow coronary flow can be related to increased oxidative stress and endothelial dysfunction (36). This is consistent with our findings indicating increased oxidative DNA damage as the coronary flow worsens.
Study limitations
The major limitation of our study is the relatively small number of study patients. Because of this small number we grouped patients into two groups instead of three according to the both SYNTAX and Gensini scores. As a result, we had two patient groups including group 1 with lowest scores and group 2 including the ones with moderate and highest scores. Therefore, we could not define the difference between the moderate and highest risk patients, which adds some limitation during interpretation of the data.
Conclusion
As a result of this study we can conclude that as the degree of atherosclerosis increases and the coronary flow worsens, the MN frequency increases and the NDI decreases. Our results may help to elucidate the relationship of the DNA damage in pathophysiology of atherosclerosis and endothelial dysfunction in patients with stable CAD.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – E.İ., H.K.; Study design – E.İ.; Supervision – H.U., N.K.; Fundings – All authors. Materials – All authors; Data collection and/or processing – E.İ., H.K.; Analysis and/or interpretation – S.D.; Literature Review – E.Y.; Writer E.İ.; Critical Review – E.E., B.D., S.Ü.
References
- 1.Botto N, Rizza A, Colombo MG, Mazzone AM, Manfredi S, Masetti S, et al. Evidence for DNA damage in patients with coronary artery disease. Mutat Res. 2001;493:23–30. doi: 10.1016/s1383-5718(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis —an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaque. Proc Natl Acad Sci USA. 1973;70:1753–6. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonassi S, El-Zein R, Bolognesi C, Fenech M. Micronuclei frequency in peripheral blood lymphocytes and cancer risk: evidence from human studies. Mutagenesis. 2011;26:93–100. doi: 10.1093/mutage/geq075. [DOI] [PubMed] [Google Scholar]
- 5.Ionescu ME, Ciocirlan M, Becheanu G, Nicolaie T, Ditescu C, Teiusanu AG, et al. Nuclear division index may predict neoplastic colorectal lesions. Maedica (Buchar) 2011;6:173–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Emond M, Mock MB, Davis KB, Fisher LD, Holmes DR, Jr, Chaitman BR, et al. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) registry. Circulation. 1994;90:2645–57. doi: 10.1161/01.cir.90.6.2645. [DOI] [PubMed] [Google Scholar]
- 7.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. Eurointervention. 2005;2:219–2. [PubMed] [Google Scholar]
- 8.Gibson CM, Cannon CP, Daley WL, Dodge JT, Alexander B, Marble SJ, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–88. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 9.Federici C, Botto N, Manfredi S, Rizza A, Del Fiandra M, Andreassi MG. Relation of increased chromosomal damage to future adverse cardiac events in patients with known coronary artery disease. Am J Cardiol. 2008;102:1296–300. doi: 10.1016/j.amjcard.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Demirbağ R, Yılmaz R, Gür M, Koçyiğit A, Çelik H, Güzel S, et al. Lymphocyte DNA damage in patients with acute coronary syndrome and its relationship with severity of acute coronary syndrome. Mutat Res. 2005;578:298–307. doi: 10.1016/j.mrfmmm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Cicchetti R, Bari M, Argentin G. Induction of micronuclei in bone marrow by two pesticides and their differentiation with CREST staining: an in vivo study in mice. Mutat Res. 1999;439:239–48. doi: 10.1016/s1383-5718(98)00185-5. [DOI] [PubMed] [Google Scholar]
- 12.Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2:1084–104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- 13.Elhajouji A, Lukamowicz M, Cammerer Z, Kirsch-Volders M. Potential thresholds for genotoxic effects by micronucleus scoring. Mutagenesis. 2011;26:199–204. doi: 10.1093/mutage/geq089. [DOI] [PubMed] [Google Scholar]
- 14.Cavallo D, Ursini CL, Perniconi B, Francesco A, Giglio M, Rubino FM, et al. Evaluation of genotoxic effects induced by exposure to antineoplastic drugs in lymphocytes and exfoliated buccal cells of oncology nurses and pharmacy employees. Mutat Res. 2005;587:45–51. doi: 10.1016/j.mrgentox.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Le Hégarat L, Mourot A, Huet S, Vasseur L, Camus S, Chesné C, et al. Performance of comet and micronucleus assays in metabolic competent HepaRG cells to predict in vivo genotoxicity. Toxicol Sci. 2014;138:300–9. doi: 10.1093/toxsci/kfu004. [DOI] [PubMed] [Google Scholar]
- 16.Fenech M. Mouse and human micronucleus models for assessing genotoxicity of whole foods in intervention studies. Mutat Res. 1993;290:119–25. doi: 10.1016/0027-5107(93)90039-i. [DOI] [PubMed] [Google Scholar]
- 17.Countryman PI, Heddle JA. The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutat Res. 1976;41:321–32. doi: 10.1016/0027-5107(76)90105-6. [DOI] [PubMed] [Google Scholar]
- 18.Andreassi MG, Barale R, Iozzo P, Picano E. The association of micronucleus frequency with obesity, diabetes and cardiovascular disease. Mutagenesis. 2011;26:77–83. doi: 10.1093/mutage/geq077. [DOI] [PubMed] [Google Scholar]
- 19.American Heart Association. Heart Disease and Stroke Statistics—2004 Update. Dallas, TX, USA: American Heart Association; 2003. [Google Scholar]
- 20.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 21.Andreassi MG. Coronary atherosclerosis and somatic mutations: an overview of the contributive factors for oxidative DNA damage. Mutat Res. 2003;543:67–86. doi: 10.1016/s1383-5742(02)00089-3. [DOI] [PubMed] [Google Scholar]
- 22.Mercer J, Mahmoudi M, Bennett M. DNA damage, p53, apoptosis and vascular disease. Mutat Res. 2007;621:75–86. doi: 10.1016/j.mrfmmm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 23.De Flora S, Izzotti A. Mutagenesis and cardiovascular diseases Molecular mechanisms, risk factors, and protective factors. Mutat Res. 2007;621:5–17. doi: 10.1016/j.mrfmmm.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Casalone R, Granata P, Minelli E, Portentoso P, Giudici A, Righi R, et al. Cytogenetic analysis reveals clonal proliferation of smooth muscle cells in atherosclerotic plaques. Hum Genet. 1991;87:139–43. doi: 10.1007/BF00204169. [DOI] [PubMed] [Google Scholar]
- 25.Tokunaga O, Satoh T, Yu S. Multinucleated variant endothelial cells (MVECs) have a greater capacity for LDL cholesterol uptake than typical mononuclear endothelial cells (TECs) J Atheroscler Thromb. 1991;9:35–41. doi: 10.5551/jat.9.35. [DOI] [PubMed] [Google Scholar]
- 26.Matturri L, Cazzullo A, Turconi P, Lavezzi AM, Vandone PL, Gabrielli L, et al. Chromosomal alterations in atherosclerotic plaques. Atherosclerosis. 2001;154:755–61. doi: 10.1016/s0021-9150(00)00488-3. [DOI] [PubMed] [Google Scholar]
- 27.Fenech M. The advantages and disadvantages of the cytokinesis-block micronucleus method. Mutat Res. 1997;392:11–8. doi: 10.1016/s0165-1218(97)00041-4. [DOI] [PubMed] [Google Scholar]
- 28.Güven M, Güven GS, Öz E, Özaydın A, Batar B, Ulutin T, et al. DNA repair gene XRCC1 and XPD polymorphisms and their association with coronary artery disease risks and micronucleus frequency. Heart Vessels. 2007;22:355–60. doi: 10.1007/s00380-007-0986-9. [DOI] [PubMed] [Google Scholar]
- 29.Andreassi MG, Botto N, Simi S, Casella M, Manfredi S, Lucarelli M, et al. Diabetes and chronic nitrate therapy as co-determinants of somatic DNA damage in patients with coronary artery disease. J Mol Med. 2005;83:279–86. doi: 10.1007/s00109-005-0634-8. [DOI] [PubMed] [Google Scholar]
- 30.Andreassi MG, Botto N, Rizza A, Colombo MG, Palmieri C, Berti S, et al. Deoxyribonucleic acid damage in human lymphocytes after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 2002;40:862–8. doi: 10.1016/s0735-1097(02)02042-9. [DOI] [PubMed] [Google Scholar]
- 31.Andreassi MG, Cioppa A, Manfredi S, Palmieri C, Botto N, Picano E. Acute chromosomal DNA damage in human lymphocytes after radiation exposure in invasive cardiovascular procedures. Eur Heart J. 2007;28:2195–9. doi: 10.1093/eurheartj/ehm225. [DOI] [PubMed] [Google Scholar]
- 32.Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, Holland N, et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2006;28:625–31. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]
- 33.Murgia E, Maggini V, Barale R, Rossi AM. Micronuclei, genetic polymorphisms and cardiovascular disease mortality in a nested case- control study in Italy. Mutat Res. 2007;621:113–8. doi: 10.1016/j.mrfmmm.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Helm A, Lee R, Durante M, Ritter S. The Influence of C-Ions and X-rays on human umbilical vein endothelial cells. Front Oncol. 2016;6:5. doi: 10.3389/fonc.2016.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dönmez-Altuntaş H, Şahin F, Bayram F, Bitgen N, Mert M, Güçlü K, et al. Evaluation of chromosomal damage, cytostasis, cytotoxicity, oxidative DNA damage and their association with body-mass index in obese subjects. Mutat Res Genet Toxicol Environ Mutagen. 2014;771:30–6. doi: 10.1016/j.mrgentox.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Tanrıverdi H, Evrengül H, Enli Y, Kuru O, Seleci D, Tanrıverdi S, et al. Effect of homocysteine-induced oxidative stress on endothelial function in coronary slow-flow. Cardiology. 2007;107:313–20. doi: 10.1159/000099068. [DOI] [PubMed] [Google Scholar]


